Abstract
Object
The neurosurgical literature has conflicting findings regarding the association between indications for CSF shunt placement and subsequent shunt surgery. The object of this study was to identify baseline factors at the time of initial CSF shunt placement that are independently associated with subsequent surgery.
Methods
This was a retrospective cohort study of children ages 0–18 years who underwent initial CSF shunt placement between January 1, 1997, and October 12, 2006, at a tertiary care children’s hospital. The outcome of interest was CSF shunt surgery (either for revision or infection) within 12 months after initial placement. Associations between subsequent CSF shunt surgery and indication for the initial shunt, adjusting for patient age and surgeon factors at the time of initial placement, were estimated using multivariate logistic regression. Medical and surgical decisions, which varied according to surgeon, were examined separately in a univariate analysis.
Results
Of the 554 children in the study cohort, 233 (42%) underwent subsequent CSF shunt surgery, either for revision (167 patients [30%]) or infection (66 patients [12%]). In multivariate logistic regression modeling, significant risk factors for subsequent CSF shunt surgery included (compared with aqueductal stenosis) intraventricular hemorrhage (IVH) secondary to prematurity (adjusted odds ratio [AOR] 2.2, 95% CI 1.1–4.5) and other unusual indications (AOR 3.7, 95% CI 1.0–13.6). The patient’s age at initial CSF shunt placement was not significantly associated with increased odds of subsequent surgery after adjusting for other associated factors.
Conclusions
The occurrence of IVH is associated with increased odds of subsequent CSF shunt surgery within 12 months after shunt placement. Families of and care providers for children with IVH should be attuned to their increased risk of shunt failure.
Keywords: hydrocephalus, cerebrospinal fluid, shunt failure, prematurity, intraventricular hemorrhage, children
Although placement of CSF shunts successfully treats hydrocephalus in children, shunt failure is common and frequently necessitates subsequent surgery.6,7,17 It is critical for families and care providers to understand which children undergoing CSF shunt placement are at highest risk for subsequent surgery. In addition, an understanding of the baseline risk factors for subsequent surgery permits investigators to account for differences in patient populations, which is necessary when comparing outcomes between neurosurgical centers in research and quality improvement efforts.
Prior studies of the association of indication for CSF shunt placement with subsequent shunt surgery14 have yielded conflicting conclusions.4,11,14,20,32,33,35,37,38,40,43–45,48 Some studies were conducted in cohorts that were selected based on procedures, rather than on patient characteristics, limiting the conclusions that can be drawn about patient-level characteristics,37,38,40,44 whereas other studies do not report the association of indication with subsequent failure. 10 In studies that have examined indications for CSF shunts in patient cohorts, some have noted more frequent shunt failure in children with obstructive hydrocephalus4,43 and IVH,11 and less frequent failure in children with congenital hydrocephalus,32,48 cerebral cyst,11 and communicating hydrocephalus.11 However, definitions of CSF shunt indication have been hampered by the vague hydrocephalus diagnosis codes available in administrative data.4,43,45 In addition, because different indications present at different ages in children, the indication for CSF shunt is confounded by age.14 Those studies considering both patient age and indication for CSF shunt have found both factors,4,11,43,48 age only,33,35 and neither factor20 to be associated with subsequent surgery. Finally, some prior studies that have examined the association of CSF shunt indication have focused on infection only as an outcome.5,8,12,13,18,29,30,34,37,38,40,41,49
Numerous additional patient and surgeon factors,2,26 as well as medical and surgical decisions at the time of initial CSF shunt placement,3,9,15,19,24,25,27,28,31,36,39,42 have been considered in studies of shunt failure. These factors must be carefully considered as potential confounders in the relationship between indication for CSF shunt and risk of subsequent surgery.
The availability of a large cohort of children who underwent initial CSF shunt placement at a large tertiary care children’s hospital presented a unique opportunity to investigate retrospectively, in a more detailed fashion than in previous work, the contribution of the indication for CSF shunt to the risk of subsequent surgery. Our objective was to identify clinically important baseline characteristics at the time of initial CSF shunt placement associated with shunt surgery over a 12-month follow-up period.
Methods
Study Design and Setting
We conducted a retrospective cohort study at PCMC, a 252-bed tertiary care children’s hospital serving Utah, Idaho, Wyoming, Nevada, and Montana. The hospital is owned and operated by Intermountain Healthcare, a regional, not-for-profit integrated health care system. At PCMC there are more than 11,000 admissions per year, and more than 95% of the pediatric CSF shunt placements in the Intermountain Healthcare system are performed here. The study was reviewed and approved by the institutional review boards at the University of Utah and Seattle Children’s Research Institute as research not requiring informed consent.
Study Population
Children younger than 18 years of age who underwent initial CSF shunt placement at PCMC, with a discharge date between January 1, 1997, and October 12, 2006, were eligible for inclusion. Children with a prior Ommaya reservoir, ventricular reservoir, and/or endoscopic third ventriculostomy were included in the cohort. Candidates were identified by a primary ICD-9-CM procedure code for placement of an extracranial ventricular shunt (02.3x). Medical record review by a trained abstractor (M.L.) confirmed that 579 children met inclusion criteria.
Primary Outcome Variable
The primary outcome was surgery for either shunt malfunction or infection within 12 months of initial CSF shunt placement. A shunt revision was defined as an operative neurosurgical intervention to the CSF shunt. A shunt infection was defined as the presence of bacteria in a culture of CSF, wound swab, and/or pseudocyst fluid; or shunt erosion (visible hardware); or abdominal pseudocyst (even without positive culture). Data from each neurosurgical admission for each child up until the time of first shunt infection were collected using Intermountain Healthcare’s database and chart review.
Baseline Characteristics at the Time of Initial CSF Shunt Placement
The main variable of interest was indication for initial shunt placement. This was usually determined by manual chart review, but when the indication was indeterminate after medical record review by a trained abstractor, a pediatric neurosurgeon (J.R.C.) reviewed the child’s initial imaging studies. Categories of indication for shunt placement included the following: myelomeningocele; IVH secondary to prematurity; aqueductal stenosis; posterior fossa, supratentorial, and midbrain tumors; posterior fossa and other intracranial cysts; head injury; congenital (including communicating congenital hydrocephalus, encephalocele, craniosynostosis, and other); spontaneous intracranial, intraventricular, and/or subarachnoid hemorrhage; infection; and other unusual causes of hydrocephalus.
Several potential confounding variables at the time of initial CSF shunt placement were evaluated based on earlier studies and included patient factors, surgeon factors, and details of medical and surgical management of initial CSF shunt placement (Table 1). Age at the time of initial CSF shunt placement was categorized (0 to < 6 months, 6 to < 12 months, 1 to < 2 years, 2 to < 9 years, and 9–18 years) and additionally, in sensitivity analyses, treated as a continuous variable (in weeks). Additional patient factors evaluated included the following: antibiotic use during hospital stay prior to initial CSF shunt placement; prior surgery; and prior neurosurgery. Comorbidities were defined using complex chronic conditions and the count for these conditions, excluding hydrocephalus. 46 The surgeon was defined as individual A, B, C, or all others (D), and surgeon volume was defined by initial CSF shunt placements per year during the study.
TABLE 1.
Literature review of patient and surgeon factors and medical and surgical decisions considered as risk factors for subsequent surgery
Details of medical and surgical management were also evaluated. In addition to use of prophylactic IV antibiotics, we examined numbers of doses and timing as documented in an operating room database called ORMIS, which is maintained by the circulating nursing staff. Surgical decisions included shunt valve brands (PS Medical, Strata, Delta, and Codman-Medos [all Medtronic]; Orbis-Sigma [Integra]; and Paedi-gav [Aesculap]) from the packaging placed in the medical chart and/or operative note dictated by the surgical staff; distal shunt location (peritoneal and nonperitoneal—including atrial and other) from the operative note; use of ultrasound from ORMIS and/or operative note; person preparing shunt site (surgeon or nonsurgeon—the latter including nurse, resident, anesthesiologist, or unknown) from ORMIS; and case priority (elective and nonelective—the latter including add-on, emergency, and urgent) from ORMIS.
Statistical Analyses
In Table 2, cohort characteristics at baseline were descriptively summarized overall and by subsequent surgery outcome in the 12 months following initial CSF shunt placement. Unadjusted odds ratios and 95% confidence intervals from univariate logistic regression models were used to summarize the bivariate associations between each variable of interest, including potential confounders, and subsequent CSF shunt surgery (Fig. 1). To describe the association between baseline characteristics and CSF shunt procedure within 12 months of placement, a multivariate logistic regression model was developed, with a priori inclusion of indication for shunt placement and patient age, sex, and race/ethnicity (Table 3). Other baseline characteristics were evaluated for inclusion in the model by using stepwise regression methodology.1,22 Sensitivity analyses of the final model were performed as detailed in the Results section to evaluate the robustness of significant associations. The study cohort provided 80% power to detect an OR of 2.50 or larger in multivariate logistic regression, assuming a rate of subsequent CSF shunt surgery of 40%, a binary independent variable with 10% or more observations in the less frequently occurring category, and the remaining variables accounting for 20% or less of the variance in the rate of subsequent surgery (PASS 2008, version 08.0.8).23 Because surgeon was collinear with several surgeon-related factors, sensitivity analyses were performed replacing surgeon with these related explanatory factors. The associations of medical and surgical decisions for all surgeons are descriptively reported (Table 4). All analyses were performed using SAS (version 9.1.3, SAS Institute, Inc.).
TABLE 2.
Baseline characteristics at time of initial CSF shunt placement for the entire cohort of 554 children and for those with and without subsequent CSF shunt surgery*
| Characteristic | Subsequent CSF Shunt Op
|
Entire Cohort | |
|---|---|---|---|
| Any | None | ||
| no. of patients | 233 | 321 | 554 |
| patient factors | |||
| median chronological age in wks (IQR) | 12 (2–69) | 16 (2–65) | 13 (2–69) |
| chronological age at initial shunt | |||
| 0 to 6 mos | 153 (66) | 191 (60) | 344 (62) |
| 6 to <12 mos | 17 (7) | 42 (13) | 59 (11) |
| 1 to <2 yrs | 12 (5) | 15 (5) | 27 (5) |
| 2 to <9 yrs | 29 (13) | 36 (11) | 65 (12) |
| 9 to 18 yrs | 22 (9) | 37 (11) | 59 (11) |
| mean gestational age in wks† | 35 ± 5 | 36 ± 5 | 35 ± 5 |
| median postconceptional age in wks (IQR)† | 1 (14 to −2) | 2 (19 to −2) | 1 (17 to −2) |
| sex | |||
| M | 140 (60) | 191 (60) | 331 (60) |
| F | 93 (40) | 130 (40) | 223 (40) |
| race/ethnicity | |||
| non-Latino white | 198 (85) | 263 (82) | 461 (83) |
| Latino | 19 (8) | 34 (11) | 53 (10) |
| other/unknown | 16 (7) | 24 (7) | 40 (7) |
| insurance | |||
| private | 152 (65) | 201 (63) | 353 (64) |
| Medicaid | 79 (34) | 116 (36) | 195 (35) |
| self-pay | 2 (1) | 4 (1) | 6 (1) |
| mean birth weight in kg‡ | 2.6 ± 1.1 | 2.6 ± 1.0 | 2.6 ± 1.0 |
| median weight at op, in kg (IQR)§ | 4.1 (3.3–10.0) | 5.5 (3.2–10.4) | 4.7 (3.3–10.2) |
| median LOS preceding shunt op, in days (IQR) | 2 (0–15) | 2 (0–14) | 2 (0–14) |
| prior inpatient antibiotics | 99 (42) | 123 (38) | 222 (40) |
| indication for shunt placement | |||
| myelomeningocele | 39 (17) | 79 (25) | 118 (21) |
| IVH due to prematurity | 48 (21) | 37 (11) | 85 (15) |
| aqueductal stenosis | 25 (11) | 42 (13) | 67 (12) |
| tumor | 33 (14) | 36 (11) | 69 (12) |
| cyst | 27 (11) | 34 (11) | 61 (11) |
| head injury | 16 (7) | 28 (9) | 44 (8) |
| congenital | 19 (8) | 24 (7) | 43 (8) |
| spontaneous hemorrhage | 5 (2) | 22 (7) | 27 (5) |
| infection | 12 (5) | 13 (4) | 25 (5) |
| other unusual cause¶ | 9 (4) | 6 (2) | 15 (3) |
| complex chronic conditions | |||
| none (except hydrocephalus) | 174 (75) | 236 (74) | 410 (74) |
| one | 32 (14) | 53 (16) | 85 (15) |
| two or more | 27 (11) | 32 (10) | 59 (11) |
| neuromuscular (except hydrocephalus) | 35 (15) | 57 (18) | 92 (17) |
| cardiac | 20 (9) | 23 (7) | 43 (8) |
| respiratory | 9 (4) | 16 (5) | 25 (5) |
| renal | 1 (0.4) | 3 (1) | 4 (1) |
| gastrointestinal | 1 (0.4) | 4 (1) | 5 (1) |
| hematological | 9 (4) | 7 (2) | 16 (3) |
| metabolic | 0 (0) | 0 (0) | 0 (0) |
| congenital/genetic | 10 (4) | 21 (7) | 31 (6) |
| malignancy | 13 (6) | 13 (4) | 26 (5) |
| prior op | 66 (28) | 73 (23) | 139 (25) |
| prior neurosurgery | 128 (55) | 194 (60) | 322 (58) |
| surgeon factors | |||
| surgeon | |||
| A | 69 (30) | 127 (40) | 196 (35) |
| B | 58 (25) | 87 (27) | 145 (26) |
| C | 57 (24) | 61 (19) | 118 (21) |
| others; Group D | 49 (21) | 46 (14) | 95 (17) |
| mean surgeon vol (initial shunts/yr) | 19 ± 8 | 20 ± 7 | 20 ± 8 |
The means are expressed ± SD throughout. Unless otherwise indicated, the values represent the number of patients, with percentages in parentheses.
Missing data for 135 children.
Missing data for 218 children.
Missing data for 1 child.
“Other” category includes 3 children with pseudomeningocele, 3 with CSF leak, 2 with pseudotumor cerebri, 2 with communicating postoperative aseptic meningitis, 1 treated to relieve pressure of baclofen pump, 1 with achondroplasia, 1 with gross macrocrania, 1 with ventriculomegaly, and 1 with acquired communicating hydrocephalus from extensive cardiac disease.
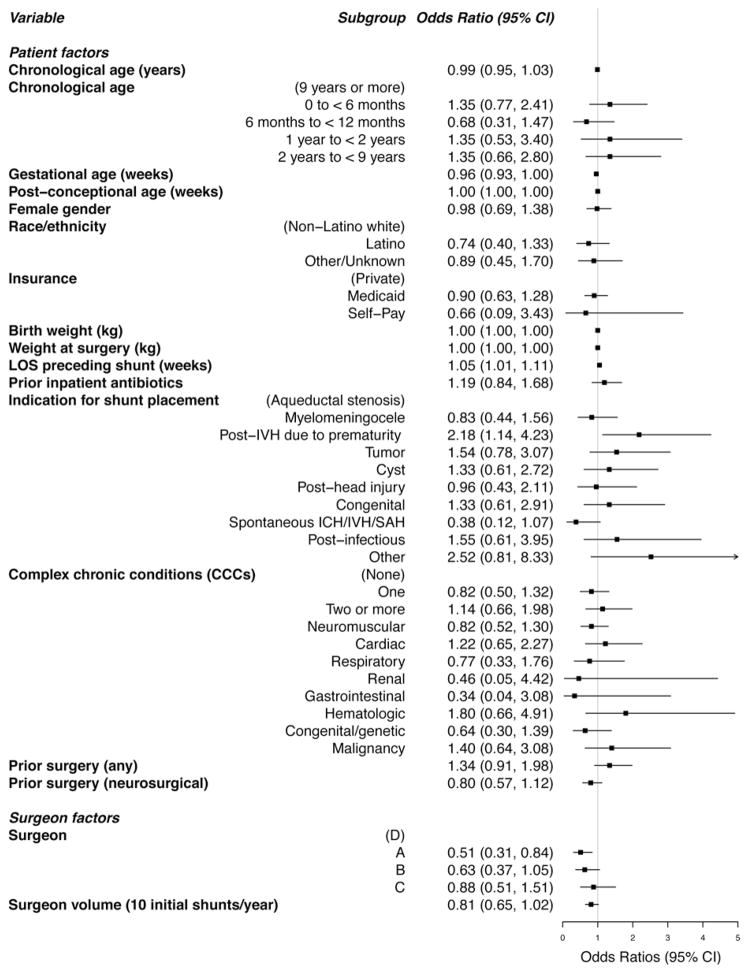
Fig. 1.
Unadjusted odds ratios for the odds of a CSF shunt procedure within 12 months of initial shunt placement, based on univariate logistic regression models.
TABLE 3.
Results of final multivariate logistic regression model showing AORs of CSF shunt procedure within 12 months of initial shunt placement
| Variable | AOR (95% CI) |
|---|---|
| chronological age | |
| 9 to 18 yrs* | |
| 0 to <6 mos | 1.9 (0.9–3.9) |
| 6 to <12 mos | 0.8 (0.4–2.0) |
| 1 to <2 yrs | 1.3 (0.5–3.4) |
| 2 to <9 yrs | 1.3 (0.6–2.7) |
| male sex | 1.0 (0.7–1.5) |
| race/ethnicity | |
| non-Latino white* | |
| Latino | 0.7 (0.4–1.3) |
| other/unknown | 0.8 (0.4–1.6) |
| indication for shunt placement | |
| aqueductal stenosis* | |
| myelomeningocele | 0.8 (0.4–1.5) |
| IVH due to prematurity | 2.2 (1.1–4.5)† |
| tumors | 2.0 (0.9–4.5) |
| cyst | 1.4 (0.7–2.9) |
| head injury | 1.3 (0.6–3.2) |
| congenital | 1.7 (0.7–3.9) |
| spontaneous hemorrhage | 0.4 (0.1–1.2) |
| infection | 1.8 (0.7–4.8) |
| other unusual | 3.7 (1.0–13.6)† |
| surgeon | |
| others; Group D* | |
| A | 0.6 (0.3–1.0) |
| B | 0.7 (0.5–1.1) |
| C | 1.0 (0.6–1.8) |
| nonelective case priority | 1.4 (0.9–2.1) |
| 10-min case duration | 1.0 (1.0–1.1) |
Reference value.
Statistically significant odds (p < 0.05).
TABLE 4.
Medical and surgical decisions at the time of initial CSF shunt placement for the entire cohort of 554 children and those with and without a subsequent CSF shunt procedure within 12 months*
| Variable | Subsequent Procedure
|
Entire Cohort | |
|---|---|---|---|
| Any | None | ||
| no. of patients | 233 | 321 | 554 |
| medical decisions | |||
| prophylactic IV antibiotic use† | 209 (90) | 289 (90) | 498 (90) |
| single dose | 205 (88) | 284 (89) | 489 (88) |
| multiple doses | 4 (2) | 5 (2) | 9 (2) |
| median IV antibiotic timing in min (IQR)‡ | −13 (−18 to −7) | −14 (−19 to −8) | −14 (−19 to −8) |
| prophylactic intrathecal antibiotic use | 4 (2) | 1 (0.3) | 5 (1) |
| surgical decisions | |||
| shunt brand§ | |||
| PS Medical/Medtronic | 152 (66) | 229 (74) | 381 (70) |
| Strata/Medtronic | 22 (10) | 27 (9) | 49 (9) |
| Delta/Medtronic¶ | 26 (11) | 19 (6) | 45 (8) |
| Codman-Medos/Medtronic | 17 (7) | 26 (8) | 43 (8) |
| Orbis-Sigma/Integra | 12 (5) | 10 (3) | 22 (4) |
| Paedi-gav/Aesculap | 1 (0.4) | 0 (0) | 1 (0.1) |
| antibiotic-impregnated tubing | 11 (5) | 27 (8) | 38 (7) |
| distal shunt location | |||
| peritoneal¶ | 220 (94) | 320 (100) | 540 (97) |
| atrial | 11 (5) | 0 (0) | 11 (2) |
| other | 2 (1) | 1 (0.3) | 3 (1) |
| use of neuroendoscope | 36 (15) | 50 (16) | 86 (16) |
| use of ultrasound | 8 (3) | 12 (4) | 20 (4) |
| person preparing site | |||
| nurse | 131 (56) | 150 (47) | 281 (51) |
| surgeon¶ | 74 (32) | 133 (41) | 207 (37) |
| other: resident, anesthesiologist, unknown | 28 (12) | 38 (12) | 66 (12) |
| case priority‡ | |||
| elective | 153 (68) | 227 (73) | 380 (71) |
| nonelective: add-on/emergency/urgent | 72 (32) | 85 (27) | 157 (29) |
| median case duration in min (IQR)† | 69 (57–87) | 64 (56–80) | 65 (56–83) |
| median no. of scrubbed personnel (IQR)** | 4 (3–4) | 4 (3–4) | 4 (3–4) |
Unless otherwise indicated, the values represent the number of patients, with percentages in parentheses.
Missing data for 1 child.
Missing data for 17 children.
Missing data for 13 children.
p < 0.05.
Not available for 2 children.
Results
Of the 579 children who underwent initial CSF shunt placement at PCMC during the study period, 554 (96%) had follow-up within Intermountain Healthcare in the subsequent 12 months. Of those 554 children, 233 (42%) underwent surgery within 12 months of initial shunt placement for either shunt malfunction (167 patients [30%]) or infection (66 [12%]). Table 2 describes the baseline characteristics of the cohort at the time of initial CSF shunt placement. The majority of children were younger than 6 months of age (62%), male (60%), and non-Latino white (83%). Indication for CSF shunt placement included the following: myelomeningocele (21%), IVH (15%), aqueductal stenosis (12%), tumor (12%), or cyst (11%). Prophylactic IV antibiotics were used in 90% of initial CSF shunt placements, with a median timing of administration 14 minutes prior to the recorded incision time (IQR −19 to −8 minutes).
Figure 1 displays UORs and corresponding 95% CIs from univariate logistic regression models used to describe the unadjusted associations between each baseline characteristic and subsequent CSF shunt surgery; the data for this figure are presented in Table 2. Baseline factors significantly associated with subsequent surgery included the following: inpatient LOS preceding shunt placement (UOR 1.05 for each additional week stayed, 95% CI 1.01–1.11); IVH secondary to prematurity (UOR 2.2, 95% CI 1.1–4.2 compared with aqueductal stenosis); and surgeon A (UOR 0.5, 95% CI 0.3–0.8 compared with a surgeon from Group D). Among the 85 children who underwent initial CSF shunt placement for IVH secondary to prematurity, 48 (56%) underwent shunt revision in the subsequent 12 months.
As shown in Table 3, in multivariate logistic regression, significant factors that remained independently associated with the odds of subsequent CSF shunt surgery included IVH secondary to prematurity (AOR 2.2, 95% CI 1.1–4.5) and other unusual indication for CSF shunt (AOR 3.7, 95% CI 1.0–13.6), both compared with aqueductal stenosis. Although not statistically significant in multivariate logistic regression, there was a trend toward decreased odds of subsequent surgery associated with surgeon A and increased odds of subsequent surgery associated with age 0–6 months, tumor, nonelective case priority, and case duration. The association between subsequent surgery and IVH secondary to prematurity remained in sensitivity analyses that included the following: 1) age as a continuous variable; and 2) exclusion of the 14 children who had a nonperitoneal distal shunt location. The exclusion was performed because these children had significantly higher odds of a subsequent surgery, and it was appropriate to test the robustness and generalizability of the model by excluding them (data not shown).
Medical and surgical decisions for all patients for the entire cohort, as well as those with and without subsequent surgery, are shown in Table 4. Conclusions about medical and surgical decisions are very limited in this small data set because such decisions vary between surgeons, as well as by surgeon volume and experience (data not shown), and thus are highly confounded by surgeon. For example, surgeon A’s practice differed from all other surgeons combined in terms of the following variables: 1) shunt brand; 2) less use of prophylactic IV antibiotics, antibiotic-impregnated shunt tubing, and ultrasound; and 3) more use of neuroendoscope and surgeon site preparation. Delta valve use, nonperitoneal distal shunt location, and a nonsurgeon preparing the surgical site were all associated with subsequent shunt surgery. Nonetheless, the association between subsequent surgery and IVH secondary to prematurity remained in sensitivity analyses, the first of which replaced surgeon with surgeon volume, shunt brand, and distal shunt location, and the second of which replaced surgeon with person preparing site and distal shunt location.
For the 85 children with IVH, the median gestational age was 27 weeks (range 22–34 weeks), and the median birth weight was 1024 g (range 430–2905 g). In these 85 children, IVH was Grade III in 32 (38%) and Grade IV in 48 (56%). Fifty-one (60%) had a previous Ommaya reservoir, 20 (24%) had a previous external ventricular drain, and 4 (5%) had a previous subgaleal shunt.
Discussion
In a large cohort of children who underwent initial CSF shunt placement and were followed for 12 months, 42% underwent surgery within 12 months of initial shunt placement for either malfunction (30%) or infection (12%). We used a deliberate assignment of the indication for CSF shunt placement in robust models that separated patient factors from surgeon factors and medical and surgical decisions. In multivariate logistic regression, IVH secondary to prematurity was independently and consistently associated with odds of subsequent surgery (AOR 2.2, 95% CI 1.1–4.5). This association persisted when the confounding variable of age was handled as both a categorical and continuous variable.
The 12-month rate of shunt failure in this large cohort of children who underwent initial CSF shunt placement was 42%. This overall failure rate is comparable to that found in previous studies, which have consistently demonstrated failure rates ranging from 27% to 40% within 1 year of CSF shunt placement.15,25,27,35,36,43 Our failure rate may be slightly higher than in previous cohorts, in part because we removed children who did not have 12 months of follow-up within our health care system from the cohort. Also, our finding of subsequent surgeries to treat shunt malfunction in 30% and infection in 12% of children is comparable to earlier work involving patient cohorts (malfunction in 35%, infection in 8%).10,15,45,47
Occurrence of IVH demonstrated an independent association with shunt failure in 2 earlier studies in which a patient cohort was used,11,48 as well as studies that found gestational age less than 40 weeks to be associated with shunt failure.33,48 Prematurity,8,30,34 younger postconceptional age,8,30 low birth weight,8,12 prolonged LOS prior to initial CSF shunt placement,30 and IVH12,45 have all demonstrated associations with shunt infection.
The association between IVH and shunt failure has biological plausibility. Clinical factors that are taken into consideration by neurosurgeons when managing IVH in premature infants include the patient’s small abdominal size, thin skin that is susceptible to breakdown, relatively immunocompromised state, and variable inflammatory response to blood in CSF. Although not all of these clinical factors are modifiable, the optimal management, including timing of shunt placement, in infants with IVH secondary to prematurity is not based on optimal evidence. Optimal timing of shunt placement in infants with IVH is a question for which further study is needed, especially given the increasing numbers of premature infants. 50
The relationship between CSF shunt indication and patient age, and their association with subsequent shunt surgery, has remained unclear based on conflicting findings in prior literature.4,11,14,20,32,33,35,37,38,40,43–45,48 We were reassured to find, as in other studies, that in our cohort young age trended toward an association with subsequent surgery. However, we were intrigued to see that after careful consideration of CSF shunt indication, young age did not remain independently associated with shunt failure.
Unlike earlier work, we did not find shunt failure to be associated with obstructive hydrocephalus,4,43 cerebral cyst,11 and communicating hydrocephalus.11 Although all 3 of these earlier studies were larger in size than our cohort, all relied on diagnosis codes to determine CSF shunt indication, whereas we performed detailed chart review to assign indication.
We are also reassured that this study replicates some earlier findings about risk factors for subsequent shunt surgery. Complication rates are reported to differ by surgeon.2,18,20 As in earlier studies, a nonperitoneal distal shunt location was associated with higher complication rates.20,48 Although we did not find an association of surgeon experience with shunt failure, this association has been inconsistent in the literature.10,11 In addition, the small number of surgeons and limited range of surgeon volumes in this cohort limit our ability to draw conclusions about surgeon factors associated with shunt failure.
This work has several limitations inherent to retrospective studies that rely on medical record review. Missing data on gestational age, postconceptional age, and birth weight limit our ability to draw conclusions about these patient factors. Any factors that were not documented in the medical record were presumed not to have occurred; in a few cases, such as antibiotic-impregnated shunt tubing, this assumption may be erroneous. We were not able to consider several additional variables, including type of skin cleanser;28,44 site preparation, including hair clipping21,28 and shampoo;16,28,51 antibiotic irrigation of hardware;28 double gloving;30 and patient positioning. In addition, the “other unusual indication” category includes predominantly children with highly heterogeneous indications for CSF shunt placement. Finally, some limitations were presented by conduct at a single center; our conclusions about medical and surgical decisions are highly confounded by the association with surgeon in this cohort.
Nonetheless, to examine patient risk factors for shunt failure within 12 months, we were able to perform a comprehensive, detailed, retrospective study in a large cohort of children undergoing initial CSF shunt placement. The occurrence of IVH associated with prematurity was significantly associated with increased odds of subsequent surgery. Eventually, development of a predictive model of CSF shunt failure would be of great utility to patients, families, and caregivers. In the interim, we hope to test this finding in a multicenter cohort to ensure that the association persists with larger numbers of surgeons and centers. This is a potentially high-risk subgroup toward which future interventional trials to prevent shunt revision should be directed. In the meantime, work comparing CSF shunt failure between centers, such as benchmarking and prospective multicenter studies, should account for differences in indication for shunt placement.
Conclusions
A comprehensive, detailed, retrospective study was performed to examine patient risk factors for shunt failure within 12 months in a large cohort of children undergoing initial CSF shunt placement. The occurrence of IVH associated with prematurity was significantly associated with increased odds of subsequent surgery. Families of and care providers for children with IVH should be aware of the children’s increased odds of CSF shunt failure. Work comparing shunt failure between centers, such as benchmarking and prospective multicenter studies, should account for differences in indication for a CSF shunt. This is a potentially high-risk subgroup toward which future interventional trials to prevent shunt revision should be directed.
Acknowledgments
The authors thank the following parties for assistance in the execution of this project: PCMC Division of Inpatient Medicine, particularly Christopher Maloney, Flory Nkoy, Gena Fletcher Lattin, and Ali Dowling (construction of the data collection tools); Neal Swensen and Jared Olson from PCMC Pharmacy (antibiotic data); Barb Nelson (medical chart retrieval); Jeff Yearley and Rene Enriquez (data management); the Pediatric Clinical and Translational Research program at the University of Utah, including Heather Keenan and Carrie Byington (project conceptualization); Laina Mercer (figure construction); as well as Stephan Nemeth for support and valuable feedback.
Abbreviations used in this paper
- AOR
adjusted odds ratio
- IQR
interquartile range
- IV
intravenous
- IVH
intraventricular hemorrhage
- LOS
length of stay
- NCRR
National Center for Research Resources
- NIH
National Institutes of Health
- NINDS
National Institute of Neurological Disorders and Stroke
- ORMIS
Operating Room Management Information System
- PCMC
Primary Children’s Medical Center
- UOR
unadjusted odds ratio
Footnotes
Portions of this work were presented in abstract and poster form at the Pediatric Academic Societies meeting in Denver, Colorado, on May 3, 2011, and at the Pediatric Hospital Medicine meeting in Kansas City, Missouri, on July 30–31, 2011.
Disclosure
Dr. Simon is supported by Award No. K23NS062900 from the NINDS; the Child Health Corporation of America via the Pediatric Research in Inpatient Setting Network Executive Council; and Seattle Children’s Center for Clinical and Translational Research. Dr. Kestle was supported by Award No. 1RC1NS068943-01 from the NINDS. This publication was supported by a PCMC Innovative Research Grant; the Children’s Health Research Center at the University of Utah; Seattle Children’s Center for Clinical and Translational Research; and Clinical and Translational Science Awards Grant No. ULI RR025014 from the NCRR, a component of the NIH. None of the sponsors participated in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. None of the authors have potential financial, personal, or professional conflicts of interest to disclose.
Author contributions to the study and manuscript preparation include the following. Conception and design: Simon, Mayer- Hamblett. Acquisition of data: Langley. Analysis and interpretation of data: Simon, Whitlock, Riva-Cambrin, Mayer-Hamblett. Drafting the article: Simon, Mayer-Hamblett. Critically revising the article: Whitlock, Riva-Cambrin, Kestle, Rosenfeld, Dean, Holubkov, Langley, Mayer-Hamblett. Reviewed submitted version of manuscript: Simon, Whitlock, Rosenfeld, Dean, Holubkov, Langley, Mayer-Hamblett. Statistical analysis: Whitlock. Administrative/technical/material support: Simon, Kestle, Langley, Mayer-Hamblett. Study supervision: Simon.
References
- 1.Agresti A. Categorical Data Analysis. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 2.Albright AL, Pollack IF, Adelson PD, Solot JJ. Outcome data and analysis in pediatric neurosurgery. Neurosurgery. 1999;45:101–106. doi: 10.1097/00006123-199907000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML. Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Childs Nerv Syst. 2005;21:56–61. doi: 10.1007/s00381-004-1052-x. [DOI] [PubMed] [Google Scholar]
- 4.Berry JG, Hall MA, Sharma V, Goumnerova L, Slonim AD, Shah SS. A multi-institutional, 5-year analysis of initial and multiple ventricular shunt revisions in children. Neurosurgery. 2008;62:445–454. doi: 10.1227/01.neu.0000316012.20797.04. [DOI] [PubMed] [Google Scholar]
- 5.Borgbjerg BM, Gjerris F, Albeck MJ, Børgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien) 1995;136:1–7. doi: 10.1007/BF01411427. [DOI] [PubMed] [Google Scholar]
- 6.Browd SR, Gottfried ON, Ragel BT, Kestle JR. Failure of cerebrospinal fluid shunts: part II: overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006;34:171–176. doi: 10.1016/j.pediatrneurol.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: part I: Obstruction and mechanical failure. Pediatr Neurol. 2006;34:83–92. doi: 10.1016/j.pediatrneurol.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Bruinsma N, Stobberingh EE, Herpers MJ, Vles JS, Weber BJ, Gavilanes DA. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect. 2000;6:202–206. doi: 10.1046/j.1469-0691.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 9.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane DD, Kestle J. Ventricular shunting for hydrocephalus in children: patients, procedures, surgeons and institutions in English Canada, 1989–2001. Eur J Pediatr Surg. 2002;12 (Suppl 1):S6–S11. doi: 10.1055/s-2002-36864. [DOI] [PubMed] [Google Scholar]
- 11.Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295–301. doi: 10.1159/000070413. [DOI] [PubMed] [Google Scholar]
- 12.Dallacasa P, Dappozzo A, Galassi E, Sandri F, Cocchi G, Masi M. Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst. 1995;11:643–649. doi: 10.1007/BF00300722. [DOI] [PubMed] [Google Scholar]
- 13.Davis SE, Levy ML, McComb JG, Masri-Lavine L. Does age or other factors influence the incidence of ventriculoperitoneal shunt infections? Pediatr Neurosurg. 1999;30:253–257. doi: 10.1159/000028806. [DOI] [PubMed] [Google Scholar]
- 14.Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst. 1994;10:321–327. doi: 10.1007/BF00335171. [DOI] [PubMed] [Google Scholar]
- 15.Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J, Jr, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294–303. 303–305. doi: 10.1097/00006123-199808000-00068. [DOI] [PubMed] [Google Scholar]
- 16.Garibaldi RA. Prevention of intraoperative wound contamination with chlorhexidine shower and scrub. J Hosp Infect. 1988;11 (Suppl B):5–9. doi: 10.1016/0195-6701(88)90149-1. [DOI] [PubMed] [Google Scholar]
- 17.Garton HJ, Kestle JR, Drake JM. Predicting shunt failure on the basis of clinical symptoms and signs in children. J Neurosurg. 2001;94:202–210. doi: 10.3171/jns.2001.94.2.0202. [DOI] [PubMed] [Google Scholar]
- 18.George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51:804–811. doi: 10.3171/jns.1979.51.6.0804. [DOI] [PubMed] [Google Scholar]
- 19.Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic- impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003;99:831–839. doi: 10.3171/jns.2003.99.5.0831. [DOI] [PubMed] [Google Scholar]
- 20.Griebel R, Khan M, Tan L. CSF shunt complications: an analysis of contributory factors. Childs Nerv Syst. 1985;1:77–80. doi: 10.1007/BF00706686. [DOI] [PubMed] [Google Scholar]
- 21.Horgan MA, Piatt JH., Jr Shaving of the scalp may increase the rate of infection in CSF shunt surgery. Pediatr Neurosurg. 1997;26:180–184. doi: 10.1159/000121187. [DOI] [PubMed] [Google Scholar]
- 22.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons; 2000. [Google Scholar]
- 23.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 2007;23:773–777. doi: 10.1007/s00381-007-0296-7. [DOI] [PubMed] [Google Scholar]
- 25.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230–236. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 26.Kestle JR, Cochrane DD, Drake JM. Shunt insertion in the summer: is it safe? J Neurosurg. 2006;105 (3 Suppl):165–168. doi: 10.3171/ped.2006.105.3.165. [DOI] [PubMed] [Google Scholar]
- 27.Kestle JR, Drake JM, Cochrane DD, Milner R, Walker ML, Abbott R, III, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284–290. doi: 10.3171/jns.2003.98.2.0284. [DOI] [PubMed] [Google Scholar]
- 28.Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB. A concerted effort to prevent shunt infection. Childs Nerv Syst. 1993;9:163–165. doi: 10.1007/BF00272269. [DOI] [PubMed] [Google Scholar]
- 29.Kontny U, Höfling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ. CSF shunt infections in children. Infection. 1993;21:89–92. doi: 10.1007/BF01710738. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 31.Lambert M, MacKinnon AE, Vaishnav A. Comparison of two methods of prophylaxis against CSF shunt infection. Z Kinderchir. 1984;39 (Suppl 2):109–110. doi: 10.1055/s-2008-1044298. [DOI] [PubMed] [Google Scholar]
- 32.Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C. Multiple shunt failures: an analysis of relevant factors. Childs Nerv Syst. 1998;14:271–275. doi: 10.1007/s003810050223. [DOI] [PubMed] [Google Scholar]
- 33.McGirt MJ, Leveque JC, Wellons JC, III, Villavicencio AT, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–255. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 34.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858–862. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 35.Piatt JH, Jr, Carlson CV. A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg. 1993;19:233–242. doi: 10.1159/000120738. [DOI] [PubMed] [Google Scholar]
- 36.Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Neurosurgery. 1999;45:1399–1411. doi: 10.1097/00006123-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 37.Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 1992;77:29–36. doi: 10.3171/jns.1992.77.1.0029. [DOI] [PubMed] [Google Scholar]
- 38.Quigley MR, Reigel DH, Kortyna R. Cerebrospinal fluid shunt infections. Report of 41 cases and a critical review of the literature. Pediatr Neurosci. 1989;15:111–120. [PubMed] [Google Scholar]
- 39.Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105:242–247. doi: 10.3171/jns.2006.105.2.242. [DOI] [PubMed] [Google Scholar]
- 40.Renier D, Lacombe J, Pierre-Kahn A, Sainte-Rose C, Hirsch JF. Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg. 1984;61:1072–1078. doi: 10.3171/jns.1984.61.6.1072. [DOI] [PubMed] [Google Scholar]
- 41.Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975;131:543–552. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- 42.Sciubba DM, Stuart RM, McGirt MJ, Woodworth GF, Samdani A, Carson B, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 2005;103 (2 Suppl):131–136. doi: 10.3171/ped.2005.103.2.0131. [DOI] [PubMed] [Google Scholar]
- 43.Shah SS, Hall M, Slonim AD, Hornig GW, Berry JG, Sharma V. A multicenter study of factors influencing cerebrospinal fluid shunt survival in infants and children. Neurosurgery. 2008;62:1095–1103. doi: 10.1227/01.neu.0000325871.60129.23. [DOI] [PubMed] [Google Scholar]
- 44.Shurtleff DB, Stuntz JT, Hayden PW. Experience with 1201 cerebrospinal fluid shunt procedures. Pediatr Neurosci. 1986;12:49–57. doi: 10.1159/000120218. [DOI] [PubMed] [Google Scholar]
- 45.Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4:156–165. doi: 10.3171/2009.3.PEDS08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131–137. doi: 10.3171/PED/2008/1/2/131. [DOI] [PubMed] [Google Scholar]
- 47.Tuan TJ, Thorell EA, Hamblett NM, Kestle JR, Rosenfeld M, Simon TD. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 2011;30:731–735. doi: 10.1097/INF.0b013e318218ac0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg. 2000;92:31–38. doi: 10.3171/jns.2000.92.1.0031. [DOI] [PubMed] [Google Scholar]
- 49.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22:692–697. doi: 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 50.Wellons JC, III, Shannon CN, Kulkarni AV, Simon TD, Riva- Cambrin J, Whitehead WE, et al. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. Clinical article. J Neurosurg Pediatr. 2009;4:50–55. doi: 10.3171/2009.2.PEDS08400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winston KR. Hair and neurosurgery. Neurosurgery. 1992;31:320–329. doi: 10.1227/00006123-199208000-00018. [DOI] [PubMed] [Google Scholar]