Abstract
1,2,4,5-Tetrazines have been established as effective dienes for inverse electron demand [4 + 2] Diels-Alder cycloaddition reactions with strained alkenes for over fifty years. Recently, this reaction pair combination has been applied to bioorthogonal labeling and cell detection applications; however, to date there has been no detailed examination and optimization of tetrazines for use in biological experiments. Here we report the synthesis and characterization of twelve conjugatable tetrazines. The tetrazines were all synthesized in a similar fashion and were screened in parallel to identify candidates most ideally suited for biological studies. In depth follow up studies revealed compounds with varying degrees of stability and reactivity that could each be useful in different bioorthogonal applications. One promising, highly stable and water soluble derivative was used in pre-targeted cancer cell labeling studies, confirming its utility as a bioorthogonal moiety.
Introduction
Bioorthogonal ‘click’ chemistry reactions are a powerful tool for exploring different aspects of biological systems. The ability to perform these chemical reactions in cellular environments (in cellulo chemistry) and host organisms (in vivo chemistry) without interference from biological components allows for selective ‘tagging’ of cellular targets and provides a means to image or track biochemical components and interactions. The most widely used and well-known bioorthogonal reaction is the azide and alkyne [3 + 2] cycloaddition.1 The use of ring strain to promote this reaction was a major development in the field allowing for the [3 + 2] cycloaddition to proceed at room temperature without the need for catalysts.2 Notable accomplishments utilizing this chemistry have involved the labeling of cell surface glycoconjugates,3 cell membrane lipids4 and glycans in living organisms5, 6 among others.7, 8 Another bioorthogonal reaction using a similar concept, but employing 1,2,4,5-tetrazines and strained alkenes for [4 + 2] inverse electron demand cycloadditions, has emerged more recently.9–11 This reaction has gained popularity due to the potential for extremely fast cycloaddition kinetics with trans-cyclooctene (TCO) as the dienophile. Applications have included fluorescent cancer cell labeling,12, 13 in vivo cancer imaging with 111In,14 18F radiolabeling,15–17 as well as cancer cell detection applications.18, 19
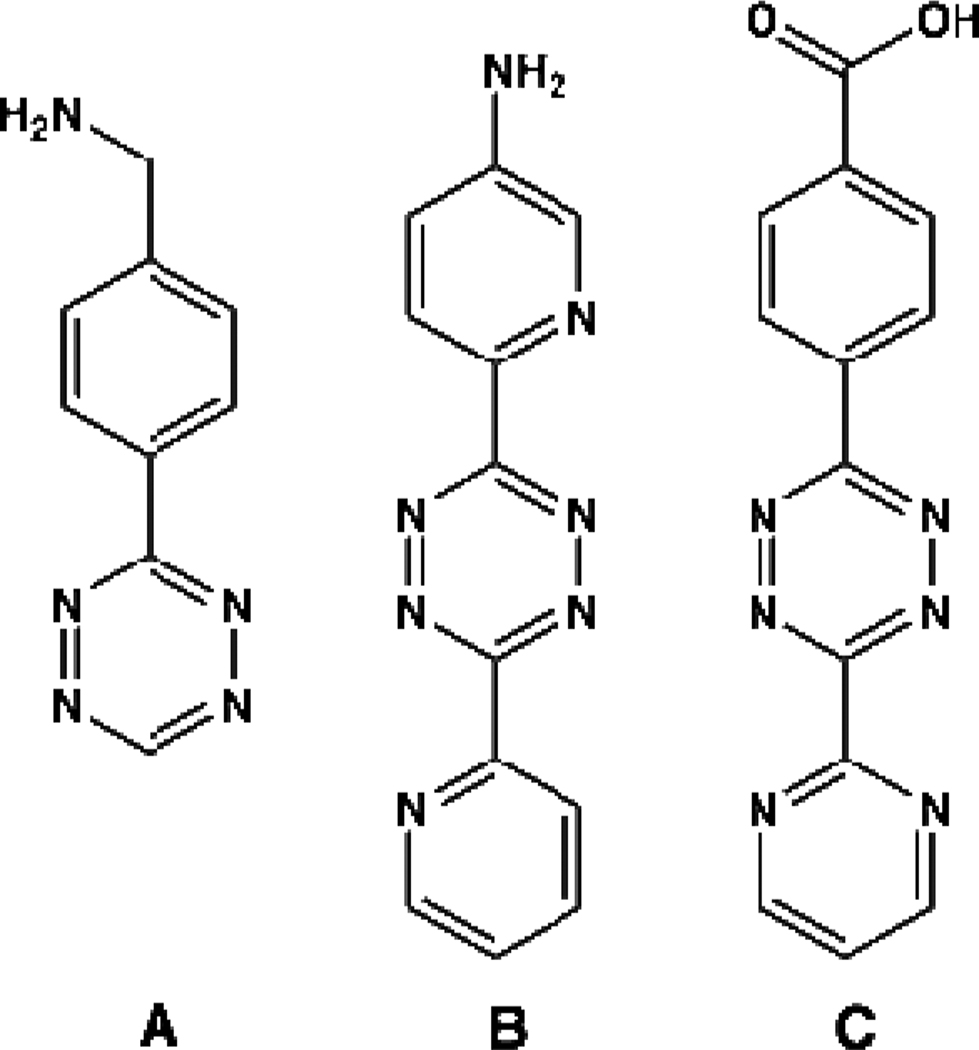
The effectiveness of the strained alkene-tetrazine reaction is clear, but there has been little detailed investigation on optimizing the reactant properties for bioorthogonal use. However, there is a wealth of reactivity data in non-aqueous media, beginning with the observation in the late 1950s that tetrazines can react with unsaturated compounds.20 Following this publication, much progress was made in synthesizing different tetrazines for reactions with various dienophiles21, 22 including kinetic analysis of this cycloaddition reaction with different dienophiles by Sauer.23–25 Sauer reported a range of [4 + 2] cycloaddition reactivity based on the nature of the dienophile that spans nine orders of magnitude with 1,2,4,5-tetrazines including the first use of norbornene and TCO, which have been the primary dienophiles used in recent bioorthogonal literature.25, 26 TCO has proven to be a much faster reactant than norbornene for bioorthogonal applications,9, 10 but the latter is more stable and commercially available. No reported attempts at improving the dienophile reactivity for bioorthogonal use with tetrazines were published until a recent article emerged describing a new derivative of TCO.27 The large cycloaddition rate differences seen by changing the chemical nature of the dienophile are equaled by changing the substituents in the 3 and 6 positions of 1,2,4,5-tetrazines.28 However, only a few tetrazines have been employed in biological environments for bioorthogonal labeling. The primary tetrazines used for this application found in recent literature are the 3,6-mono-aryl as well as the diaryl-s-tetrazines shown in Figure 1. Tetrazine A and carboxylic acid modified versions of this compound shown in Figure 1 have been employed for many applications by our lab,12, 13, 15, 17–19 whereas tetrazines B and C have been reported elsewhere and were used for various purposes including the post-synthetic modification of DNA as well as for the synthesis of a ‘BioShuttle’ that effectively assists in transporting cargo into cells.9, 11, 14, 29–31 These tetrazines fall within the mid-range of reported cycloaddition reactivity with dienophiles mainly because some of the tetrazines with the fastest kinetics are not stable in water.32 With such a large range of reactivity, however, many substituents are likely to be suitable for bioorthogonal use that could have a significant impact on the kinetics of the reaction. In dealing with bioorthogonal reactions, another parameter besides the rate constant that should be considered is aqueous solution stability. Here we report the design, synthesis and characterization of a series of twelve conjugatable tetrazines with varying functional groups. We demonstrate a variety of solution stabilities and reaction rates and validate the bioorthogonal use of a new highly stable and water soluble tetrazine.
Figure 1.
Chemical structures of tetrazines used in reported bioorthogonal applications. A) (4-(1,2,4,5-tetrazin-3-yl)phenyl)methanamine, B) 6-(6-(pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)pyridin-3-amine, C) 4-(6-(pyrimidin-2-yl)-1,2,4,5-tetrazin-3-yl)benzoic acid.
Experimental Procedures
General Considerations
All chemicals and reagents were purchased from commercial sources and used without further purification. (E)-cyclooct-4-en-1-yl (2,5- dioxopyrrolidin-1-yl) carbonate (TCO-carbonate) was synthesized as previously described.13 NMR spectra were collected on a Varian 500 MHz spectrometer. 1H and 13C NMRs were referenced to residual solvent peaks or TMS (0.00 ppm). All phosphate buffered saline (PBS) solutions used were composed of 0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride, pH 7.4. HyClone DBPS/Modified was used for DPBS. HyClone USDA tested fetal bovine serum was used in FBS stability studies. Herceptin (trastuzumab) was purchased from Genentech. Analytical HPLC and LCMS were performed on a Waters 2695 HPLC equipped with a 2996 diode array detector, a Micromass ZQ4000 ESI-MS module, and a Varian 100 × 2.0 mm RPC18 column at a flow rate of 0.3 mL/min. Preparative HPLC was performed on a Varian ProStar model 210 instrument equipped with a model 335 diode array detector, a model 701 fraction collector, and a Varian 250×21.2 mm RPC18 column at a flow rate of 21 mL/min. HPLC buffers contained: buffer A (0.1% TFA in H2O) and buffer B (acetonitrile with 10% H2O and 0.1% TFA). Three to five mg portions of all compounds were weighed 3X on a Mettler Toledo AB265-S/Fact balance with an accuracy of 0.03 mg in the preparation of stock solutions. Mass spectrometry measurements for exact mass were performed by the Department of Chemistry Instrumentation Facility at the Massachusetts Institute of Technology using a Bruker Daltonics 4.7 Tesla Fourier Transform Ion Cyclotron Mass Spectrometer (FTMS).
Synthesis
General Tetrazine Synthesis Procedure
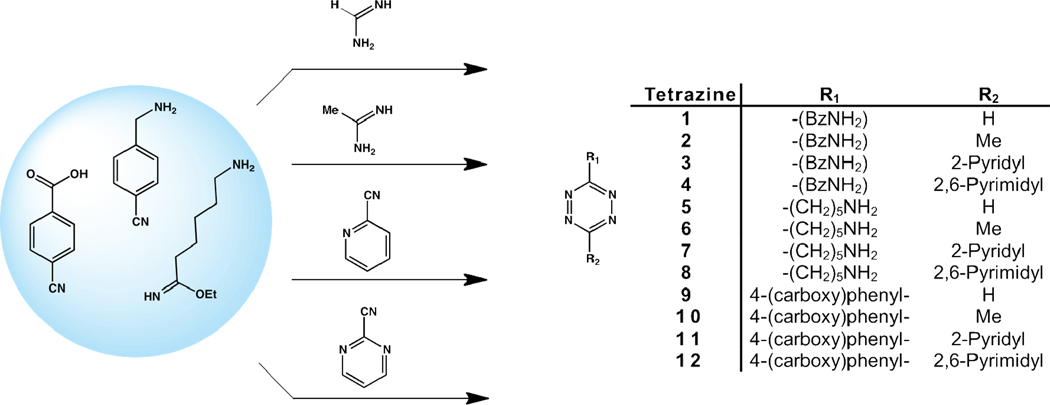
Compounds 1–12 were synthesized by mixing 2 mmol of 4-(aminomethyl)benzonitrile, ethyl 6-aminohexanimidate dihydrochloride, or 4-cyanobenzoic acid with 10 mmol of formamidine acetate, acetamidine hydrochloride, 2-cyanopyridine, or pyrimidine-2-carbonitrile under N2. Anhydrous hydrazine (2 mL) was then added slowly to the solid mixture with stirring (Note: This procedure should be carried out in a well-ventilated fume hood due to the formation of ammonia gas). Reactions were then stirred at room temperature or with heat for 30 min to 2 h. Sodium nitrite (10 mmol) in water was added to the reaction mixture followed by drop-wise addition of 2% aqueous HCl until the solution reached approximately pH 3. The solution turned red and stopped bubbling indicating that the dihydrotetrazines were oxidized to the tetrazines (Note: This procedure should be carried out in a well-ventilated fume hood due to the formation of nitrogen oxide gases). The crude aqueous mixtures were then subjected to one of two general workups.
Workup A (compounds 1–8)
The oxidized acidic solutions were extracted with DCM until the organic layer was colorless. The organic fractions were discarded and the aqueous layer was then saturated with NaCl and basified by addition of solid NaHCO3 (compounds 1–4) or Na2CO3 (compounds 5–8) and immediately extracted with DCM. The organic layers were then dried with MgSO4, filtered, and the solvent was removed by rotary evaporation (alternatively, approximately 300 µL TFA was added prior to rotary evaporation) to yield a crude product mixture.
Workup B (compounds 9–12)
The oxidized acidic solutions yielded a pink/red precipitate, which was filtered or separated by centrifugation and washed with 2% aqueous HCl to afford a crude product mixture.
The crude mixtures of all compounds (1–12) were then purified by HPLC, using the appropriate gradient of buffer A and buffer B, and lyophilized (Note: in some cases, especially with the pyridyl and pyrimidyl substituted compounds, multiple rounds of HPLC purification were necessary due to poor resolution of the product peak). The resulting TFA salts of amine containing compounds 1–8 were then dissolved in 0.1% HCl, loaded onto Waters Sep-Pak® Vac 10 g C18 cartridges and flushed with approximately 10 column volumes of 0.1% HCl. The hydrochloride salts of purified compounds 1–8 were then eluted with a mixture of 0.1% HCl and MeCN and either dried in vacuo or lyophilized to dryness.
Synthesis of ethyl 6-aminohexanimidate dihydrochloride
6-Aminohexanenitrile (50 mmol) was dissolved in anhydrous EtOH (30 mL). Excess HCl gas was bubbled into the solution on an ice bath with stirring for 1 h. The ice bath was removed and HCl bubbling was continued for 24 h. The solvent was then removed by rotary evaporation giving a crystalline solid that was then stirred in cold iPrOH (30 ml), filtered and washed with cold iPrOH (2 × 10 mL). After drying in vacuo, pure ethyl 6-aminohexanimidate dihydrochloride salt (9.05 g, 78%) was obtained as a white solid. 1H NMR (500 MHz, DMSO d6) δ 1.35 (5H, pentet, J = 15.5 Hz), 1.54–1.66 (4H, m), 2.63 (2H, t, J = 7 Hz), 2.72–2.78 (2H, m), 4.42 (2H, quartet, J = 21 Hz), 8.11 (3H, s, N-H), 13C NMR (125 MHz, DMSO d6) δ 179.37, 69.33, 38.75, 32.55, 26.76, 25.32, 24.72, 13.84 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C8H19N2O]+ 159.1492, found 159.1489.
(4-(1,2,4,5-Tetrazin-3-yl)phenyl)methanamine hydrochloride (1)
Reaction time was 30 min at 80°C. The title compound as the TFA salt was purified by HPLC using a 0–50% buffer B gradient. A red crystalline solid was obtained after anion exchange to the hydrochloride salt. (17% yield, 76 mg). 1H NMR (500 MHz, D2O) δ 4.37 (2H, s), 7.75 (2H, d, J = 8.0 Hz), 8.54 (2H, d, J = 8.0 Hz), 10.43 (1H, s) 13C NMR (125 MHz, D2O) δ 169.11, 160.39, 140.49, 134.95, 132.62, 131.84, 45.60 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C9H10N5]+ 188.0931, found 188.0934.
(4-(6-Methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride (2)
Reaction time was 30 min at 80°C. The title compound as the TFA salt was purified by HPLC using a 0–50% buffer B gradient. A red/purple crystalline solid was obtained after anion exchange to the hydrochloride salt. (17% yield, 81 mg). 1H NMR (500 MHz, D2O) δ 3.11 (3H, s), 4.36 (2H, s), 7.74 (2H, d, J = 8.5 Hz), 8.48 (2H, d, J = 8.0 Hz), 13C NMR (125 MHz, D2O) δ 170.36, 166.71, 140.06, 134.89, 132.61, 131.44, 45.59, 23.00 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C10H12N5]+ 202.1087, found 202.1090.
(4-(6-(Pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride (3)
Reaction time was 2 h at 80°C. The cooled reaction mixture was suspended in a mixture of 25 mL MeCN,10 mL MeOH and 4 mL TFA giving a clear yellow-orange solution. Solid NaNO2 (10 mmol) was then added and the reaction was allowed to stir for 15 min. After removing the solvent by rotary evaporation, the residue was washed with DCM and the organics were discarded, leaving an oil. The oil was then dissolved in water (50 ml), saturated with solid NaHCO3, and extracted with DCM. The combined DCM fractions were concentrated and the title compound as the TFA salt was purified by HPLC using a 0–25% buffer B gradient. A red crystalline solid was obtained after anion exchange to the hydrochloride salt. (22% yield, 132 mg). 1H NMR (500 MHz, DMSO d6) δ 4.19 (2H, s), 7.53 (1H, t, J = 5 Hz), 7.83 (2H, d, J = 8 Hz), 8.19 (1H, t, J = 8 Hz), 8.56–8.64 (3H, m), 8.95 (1H, d, J = 5 Hz), 13C NMR (125 MHz, DMSO d6) δ 163.17, 163.03, 150.33, 149.87, 138.85, 137.99, 131.55, 129.85, 127.89, 126.57, 124.01, 41.73 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C14H13N6]+ 265.1196, found 265.1190.
(4-(6-(Pyrimidin-2-yl)-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride (4)
Reaction time was 2 h at 80°C. The cooled reaction mixture was treated as in 3. The title compound as the TFA salt was purified by HPLC using a 0–25% buffer B gradient. A red crystalline solid was obtained after anion exchange to the hydrochloride salt. (17% yield, 102 mg). 1H NMR (500 MHz, D2O) δ 4.35 (2H, s), 7.74 (2H, d, J = 8.5 Hz), 7.86 (1H, t, J = 5 Hz), 8.58 (2H, d, J = 8.0 Hz), 9.13 (2H, d, J = 5.5 Hz) 13C NMR (125 MHz, D2O) δ 167.32, 164.79, 161.60, 160.45, 141.00, 134.30, 132.78, 132.14, 126.75, 45.60 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C13H12N7]+ 266.1149, found 266.1140.
5-(1,2,4,5-Tetrazin-3-yl)pentan-1-amine hydrochloride (5)
Reaction time was 2 h at room temperature. The title compound as the TFA salt was purified by HPLC using a 0–25% buffer B gradient. A pink crystalline solid was obtained after anion exchange to the hydrochloride salt. (6% yield, 24 mg). 1H NMR (500 MHz, D2O) δ 1.53 (2H, pentet, J = 15.5 Hz) 1.75 (2H, pentet, J = 15.5 Hz), 1.99 (2H, pentet, J = 15.5 Hz) 3.02 (2H, t, J = 7.5 Hz), 3.39 (2H, t, J = 7.5 Hz), 10.36 (1H, s) 13C NMR (125 MHz, D2O) δ 175.63, 160.63, 42.25, 37.13, 29.71, 29.35, 27.99 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C7H14N5]+ 168.1244, found 168.1241.
5-(6-Methyl-1,2,4,5-tetrazin-3-yl)pentan-1-amine hydrochloride (6)
Reaction time was 2 h at room temperature. The title compound as the TFA salt was purified by HPLC using a 0–100% buffer B gradient. A purple crystalline solid was obtained after anion exchange to the hydrochloride salt. (21% yield, 92 mg). 1H NMR (500 MHz, D2O) δ 1.51 (2H, pentet, J = 15.5 Hz) 1.73 (2H, pentet, J = 15 Hz), 1.95 (2H, pentet, J = 15 Hz) 3.02 (2H, t, J = 7.5 Hz), 3.34 (2H, t, J = 7.5 Hz), 4.77 (3H, s) 13C NMR (125 MHz, D2O) δ 172.49, 170.45, 42.21, 36.42, 29.78, 29.29, 27.91, 22.97 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C8H16N5]+ 182.1400, found 182.1405.
5-(6-(Pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)pentan-1-amine hydrochloride (7)
Reaction time was 30 min at 80°C. The title compound as the TFA salt was purified by HPLC using a 0–50% buffer B gradient. An orange/red crystalline solid was obtained after anion exchange to the hydrochloride salt. (10% yield, 55 mg). 1H NMR (500 MHz, DMSO d6) δ 1.48 (2H, pentet, J = 15 Hz), 1.66 (2H, pentet, J = 15.5 Hz), 1.96 (2H, pentet, J = 15 Hz), 2.72–2.82 (2H, m), 3.35 (2H, t, J = 7), 7.70–7.76 (1H, m), 8.16 (1H, t, J = 7.5 Hz), 8.53 (1H, d, J = 7.5 Hz), 8.91 (1H, d, J = 5 Hz), 13C NMR (125 MHz, DMSO d6) δ 169.78, 163.19, 150.23, 150.00, 137.98, 126.46, 123.83, 38.39, 33.82, 26.60, 26.54, 25.11 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C12H17N6]+ 245.1509, found 245.1503.
5-(6-(Pyrimidin-2-yl)-1,2,4,5-tetrazin-3-yl)pentan-1-amine hydrochloride (8)
Reaction time was 30 min at 80°C. The title compound as the TFA salt was purified by HPLC using a 0–50% buffer B gradient. A red crystalline solid was obtained after anion exchange to the hydrochloride salt. (2% yield, 12 mg). 1H NMR (500 MHz, D2O) δ 1.59 (2H, pentet, J = 15 Hz) 1.80 (2H, pentet, J = 15.5 Hz), 2.08 (2H, pentet, J = 15.5 Hz) 3.05 (2H, t, J = 8 Hz), 3.52 (2H, t, J = 8 Hz), 7.86 (1H, t, J = 5.5 Hz), 9.14 (2H, d, J = 5.5 Hz), 13C NMR (125 MHz, D2O) δ 174.31, 165.18, 161.60, 160.45, 126.71, 42.19, 36.87, 29.68, 29.30, 27.99 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C11H16N7]+ 246.1462, found 246.1465.
4-(1,2,4,5-Tetrazin-3-yl)benzoic acid (9)
Reaction time was 30 min at 80°C. The title compound was purified as a pink solid by HPLC using a 0–100% buffer B gradient. (18% yield, 73 mg). 1H NMR (500 MHz, DMSO d6) δ 8.22 (2H, d, J = 8.0 Hz), 8.62 (2H, d, J = 8.5 Hz), 10.66 (1H, s) 13C NMR (125 MHz, DMSO d6) δ 166.58, 164.98, 158.16, 135.62, 134.22, 130.11, 127.88 ppm. HRMS-ESI [M-H]− m/z calcd. for [C9H5N4O2]− 201.0418, found 201.0416.
4-(6-Methyl-1,2,4,5-tetrazin-3-yl)benzoic acid (10)
Reaction time was 30 min at 80°C. The title compound was purified as a purple solid by HPLC using a 0–100% buffer B gradient. (11% yield, 48 mg). 1H NMR (500 MHz, DMSO d6) δ 3.03 (3H, s), 8.20 (2H, d, J = 9.0 Hz), 8.58 (2H, d, J = 8 Hz), 13C NMR (125 MHz, DMSO d6) δ 167.84, 167.17, 163.30, 136.18, 134.43, 130.67, 128.06, 21.36 ppm. HRMS-ESI [M-H]− m/z calcd. for [C10H7N4O2]− 215.0574, found 215.0574.
4-(6-(Pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)benzoic acid (11)
Reaction time was 90 min at 80°C. The title compound was purified as a purple solid by HPLC using a 0–100% buffer B gradient. (3% yield, 17 mg). 1H NMR (500 MHz, DMSO d6) δ 7.74 (1H, t, J = 6.5 Hz), 8.17 (1H, d, J = 7.5 Hz), 8.24 (2H, d, J = 9.0 Hz), 8.62 (1H, d, J = 8.0 Hz), 8.69 (2H, d, J = 8.5 Hz), 8.95 (1H, d, J = 3.5), 13C NMR (125 MHz, DMSO d6) δ 165.57, 162.02, 161.99, 149.46, 148.89, 136.78, 134.39, 133.16, 129.14, 126.92, 125.57, 123.09 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C14H10N5O2]+ 280.0829, found 280.0827.
4-(6-(Pyrimidin-2-yl)-1,2,4,5-tetrazin-3-yl)benzoic acid (12)
Reaction time was 90 min at 80°C. The title compound was purified as a red solid by HPLC using a 0–100% buffer B gradient. (2% yield, 12 mg). 1H NMR (500 MHz, DMSO d6) δ 7.78 (1H, t, J = 5.0 Hz), 8.19 (2H, d, J = 8.5 Hz), 8.64 (2H, d, J = 8.5 Hz), 9.14 (2H, d, J = 5.0 Hz), 13C NMR (125 MHz, DMSO d6) δ 167.15, 163.64, 163.36, 159.48, 159.00, 135.82, 134.94, 130.73, 128.83, 123.51 ppm. HRMS-ESI [M+H]+ m/z calcd. for [C13H9N6O2]+ 281.0781, found 281.0784.
Synthesis of AF750-Tetrazine Conjugate (AF750-6)
The succinimidyl ester of AlexaFluor-750 (AF750, Invitrogen, 2.5 mg) was dissolved in 10 mM phosphate buffer, pH 8.3. Excess (approximately 10-fold molar excess, 4 mg) tetrazine 6 was then added immediately and the solution was allowed to stir at room temperature in the dark for 5 h. The product was identified by LCMS and purified by HPLC using a 0–100% buffer B gradient. The resulting fractions containing the desired compound were neutralized with NaOH, desalted by flushing with deionized water after loading on Waters Sep-Pak® Vac 10 g C18 cartridges, eluted with a mixture of water/MeCN, frozen and lyophilized to yield purified product as determined by analytical HPLC (see Figure S1).
Kinetics
Tetrazine Kinetics with Norbornene
Cycloaddition kinetics were monitored using a Tecan Safire2 microplate reader in clear flat-bottomed 96-well plates. Norbornene carboxylic acid ((1S,2S,4S)-bicyclo[2.2.1]hept-5-en-2-ylacetic acid) (NB) was used in excess as the dienophile at 1, 2, 3, 4 and 5 mM concentrations with each of the twelve tetrazines (100 µM) in triplicate. Stock solutions of reactants in DMSO were diluted in PBS pH 7.4 to a final concentration of 5% DMSO. The decrease of the tetrazine absorbance measured at 515 nm was monitored at 37°C (NB PBS solutions were allowed to pre-equilibrate at 37°C in the instrument for 10 min before addition of the tetrazine). The kobs (s−1) values were then calculated using the Prism software package, averaged and plotted against the concentration of NB to yield the second order rate constants (kobs, M−1s−1) from the slope of the line. Trials for each tetrazine were done in duplicate. The relative (to tetrazine 1) rate constants for each of the twelve tetrazines can be seen in Figure S2.
Tetrazine Kinetics with TCO
Kinetics were performed with the major isomer of (E)-cyclooct-4-enol (TCO) and selected tetrazines using an Applied Photophysics Stopped-Flow spectrophotometer. Stock solutions of reactants in DMSO were diluted in PBS pH 7.4 to a final concentration of 1% DMSO. Solutions of TCO and tetrazine were loaded into the individual chambers of the spectrophotometer and equilibrated to 37°C for 10 min. Samples were then mixed by the instrument in a 1:1 v/v ratio and the decrease of the tetrazine absorbance measured at 515 nm was monitored at regular intervals. Final concentrations of reactants were 1.0, 1.5, 2.0 and 2.5 µM for TCO and 50 µM for the tetrazines. Baseline corrected kobs (s−1) values were calculated using Prism and the results of 4 runs were averaged for each concentration of TCO using the standard deviation as the error. The average kobs values were then plotted against the concentration of TCO to yield the second order rate constants (k2, M−1s−1) from the slope of the line and the error from the standard deviation in the slope calculated in Prism.
Tetrazine Stability Studies
Stability in phosphate buffered saline
The stability of tetrazines 1–12 in PBS was measured using a Tecan Safire2 microplate reader in clear flat-bottomed 96-well plates. DMSO stocks of tetrazines were diluted in PBS pH 7.4 to 0.2 mM and a final DMSO concentration of 1%. The decrease of the tetrazine absorbance measured at 515 nm was monitored for 14 h at 37°C. Three independent trials were conducted and the average of three wells for each tetrazine was calculated for each trial. The PBS corrected averages from each of the three trials were themselves averaged and the relative (to tetrazine 1) percent tetrazine remaining can be seen in Figure S3 where the error is the standard deviation of the three trials.
Stability in serum (FBS)
The stability of selected tetrazines in 100% fetal bovine serum was measured using an Agilent 8453 UV-visible spectrophotometer. The compounds were added to pre-equilibrated serum at 37°C in a capped quartz cuvette at a final concentration of 1 mM and 1% DMSO. A higher concentration of tetrazine was used as compared to the PBS stability measurements due to a small but significant baseline drift over time from the FBS resulting in absorbance that overlaps with the tetrazine absorbance at 515 nm. The decrease of the tetrazine absorbance measured at 515 nm was monitored for 10 h at 37°C. This procedure was repeated three times and the serum only corrected percent decreases were averaged and the standard deviation was used as the error.
Antibody Labeling
Herceptin (5 mg) was dissolved in 10 mM NaHCO3 pH 8.4 buffer before adding approximately 3 molar equivalents (0.25 mg) AlexaFluor-568 succinimidyl ester (AF568, Invitrogen). The reaction was allowed to shake at room temperature overnight in the dark. HerceptinAF568 was then purified from unconjugated fluorophore using an Amicon centrifugal filter (3K MWCO, Millipore) and determined to have approximately 1–2 fluorophores/ antibody by absorbance using 91,300 M−1cm−1 as the extinction coefficient for AF568. Fluorophore labeled Herceptin was further labeled with excess TCO-succinimidyl carbonate as described previously.19
Cell Studies
Flow Cytometry
Confluent SKBR-3 cells were suspended using 0.05% Trypsin/0.53 mM EDTA, washed by centrifugation with DPBS and placed in microcentrifuge tubes. HerceptinAF568 only (control) or HerceptinAF568-TCO were then added separately to individual tubes to a final concentration of 10 µg/mL in 100 µL total volume of DBPS. After incubating for 30 min at room temperature, cells were washed by centrifugation with DPBS (3x 1mL). AF750-6 in DMSO was then added to give a final concentration of 10 µM in 100 µL DBPS and was allowed to incubate for 30 min at room temperature (the final DMSO concentration was 1%). Cells were then washed by centrifugation with DBPS (4 × 1 mL) before resuspension in DPBS containing 5% FBS. AF568 and AF750 fluorescence was then assessed with a Becton Dickinson LSRII flow cytometer using the PE and APC-Cy7 channels respectively. Data collected was analyzed using the FlowJo software package.
Microscopy
SKBR-3 Human breast cancer cells were cultured in breakaway glass chamber slides. The antibodies HerceptinAF568-TCO (HerceptinAF568 only for controls) were incubated with the cells at a final concentration of approximately 10 µg/mL for 30 min in growth media (McCoy’s 5A containing 10% FBS 1% L-Glutaimine 1% Penstrep and 2% NaHCO3). Cells were then washed 4× with media before incubating with 10 µM AF750-6 for 30 min in growth media. The cells were then washed 4× with DPBS and fixed by incubating with Cytofix fixation buffer (BD Biosciences) for 20 min. After washing 4× with DPBS, the chambers were removed and the cells were preserved with ProLong Gold (Invitrogen). Images were taken using a Nikon Eclipse 80i fluorescence microscope and imaged in the Y-2E/C and INDO CY GR channels for AF568 and AF750 respectively. Identical image acquisition settings were used for both the control and experimental data sets. Images were analyzed using ImageJ applying identical leveling adjustments to control and experimental data across the individual channels.
Results and Discussion
Synthesis of Tetrazines
A series of 1,2,4,5-tetrazines was synthesized with the goal of testing the effects different substituents have on the solution stability and cycloaddition kinetic properties of these compounds. A total of twelve tetrazines were synthesized based on three conjugatable scaffolds and four different substituent groups. The substituents used varied in both size and electronic properties. Tetrazines 1–12 were all synthesized in a similar manner as shown in Scheme 1. After the appropriate carbonitrile/imidate ester/amidine pair was allowed to react in hydrazine, the crude reaction mixtures containing the dihydrotetrazine intermediates were oxidized with NaNO2 in water and 2% aqueous HCl. The crude tetrazine mixtures were then purified and characterized by NMR (1H and 13C) and HRMS in yields ranging from 2–22%. Several of the pyrimidyl and pyridyl substituted tetrazines proved difficult to purify resulting in lower reported yields. It was often the case that material of approximately 80–85% purity could be obtained in much higher yields, but subsequent and often multiple rounds of chromatography to obtain ≥95% purity substantially lowered the overall yield of several of these compounds.
Scheme 1.
Synthesis of tetrazines 1–12.
Tetrazine Kinetics with Norbornene and PBS stability
With the goal of narrowing down the number of tetrazines used for in depth follow up studies, the synthesized compounds 1–12 were screened initially in parallel. In 96-well plates, tetrazines 1–12 were incubated with norbornene carboxylic acid ((1S,2S,4S)-bicyclo[2.2.1]hept-5-en-2-ylacetic acid) (NB) to determine their relative inverse electron demand [4+2] cycloaddition kinetics (Scheme S1 depicts the generic reaction). NB was used in these initial screens due to its previous bioorthogonal utility in this reaction,10, 33 as well as its commercial availability. In all cases, NB was used in excess (at least 10-fold) and the disappearance of the visible absorption band of the tetrazine ring was monitored spectrophotometrically to obtain the pseudo first order rate constants (kobs s−1). Reactions had varying half-lives ranging from 1.5 to 60 min and were monitored until completion of the cycloaddition at 37°C. The kobs values at each concentration of NB were then used to calculate the relative second order rate constants for all tetrazines. In general, tetrazines with stronger electron withdrawing groups (4 and 8) attached to the tetrazine ring showed faster kinetics, whereas electron donating groups (2, 6 and 10) resulted in slower cycloaddition reactions when compared to hydrogen substituted tetrazine 1 (see supplementary Figure S2). This is consistent with the general [4 + 2] inverse electron demand reaction in that more electron deficient dienes result in faster cycloadditions.28 However, it is also important to note that hydrogen substituted tetrazines resulted in faster kinetics than would be predicted based on their ‘neutral’ character suggesting other parameters such as sterics may play an important role in the rate of this reaction.
The solution stability of the tetrazines in PBS pH 7.4 at 37°C was investigated. This screen was done in parallel in which the compounds were incubated in 96-well plates and the absorbance of the tetrazine at 515 nm was used to determine the percent tetrazine remaining after 14 h. The relative stabilities compared to tetrazine 1 can be seen in supplementary Figure S3. In general, compounds with stronger electron withdrawing groups showed lower stability than hydrogen substituted tetrazines and the electron donating alkyl substituted tetrazines exhibited the highest stability.
In addition to these observed trends in kinetics and stability, other factors such as aqueous solubility and ease of synthesis/purification were also considered before selecting tetrazines for more in depth follow-up studies. Overall, 3,6-diaryl tetrazines, demonstrate lower aqueous solubility than those with methyl or hydrogen substituents. For example, 3,6-diphenyl tetrazine is not soluble to any measurable amount in 100% water and 12 is not soluble above 0.2 mM, whereas 6 readily dissolves at concentrations above 500 mM. Taking all these data into account, tetrazines 1, 2, 3, 4, 5, 6, 7, 8 and 9 were chosen for further evaluation.
Tetrazine Kinetics with TCO and Serum Stability
The selected tetrazines were tested for their cycloaddition kinetics against the highly reactive dienophile TCO. A stopped-flow spectrophotometer was used to follow the decrease of the absorption at 515 nm of each tetrazine when mixed with excess TCO at 37°C in PBS pH 7.4. Reactions had varying half-lives ranging from 9 to 3000 milliseconds and were monitored until completion of the cycloaddition at 37°C. As with NB, second order rate constants were calculated for cycloaddition reactions of the tetrazines with TCO (Table 1). These data again show that hydrogen substituted tetrazines demonstrate exceptionally fast kinetics up to 30,000 M−1s−1 for 9. Interestingly, tetrazine 4, which displayed faster kinetics than 1 and 9 with NB, while still relatively fast, was slightly slower than these two tetrazines with TCO as the dienophile. This is perhaps due to steric interference between the 3,6-diaryl 4 and TCO that was not encountered with mono-substituted 1 and 9. Even the slowest measured tetrazine 6 at 210 M−1s−1 is still much faster than any of the reactions with NB, which was previously shown to be sufficient for pre-targeted cell labeling studies.10 Together with the NB kinetics, these data suggest a rate advantage for hydrogen substituted tetrazines over more bulky aryl substituents. In order to test the effect pH has on the reaction kinetics, another physiologically relevant pH was chosen, pH 5.0, approximating what is expected to occur within lysosomes. At this pH, representative compounds 1 and 3 showed no significant change in second order rate constant compared to pH 7.4.
Table 1.
Second order [4+2] cycloaddition rate constants of selected tetrazines with TCO in PBS at 37°C.
| Tetrazine | k2 (M−1s−1) | Error |
|---|---|---|
| 1 | 26,000a | 500 |
| 2 | 820 | 70 |
| 3 | 5,300 | 400 |
| 4 | 22,000 | 2,000 |
| 5 | 4,900 | 100 |
| 6 | 210 | 20 |
| 7 | 2,300 | 300 |
| 8 | 4,400 | 300 |
| 9 | 30,000 | 3,000 |
Discrepancies between the rate constant reported here for 1 and TCO of 26,000 M−1s−1 and a previous report from our lab13 of 6,000 M−1s−1 may be explained by the methods used. The current method herein evaluated the kinetics of the free molecules in solution directly by absorbance of the tetrazine whereas the previous study was conducted using TCO modified antibody physically absorbed to a surface and fluorescence readout after incubation with a tetrazine-fluorophore conjugate.
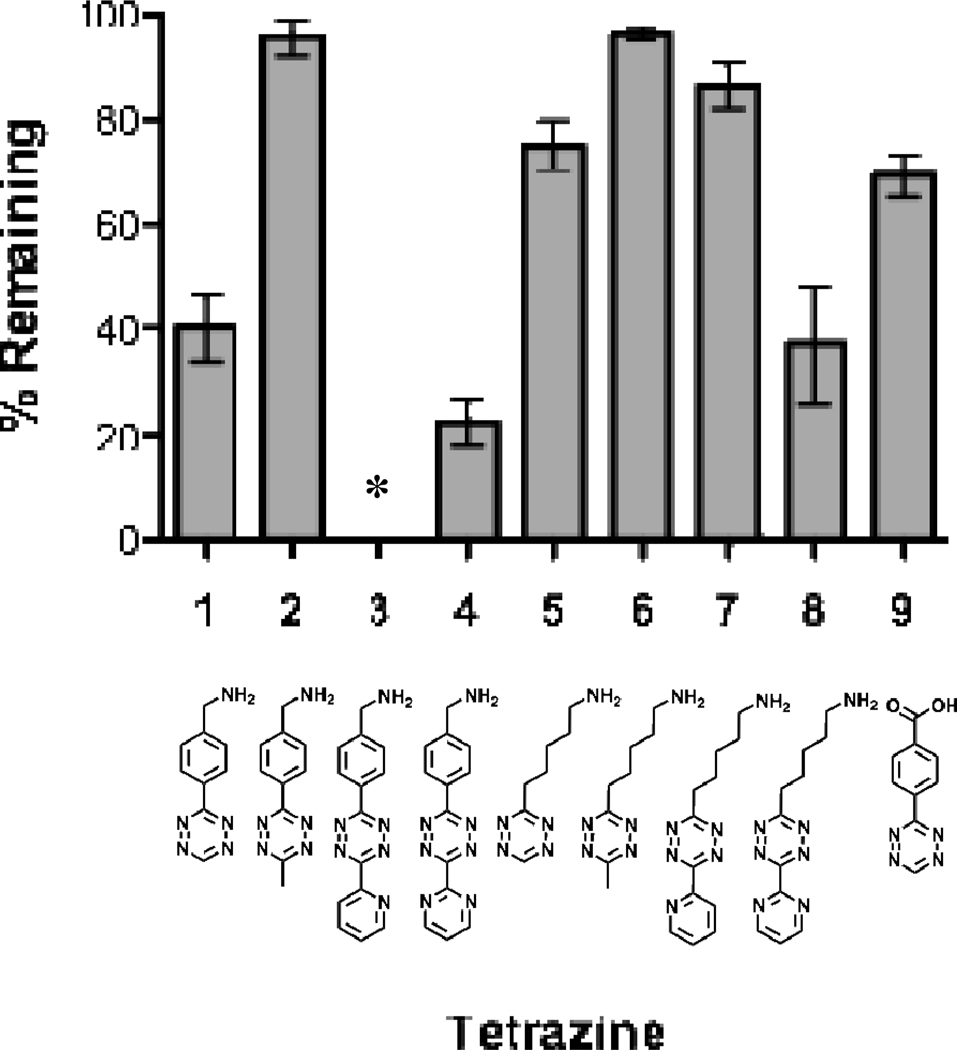
As tetrazines continue to become increasingly important moieties in biological labeling/detection studies, a more physiologically relevant stability experiment was conducted with the selected compounds. Tetrazines were incubated in pure fetal bovine serum (FBS) at 37°C and the decrease in absorbance at 515 nm was measured. The average percent remaining over 10 h can be seen in Figure 2. The most stable tetrazine was 6, with greater than 96% remaining after 10 h, followed by the other alkyl substituted tetrazines and ending with the least stable electron withdrawing group containing tetrazine 4. These results mimic the data observed in the previous PBS stability studies in that tetrazines with electron donating groups were the most stable. Accounting for the speed of the [4 + 2] cycloaddition reaction of these molecules with TCO and even NB, the stability of many of these tetrazines could be considered more than adequate for many biological labeling experiments. Depending on the individual application, any one of the tetrazines analyzed in this manuscript could be of importance. For example, if the tetrazine is to be employed where rapid reaction kinetics are desired, a faster compound that also has a good balance of chemical stability such as 1 or 9 would be a logical choice. Many applications of 1 have already been demonstrated (vide supra) including conjugation to proteins and nanoparticles.19, 33 However, in applications where rapid cycloaddition kinetics may not be as critical and improved chemical stability would be more beneficial, a tetrazine able to withstand more harsh chemical environments or endure long-term solution storage such as 2 or 6 may be of more value.
Figure 2.
Stability of selected tetrazines in FBS at 37°C after 10 h.
* It was not possible to measure the serum stability of tetrazine 3 due to formation of a precipitate after incubation in FBS for approximately 1.5–2h.
Cancer Cell Labeling
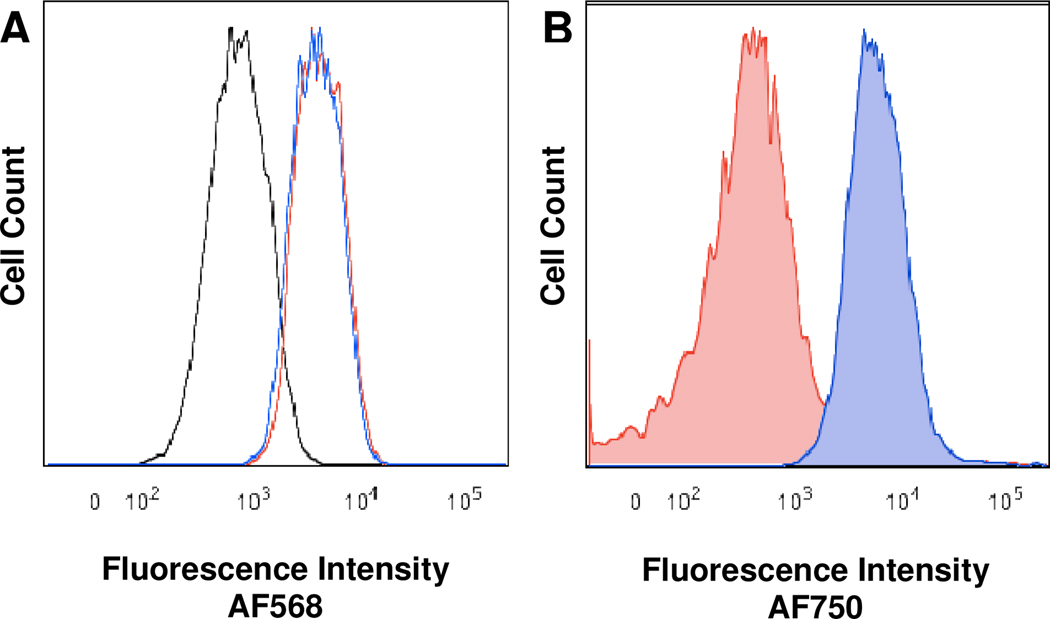
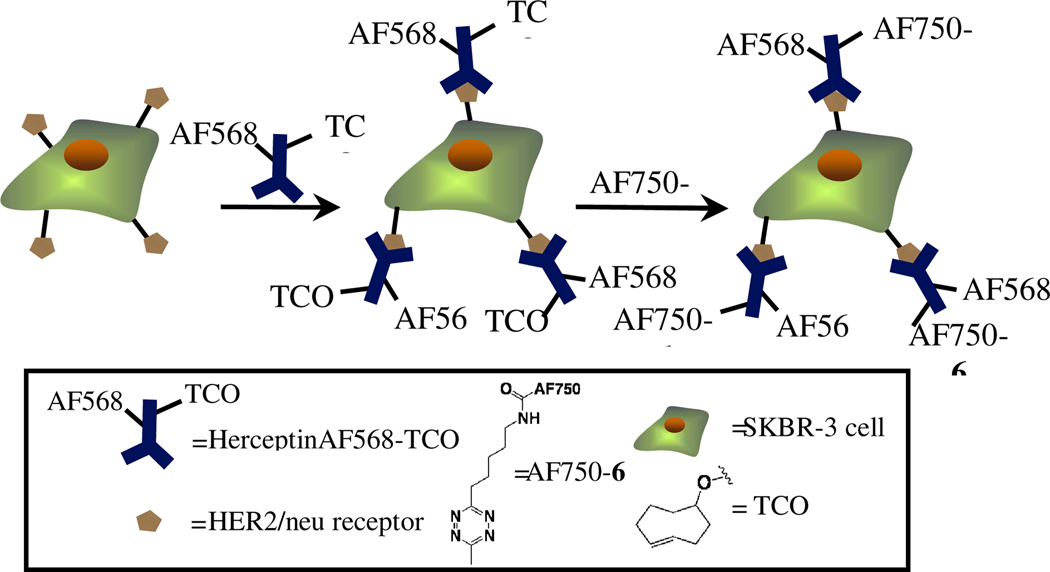
To confirm the biological utility of the new tetrazine derivatives, 6 was chosen as a representative compound for validation in pre-targeted labeling studies with live SKBR-3 human breast cancer cells. Since we have already used the much faster tetrazine 1 in a similar experiment,13 we wanted to demonstrate that the slowest tetrazine from this study was still a practical cycloaddition partner with TCO for bioorthogonal use. Despite showing the slowest reaction kinetics, tetrazine 6 demonstrated the best serum stability, was extremely water soluble and was synthesized/purified in relatively high yield. SKBR3 cells over-express HER2/neu receptors and have been shown previously to be useful in pre-targeted cell labeling assays with a tetrazine-norbornene system.10 The HER2/neu antibody, Herceptin, was labeled with AF568 and TCO through sequential incubations with the dye succinimidyl ester and then TCO-succinimidyl carbonate. The fluorescent labeling allows for tracking of the antibody and in order to follow the cycloaddition reaction, the free amine tetrazine 6 was conjugated with the succinimidyl ester of AF750 to afford AF750-6. For confirmation of cycloaddition reactivity in a biological environment, SKBR3 cells were incubated with labeled antibodies HerceptinAF568-TCO or HerceptinAF568 (control). After washing, antibody labeled cells were then incubated with AF750-6, washed again and analyzed by flow cytometry. Histograms showing the antibody labeling of cells incubated with both the control and TCO labeled Herceptin can be seen in Figure 3A. This figure indicates that the TCO loaded antibody shows identical affinity for the cells as the AF568 only loaded Herceptin. Figure 3B demonstrates that cells labeled with HerceptinAF568-TCO react with AF750-6 through the significant shift in near infrared fluorescence of these cells compared to the HerceptinAF568 labeled control cells. This study confirms the effectiveness of our selected tetrazine for bioorthogonal labeling.
Figure 3.
Flow cytometry histograms of SKBR3 cells. A) AF568 fluorescence of cells only (black), cells incubated with HerceptinAF568 (red), or HerceptinAF568-TCO (blue) followed by AF750-6. B) AF750 fluorescence of cells incubated with HerceptinAF568 (red) or HerceptinAF568-TCO (blue) followed by AF750-6.
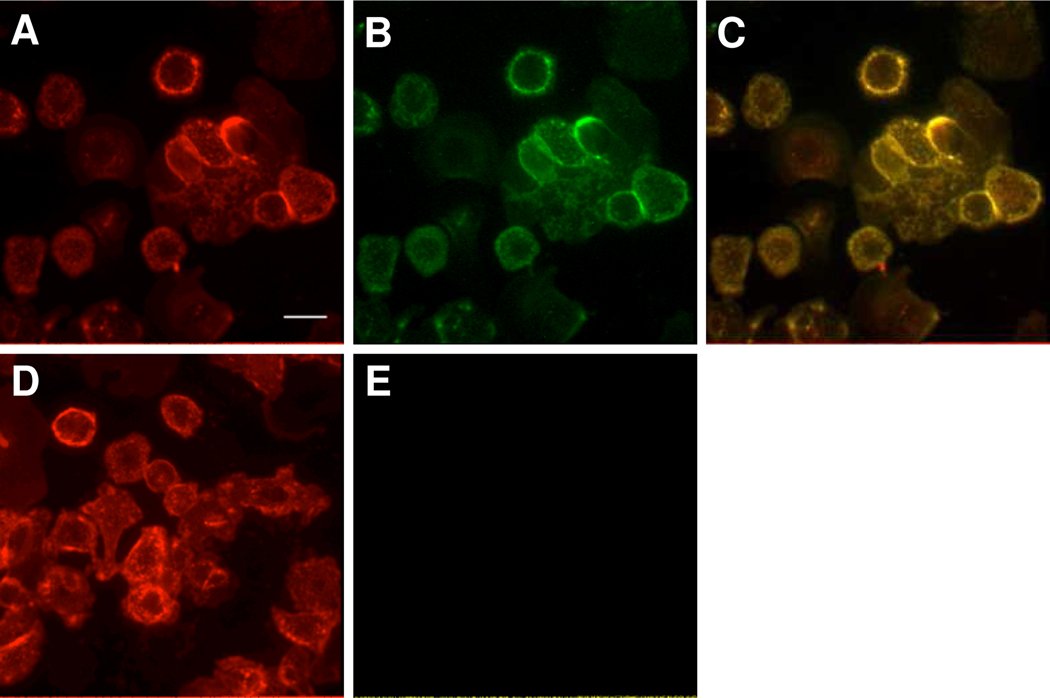
In order to corroborate the flow cytometry data, we looked at the same HerceptinAF568-TCO reaction but using AF750-6 under fluorescence microscopy. The antibodies from the flow cytometry experiment were used along with AF750-6 as the cycloaddition partner (See Scheme 2). SKBR3 cells were incubated individually with the labeled antibodies, followed by washing, subsequent incubation with AF750-6, and imaging (Figure 4, A–E). As can be seen in this figure, cells labeled with HerceptinAF568-TCO are also labeled with AF750-6, but cells incubated with control HerceptinAF568 showed no fluorescence in the 750 nm channel. This study further demonstrates that our selected tetrazine is useful in bioorthogonal labeling on par with previously reported compounds.
Scheme 2.
Cell labeling experiment.
Figure 4.
Fluorescent microscopy images of SKBR3 cells. Cells were treated with HerceptinAF568-TCO (A–C) or control HerceptinAF568 (D,E) followed by AF750-6 for 30 min. Displayed is fluorescence from A) AF568, B) AF750, C) AF568/AF750 merge, D) AF568, E) AF750. Scale bar (top left): 50 µm
Conclusions
A series of twelve 1,2,4,5-tetrazines was synthesized with a combination of four substituent groups and three conjugatable handles. Upon initial screening for reactivity and solution stability of these compounds, several were chosen for more in depth follow up studies. These studies revealed a considerable range of serum stabilities and cycloaddition reactivities with TCO. Overall, tetrazines substituted with electron donating groups tended to be more stable, but had slower cycloaddition kinetics, and hydrogen substituted tetrazines, such as 1 and 9, demonstrated a good balance of solution stability and fast reaction kinetics compared to compounds that would electronically be more favorable suggesting these tetrazines may react faster due to less steric interference with the dienophile. Based on these data along with ease of synthesis/purification, a tetrazine (6) was selected with exceptional chemical stability for biological evaluation. This selected tetrazine, despite demonstrating relatively slow kinetics, was then shown to be suitable for bioorthogonal use based on pre-targeted cancer cell labeling studies using flow cytometry and fluorescence microscopy. The presented tetrazines add more compounds to the inverse electron demand [4 + 2] cycloaddition arsenal that can be chosen from for different bioorthogonal labeling applications depending on the desired chemical properties.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Jack Szostak at MGH for the use of the Stopped-Flow spectrophotometer. The authors would also like to thank Alex Chudnovskiy and Elizabeth Tiglao of CSB for technical assistance with cell culture and flow cytometry and Neal K. Devaraj for helpful discussions. This research is supported in part by NIH grants RO1EB010011 and P50CA86355.
Footnotes
Supporting Information
Supporting information available: analytical HPLC trace of AF750-6 and initial tetrazine screens. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Moses JE, Moorhouse AD. The Growing Applications of Click Chemistry. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 2.Agard NJ, Prescher JA, Bertozzi CR. A Strain-Promoted [3+2] Azid-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 3.Ning X, Guo J, Wolfert MA, Boons G-J. Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neef AB, Schultz C. Selective Fluorescence Labeling of Lipids in Living Cells. Angew. Chem. Int. Ed. 2009;48:1498–1500. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]
- 5.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Copper-free Click Chemistry in Living Animals. Proc. Natl. Acad. Sci. USA. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In Vivo Imaging of Membrane-Associated Glycans in Developing Zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewett JC, Bertozzi CR. Cu-free Click Cycloaddition Reactions in Chemical Biology. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackman ML, Royzen M, Fox JM. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaraj NK, Weissleder R, Hilderbrand SA. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjugate Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldeck W, Wiessler M, Ehemann V, Pipkorn R, Spring H, Debus J, Didinger B, Mueller G, Langowski J, Braun K. TMZ-BioShuttle - A Reformulated Temozolomide. IntJ. Med. Sci. 2008;5:273–284. doi: 10.7150/ijms.5.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Bioorthogonal Turn-On Probes for Imaging Small Molecules Inside Living Cells. Angew. Chem. Int. Ed. 2010;49:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition. Angew. Chem. Int. Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossin R, Verkerk PR, Bosch SMvd, Vulders RCM, Verel I, Lub J, Robillard MS. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem. Int. Ed. 2010;122:3447–3450. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 15.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-yielding, Two-step 18F Labeling Strategy for 18F-PARP1 Inhibitors. ChemMedChem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Tetrazine-trans-Cyclooctene Ligation for the Rapid Construction of 18F Labeled Probes. Chem. Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and In Vivo Imaging of a 18F-Labeled PARP1 Inhibitor Using a Chemically Orthogonal Scavenger-assisted High-performance Method. Angew. Chem. Int. Ed. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haun JB, Castro CM, Wang R, Peterson VM, Marinelli BS, Lee H, Weissleder R. Micro-NMR for Rapid Molecular Analysis of Human Tumor Samples. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002048. 71ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Bioorthogonal Chemistry Amplifies Nanoparticle Binding and Enhances the Sensitivity of Cell Detection. Nat. Nanotechol. 2010;5:660–665. doi: 10.1038/nnano.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carboni RA, Lindsey RA., Jr Reactions of Tetrazines with Unsaturated Compounds. A New Synthesis of Pyridazines. J. Am. Chem. Soc. 1959;81:4342–4346. [Google Scholar]
- 21.Boger DL, Sakya SM. Inverse Electron Demand Diels-Alder Reactions of 3,6-Bis(methylthio)-1,2,4,5-tetrazine: 1,2-Diazine Introduction and Direct Implementation of a Divergent 1,2,4,5-Tetrazine - 1,2-Diazine - Benzene (Indoline/Indole) Diels-Alder Strategy. J. Org. Chem. 1988;53:1415–1423. [Google Scholar]
- 22.Soenen DR, Zimpleman JM, Boger DL. Synthesis and Inverse Electron Demand Diels-Alder Reactions of 3,6-Bis(3,4-dimethoxybenzoyl)-1,2,4,5-tetrazine. J. Org. Chem. 2003;68:3593–3598. doi: 10.1021/jo020713v. [DOI] [PubMed] [Google Scholar]
- 23.Sauer J, Lang D. Diels-Alder-Reaktionen der 1.2.4.5-Tetrazine. Angew. Chem. 1964;76:603. [Google Scholar]
- 24.Sauer J, Lang D, Mielert A. Reaktivitaetsfolge von Dienen gegenueber Maleinsaeureanhydrid bei der Diels-Alder-Reaktion. Angew. Chem. 1962;74:352–353. [Google Scholar]
- 25.Sauer J, Wiest H. Diels-Alder-additionen mit "inversem" Elektronenbedarf. Angew. Chem. 1962;74:353. [Google Scholar]
- 26.Thalhammer F, Wallfahrer U, Sauer J. Reaktivitaet Einfacher Offenkettiger und Cyclischer Dienophile bei Diels-Alder-Reaktionen mit Inversem Elektronenbedarf. Tetrahedron Lett. 1990;31:6851–6854. [Google Scholar]
- 27.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. Design and Synthesis of Highly Reactive Dienophiles for the Tetrazine-trans-Cyclooctene Ligation. J. Am. Chem. Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer J. Structure-reactivity Problem in Cycloaddition Reactions to Form Heterocyclic Compounds. Chem. Heterocycl. Compd. 1995;31:1140–1154. [Google Scholar]
- 29.Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. Inverse-electron-demand Diels-Alder Reaction as a Highly Efficient Chemoselective Ligation Procedure: Synthesis and Function of a BioShuttle for Temozolomide Transport into Prostate Cancer Cells. J. Pept. Sci. 2009;15:235–241. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]
- 30.Schoch J, Wiessler M, Jaeschke A. Post-synthetic Modification of DNA by Inverse-electron-demand Diels-Alder Reaction. J. Am. Chem. Soc. 2010;132:8846–8847. doi: 10.1021/ja102871p. [DOI] [PubMed] [Google Scholar]
- 31.Wiessler M, Waldeck W, Kliem C, Pipkorn R, Braun K. The Diels-Alder-Reaction with Inverse-Electron-Demand, a Very Efficient Versatile Click-Reaction Concept for Proper Ligation of Variable Molecular Partners. Int. J. Med. Sci. 2010;7:19–28. doi: 10.7150/ijms.7.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaempchen T, Massa W, Overheu W, Schmidt R, Seitz G. Zur Kenntnis von Reaktionen des 1,2,4,5-Tetrazin-3,6-dicarbonsaeure-dimethylesters mit Nucleophilen. Chem. Ber. 1982;115:683–694. [Google Scholar]
- 33.Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. Development of a Bioorthogonal and Highly Efficient Conjugation Method for Quantum Dots Using Tetrazine-norbornene Cycloaddition. J. Am. Chem. Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.