Abstract
The synthesis, growth inhibition and radioprotective activity of the PrC-210 aminothiol, 3-(methylamino)-2-((methylamino)methyl)propane-1-thiol, and its polyamine and thiolated polyamine progenitors are reported. All of the molecules significantly inhibited growth of cultured normal human fibroblasts. The combination of an ROS-scavenging thiol group and a positively charged alkyl-amine backbone provided the most radioprotective aminothiol molecule.
Keywords: 3-(methylamino)-2-((methylamino)methyl)propane-1-thiol, aminothiol, growth regulator, radioprotector, polyamine, ROS-scavenger
Radiotherapy-induced dermatitis is a common side effect seen in up to 85% of patients who receive a course of radiotherapy as part of their cancer therapy regimen1. A topically administered radioprotector that could be applied prior to radiotherapy on each of the 30 irradiation days would reduce pain and long term scarring and would improve patient compliance in receiving all 30 days of treatment. Skipped radiotherapy days have a discernible risk for the patient as measured by a decrease in 5 year survival rate for breast cancer2.
There is also a need for new systemically administered radioprotectors that lack the side effects of nausea/vomiting and hypotension/fainting that have hampered the use of current generation aminothiol radioprotectors, most notably the five carbon aminothiophosphonate, amifostine3.
The aminothiol radioprotector design concepts of: i) a flexible alkyl chain backbone, which carries a positive charge due to one or more amine groups, to achieve ionic interaction and concentration around negatively charged DNA in cells, and ii) the presence of a free or capped thiol group to scavenge oxygen free radicals formed from ionizing radiation, have been used before in programs to build radioprotective molecules within both the U.S.4 and the former Soviet Union5.
In the present investigation, we disclose a process in which: i) the number of alkyl-amine segments in the aminothiol backbone is systematically increased to increase drug-DNA affinity and ionic interaction, resulting in increased growth inhibition that is associated with this enhanced drug-DNA interaction, and ii) the placement or “display” of a free thiol reactive oxygen species (ROS) scavenger at the end of a short alkyl side chain that displaces or “displays” the scavenger moiety away from the DNA backbone to theoretically enable ROS scavenging before ROS attack on dG bases within cellular DNA. This work has resulted in a small family of new aminothiol molecules6,7, the prototype of which, PrC-210, is described in initial detail here.
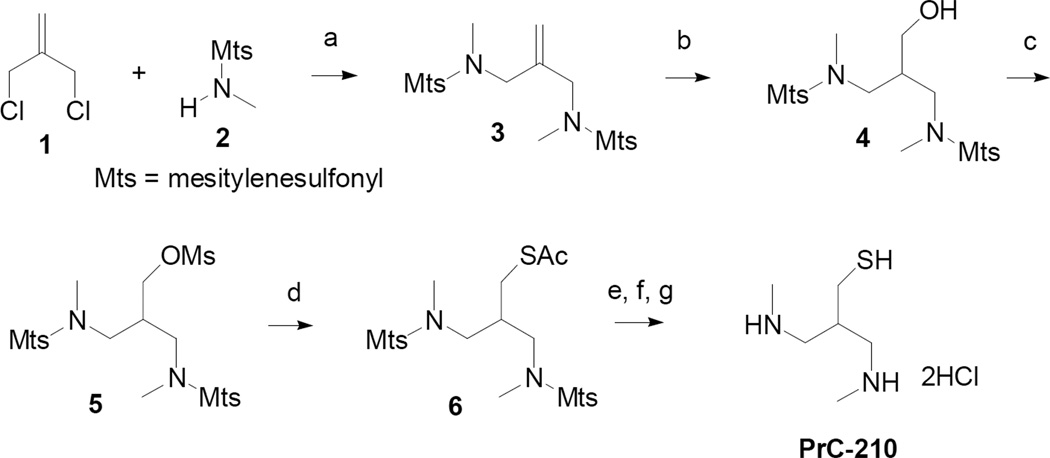
The synthesis of PrC-210, shown in Scheme 1, started with a double displacement of chloride from 1 using N-methyl mesitylenesulfonamide (2) and sodium hydride, to form allylic sulfonamide 3. Hydroboration of 3 afforded clean conversion to sulfonamide alcohol 4. Using standard conditions, 4 was converted to mesylate 5 which was immediately treated with potassium thioacetate to form 6. Following an established procedure,8 the mesitylene (Mts) protecting groups were removed with HBr/HOAc, in the presence of excess phenol. The deblocking procedure also hydrolyzed the thioacetate group. Work up resulted in a mixture of PrC-210 and the corresponding disulfide (dimer). The mixture was treated with 2-mercaptoethanol to cleave the disulfide and the product, PrC-210, was precipitated from EtOH as the HCl salt. Subsequent recrystallizations removed the sulfurous odor.
Scheme 1.
Reagents and conditions: (a) NaH, DMF, THF; (b) BH3-THF; EtOH, H2O2, NaOH (aq); (c) MsCl, Et3N, CH2Cl2; (d) KSAc, DMF; (e) HBr, PhOH, HOAc, CH2Cl2; (f) K2CO3 (aq); (g) HCl (aq), 2-mercaptoethanol, EtOH; recrystallize.
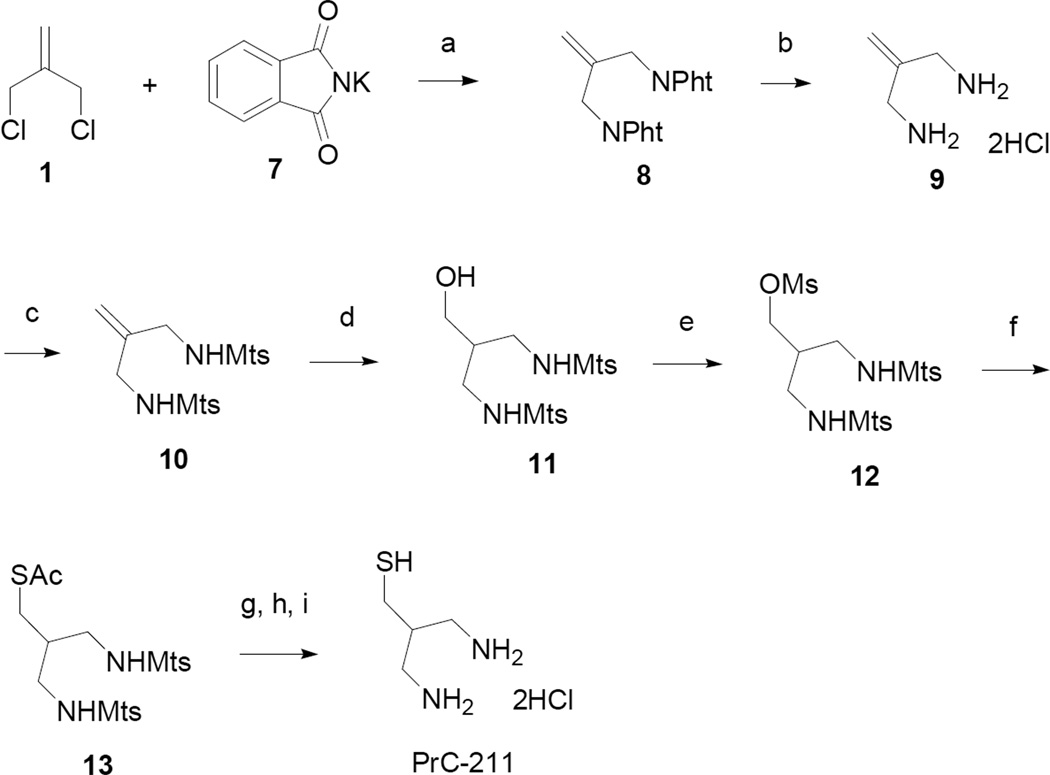
The synthesis of PrC-211, shown in Scheme 2, employed a modification of the route used for PrC-210. An attempt was made to form sulfonamide 10 directly by displacement of chloride from 1 using mesitylenesulfonamide, activated with sodium hydride. A complex mixture formed, from which 10 could not be isolated in pure form. Alternatively, 1 was treated with potassium phthalimide to form allylic phthalimide 8.9 Removal of the phthalate groups with hydrazine provided 9, which upon treatment with mesitylenesulfonyl chloride afforded the bis-sulfonamide 10 in good yield. Using the sequence from the PrC-210 preparation, hydroboration, mesylation, thioacetate displacement and deblocking, PrC-211 was obtained as the HCl salt and subsequently recrystallized.
Scheme 2.
Reagents and conditions: (a) 2 eq. potassium phthalimide, DMF; (b) 1. NH2NH2-H2O, 2. HCl; (c) NaOH, mesitylenesulfonyl chloride; (d) 1. BH3-THF, 2. H2O2, NaOH; (e) MsCl, Et3N, CH2Cl2; (f) KSAc, DMF; (g) HBr, PhOH, HOAc, CH2Cl2; (h) K2CO3 (aq); (i) HCl (aq), 2-mercaptoethanol; recrystallize.
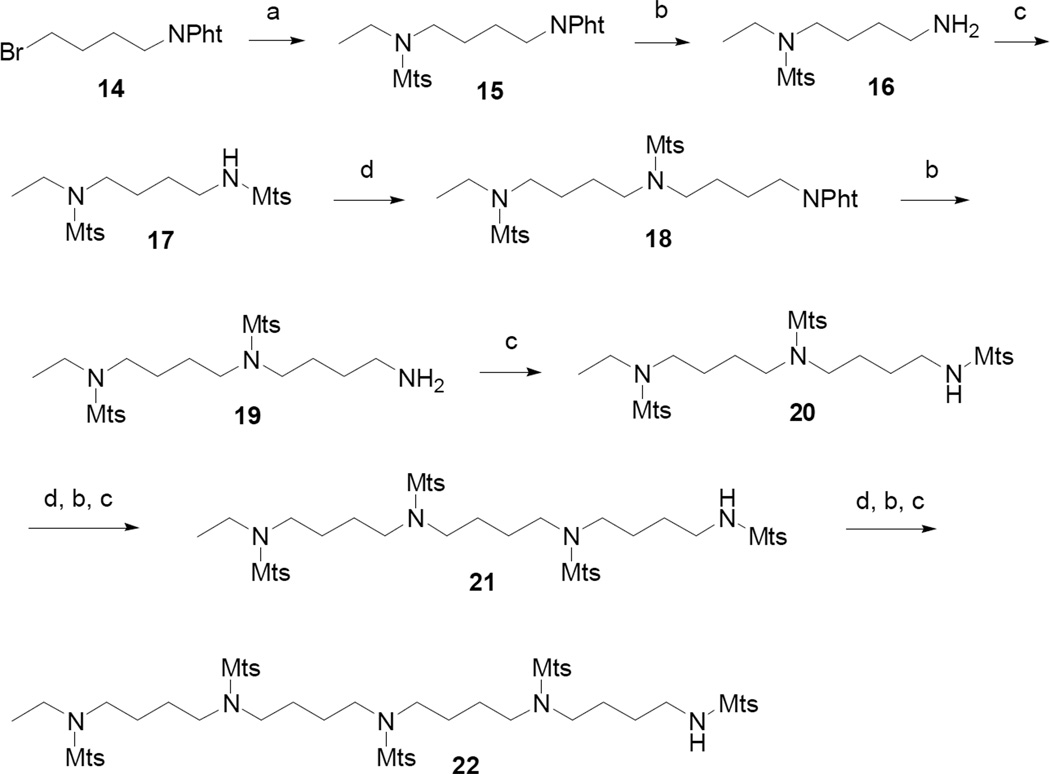
The amine side chains, synthesized according to the route illustrated in Scheme 3, were constructed as sulfonamide-protected intermediates, each with a single point of attachment (N-H), at one terminus, for coupling to the olefinic core (Fig. 1E). Preparations for sulfonamides 17 and 20 have previously been described.10 A convenient alternative approach was found that employed a modification of a reported method,11 starting with N-(4-bromobutyl)phthalimide (14). Displacement with N-ethyl mesitylenesulfonamide and sodium hydride, afforded 15, which upon treatment with hydrazine, was converted to 16. Amidation with mesitylenesulfonyl chloride, under standard conditions, gave 17. Sulfonamide 17, a protected diamine, represents the smallest amine side chain unit in the series, which includes 20, 21 and 22. Sulfonamide 17 was systematically elaborated to the subsequent side chain units using the same methodology as described for 17.
Scheme 3.
Reagents and conditions: (a) N-ethyl mesitylenesulfonamide, NaH, DMF; (b) N2H4-H2O; (c) mesitylenesulfonyl chloride, Et3N, CH2Cl2; (d) 4-bromobutylphthalimide, NaH, DMF
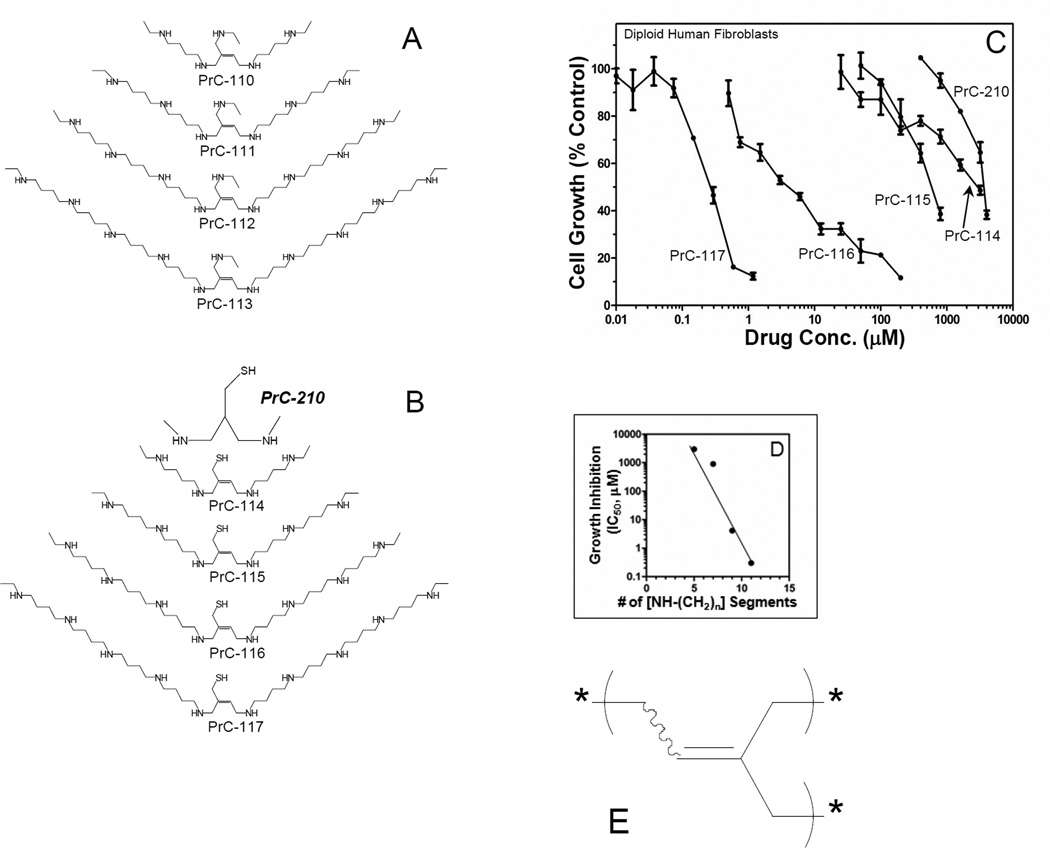
Figure 1.
Growth inhibition of normal human cells in culture by polyamine and aminothiol molecules. Structures of (A) first generation synthetic polyamines. (B) first generation “thiolated polyamines” and the next generation aminothiol, PrC-210. (C) Molecules were added at indicated concentrations to log phase, growing cultures of diploid human fibroblasts, and cell numbers were measured 4 days later. (D) Growth inhibition is directly related to the number of [NH-(CH2)] segments present in the polyamine or aminothiol molecule. (E) Olefinic core – common to polyamine and polyamine-thiol series.
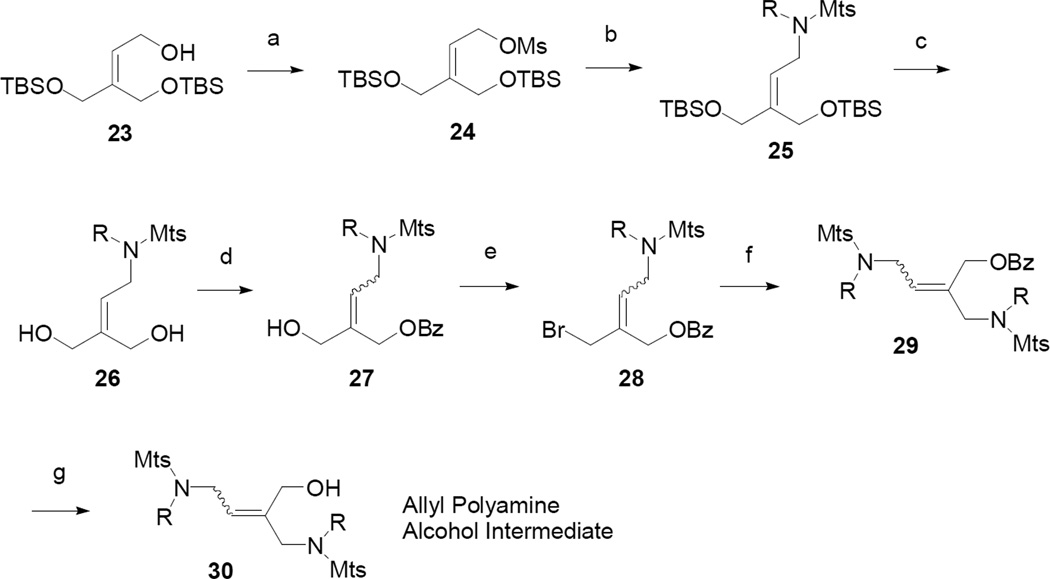
Coupling of the various amine side chains to the olefinic core is shown in Scheme 4. The synthesis commenced with the known TBS-protected allylic alcohol 23.12 Mesylation, using standard conditions, provided the activated intermediate 24. Coupling with a sulfonamide side chain and sodium hydride provided 25. Removal of the TBS-protecting groups with HCl afforded diol 26. Treatment of 26 with 1 equivalent of benzoyl chloride, in the presence of pyridine, afforded alcohol 27 as a mixture of isomers. Activation of the allylic alcohol 27, for amine side chain coupling, was attempted with methanesulfonyl chloride, resulting in a complex mixture. Alternatively, the alcohol was converted to bromide 28, with phosphorous tribromide, and immediately coupled with a second side chain unit to form 29. Hydrolysis of the benzoate group was carried out under standard conditions to afford the versatile polyamine alcohol intermediate 30, which was a common intermediate for the formation of both polyamines and polyamine thiols.
Scheme 4.
Reagents and conditions: (a) MsCl, Et3N, CH2Cl2; (b) Amine Side Chain, NaH, DMF; (c) HCl, MeOH; (d) BzCl, Pyd; (e) PBr3, Toluene; H2O; (f) Amine Side Chain, NaH, DMF; (g) NaOH.
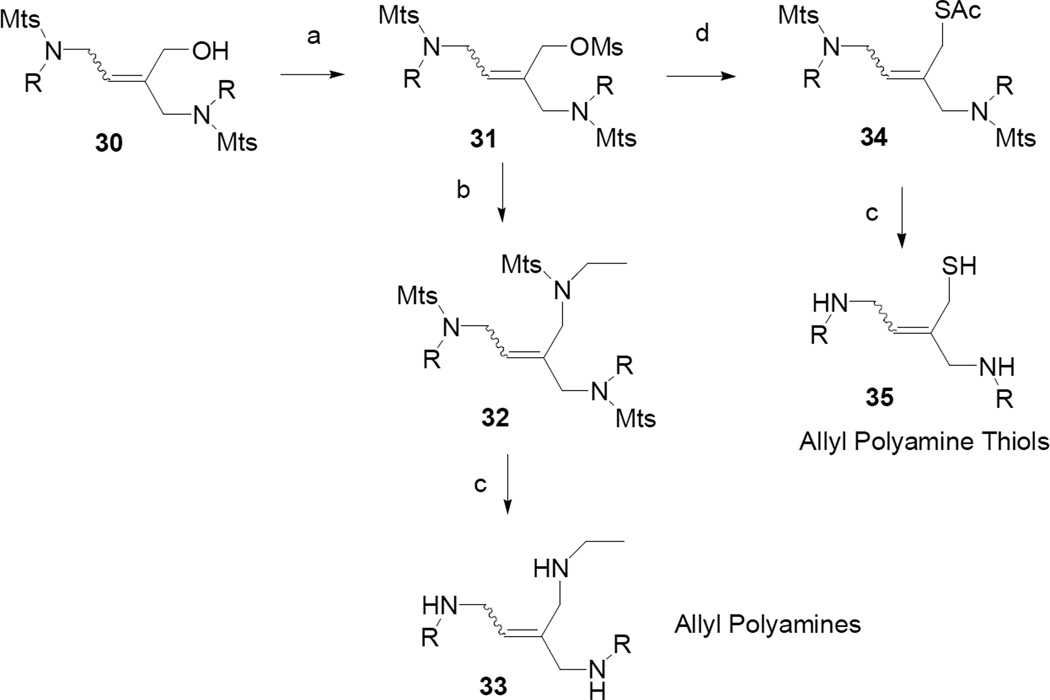
Conversion of the versatile intermediate 30 to polyamines and polyamine thiols is shown in Scheme 5. Treatment of 30 with methanesulfonyl chloride and triethylamine afforded the mesylate 31. Displacement with N-ethyl mesitylenesulfonamide, activated with sodium hydride, provided 32 which was deblocked with HBr/HOAc and phenol, to afford crude polyamine 33. Free-basing with potassium carbonate followed by HCl treatment gave 33 hydrochloride salt as a mixture of cis/trans isomers. Mesylate intermediate 31 was treated with potassium thioacetate to form 34 which was deblocked, using the same method as for 32, to form 35. Free-basing and treatment with HCl provided 35 as the hydrochloride salt.
Scheme 5.
Reagents and conditions: (a) MsCl, Et3N, CH2Cl2; (b) N-Et mesitylenesulfonamide, NaH, DMF; (c) HBr, PhOH, HOAc, CH2Cl2; K2CO3 (aq); HCl (aq); (d) KSAc, DMF.
Fig. 1A provides a summary of the first set of polyamine structures that was synthesized using the strategies outlined in Schemes 3–5. We anticipated that these drug molecules would provide radio- and chemoprotection to human cells by inducing a cell cycle block in mammalian cells at the G1/S cell cycle border because of tight binding of the (+) charged polyamine backbones to the (−) charged DNA backbone13. Such a block can provide time for DNA repair in radiation- or mutagen-treated cells before washout of the polyamine drug and restoration of cell cycle progression14. Fig. 1B shows a nearly identical set of polyamines now with the addition of a thiol group to each molecule. The goal in synthesizing these molecules was to achieve the same cell cycle inhibition anticipated for the Fig. 1A molecules, but now combined with the addition of a thiol group that could serve as a scavenger for the burst of short-lived ROS that is generated when mammalian tissue is irradiated15. The aminothiol and polyamine structures in Fig. 1B are growth inhibitory when added to rapidly growing, normal human fibroblasts, and the potency of growth inhibition was directly related to the number of (NH-(CH2)n) segments present within the molecule (Fig. 1D).
Early in vitro studies of radioprotection with cultured cells demonstrated that the long, polyamine structures were so growth-inhibitory (Fig. 1C) that we could not add sufficient moles of the thiolated polyamines (e.g., PrC-117) to cell cultures that would enable the thiol groups to significantly scavenge and radioprotect when the tissue culture cells were irradiated. This provided the impetus to design and synthesize the PrC-200 series of small aminothiols, of which PrC-210 is the prototype.
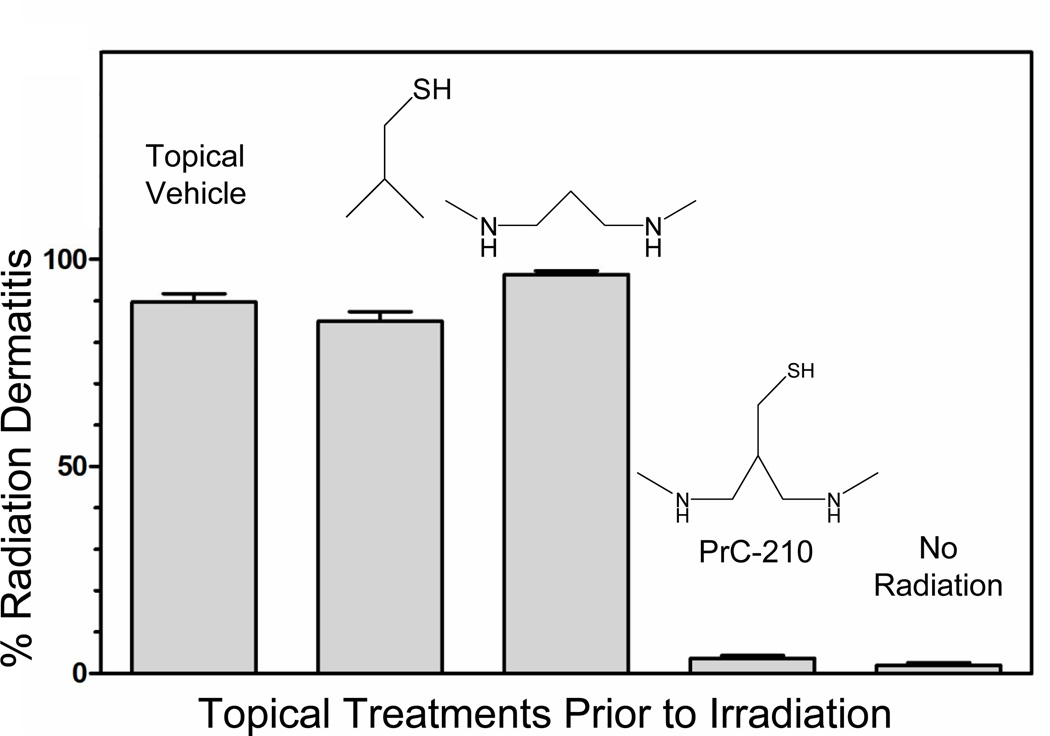
To determine if PrC-210 could function as a topical radioprotector that could prevent radiation dermatitis when applied prior to a cancer patient’s daily radiotherapy, a rat assay that realistically mimics human radiation dermatitis was created16. This assay quantifies the severity of radiation dermatitis in rat skin 13 days after a single radiation dose of 17.3 Gy. In this study, PrC-210 was applied to rat skin in an ethanol:water delivery vehicle four times in the two hours before irradiation, and control rats received only topical vehicle before irradiation. Additional drug/irradiation groups of three rats each received either the alkyl-thiol moiety or the alkyl-diamine moiety from PrC-210 in the same delivery vehicle. Following the 17.3 Gy irradiation of a 1.5 × 3.0 cm patch of dorsal skin on the rats’ backs, rats were scored 13 days later. The ability of the PrC-210 aminothiol to completely prevent radiation dermatitis was striking. Other radiodermatitis test groups exploring efficacy of thiolated polyamines or other PrC-200 series aminothiols have shown that PrC-210 is the most potent and most effective topical aminothiol to date.
Figure 2.
Prevention of radiation-induced dermatitis in rat model by prior topical application of the PrC-210 aminothiol or its component structures. Molecules were applied to skin in an alcohol:water delivery vehicle prior to receiving a 17.33 Gy radiation dose to the 1.5 cm × 3.0 cm skin site. Radiation dermatitis severity (i.e., % of the site covered by scab material) was scored 13 days following irradiation.
Acknowlegements
This work was supported by grant #CA-22484 from NCI as well as research funds from ProCertus BioPharm, Inc.
References and notes
- 1.Salvo N, Barnes E, van Draanen J. Curr. Oncol. 2010;17:94. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bese NS, Hendry J, Jeremic B. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:654. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG. Semin. Oncol. 1996;23(Suppl 8):83. [PubMed] [Google Scholar]
- 4.Weiss JF, Landauer MR. Int. J. Radiat. Biol. 2009;85:539. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 5.Plotnikova ED, Levitman MK, Shaposhnikova VV, Koshevoj JV, Eidus LK. Int. J. Radiat. Oncol. Biol. Phys. 1988;15:1197. doi: 10.1016/0360-3016(88)90204-0. [DOI] [PubMed] [Google Scholar]
- 6.Fahl WE, Peebles DD, Copp RR. 7,314,959. United States patent US. See http://patft.uspto.gov.
- 7.Soref CM, Fahl WE. Intl. J. Radiat. Oncol. Biol. Phys. (in press). [Google Scholar]
- 8.Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JR, Kramer D. J. Med. Chem. 1994;37:3464. doi: 10.1021/jm00047a004. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Guo X, Li Z, Yao L. Hecheng Huaxue. 1997;5:291. [Google Scholar]
- 10.Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B. J. Med. Chem. 2001;44:390. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron RJ, Weimar WR, Wu Q, Feng Y, McManis JS. J. Med. Chem. 1996;39:5257. doi: 10.1021/jm960545x. [DOI] [PubMed] [Google Scholar]
- 12.Moon HR, Siddiqui MA, Sun G, Filippov IV, Landsman NA, Lee YC, Adams KM, Barchi JJ, Deschamps JR, Nicklaus MC, Kelley JA, Marquez VE. Tetrahedron. 2010;66:6707. doi: 10.1016/j.tet.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuerstein BG, Williams LD, Basu HS, Marton LJ. J. Cellular Biochem. 1991;46:37. doi: 10.1002/jcb.240460107. [DOI] [PubMed] [Google Scholar]
- 14.Kramer DL, Chang BD, Chen Y, Diegelman P, Alm K, Black AR, Roninson IB, Porter CW. Cancer Res. 2001;61:7754. [PubMed] [Google Scholar]
- 15.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelson RB. Cancer Res. 2001;61:3894. [PubMed] [Google Scholar]
- 16.Peebles DD, Soref CM, Copp RR, Fahl WE. Rad. Res. (in press). [Google Scholar]