Abstract
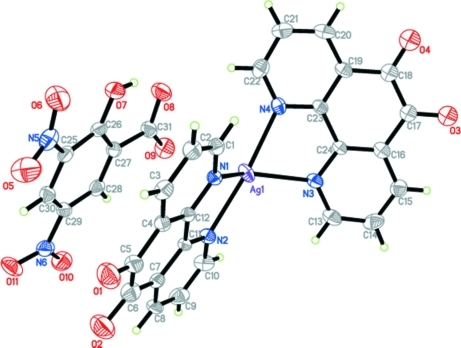
In the cation of the title salt, [Ag(C12H6N2O2)2](C7H3N2O7), the AgI atom is coordinated in a distorted tetrahedral geometry by four N atoms from two 1,10-phenanthroline-5,6-dione ligands, while the 3,5-dinitrosalicylate anion has only a short contact [2.847 (6) Å] between one of its O atoms and the AgI atom. The dihedral angle between the two 1,10-phenanthroline-5,6-dione ligands is 58.4 (1)°. There is an intramolecular O—H⋯O hydrogen bond in the 3,5-dinitrosalicylate anion.
Related literature
For general background to the structures and potential applications of supramolecular architectures with 1,10-phenantroline-5,6-dione and 3,5-dinitrosalicylic acid, see: Hiort et al. (1993 ▶); Song et al. (2007 ▶); Che et al. (2008 ▶); Onuegbu et al. (2009 ▶). For the synthesis of the 1,10-phenantroline-5,6-dione ligand, see: Dickeson & Sumers (1970 ▶).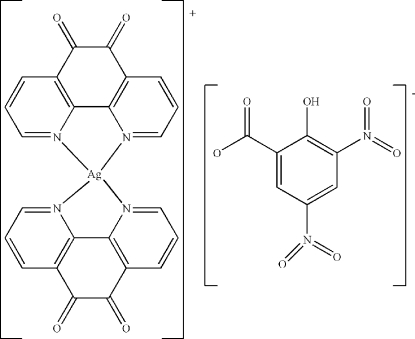
Experimental
Crystal data
[Ag(C12H6N2O2)2](C7H3N2O7)
M r = 755.36
Monoclinic,
a = 11.757 (2) Å
b = 18.297 (4) Å
c = 13.223 (3) Å
β = 103.91 (3)°
V = 2761.1 (11) Å3
Z = 4
Mo Kα radiation
μ = 0.81 mm−1
T = 174 K
0.30 × 0.24 × 0.20 mm
Data collection
Bruker SMART diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2002 ▶) T min = 0.780, T max = 0.910
12726 measured reflections
5059 independent reflections
3914 reflections with I > 2σ(I)
R int = 0.052
Refinement
R[F 2 > 2σ(F 2)] = 0.083
wR(F 2) = 0.163
S = 1.11
5013 reflections
442 parameters
22 restraints
H-atom parameters constrained
Δρmax = 1.11 e Å−3
Δρmin = −0.72 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶) and DIAMOND (Brandenburg, 1999 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811053785/vn2025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811053785/vn2025Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Ag1—N1 | 2.400 (6) |
| Ag1—N2 | 2.351 (6) |
| Ag1—N3 | 2.337 (6) |
| Ag1—N4 | 2.377 (6) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O7—H7⋯O8 | 0.82 | 1.71 | 2.457 (9) | 151 |
Acknowledgments
The authors thank Jiangsu University for supporting this work.
supplementary crystallographic information
Comment
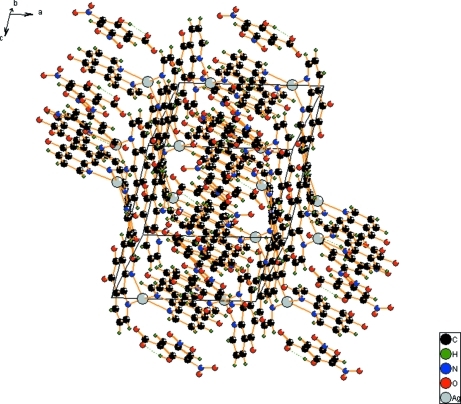
The design and construction of supramolecular architectures have received considerable attention in recent years, mostly motivated by their intriguing structural features and potential applications in molecular adsorption, molecular sensing, magnetism, catalysis and non-linear optics (Che et al., 2008). Metal complexes with 1,10-phenantroline-5,6-dione (L) and 3,5-dinitrosalicylic acid as important ligands for the construction of metal-organic supramolecular architectures have been increasingly studied over recent years (Hiort et al., 1993; Onuegbu et al., 2009; Song et al., 2007). We report herein on the crystal structure of the title compound (Fig. 1). The molecular structure of the title compound, is made up of one [AgL2]+ cation and one 3,5-dinitrosalicylate anion. The AgI atom is surrounded by four N atoms from two 1,10-phenanthroline-5,6-dione ligands, while the 3,5-dinitrosalicylate ligand is uncoordinated. In the compound the dihedral angle between the phendione ligand A (C1-C12, N1, N2, O1 and O2) and B (C13-C24, N3, N4, O3, and O4) is 58.4 (1)°. The dihedral angle between B and 3,5-dinitrosalicylate ligand C (C25-C31, N5, N6, O5-O11) is 56.1 (2)°. The dihedral angle between A and C is 2.4 (9)°, suggesting that the planes of rings A and C are almost parallel. In addition, in the 3,5-dinitrosalicylate ligand, there is one intramolecular O–H···O hydrogen bond (Fig. 2).
Experimental
The L ligand was synthesized according to the literature method (Dickeson & Sumers, 1970). The title compound was synthesized under hydrothermal conditions. A mixture of L (0.042 g, 0.2 mmol), 3,5-dinitrosalicylic acid (0.046 g, 0.2 mmol), AgNO3 (0.034 g, 0.2 mmol) and water (10 mL) was placed in a 25 mL Teflon-lined autoclave and heated for 3 days at 433 K under autogenous pressure. Upon cooling and opening the bomb, yellow block-shaped crystals were obtained, then washed with water and dried in air.
Refinement
All H atoms on C atoms were positioned geometrically and refined as riding atoms, with (C—H = 0.93 Å) and refined as riding, with Uiso(H) = 1.2 Ueq(C). The hydrogen atom of the hydroxyl group was located in a difference Fourier map, and was refined with a suitable O—H distance restraint; Uiso(H) = 1.5 Ueq(O). The geometry of the aromatic rings was regularized using distance and planariety restraints.
Figures
Fig. 1.
The molecular structure of the title compound with the atom numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. All H atoms are presented as a small spheres of arbitrary radius.
Fig. 2.
A view of the crystal packing of the title compound, showing the O–H···O hydrogen bonds interaction.
Crystal data
| C24H12AgN4O4·C7H3N2O7 | F(000) = 1512 |
| Mr = 755.36 | Dx = 1.817 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 5197 reflections |
| a = 11.757 (2) Å | θ = 3.2–25.4° |
| b = 18.297 (4) Å | µ = 0.81 mm−1 |
| c = 13.223 (3) Å | T = 174 K |
| β = 103.91 (3)° | Prism, yellow |
| V = 2761.1 (11) Å3 | 0.3 × 0.24 × 0.2 mm |
| Z = 4 |
Data collection
| Oxford Diffraction Gemini R Ultra diffractometer | 5059 independent reflections |
| Radiation source: fine-focus sealed tube | 3914 reflections with I > 2σ(I) |
| graphite | Rint = 0.052 |
| ω scans | θmax = 25.4°, θmin = 3.2° |
| Absorption correction: multi-scan SADABS (Bruker, 2002) | h = −14→11 |
| Tmin = 0.780, Tmax = 0.910 | k = −17→22 |
| 12726 measured reflections | l = −15→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.083 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.163 | H-atom parameters constrained |
| S = 1.11 | w = 1/[σ2(Fo2) + (0.0354P)2 + 13.4705P] where P = (Fo2 + 2Fc2)/3 |
| 5013 reflections | (Δ/σ)max < 0.001 |
| 442 parameters | Δρmax = 1.11 e Å−3 |
| 22 restraints | Δρmin = −0.72 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 0.21081 (6) | 0.44038 (3) | 0.52487 (5) | 0.0540 (2) | |
| C1 | 0.4793 (7) | 0.5137 (4) | 0.6222 (5) | 0.0435 (18) | |
| H1 | 0.4399 | 0.5580 | 0.6074 | 0.052* | |
| C2 | 0.5976 (7) | 0.5157 (4) | 0.6690 (6) | 0.048 (2) | |
| H2 | 0.6371 | 0.5599 | 0.6845 | 0.058* | |
| C3 | 0.6552 (7) | 0.4497 (4) | 0.6920 (6) | 0.0470 (19) | |
| H3 | 0.7345 | 0.4488 | 0.7248 | 0.056* | |
| C4 | 0.5941 (6) | 0.3844 (4) | 0.6659 (5) | 0.0380 (16) | |
| C5 | 0.6515 (9) | 0.3155 (4) | 0.6883 (7) | 0.062 (2) | |
| C6 | 0.5895 (7) | 0.2505 (5) | 0.6710 (7) | 0.063 (2) | |
| C7 | 0.4678 (6) | 0.2544 (4) | 0.6244 (5) | 0.0431 (18) | |
| C8 | 0.3994 (8) | 0.1885 (4) | 0.6037 (6) | 0.053 (2) | |
| H8 | 0.4340 | 0.1433 | 0.6229 | 0.064* | |
| C9 | 0.2836 (9) | 0.1930 (5) | 0.5557 (7) | 0.063 (2) | |
| H9 | 0.2388 | 0.1508 | 0.5403 | 0.075* | |
| C10 | 0.2342 (7) | 0.2594 (5) | 0.5305 (7) | 0.055 (2) | |
| H10 | 0.1552 | 0.2613 | 0.4966 | 0.066* | |
| C11 | 0.4090 (6) | 0.3201 (3) | 0.5978 (5) | 0.0338 (16) | |
| C12 | 0.4738 (5) | 0.3869 (3) | 0.6196 (5) | 0.0335 (16) | |
| C13 | 0.0848 (7) | 0.4147 (4) | 0.2775 (7) | 0.055 (2) | |
| H13 | 0.1080 | 0.3666 | 0.2933 | 0.066* | |
| C14 | 0.0374 (8) | 0.4324 (5) | 0.1743 (7) | 0.061 (2) | |
| H14 | 0.0287 | 0.3972 | 0.1223 | 0.073* | |
| C15 | 0.0034 (7) | 0.5036 (5) | 0.1506 (6) | 0.053 (2) | |
| H15 | −0.0298 | 0.5170 | 0.0820 | 0.064* | |
| C16 | 0.0190 (6) | 0.5564 (4) | 0.2312 (5) | 0.0432 (18) | |
| C17 | −0.0122 (5) | 0.6312 (4) | 0.2098 (5) | 0.052 (2) | |
| C18 | −0.0077 (7) | 0.6805 (4) | 0.2948 (6) | 0.055 (2) | |
| C19 | 0.0460 (6) | 0.6571 (4) | 0.3993 (6) | 0.0447 (18) | |
| C20 | 0.0688 (7) | 0.7058 (4) | 0.4853 (7) | 0.055 (2) | |
| H20 | 0.0483 | 0.7548 | 0.4749 | 0.067* | |
| C21 | 0.1205 (8) | 0.6816 (5) | 0.5829 (7) | 0.059 (2) | |
| H21 | 0.1348 | 0.7131 | 0.6398 | 0.071* | |
| C22 | 0.1506 (7) | 0.6090 (5) | 0.5945 (6) | 0.056 (2) | |
| H22 | 0.1865 | 0.5925 | 0.6611 | 0.067* | |
| C23 | 0.0809 (6) | 0.5844 (3) | 0.4175 (5) | 0.0369 (16) | |
| C24 | 0.0632 (5) | 0.5330 (3) | 0.3334 (5) | 0.0322 (15) | |
| C25 | 0.6149 (7) | 0.4146 (4) | 0.9278 (5) | 0.0419 (18) | |
| C26 | 0.5083 (7) | 0.4530 (4) | 0.8867 (5) | 0.0411 (18) | |
| C27 | 0.4075 (6) | 0.4103 (4) | 0.8430 (5) | 0.0388 (17) | |
| C28 | 0.4092 (7) | 0.3351 (4) | 0.8460 (5) | 0.0433 (18) | |
| H28 | 0.3421 | 0.3080 | 0.8181 | 0.052* | |
| C29 | 0.5165 (8) | 0.3002 (4) | 0.8928 (5) | 0.0434 (19) | |
| C30 | 0.6177 (7) | 0.3387 (4) | 0.9327 (6) | 0.0435 (18) | |
| H30 | 0.6871 | 0.3143 | 0.9625 | 0.052* | |
| C31 | 0.2956 (8) | 0.4467 (5) | 0.7881 (6) | 0.050 (2) | |
| N1 | 0.4178 (5) | 0.4524 (3) | 0.5970 (4) | 0.0347 (13) | |
| N2 | 0.2931 (5) | 0.3228 (3) | 0.5518 (5) | 0.0399 (14) | |
| N3 | 0.0992 (5) | 0.4629 (3) | 0.3562 (5) | 0.0422 (15) | |
| N4 | 0.1319 (5) | 0.5605 (3) | 0.5164 (5) | 0.0423 (14) | |
| N5 | 0.7268 (7) | 0.4516 (5) | 0.9629 (5) | 0.0587 (19) | |
| N6 | 0.5177 (8) | 0.2213 (4) | 0.9005 (5) | 0.0560 (19) | |
| O1 | 0.7645 (7) | 0.3121 (4) | 0.7270 (6) | 0.088 (2) | |
| O2 | 0.6419 (7) | 0.1890 (4) | 0.6988 (6) | 0.096 (2) | |
| O3 | −0.0430 (5) | 0.6542 (3) | 0.1162 (4) | 0.0700 (18) | |
| O4 | −0.0502 (6) | 0.7445 (3) | 0.2780 (5) | 0.080 (2) | |
| O5 | 0.8165 (6) | 0.4147 (4) | 0.9745 (6) | 0.091 (2) | |
| O6 | 0.7295 (6) | 0.5175 (4) | 0.9763 (6) | 0.085 (2) | |
| O7 | 0.5032 (6) | 0.5236 (3) | 0.8852 (4) | 0.0608 (16) | |
| H7 | 0.4365 | 0.5366 | 0.8565 | 0.091* | |
| O8 | 0.2947 (6) | 0.5172 (3) | 0.7949 (5) | 0.0684 (17) | |
| O9 | 0.2133 (5) | 0.4109 (4) | 0.7368 (5) | 0.0641 (16) | |
| O10 | 0.4296 (7) | 0.1876 (3) | 0.8584 (5) | 0.0713 (19) | |
| O11 | 0.6097 (6) | 0.1916 (3) | 0.9511 (5) | 0.0684 (18) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.0472 (4) | 0.0491 (4) | 0.0609 (4) | 0.0150 (3) | 0.0036 (3) | 0.0149 (3) |
| C1 | 0.056 (5) | 0.038 (4) | 0.039 (4) | −0.007 (4) | 0.015 (4) | 0.001 (3) |
| C2 | 0.054 (5) | 0.046 (5) | 0.048 (5) | −0.011 (4) | 0.019 (4) | −0.001 (4) |
| C3 | 0.033 (4) | 0.065 (6) | 0.042 (4) | −0.003 (4) | 0.008 (3) | −0.006 (4) |
| C4 | 0.036 (4) | 0.043 (4) | 0.037 (4) | 0.007 (3) | 0.013 (3) | 0.001 (3) |
| C5 | 0.084 (7) | 0.059 (5) | 0.049 (5) | 0.022 (5) | 0.028 (5) | 0.008 (4) |
| C6 | 0.067 (5) | 0.068 (6) | 0.057 (5) | 0.007 (5) | 0.019 (4) | −0.001 (5) |
| C7 | 0.056 (4) | 0.045 (4) | 0.033 (4) | 0.005 (4) | 0.020 (3) | 0.001 (3) |
| C8 | 0.085 (5) | 0.031 (4) | 0.049 (5) | 0.014 (4) | 0.026 (4) | 0.008 (4) |
| C9 | 0.077 (5) | 0.053 (5) | 0.064 (6) | −0.012 (5) | 0.027 (4) | −0.010 (5) |
| C10 | 0.043 (5) | 0.050 (5) | 0.072 (6) | −0.002 (4) | 0.014 (4) | −0.009 (4) |
| C11 | 0.044 (4) | 0.027 (4) | 0.031 (4) | 0.008 (3) | 0.011 (3) | 0.001 (3) |
| C12 | 0.041 (4) | 0.031 (4) | 0.030 (4) | −0.001 (3) | 0.011 (3) | 0.002 (3) |
| C13 | 0.051 (5) | 0.036 (4) | 0.072 (6) | −0.001 (4) | 0.005 (4) | −0.002 (4) |
| C14 | 0.056 (6) | 0.057 (6) | 0.063 (6) | −0.002 (4) | 0.002 (4) | −0.024 (5) |
| C15 | 0.047 (5) | 0.060 (6) | 0.047 (5) | −0.001 (4) | 0.003 (4) | −0.002 (4) |
| C16 | 0.032 (4) | 0.042 (4) | 0.053 (5) | 0.000 (3) | 0.006 (3) | 0.004 (4) |
| C17 | 0.041 (5) | 0.053 (5) | 0.058 (5) | 0.003 (4) | 0.002 (4) | 0.019 (4) |
| C18 | 0.041 (5) | 0.049 (5) | 0.071 (6) | −0.004 (4) | 0.004 (4) | 0.006 (5) |
| C19 | 0.038 (4) | 0.037 (4) | 0.058 (5) | 0.001 (3) | 0.008 (4) | 0.001 (4) |
| C20 | 0.045 (5) | 0.035 (4) | 0.084 (7) | 0.001 (4) | 0.012 (5) | −0.008 (4) |
| C21 | 0.052 (5) | 0.055 (5) | 0.069 (6) | 0.003 (4) | 0.012 (5) | −0.017 (5) |
| C22 | 0.047 (5) | 0.070 (6) | 0.047 (5) | −0.002 (4) | 0.004 (4) | −0.005 (4) |
| C23 | 0.028 (4) | 0.036 (4) | 0.045 (4) | 0.003 (3) | 0.005 (3) | 0.002 (3) |
| C24 | 0.022 (3) | 0.029 (4) | 0.045 (4) | 0.000 (3) | 0.006 (3) | 0.004 (3) |
| C25 | 0.056 (5) | 0.040 (4) | 0.029 (4) | −0.010 (4) | 0.008 (3) | −0.003 (3) |
| C26 | 0.061 (5) | 0.033 (4) | 0.033 (4) | 0.001 (4) | 0.018 (4) | −0.003 (3) |
| C27 | 0.045 (5) | 0.039 (4) | 0.035 (4) | 0.001 (3) | 0.016 (3) | 0.003 (3) |
| C28 | 0.061 (5) | 0.042 (4) | 0.032 (4) | −0.008 (4) | 0.019 (4) | −0.002 (3) |
| C29 | 0.071 (6) | 0.030 (4) | 0.035 (4) | 0.006 (4) | 0.024 (4) | 0.006 (3) |
| C30 | 0.047 (5) | 0.046 (5) | 0.038 (4) | 0.004 (4) | 0.013 (3) | 0.004 (3) |
| C31 | 0.060 (6) | 0.049 (5) | 0.048 (5) | 0.014 (4) | 0.024 (4) | 0.011 (4) |
| N1 | 0.036 (3) | 0.032 (3) | 0.035 (3) | 0.002 (3) | 0.008 (3) | 0.000 (3) |
| N2 | 0.038 (4) | 0.037 (3) | 0.045 (3) | 0.000 (3) | 0.009 (3) | −0.001 (3) |
| N3 | 0.038 (4) | 0.032 (3) | 0.053 (4) | 0.000 (3) | 0.004 (3) | −0.002 (3) |
| N4 | 0.037 (3) | 0.043 (4) | 0.046 (4) | 0.003 (3) | 0.007 (3) | 0.006 (3) |
| N5 | 0.059 (5) | 0.071 (6) | 0.042 (4) | −0.011 (4) | 0.006 (3) | −0.007 (4) |
| N6 | 0.091 (6) | 0.039 (4) | 0.046 (4) | 0.003 (4) | 0.032 (4) | 0.003 (3) |
| O1 | 0.081 (5) | 0.099 (6) | 0.084 (5) | 0.033 (4) | 0.020 (4) | 0.021 (4) |
| O2 | 0.086 (6) | 0.079 (5) | 0.110 (6) | 0.022 (4) | −0.003 (4) | 0.000 (4) |
| O3 | 0.067 (4) | 0.071 (4) | 0.067 (4) | 0.003 (3) | 0.005 (3) | 0.024 (3) |
| O4 | 0.097 (5) | 0.040 (4) | 0.094 (5) | 0.018 (3) | 0.005 (4) | 0.016 (3) |
| O5 | 0.055 (5) | 0.099 (6) | 0.113 (6) | −0.004 (4) | 0.008 (4) | −0.033 (5) |
| O6 | 0.089 (5) | 0.050 (4) | 0.098 (5) | −0.024 (4) | −0.016 (4) | 0.002 (4) |
| O7 | 0.086 (5) | 0.039 (3) | 0.058 (4) | 0.000 (3) | 0.019 (3) | −0.001 (3) |
| O8 | 0.080 (5) | 0.057 (4) | 0.073 (4) | 0.022 (3) | 0.028 (3) | 0.010 (3) |
| O9 | 0.041 (4) | 0.081 (4) | 0.069 (4) | 0.004 (3) | 0.012 (3) | −0.002 (3) |
| O10 | 0.110 (6) | 0.039 (3) | 0.066 (4) | −0.015 (4) | 0.025 (4) | −0.003 (3) |
| O11 | 0.088 (5) | 0.042 (3) | 0.083 (4) | 0.017 (3) | 0.036 (4) | 0.021 (3) |
Geometric parameters (Å, °)
| Ag1—N1 | 2.400 (6) | C16—C24 | 1.394 (7) |
| Ag1—N2 | 2.351 (6) | C16—C17 | 1.428 (7) |
| Ag1—N3 | 2.337 (6) | C17—O3 | 1.275 (7) |
| Ag1—N4 | 2.377 (6) | C17—C18 | 1.432 (8) |
| Ag1—O9 | 2.847 (6) | C18—O4 | 1.272 (9) |
| C1—N1 | 1.333 (9) | C18—C19 | 1.438 (8) |
| C1—C2 | 1.380 (11) | C19—C23 | 1.397 (7) |
| C1—H1 | 0.9300 | C19—C20 | 1.419 (11) |
| C2—C3 | 1.383 (11) | C20—C21 | 1.361 (12) |
| C2—H2 | 0.9300 | C20—H20 | 0.9300 |
| C3—C4 | 1.394 (10) | C21—C22 | 1.374 (12) |
| C3—H3 | 0.9300 | C21—H21 | 0.9300 |
| C4—C12 | 1.400 (7) | C22—N4 | 1.340 (10) |
| C4—C5 | 1.426 (8) | C22—H22 | 0.9300 |
| C5—O1 | 1.304 (11) | C23—N4 | 1.373 (8) |
| C5—C6 | 1.385 (8) | C23—C24 | 1.433 (7) |
| C6—O2 | 1.293 (10) | C24—N3 | 1.362 (8) |
| C6—C7 | 1.417 (8) | C25—C30 | 1.390 (10) |
| C7—C11 | 1.390 (7) | C25—C26 | 1.426 (10) |
| C7—C8 | 1.438 (11) | C25—N5 | 1.454 (10) |
| C8—C9 | 1.358 (12) | C26—O7 | 1.292 (8) |
| C8—H8 | 0.9300 | C26—C27 | 1.421 (10) |
| C9—C10 | 1.353 (12) | C27—C28 | 1.377 (10) |
| C9—H9 | 0.9300 | C27—C31 | 1.498 (11) |
| C10—N2 | 1.347 (10) | C28—C29 | 1.416 (11) |
| C10—H10 | 0.9300 | C28—H28 | 0.9300 |
| C11—N2 | 1.353 (8) | C29—C30 | 1.374 (11) |
| C11—C12 | 1.432 (7) | C29—N6 | 1.448 (10) |
| C12—N1 | 1.365 (8) | C30—H30 | 0.9300 |
| C13—N3 | 1.343 (10) | C31—O9 | 1.228 (10) |
| C13—C14 | 1.383 (12) | C31—O8 | 1.293 (10) |
| C13—H13 | 0.9300 | N5—O6 | 1.218 (9) |
| C14—C15 | 1.376 (11) | N5—O5 | 1.230 (9) |
| C14—H14 | 0.9300 | N6—O10 | 1.218 (9) |
| C15—C16 | 1.417 (10) | N6—O11 | 1.252 (9) |
| C15—H15 | 0.9300 | O7—H7 | 0.8200 |
| N3—Ag1—N2 | 115.0 (2) | O4—C18—C19 | 120.4 (7) |
| N3—Ag1—N4 | 70.7 (2) | C17—C18—C19 | 119.4 (7) |
| N2—Ag1—N4 | 174.1 (2) | C23—C19—C20 | 117.9 (7) |
| N3—Ag1—N1 | 130.3 (2) | C23—C19—C18 | 119.5 (7) |
| N2—Ag1—N1 | 71.5 (2) | C20—C19—C18 | 122.5 (7) |
| N4—Ag1—N1 | 106.3 (2) | C21—C20—C19 | 120.8 (7) |
| N3—Ag1—O9 | 147.53 (19) | C21—C20—H20 | 119.6 |
| N2—Ag1—O9 | 76.74 (19) | C19—C20—H20 | 119.6 |
| N4—Ag1—O9 | 97.64 (19) | C20—C21—C22 | 117.7 (8) |
| N1—Ag1—O9 | 81.75 (18) | C20—C21—H21 | 121.2 |
| N1—C1—C2 | 124.2 (7) | C22—C21—H21 | 121.2 |
| N1—C1—H1 | 117.9 | N4—C22—C21 | 124.4 (8) |
| C2—C1—H1 | 117.9 | N4—C22—H22 | 117.8 |
| C1—C2—C3 | 117.6 (7) | C21—C22—H22 | 117.8 |
| C1—C2—H2 | 121.2 | N4—C23—C19 | 120.7 (6) |
| C3—C2—H2 | 121.2 | N4—C23—C24 | 118.6 (6) |
| C2—C3—C4 | 119.9 (7) | C19—C23—C24 | 120.6 (6) |
| C2—C3—H3 | 120.0 | N3—C24—C16 | 121.9 (6) |
| C4—C3—H3 | 120.0 | N3—C24—C23 | 117.9 (6) |
| C3—C4—C12 | 119.1 (6) | C16—C24—C23 | 120.1 (6) |
| C3—C4—C5 | 121.0 (7) | C30—C25—C26 | 121.4 (7) |
| C12—C4—C5 | 119.9 (7) | C30—C25—N5 | 116.1 (7) |
| O1—C5—C6 | 118.0 (7) | C26—C25—N5 | 122.5 (7) |
| O1—C5—C4 | 120.7 (8) | O7—C26—C27 | 120.8 (7) |
| C6—C5—C4 | 121.3 (9) | O7—C26—C25 | 122.2 (7) |
| O2—C6—C5 | 120.3 (8) | C27—C26—C25 | 117.0 (6) |
| O2—C6—C7 | 122.0 (8) | C28—C27—C26 | 122.2 (7) |
| C5—C6—C7 | 117.8 (8) | C28—C27—C31 | 117.6 (7) |
| C11—C7—C6 | 122.8 (7) | C26—C27—C31 | 120.2 (7) |
| C11—C7—C8 | 117.1 (7) | C27—C28—C29 | 117.9 (7) |
| C6—C7—C8 | 120.1 (7) | C27—C28—H28 | 121.0 |
| C9—C8—C7 | 119.4 (7) | C29—C28—H28 | 121.0 |
| C9—C8—H8 | 120.3 | C30—C29—C28 | 122.3 (7) |
| C7—C8—H8 | 120.3 | C30—C29—N6 | 119.4 (8) |
| C10—C9—C8 | 119.5 (8) | C28—C29—N6 | 118.2 (8) |
| C10—C9—H9 | 120.3 | C29—C30—C25 | 119.0 (7) |
| C8—C9—H9 | 120.3 | C29—C30—H30 | 120.5 |
| N2—C10—C9 | 123.6 (8) | C25—C30—H30 | 120.5 |
| N2—C10—H10 | 118.2 | O9—C31—O8 | 123.5 (8) |
| C9—C10—H10 | 118.2 | O9—C31—C27 | 120.9 (8) |
| N2—C11—C7 | 122.1 (6) | O8—C31—C27 | 115.5 (8) |
| N2—C11—C12 | 119.3 (5) | C1—N1—C12 | 118.7 (6) |
| C7—C11—C12 | 118.6 (6) | C1—N1—Ag1 | 127.8 (5) |
| N1—C12—C4 | 120.6 (6) | C12—N1—Ag1 | 113.4 (4) |
| N1—C12—C11 | 119.9 (6) | C10—N2—C11 | 118.3 (6) |
| C4—C12—C11 | 119.5 (6) | C10—N2—Ag1 | 125.8 (5) |
| N3—C13—C14 | 123.8 (8) | C11—N2—Ag1 | 115.8 (4) |
| N3—C13—H13 | 118.1 | C13—N3—C24 | 118.2 (6) |
| C14—C13—H13 | 118.1 | C13—N3—Ag1 | 124.2 (5) |
| C15—C14—C13 | 118.2 (8) | C24—N3—Ag1 | 116.8 (4) |
| C15—C14—H14 | 120.9 | C22—N4—C23 | 118.5 (6) |
| C13—C14—H14 | 120.9 | C22—N4—Ag1 | 125.9 (5) |
| C14—C15—C16 | 119.8 (7) | C23—N4—Ag1 | 114.5 (4) |
| C14—C15—H15 | 120.1 | O6—N5—O5 | 122.3 (8) |
| C16—C15—H15 | 120.1 | O6—N5—C25 | 119.8 (8) |
| C24—C16—C15 | 118.0 (6) | O5—N5—C25 | 117.8 (8) |
| C24—C16—C17 | 120.3 (6) | O10—N6—O11 | 123.8 (7) |
| C15—C16—C17 | 121.7 (6) | O10—N6—C29 | 118.7 (8) |
| O3—C17—C18 | 120.3 (7) | O11—N6—C29 | 117.6 (8) |
| O3—C17—C16 | 120.4 (7) | C26—O7—H7 | 109.5 |
| C18—C17—C16 | 119.3 (6) | C31—O9—Ag1 | 105.3 (5) |
| O4—C18—C17 | 120.2 (7) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O7—H7···O8 | 0.82 | 1.71 | 2.457 (9) | 151. |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: VN2025).
References
- Brandenburg, K. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2002). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2007). SAINT and SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Che, G. B., Liu, C. B., Liu, B., Wang, Q. W. & Xu, Z. L. (2008). CrystEngComm, 10, 184–191.
- Dickeson, J. E. & Sumers, L. A. (1970). Aust. J. Chem 23, 1023–1027.
- Hiort, C., Lincoln, P. & Norden, B. (1993). J. Am. Chem. Soc 115, 3448–3454.
- Onuegbu, J., Butcher, R. J., Hosten, C., Udeochu, U. C. & Bakare, O. (2009). Acta Cryst. E65, m1119–m1120. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Song, W.-D., Guo, X.-X. & Zhang, C.-H. (2007). Acta Cryst. E63, m399–m401.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811053785/vn2025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811053785/vn2025Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report