Summary
Aim.
The aim of this study is to evaluate the capability of Hyaloss™ matrix (Fab – Fidia Advanced Biopolymers – Pd – Italy), a biomaterial based on hyaluronic acid, used as organic scaffold in bone repair in post-extractive defects.
Materials and methods:
20 post-extractive sockets were selected, with similar size defects in the same patient and in the same hemiarch. Hyaluronic acid with high molecular weight (Hyaloss™ matrix, Fab – Pd – Italy) was mixed with autologous bone obtained using Safescraper® curve (Meta – Re – Italy) to repair post-extractive sites. Safescraper® is a cutting edge system that allows to the collection of autologous bone without using traditional, incision-based collection techniques, which could cause discomfort to the patient.
Results:
Clinical and hystological evaluations were performed, four months after grafting, in the maxilla and in the mandible. From a clinical point of view Hyaloss™ matrix mixed with autologous bone and patient’s blood becomes a substance similar to gel, which is easy to insert in to the defect. From a hystological point of view, in the treated site there is the presence of an erosive activity, with accelerated angiogenetic and bone remodelling activities.
Conclusions:
The preliminary results show an acceleration of the bone deposit process and of its remodelling due to the presence of Hyaloss™ matrix, which, from a clinical point of view, improves the handling and application of the bone matrix inside the defects and, from a hystologic point of view makes it possible to obtain bone regeneration in less time when it is used with autologous bone.
Keywords: autologous bone, hyaluronic acid, bone, bone defect healing, bone graft
Introduction
Numerous biologic processes could take place following a tooth extraction, with important changes of the anatomy of the post-extractive site (Trombelli, 2008).
Processes involved in the healing of the extraction socket have been evaluated in animal experimental situations (Lekic, 2001; Sato, 2007) and in human models (Boyne, 1966).
The anatomo-morphologic situation of the empty socket involves a vertical and horizontal bone reabsorption which modifies the morphologic aspect with a decrease in height, in the thickness of the alveolar bone and gingival collapse (Schropp, 2003; Trombelli, 2008).
At the end of the involutional processes the socket could result unsuitable for the implant diameter. At this point the clinician has to deal with surgical problems difficult to solve and, the insertion of the implant could be unsatisfying from an aesthetic point of view (Carlino, 2008).
For instance the positioning of graft material or the use of membranes to cover and prevent outer communication (Adriaens, 1999). Some authors do not agree with the insertion of graft material in post-extractive sites because it seems to interfere with the normal healing process of the bone in the areas where the implant is to be inserted (Becker, 1996; Buser, 1998; Artzi, 2000).
Much research, performed on human models using demineralized freeze-dried bone allograft (DFDBA) (Becker, 1996) and deproteinized natural bovine bone mineral (Bio-Oss) (Carmagnola, 2001), have demonstrated the presence of particles of grafted material in the alveolar sockets even 6–9 months after their insertion. Other authors (Lekovic, 1997) have demonstrated that using reabsorbable membranes made of polylactic and polyglicolic acid material can lower the reabsorption of the bone.
Bone grafting is a common option in treating bone defects and reconstructing alveolar bone before an implant insertion. Every grafting material, homologous, xenologous, heterologous or synthetic, has some drawbacks, even if they have a distinctive feature: availability on demand. Autologous bone is “gold standard” for bone grafting (Jakse, 2001) since it does not produce adverse reactions and has optimal biocompatible remodelling patterns and osteoinductive capabilities (Bunger, 2003; Hu, 2004). Bone has been used in blocks (Misch, 1992) or in particulates (Missori, 2002), alone (Dahlin, 1988) or under a membrane protected space in guided bone regeneration (GBR) procedure (Simion, 1998) or mixed with other graft materials (Hallman, 2002). Controversy remains as to whether cortical or spongy bone is the material of choice for autologous bone graft.
Particulated bone can be harvested in many ways. The most common method is to mill large bone portions (Springer, 2004). Treatment of transplants with the bone mill or lifting transplants by rotating electrical instruments seem to reduce the amount of viable bone cells supplied. Some authors collect bone during implant surgery (Zaffe, 2007), however they need an implant site. The use of a bone collector represents an uncommon technique. Bone collectors were proposed many years ago (Widmark, 2000), but they have been continuously redesigned, renewed, studied and proposed to achieve the most effective and practical use (Zaffe, 2007).
The aim of this study has been to evaluate, from a clinical perspective, if the hyaluronic acid mixed with cortical autologous bone particles harvested from intraoral sites can prevent or reduce the bone reabsorption after tooth avulsion, compared with control cases when only autologous bone is inserted, and make a hystological evaluation of the quantity and the quality of the bone tissue developed in the post-extractive socket 4 months after the graft insertion with microradiography and Sem analysis.
Materials and methods
Selection of the patients: we selected 10 patients (6 females and 4 males), aged between 25 and 55, who needed tooth extractions due to periodontal or endodontic problems. It was necessary to insert in their sockets osteointegrated implants to substitute at least two teeth. In particular we decided to consider sockets in the same hemiarch which could allow the insertion of 10 mm long implants. So there was no need to resort to GBR (Guided Bone Regeneration) to correct bone defects following tooth extractions, to favour a correct insertion of the implant in the socket after the avulsion of the compromised teeth.
Patients should not have systemic disorders. We also excluded from this study patients who showed, through anamnesis or radiography, symptoms or pathologies of nasal and paranasal sinuses, in case of implant insertion near the maxillary sinus. Before being inserted in to the protocol, we informed the patients about the kind of research they were going to be subdued to and they signed the informed consent. Every patient received basic information for adeguate control of bacterial plaque and oral hygiene. We evaluated the general state of health through anamnesis: an eventual hypothesis regarding implant rehabilitating treatment was considered through a clinical evaluation of the stomatognathic apparatus. The subjects representing potential candidates for this study were subjected to the following controls:
standard haematochemical evaluation, to rule out every systhemic pathology which was unknown at the moment of the anamnesis;
radiographic evaluation through orthopantomography (OPT), teleradiography of the skull in latero-lateral projection (Tele L-L) and computerized axial tomography plus dentalscan (TAC – Dentalscan), to identify the atrophy class, the possible pathologies of the maxillary sinus and to plan a detailed surgical excision.
The aim of this research has been to study the activity of a biopolymer, based on hyaluronic acid, used as scaffold to repair bone defects.
Hyaluronic acid is a natural polysaccharide with high molecular weight (>5MDa), which is normally present in great quantities in amorphous, extracellular matrixes such as basal laminae, connective matrixes and synovial fluid (Fig. 1). It has a simple, linear structure, due to the repetition of the dimer glucuronic acid/N-acetylglucosamin. Numerous studies (Toole, 2001; Huang, 2003; Chen, 1999; Weigel, 1988; Peattle, 2004; Peattle, 2002) highlighted the roles of hyaluronic acid in the inflammatory response, in antimicrobic and osteoinductive activities. These activities are particularly important in bone regeneration, especially in repairing bone defects (Slevin, 2002; Pilloni, 2003; Graber, 1999; Sasaki, 1995; Savani 2001). Hyaluronic acid, once it is inserted, is quickly metabolized and therefore its effect lasts only a short time.
Figure 1.
Hyaluronic acid.
HYAFF®-11 (Fidia- Abano Terme- Pd) has been created to stabilize this polymer, which derives from bacterial hyaluronic acid. From HYAFF®-11 it is possible to obtain Hyaloss™matrix, which is the subject of our study and which, once it has been dehydrated, becomes similar to a fibrous felt (Fig. 2).
Figure 2.
Hyaff’s molecule.
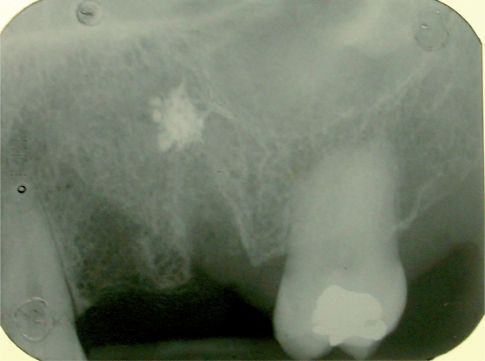
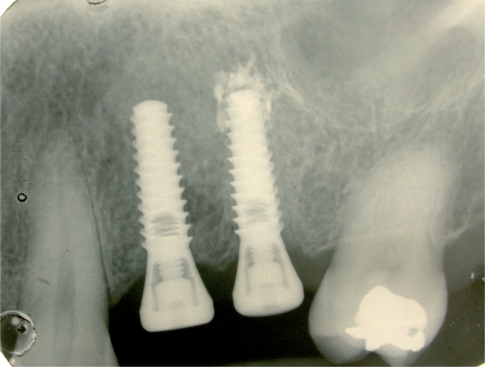
Surgery: following a local anaesthesia and elevation of a vestibular and palatal or lingual flap, the teeth are delicately extracted. The sockets, after the extraction of radicular rests, have been carefully cleaned using alveolar spoons to remove the granulation tissue (Fig. 3). In this research we decided to perform a surgical extraction and to insert Hyaloss™matrix and autologous bone and suture in the test site and simple suture and the application of autologous bone, collected using safescraper, in the control site.
Figure 3.
Radiographic control of two post extractive sites before the grafting.
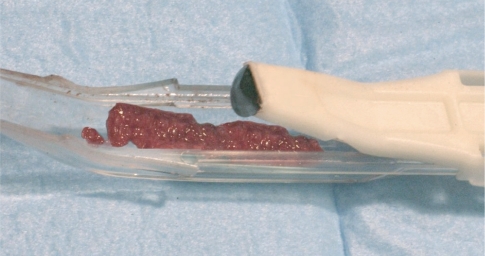
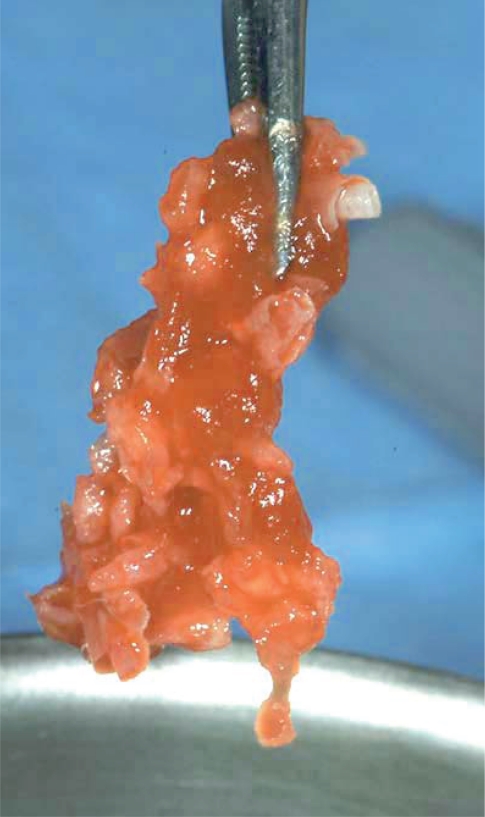
In this study Hyaloss™matrix was mixed with autologous bone to fill post-extractive cavities. The autologous bone was collected using Safescraper® (Meta – RE), a new device which allows the possibility to obtain large quantities of bone particulate with a slightly invasive surgical operation. The Safescraper®curve (Meta – RE – Italy) is used to repair post-extractive sites. Safescraper® is a cutting edge system to collect autologous cortical bone with a slightly invasive method, without using techniques which can cause great discomforts to the patient. With this method allows to the collection of great quantities of cortical and autologous bone (from 2 to 5 cc) (Fig. 4). Using Hyaloss™matrix as adjuvant of the autologous bone in the regeneration process improves the graft handling and the application of bone matrix obtained inside the defects. During the collection process the intertwined lamellae of bone, mix directly with blood, allowing Hyaloss™matrix to hydrate and to form a high density bone concentrate which can be used as a very good filler in regeneration therapy (Fig. 5). Patients were treated with chlorhexidine-dicluconate 0,2% twice a day for two weeks. They were also asked to undergo antibiotic therapy for 6 days, while an analgesic was suggested only in case of post-operative pain. Stitches were removed a week after the excision. Four months after bone and Hyaloss™matrix graft, after local anaesthesia and elevation of a buccal and lingual flap, fixtures were inserted in post-extractive sites and in control sites treated only with autologous bone (Fig. 6).
Figure 4.
Safescraper device for bone harvesting near the bone defect.
Figure 5.
Autologous cortical bone mixed with hyaluronic acid ready to be inserted in to the bone socket.
Figure 6.
The implants (Swiss Plus 3,75x12 mm) at 3 months grafting. The mesial implant is the control case (only cortical autologous bone), the distal implant is the treated case (autologous cortical bone and hyaluronic acid).
Biopsies: during the preparation of the implant site a surgical trephine of 3 mm diameter and 6 mm long was used to collect bone cores. Following the surgical protocol to prepare the implant site for the insertion of fixtures, the site was widened to receive implants of 3,7 mm diameter and 10 mm or more long. Biopsies were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.2 for 1 h at room temperature and then dehydrated and embedded in methyl methacrylate (PMMA) at 4°C, as reported elsewhere. Longitudinal thin (5-μm-thick) and thick (200-μm-thick) sections were obtained from biopsies using an Autocut 1150 bone microtome (Reichert-Jung-D) and a diamond saw microtome (1600, Leica, D), respectively. Thick sections were reduced to 100 μm and X-rayed (microradiographed). Thin sections have been stained with toluidine blue, trypanblue, solochrome cyanin/nuclear fast red as reported elsewhere. Microradiographs and thin sections were analyzed and photographed using an Axiophot Zeiss microscope under ordinary or polarized light.
Results
From a clinical point of view Hyaloss™matrix mixed with autologous bone and patient’s blood becomes a substance similar to gel, which is easy to insert in to the defect. The bone collection procedure allows the harvesting of great quantities of cortical autologous bone (2 to 5 cc). Therefore the use of Hyaloss™matrix in autologous bone grafting improves handling and application inside the defect. During the collection process the intertwined lamellae of bone, mixed with blood and Hyaloss™matrix, form a high density bone concentrate which can be used as a very good filler in regeneration therapy. Hyaluronic acid, thanks to its own features, helps to improve the tissue repairing processes, creating an ideal environment for the recovery and restoration of connective and bony tissues.
During sutures removal, no important tissue inflammation was observed. A 10 day follow-up post-operative clinical assessment demonstrated a gingivitis grade of 0 or 1. Thanks to the bacteriostatic properties of the tested polymer, a more effective control of the surgical wound and non bacterial contamination of the surgical site were observed in all instances (Pilloni, 2003).
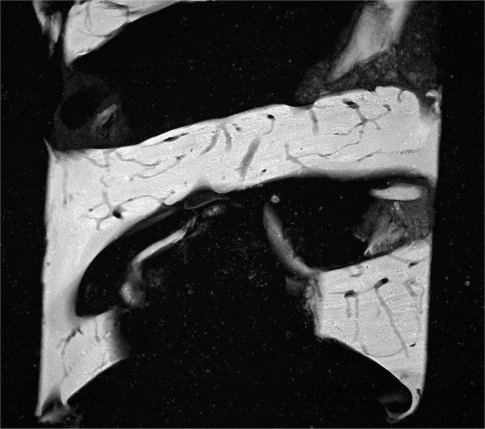
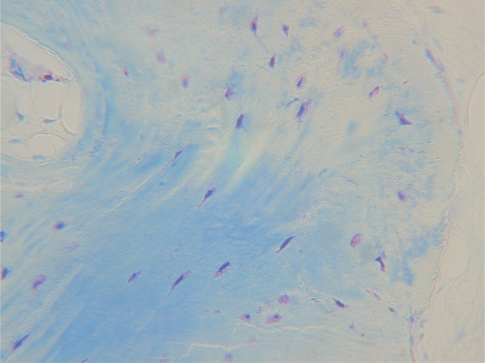
Post operative radiographs showed absence of bone remodelling and satisfactory filling of the defects with Hyaloss™ and autologous bone in all the post-extractive sites. From a hystological point of view the first results showed the presence of a rich network of bone trabeculae, having a very high TBV. The newly formed bone was made mainly of woven structures and showed few erosive activities. Comparison with control sites indicated a preliminary prevail of bone amount produced by Hyaloss™matrix (Figs 7–8).
Figures 7 and 8.
(Hyaloss) Microradiographies of biopsies from maxillary post-extractive sites of the same patient, 4 months after grafting. The control shows an amount of bone higher than the Hyaloss treated site. Microradiography of the biopsy of the Hyaloss treated site: we can observe that the bone trabeculae seem to be particularly thick but show a large amount of vascular neo-cavities.
A total amount of 20 fixtures were placed with a 100% success rate at the moment of prosthetic delivery.
Discussion
Thanks to its complex interactions with matrix components and cells, hyaluronic acid has played multifaceted roles in biology, using both its physiochemical and biological properties. It interacts with other macromolecules and plays a predominant role in tissue morphogenesis, cell migration, differentiation and adhesion (Toole, 1991; Turley, 1989; Toole, 2001). Up to today hyaluronic acid has been used prevalently in periodontology and oral pathology (Ballini, 2009; Vandebogaerde, 2009).
In his protocol of research Ballini (Ballini, 2009) analyzed the osteoinductive effect of hyaluronic acid as an adjuvant in the grafting processes to produce bone-like tissue, employing autologous bone obtained from intraoral sites, to treat intrabony defects without covering membrane, in 9 patients. The clinical results showed an average increase in clinical attachment and suggest that autologous bone combined with hyaluronic acid seems to have good capabilities in accelerating new bone formation in the intrabony defects.
In the Vandebogaerde work (Vandebogaerde, 2009) 19 deep periodontal defects were analyzed. One year after the treatment, the average PPD has been reduced to 5,8 mm, gingival recession increased to 2.0 mm and the attachment increase was to 3.8 mm, using esterified hyaluronic acid (Hyaloss™ matrix).
In our protocol we wanted to evaluate the clinical effectiveness of the use of Hyaloss™matrix with autologous bone in filling post-extractive defects and the following insertion of implants. The clinical criteria of reference to evaluate the success of the osteointegration are those suggested by Albrektsson (Albrektsson, 1986) and modified by Buser (Buser, 1997). In particular: lack of inflammatory signs or infection during clinical examination, lack of mobility, lack of peri-implantar radio-transparency. In all the evaluated sites we successfully inserted implants following the above-mentioned criteria.
As a matter of fact the clinical and biological characteristics of these defects favour the bone filling of the post-extractive sites, while good regenerative activity was obtained in all situations. Our results confirm that bone harvesting with a manual collector achieves good clinical success in extraction socket healing (Zaffe, 2007). Also very interesting is the use of hyaluronic acid (Hyaloss™ matrix) which, as confirmed by hystologic evaluations, allows a better and faster healing process. The preliminary results of our study seem to confirm the ones obtained through an experimental protocol performed on animals, which has a similar approach (Muzaffer, 2006).
In this study, two cavities of 3 mm diameter and depth were created in the right tibia of 30 adult rabbits. Following the protocol, one of the cavities of the tibia is filled with hyaluronic acid and bone graft and the other is filled only with spongiosal bone graft. At different times, after 20, 30 and 40 days, rabbits were sacrificed in equal numbers and defective regions were extracted. The cavities that were filled with hyaluronic acid and bone graft showed higher scores than the control group during every period of the study.
The preliminary results of our protocol seems to point out an acceleration of the bone deposition activity and of its remodelling thanks to the presence of Hyaloss™matrix which, from a clinical point of view, improves the handling and application of the bony matrix inside the defects; there was no intolerance of the biomaterial (Baldini, 2004).
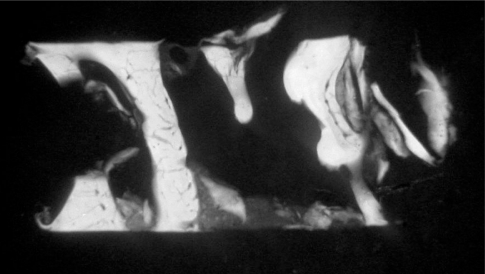
From a hystological point of view Hyaloss™ matrix (Baldini, 2004) allows the reaching of bone regeneration in shorter time when it is used only with autologous bone, with important benefits for the clinical situation because it allows the reduction of the period of time maintained after bone grafts (Figs 9–10).
Figure 9.
Toluidine blue staining highlights living osteocytes (black arrow) both in newly formed bone and in grafted autologous bone.
Figure 10.
Microradiography of the biopsy of the Hyaloss treated site. Note the several bone layers formed on the grafted autologous cortical bone. Osteoclasts resorb the new, old or recently formed bone and the grafted autologous bone.
Microradiographies of the external part of bone trabecula in a biopsy of the Hyaloss treated site. The solochrome cyanine/nuclear fast red staining highlights living osteocytes both in the grafted autologous bone (arrows – bottom) and in the newly formed bone. In the control sites, on the contrary, the new bone, formed in apposition to the grafted autologous bone, shows many parts with lamellar structures.
Conclusions
The properties of hyaluronic acid are very useful in the regeneration therapy as adjuvant of autologous bone grafting. When it comes into contact with the patient’s blood or a saline solution, Hyaloss™matrix becomes a substance similar to gel, which is easy to insert in to bone defects
Hyaloss™ matrix is easy to use because the clinician can adjust it to suit every bone defect and every size to fill. The biochemical properties of this material allows a better healing and post-operative follow-up.
This preliminary study, with clinical and histological evaluations, shows an acceleration of bone deposition activities and of bone remodelling due to the presence of hyaluronic acid, which can reduce the time required for bone regeneration when associated with autologous cortical bone.
References
- 1.Adriaens PA. Lang NP, Karring T, Lindhe J. Implant Dentistry. Chicago: Quintessence Pubblishing Co. Inc; 1999. Preservations of bony sites; Proccedings of the Third European workshop on Periodontology; pp. 266–280. [Google Scholar]
- 2.Albrektsson R, et al. The long term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986:34–42. [PubMed] [Google Scholar]
- 3.Baldini A, Zaffe D, et al. Bone defects healing by high –molecular hyaluronic acid:preliminary results. Acts of the 4 th World Congress of Osteointegration Venezia. Italy Ottobre 2004. Italian Journal of Osseointegration 2004. 2004;4:95. [Google Scholar]
- 4.Baldini A, Zaffe D, et al. Evaluation of bone defects healing by high molecular hyaluronic acid. 2004. p. 222. Abstract del LVIII Meeting of the Italian Society of Anatomy, Italian Journal of Anatomy and Embriology, vol 109, Supplemento n.1 al Fasc. 3, July–September 2004, Mozzon Giuntina S.P..A., Il Sedicesimo, Firenze. [PMC free article] [PubMed]
- 5.Ballini A, Cantore S, Capodiferro S, Grassi FR. Esterified hyaluronic acid and autologous bone in the surgical correction of the infrabone defects. Int J Med Sci. 2009;6(2):65–71. doi: 10.7150/ijms.6.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker W, Urist M, Vincenzi G, De Georges D, Niederwanger M. Clinical and Histological observations of sites implanted with intraoral autologous bone graft and allograft.15 human case reports. Journal of Periodontology. 1996;67:1025–1033. doi: 10.1902/jop.1996.67.10.1025. [DOI] [PubMed] [Google Scholar]
- 7.Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histological findings after implantation and evaluation of different grafting materials and titanium microscrews into extraction sockets :case reports. Journal of Periodontology. 1998;69:414–421. doi: 10.1902/jop.1998.69.4.414. [DOI] [PubMed] [Google Scholar]
- 8.Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Medicine Oral Pathology. 1966;17:785–796. doi: 10.1016/0030-4220(66)90104-6. [DOI] [PubMed] [Google Scholar]
- 9.Bunger MH, Langdahl BL, Andersen T, Husted L. Semi-quantitative mRNA measurements of osteoinductive growth factors in human iliac crest bone: expression of LMP splice variants in human bone. Calcified –Tissue –International. 2003;73:446–454. doi: 10.1007/s00223-002-2109-z. [DOI] [PubMed] [Google Scholar]
- 10.Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler Evaluation of filling materials in membrane-protected defect. Clinical Oral Implants Research. 1998;3:137–150. doi: 10.1034/j.1600-0501.1998.090301.x. [DOI] [PubMed] [Google Scholar]
- 11.Buser, et al. Long term evaluation of non-submerged ITI implants; Part 1: 8-year life table analysis of a prospective multicenter study with 2359 implants. Clin Oral Implants Res. 1997;4:54–63. doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 12.Cafiero C, Annibali S, Gherlone E, Grassi FR, Guadini F, Magliano A, Romeo E, Tonelli P, Lang NP, Salvi GE, ITI Study Group Italia Immediate transmucosal implant placement in molar extraction sites: a 12 month prospective multicenter cohort study. Clin. Oral Implants Res. 2008 May;19(5):476–82. doi: 10.1111/j.1600-0501.2008.01541.x. [DOI] [PubMed] [Google Scholar]
- 13.Carlino P, Pepe V, Pollice G, Grassi FR. Immediate transmucosal implant placement in fresh maxillary and mandibular molar extraction sockets:description of tecnique and preliminary results. Minerva Stomatol. 2008 Oct;57(10):471–83. [PubMed] [Google Scholar]
- 14.Cardaropoli G, Araujo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced augmented and non augmented defects in the alveolar ridge. An experimental study in the dog. Journal of Clinical Periodontology. 2005;32:435–440. doi: 10.1111/j.1600-051X.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 15.Carmagnola D, Adriaens P, Berglungh . Bone Tissue Reaction at Sites Grafted with Bio-Oss, Thesis. Goteborg, Sweden: Goteborg University; 2001. Healing of human extraction sockets filled with Bio-Oss. [Google Scholar]
- 16.Casap N, Zeltser C, Wexler A, Tarazi E, Zeltser R. Immediate placement of dental intodebrided infected dentoalveolar sockets. J Oral Maxillofac Surg. 2008 May;66(5):1081. doi: 10.1016/j.joms.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Christopher ER. Histological study of bone healing in relation to the extraction of teeth. Northwestern University Bulletin. 1942. 45, 5.
- 18.Dahlin C, Lindhe A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plastic and Reconstructive Surgery. 1988;81:672–676. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 19.De Leonardis D. Impianti post-estrattivi :descrizione tecnica e revisione critica. Quintessence Int. 1992;10:615–622. [Google Scholar]
- 20.Hallman M, Sennerby L. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. International Journal of Oral Maxillofacial Implants. 2002;17:635–643. [PubMed] [Google Scholar]
- 21.Hu ZM, Peel SA, Sandor GK. The osteoinductive activity of bone morphogenetic protein(BMP) purified by repeated extracts of bovine bone. Growth Factors. 2004;22:29–33. doi: 10.1080/08977190410001682854. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Cheng YY, Koo PL, Lee KM, Qin L, Cheng JC, Kumpta SM. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial –derived cell cultures. J Biomed Mater Res. 2003 Sep;66A(4):880–4. doi: 10.1002/jbm.a.10535. 15. [DOI] [PubMed] [Google Scholar]
- 23.Jakse N, Seibert FJ, Lorenzoni M, Eskici A. A modified technique of harversting tibial cancellous bone and its use for sinus grafting. Clinical Oral Implants Research. 2001;12:488–494. doi: 10.1034/j.1600-0501.2001.120509.x. [DOI] [PubMed] [Google Scholar]
- 24.Krump JL, Barnett BG. The immediate implant :a treatment alternative. Int. J. Oral. Maxillofac. Implants. 1991;6:19–23. [PubMed] [Google Scholar]
- 25.Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA. Phenotype comparison of periodontal ligament cells in vivo and in vitro. Journal of Periodontal Research. 2001;36:71–79. doi: 10.1034/j.1600-0765.2001.360202.x. [DOI] [PubMed] [Google Scholar]
- 26.Lekovic V, Kenney E, BWeinlaender M, HanTKlakkevold P, Nedic M. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 years. Journal of Periodontology. 1997;68:563–570. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 27.Misch CM, Misch CE, Reisnik RR, Ismail YH. Reconstruction of maxillary alveolar defects with mandibular synphisis grafts for dental ipmplants. A preliminary procedural report. International Journal of Oral and maxillofacial Implants. 1992;7:360–366. [PubMed] [Google Scholar]
- 28.Missori P, Rastelli E, Polli FM, Tarantino R. Reconstruction of suboccipital cranietomy with autologous bone chips. Acta Neurochirurgica. 2002;144:917–920. doi: 10.1007/s00701-002-0988-4. [DOI] [PubMed] [Google Scholar]
- 29.Peattle RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine – loaded hyaluronic acid hydrogel implants. Biomaterials. 2004 Jun;25(14):2789–98. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 30.Pilloni A, Rimondini L, De Luca M, Bernard GW. Effect of hyaluronan on calcification –nodule formation from human periodontal ligament cell culture. Journal of Applied Biomaterials and biomechanics. 2003. [PubMed]
- 31.Quirynem M, Van Assche N, Botticelli D, Berglundh T. How does the timing of implant placement to excraction affect outcome? Int J Oral. Maxillofac Implants. 2007;22(Suppl):203–23. Review. [PubMed] [Google Scholar]
- 32.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, Delisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for Ha- mediated motility in endhotelial cell function and angiogenesis. J Biol. 2001 Sep;276(39):36770–8. doi: 10.1074/jbc.M102273200. 28. Epub Jul 11. [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Takeda Y. Proliferative activity, apoptosis, and histogenesis in the early stages of rat tooth extraction wound healing. Cells, Tissues, Organs. 2007;186:104–111. doi: 10.1159/000103513. [DOI] [PubMed] [Google Scholar]
- 34.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour study. International Journal of Periodontics and Restorative Dentistry. 2003;23:313–323. [PubMed] [Google Scholar]
- 35.Simion M, Jovanovic S, Trisi P, Scarano A, Piattelli A. Vertical ridge augmentation around dental implants using a membrane tecnique and autogenous bone or allografts in humans. International Journal of Periodontics and restorative Dentistry. 1998;18:8–23. [PubMed] [Google Scholar]
- 36.Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endhotelial cell mitogenic and wound healing responses. J Biol Chem. 2002 Oct;277(43):41046–59. doi: 10.1074/jbc.M109443200. 25. Epub Aug 22. [DOI] [PubMed] [Google Scholar]
- 37.Smith JD, Abramson M. Membranous vs. endochondral bone autografts. Archives of Otolaringology. 1974;99:203–205. doi: 10.1001/archotol.1974.00780030211011. [DOI] [PubMed] [Google Scholar]
- 38.Springer IN, Terheyden H, Geiss S, Harle F, Hedderich J. Particulated bone grafts –effectiveness of bone cell supply. clinical Oral Implant Research. 2004;15:205–212. doi: 10.1111/j.1600-0501.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 39.Toole BP. Proteoglycans and hyaluronan in morphogenesis and differentiation. In: Hay ED, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1991. pp. 305–41. [Google Scholar]
- 40.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001 Apr;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]; Turley EA. The role of cell-associated hyaluronan binding protein in fibroblast behaviour. In: Evered D, Whelan J, editors. The Biology of Hyaluronan. Chichester: J Wiley; 1989. pp. 121–137. [DOI] [PubMed] [Google Scholar]
- 41.Trombelli L, Farina R, Marzola A, Bozzi L, Lilljenberg B, Linde J. Modeling and remodeling of human extraction sockets. JClin Periodontol. 2008 Jul;(35):630–9. doi: 10.1111/j.1600-051X.2008.01246.x. Epub 2008 May 21. [DOI] [PubMed] [Google Scholar]
- 42.Krump JL, Barnett BG. The immediate implant :a treatment alternative. Int JOral Maxillofac Implants. 1991;6:19–23. [PubMed] [Google Scholar]
- 43.Kuboki Y, Hashimoto F, Ishibashi K. Time –dependent changes of collagen crosslinks in the socket after tooth extraction in rabbits. Journal of Dental Research. 1998;67:944–94. doi: 10.1177/00220345880670061101. [DOI] [PubMed] [Google Scholar]
- 44.Wanden Bogaerde L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: clinical report of 19 consecutive lesions. Int J Periodontics Restorative Dent. 2009 Jun;29(3):315–23. [PubMed] [Google Scholar]
- 45.Widmark G, Ivanoff CJ. Augmentation of exposed implant threads with autogenous bone chips: prospective clinical study. Clinical Implant Dentistry and Related Research. 2000;2:178–183. doi: 10.1111/j.1708-8208.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 46.Zaffe D, D’Avenia F. A novel bone scraper for intraoral harversting :a device for filling small bone defects. Clin Oral Impl Res. 2007;18:525–533. doi: 10.1111/j.1600-0501.2007.01368.x. [DOI] [PubMed] [Google Scholar]