Abstract
In the title compound, C14H12ClNO3S·H2O, the dihedral angle between the sulfonyl and benzoyl benzene rings is 84.4 (2)°. In the crystal, every water molecule forms four hydrogen bonds with three different molecules of 4-chloro-N-(3-methylbenzoyl)benzenesulfonamide. One of the water H atoms forms a bifurcated hydrogen bond with both the sulfonyl and the carbonyl O atoms of the same molecule. Molecules are linked into layers in the ab plane through N—H⋯O and O—H⋯O hydrogen bonds.
Related literature
For our studies on the effects of substituents on the structures and other aspects of N-(aryl)-amides, see: Gowda et al. (2004 ▶), on N-(aryl)-methanesulfonamides, see: Jayalakshmi & Gowda (2004 ▶), on N-(aryl)-arylsulfonamides, see: Gowda et al. (2003 ▶), on N-(substitutedbenzoyl)-arylsulfonamides, see: Suchetan et al. (2011 ▶) and on N-chloroarylamides, see: Gowda et al. (1996 ▶).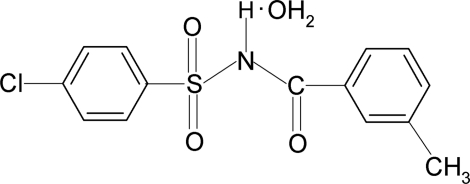
Experimental
Crystal data
C14H12ClNO3S·H2O
M r = 327.77
Orthorhombic,
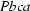
a = 5.0148 (6) Å
b = 12.864 (2) Å
c = 46.314 (5) Å
V = 2987.7 (7) Å3
Z = 8
Mo Kα radiation
μ = 0.41 mm−1
T = 293 K
0.46 × 0.14 × 0.06 mm
Data collection
Oxford Diffraction Xcalibur diffractometer with a Sapphire CCD detector
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▶) T min = 0.834, T max = 0.976
5972 measured reflections
2684 independent reflections
1959 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.105
wR(F 2) = 0.185
S = 1.35
2684 reflections
200 parameters
3 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.33 e Å−3
Δρmin = −0.34 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2009 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811051932/bt5739sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811051932/bt5739Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811051932/bt5739Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O4 | 0.86 (2) | 1.93 (2) | 2.771 (8) | 169 (6) |
| O4—H41⋯O1i | 0.84 (2) | 2.29 (7) | 2.916 (7) | 131 (8) |
| O4—H41⋯O3i | 0.84 (2) | 2.42 (6) | 3.117 (8) | 140 (8) |
| O4—H42⋯O2ii | 0.84 (2) | 2.35 (6) | 3.022 (8) | 137 (8) |
Symmetry codes: (i) 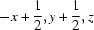 ; (ii)
; (ii) 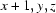 .
.
Acknowledgments
BTG thanks the University Grants Commission, Government of India, New Delhi, for a special grant under the UGC–BSR one-time grant to faculty.
supplementary crystallographic information
Comment
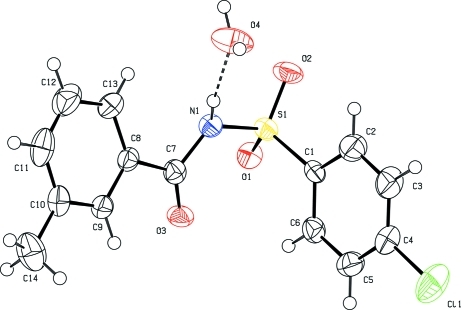
Diaryl acylsulfonamides are known as potent antitumor agents. As part of our studies on the substituent effects on the structures and other aspects of N-(aryl)-amides (Gowda et al., 2004), N-(aryl)-methanesulfonamides (Jayalakshmi & Gowda, 2004), N-(aryl)-arylsulfonamides (Gowda et al., 2003); N-(substitutedbenzoyl)-arylsulfonamides (Suchetan et al., 2011) and N-chloro-arylsulfonamides (Gowda et al., 1996), in the present work, the crystal structure of 4-Chloro-N-(3-methylbenzoyl)-benzenesulfonamide monohydrate (I) has been determined (Fig.1).
The conformations of the N—H and C=O bonds in the C—SO2—NH—C(O) segment are anti to each other (Fig.1), similar to that observed in 4-Chloro-N-(2-methylbenzoyl)-benzenesulfonamide monohydrate (II) (Suchetan et al., 2011). The molecule is twisted at the S- atom with the torsional angle of -70.67 (55)°, compared to the value of -69.2 (2)° in (II).
The dihedral angle between the sulfonyl benzene ring and the —SO2—NH—C—O segment is 78.4 (2)°, compared to the value of 87.2 (1)° in (II). Furthermore, the dihedral angle between the sulfonyl and the benzoyl benzene rings is 84.4 (2)°, compared to the value of 57.7 (1)° in (II).
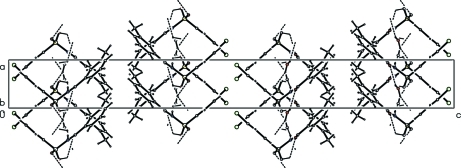
Further, the crystal structure shows interesting H-bonding. Every water molecule forms four H-bonds with three different molecules of the title compound. One of the H-atoms of the water molecule forms simultaneous H-bonding with both the sulfonyl and the carbonyl oxygen atoms of the same molecule.
The packing of molecules through N1—H1N···O4, O4—H41···O1, O4—H41···O3 and O4—H42···O2 hydrogen bonds (Table 1) is shown in Fig. 2.
Experimental
The title compound was prepared by refluxing a mixture of m-methyl benzoic acid (0.02 mole), 4-chlorobenzenesulfonamide (0.02 mole) and excess phosphorous oxy chloride for 3 h on a water bath. The resultant mixture was cooled and poured into crushed ice. The solid, 4-Chloro-N-(3-methylbenzoyl)-benzenesulfonamide monohydrate, obtained was filtered, washed thoroughly with water and then dissolved in sodium bicarbonate solution. The compound was later reprecipitated by acidifying the filtered solution with dilute HCl. It was filtered, dried and recrystallized.
Needle like colourless single crystals of the title compound used in X-ray diffraction studies were obtained by slow evaporation of its ethanol-tetrahydrofuran solution at room temperature.
Refinement
The H atoms of the NH group and of the water molecule were located in a difference map and later restrained to N—H = 0.86 (2)Å and O—H = 0.85 (2) Å. The other H atoms were positioned with idealized geometry using a riding model with the aromatic C—H = 0.93 Å and methyl C—H = 0.96 Å. All H atoms were refined with isotropic displacement parameters set to 1.2 times of the Ueq of the parent atom.
Figures
Fig. 1.
Molecular structure of the title compound, showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Molecular packing in the title compound. Hydrogen bonds are shown as dashed lines.
Crystal data
| C14H12ClNO3S·H2O | F(000) = 1360 |
| Mr = 327.77 | Dx = 1.457 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 1913 reflections |
| a = 5.0148 (6) Å | θ = 2.6–27.8° |
| b = 12.864 (2) Å | µ = 0.41 mm−1 |
| c = 46.314 (5) Å | T = 293 K |
| V = 2987.7 (7) Å3 | Needle, colourless |
| Z = 8 | 0.46 × 0.14 × 0.06 mm |
Data collection
| Oxford Diffraction Xcalibur diffractometer with a Sapphire CCD detector | 2684 independent reflections |
| Radiation source: fine-focus sealed tube | 1959 reflections with I > 2σ(I) |
| graphite | Rint = 0.037 |
| Rotation method data acquisition using ω and phi scans | θmax = 25.4°, θmin = 2.6° |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | h = −3→6 |
| Tmin = 0.834, Tmax = 0.976 | k = −9→15 |
| 5972 measured reflections | l = −55→55 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.105 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.185 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.35 | w = 1/[σ2(Fo2) + (0.P)2 + 17.7362P] where P = (Fo2 + 2Fc2)/3 |
| 2684 reflections | (Δ/σ)max = 0.001 |
| 200 parameters | Δρmax = 0.33 e Å−3 |
| 3 restraints | Δρmin = −0.34 e Å−3 |
Special details
| Experimental. CrysAlis RED (Oxford Diffraction, 2009) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3419 (12) | −0.0253 (5) | 0.07777 (12) | 0.0283 (14) | |
| C2 | 0.4759 (15) | 0.0400 (6) | 0.05920 (14) | 0.0469 (19) | |
| H2 | 0.4533 | 0.1117 | 0.0604 | 0.056* | |
| C3 | 0.6448 (16) | −0.0027 (6) | 0.03877 (15) | 0.051 (2) | |
| H3 | 0.7378 | 0.0403 | 0.0262 | 0.061* | |
| C4 | 0.6751 (15) | −0.1085 (6) | 0.03706 (14) | 0.0423 (18) | |
| C5 | 0.5420 (15) | −0.1736 (6) | 0.05504 (14) | 0.0437 (18) | |
| H5 | 0.5650 | −0.2451 | 0.0536 | 0.052* | |
| C6 | 0.3717 (14) | −0.1325 (5) | 0.07558 (13) | 0.0367 (16) | |
| H6 | 0.2776 | −0.1764 | 0.0879 | 0.044* | |
| C7 | 0.4414 (13) | −0.0406 (5) | 0.14890 (13) | 0.0323 (15) | |
| C8 | 0.6298 (13) | −0.0066 (5) | 0.17281 (13) | 0.0308 (15) | |
| C9 | 0.7972 (13) | −0.0844 (5) | 0.18361 (13) | 0.0335 (16) | |
| H9 | 0.7884 | −0.1511 | 0.1759 | 0.040* | |
| C10 | 0.9761 (14) | −0.0630 (6) | 0.20566 (14) | 0.0403 (18) | |
| C11 | 0.9834 (16) | 0.0360 (7) | 0.21646 (15) | 0.055 (2) | |
| H11 | 1.1054 | 0.0517 | 0.2309 | 0.066* | |
| C12 | 0.8156 (18) | 0.1131 (7) | 0.20656 (16) | 0.059 (2) | |
| H12 | 0.8217 | 0.1791 | 0.2148 | 0.071* | |
| C13 | 0.6372 (16) | 0.0924 (5) | 0.18426 (14) | 0.0437 (18) | |
| H13 | 0.5253 | 0.1441 | 0.1772 | 0.052* | |
| C14 | 1.1601 (16) | −0.1458 (7) | 0.21706 (17) | 0.063 (2) | |
| H14A | 1.2381 | −0.1830 | 0.2012 | 0.075* | |
| H14B | 1.0610 | −0.1933 | 0.2289 | 0.075* | |
| H14C | 1.2986 | −0.1141 | 0.2283 | 0.075* | |
| N1 | 0.3383 (10) | 0.0427 (4) | 0.13292 (11) | 0.0302 (12) | |
| H1N | 0.429 (11) | 0.099 (3) | 0.1325 (14) | 0.036* | |
| O1 | −0.0632 (9) | −0.0465 (3) | 0.11239 (9) | 0.0360 (11) | |
| O2 | 0.0653 (10) | 0.1296 (3) | 0.09642 (10) | 0.0441 (13) | |
| O3 | 0.3853 (10) | −0.1289 (3) | 0.14414 (9) | 0.0387 (12) | |
| O4 | 0.5930 (12) | 0.2319 (4) | 0.12531 (16) | 0.0649 (17) | |
| H41 | 0.533 (17) | 0.289 (4) | 0.1312 (18) | 0.078* | |
| H42 | 0.756 (6) | 0.234 (7) | 0.1210 (18) | 0.078* | |
| Cl1 | 0.8942 (5) | −0.1606 (2) | 0.01180 (5) | 0.0729 (7) | |
| S1 | 0.1393 (3) | 0.02651 (13) | 0.10489 (3) | 0.0315 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.031 (3) | 0.027 (3) | 0.027 (3) | −0.002 (3) | −0.003 (3) | 0.006 (3) |
| C2 | 0.056 (5) | 0.040 (4) | 0.045 (4) | −0.006 (4) | 0.008 (4) | −0.001 (4) |
| C3 | 0.059 (5) | 0.051 (5) | 0.043 (4) | −0.020 (4) | 0.021 (4) | 0.001 (4) |
| C4 | 0.043 (4) | 0.049 (5) | 0.035 (4) | −0.003 (4) | 0.004 (3) | −0.008 (3) |
| C5 | 0.053 (5) | 0.035 (4) | 0.044 (4) | −0.001 (4) | 0.005 (4) | −0.004 (3) |
| C6 | 0.042 (4) | 0.037 (4) | 0.031 (3) | −0.005 (4) | 0.005 (3) | 0.005 (3) |
| C7 | 0.030 (3) | 0.037 (4) | 0.030 (3) | 0.002 (3) | 0.005 (3) | 0.002 (3) |
| C8 | 0.035 (4) | 0.032 (4) | 0.026 (3) | −0.003 (3) | 0.004 (3) | −0.002 (3) |
| C9 | 0.040 (4) | 0.032 (4) | 0.028 (3) | 0.000 (3) | 0.004 (3) | −0.001 (3) |
| C10 | 0.036 (4) | 0.056 (5) | 0.029 (3) | −0.001 (4) | 0.001 (3) | 0.002 (4) |
| C11 | 0.045 (5) | 0.087 (7) | 0.033 (4) | −0.009 (5) | −0.011 (4) | −0.010 (4) |
| C12 | 0.072 (6) | 0.058 (5) | 0.048 (5) | −0.011 (5) | −0.004 (5) | −0.017 (4) |
| C13 | 0.055 (5) | 0.038 (4) | 0.038 (4) | −0.001 (4) | −0.005 (4) | −0.009 (3) |
| C14 | 0.045 (5) | 0.088 (7) | 0.055 (5) | 0.005 (5) | −0.008 (4) | 0.018 (5) |
| N1 | 0.029 (3) | 0.028 (3) | 0.033 (3) | −0.006 (3) | −0.005 (2) | −0.002 (2) |
| O1 | 0.032 (2) | 0.039 (3) | 0.037 (2) | −0.006 (2) | 0.002 (2) | −0.001 (2) |
| O2 | 0.047 (3) | 0.030 (3) | 0.055 (3) | 0.012 (2) | −0.009 (3) | 0.004 (2) |
| O3 | 0.050 (3) | 0.023 (3) | 0.043 (3) | −0.004 (2) | −0.012 (2) | −0.001 (2) |
| O4 | 0.051 (4) | 0.036 (3) | 0.107 (5) | −0.003 (3) | −0.004 (4) | 0.009 (3) |
| Cl1 | 0.0690 (15) | 0.0909 (17) | 0.0590 (12) | −0.0093 (14) | 0.0315 (12) | −0.0208 (13) |
| S1 | 0.0309 (8) | 0.0296 (8) | 0.0338 (8) | 0.0019 (8) | −0.0033 (7) | 0.0031 (8) |
Geometric parameters (Å, °)
| C1—C2 | 1.377 (9) | C9—H9 | 0.9300 |
| C1—C6 | 1.392 (9) | C10—C11 | 1.369 (10) |
| C1—S1 | 1.747 (6) | C10—C14 | 1.505 (10) |
| C2—C3 | 1.384 (10) | C11—C12 | 1.380 (11) |
| C2—H2 | 0.9300 | C11—H11 | 0.9300 |
| C3—C4 | 1.371 (10) | C12—C13 | 1.392 (10) |
| C3—H3 | 0.9300 | C12—H12 | 0.9300 |
| C4—C5 | 1.357 (10) | C13—H13 | 0.9300 |
| C4—Cl1 | 1.739 (7) | C14—H14A | 0.9600 |
| C5—C6 | 1.383 (9) | C14—H14B | 0.9600 |
| C5—H5 | 0.9300 | C14—H14C | 0.9600 |
| C6—H6 | 0.9300 | N1—S1 | 1.651 (5) |
| C7—O3 | 1.192 (7) | N1—H1N | 0.86 (2) |
| C7—N1 | 1.400 (8) | O1—S1 | 1.426 (4) |
| C7—C8 | 1.520 (9) | O2—S1 | 1.432 (5) |
| C8—C13 | 1.380 (9) | O4—H41 | 0.84 (2) |
| C8—C9 | 1.398 (9) | O4—H42 | 0.84 (2) |
| C9—C10 | 1.387 (9) | ||
| C2—C1—C6 | 120.5 (6) | C11—C10—C14 | 120.9 (7) |
| C2—C1—S1 | 120.0 (5) | C9—C10—C14 | 121.0 (7) |
| C6—C1—S1 | 119.5 (5) | C10—C11—C12 | 122.1 (7) |
| C1—C2—C3 | 118.9 (7) | C10—C11—H11 | 118.9 |
| C1—C2—H2 | 120.6 | C12—C11—H11 | 118.9 |
| C3—C2—H2 | 120.6 | C11—C12—C13 | 120.0 (7) |
| C4—C3—C2 | 120.1 (7) | C11—C12—H12 | 120.0 |
| C4—C3—H3 | 119.9 | C13—C12—H12 | 120.0 |
| C2—C3—H3 | 119.9 | C8—C13—C12 | 118.6 (7) |
| C5—C4—C3 | 121.5 (7) | C8—C13—H13 | 120.7 |
| C5—C4—Cl1 | 119.0 (6) | C12—C13—H13 | 120.7 |
| C3—C4—Cl1 | 119.4 (6) | C10—C14—H14A | 109.5 |
| C4—C5—C6 | 119.4 (7) | C10—C14—H14B | 109.5 |
| C4—C5—H5 | 120.3 | H14A—C14—H14B | 109.5 |
| C6—C5—H5 | 120.3 | C10—C14—H14C | 109.5 |
| C5—C6—C1 | 119.7 (6) | H14A—C14—H14C | 109.5 |
| C5—C6—H6 | 120.2 | H14B—C14—H14C | 109.5 |
| C1—C6—H6 | 120.2 | C7—N1—S1 | 122.9 (4) |
| O3—C7—N1 | 123.0 (6) | C7—N1—H1N | 118 (4) |
| O3—C7—C8 | 123.7 (6) | S1—N1—H1N | 114 (4) |
| N1—C7—C8 | 113.3 (6) | H41—O4—H42 | 113 (9) |
| C13—C8—C9 | 120.4 (6) | O1—S1—O2 | 119.5 (3) |
| C13—C8—C7 | 124.2 (6) | O1—S1—N1 | 108.8 (3) |
| C9—C8—C7 | 115.4 (6) | O2—S1—N1 | 104.8 (3) |
| C10—C9—C8 | 120.7 (6) | O1—S1—C1 | 109.8 (3) |
| C10—C9—H9 | 119.7 | O2—S1—C1 | 107.9 (3) |
| C8—C9—H9 | 119.7 | N1—S1—C1 | 105.2 (3) |
| C11—C10—C9 | 118.1 (7) | ||
| C6—C1—C2—C3 | −1.4 (11) | C9—C10—C11—C12 | 1.5 (11) |
| S1—C1—C2—C3 | 177.0 (6) | C14—C10—C11—C12 | −179.5 (7) |
| C1—C2—C3—C4 | 0.5 (12) | C10—C11—C12—C13 | −2.1 (12) |
| C2—C3—C4—C5 | 0.2 (13) | C9—C8—C13—C12 | 0.5 (11) |
| C2—C3—C4—Cl1 | −178.5 (6) | C7—C8—C13—C12 | 178.7 (6) |
| C3—C4—C5—C6 | 0.0 (12) | C11—C12—C13—C8 | 1.0 (12) |
| Cl1—C4—C5—C6 | 178.6 (5) | O3—C7—N1—S1 | −2.9 (9) |
| C4—C5—C6—C1 | −0.8 (11) | C8—C7—N1—S1 | 177.3 (4) |
| C2—C1—C6—C5 | 1.5 (10) | C7—N1—S1—O1 | 46.8 (6) |
| S1—C1—C6—C5 | −176.9 (5) | C7—N1—S1—O2 | 175.7 (5) |
| O3—C7—C8—C13 | −159.8 (7) | C7—N1—S1—C1 | −70.7 (5) |
| N1—C7—C8—C13 | 19.9 (9) | C2—C1—S1—O1 | 153.9 (5) |
| O3—C7—C8—C9 | 18.5 (9) | C6—C1—S1—O1 | −27.6 (6) |
| N1—C7—C8—C9 | −161.7 (5) | C2—C1—S1—O2 | 22.2 (6) |
| C13—C8—C9—C10 | −1.0 (10) | C6—C1—S1—O2 | −159.4 (5) |
| C7—C8—C9—C10 | −179.4 (6) | C2—C1—S1—N1 | −89.2 (6) |
| C8—C9—C10—C11 | 0.1 (10) | C6—C1—S1—N1 | 89.2 (6) |
| C8—C9—C10—C14 | −179.0 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O4 | 0.86 (2) | 1.93 (2) | 2.771 (8) | 169 (6) |
| O4—H41···O1i | 0.84 (2) | 2.29 (7) | 2.916 (7) | 131 (8) |
| O4—H41···O3i | 0.84 (2) | 2.42 (6) | 3.117 (8) | 140 (8) |
| O4—H42···O2ii | 0.84 (2) | 2.35 (6) | 3.022 (8) | 137 (8) |
Symmetry codes: (i) −x+1/2, y+1/2, z; (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5739).
References
- Gowda, B. T., Dou, S. Q. & Weiss, A. (1996). Z. Naturforsch. Teil A, 51, 627–636.
- Gowda, B. T., Jyothi, K., Kozisek, J. & Fuess, H. (2003). Z. Naturforsch. Teil A, 58, 656–660.
- Gowda, B. T., Svoboda, I. & Fuess, H. (2004). Z. Naturforsch. Teil A, 59, 845–852.
- Jayalakshmi, K. L. & Gowda, B. T. (2004). Z. Naturforsch. Teil A, 59, 491–500.
- Oxford Diffraction (2009). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suchetan, P. A., Foro, S. & Gowda, B. T. (2011). Acta Cryst. E67, o22. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811051932/bt5739sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811051932/bt5739Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811051932/bt5739Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report