Abstract
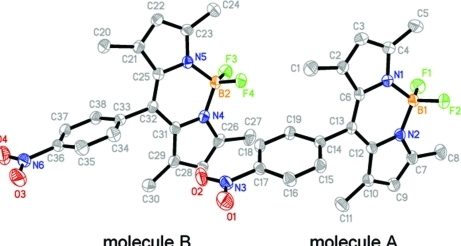
In an effort to discover novel and potential boron–dipyrromethene (BODIPY) dyes, the title compound, C19H18BF2N3O2, was prepared from 2,4-dimethylpyrrole, 4-nitrobenzaldehyde and BF3·Et2O in a one-pot reaction. There are two independent molecules, A and B, in the asymmetric unit in which the dihedral angles between the benzene ring and boron–dipyrromethene mean plane have significantly different values [82.71 (8)° for molecule A and 73.16 (8)° for molecule B]. Intermolecular C—H⋯π interactions help to stabilize the crystal structure.
Related literature
For the use of related compounds in fluorescence analysis, see: Weiner et al. (2001 ▶); Gabe et al. (2004 ▶). For related structures, see: Euler et al. (2002a
▶,b
▶); Cui et al. (2006 ▶). For the synthetic procedure, see: Kollmannsberger et al. (1998 ▶).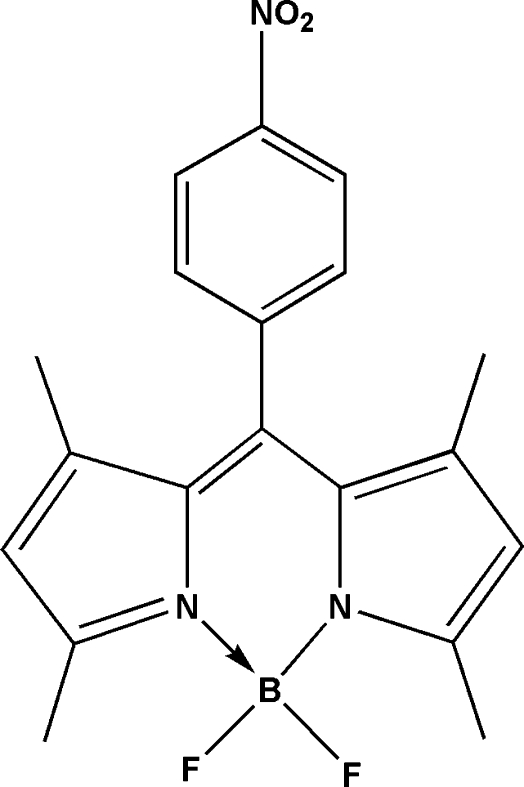
Experimental
Crystal data
C19H18BF2N3O2
M r = 369.17
Monoclinic,
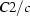
a = 30.5729 (6) Å
b = 11.8625 (2) Å
c = 19.8975 (5) Å
β = 96.732 (1)°
V = 7166.5 (3) Å3
Z = 16
Mo Kα radiation
μ = 0.10 mm−1
T = 295 K
0.60 × 0.31 × 0.12 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2003 ▶) T min = 0.961, T max = 0.989
10802 measured reflections
6278 independent reflections
4790 reflections with I > 2σ(I)
R int = 0.027
Refinement
R[F 2 > 2σ(F 2)] = 0.083
wR(F 2) = 0.193
S = 1.26
6278 reflections
492 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.26 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: APEX2 and SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811052196/im2338sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811052196/im2338Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the N4/C26/C28/C29/C31 and N5/C21–C23/C25 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C18—H18A⋯Cg1 | 0.93 | 2.93 | 3.784 (4) | 154 |
| C35—H35A⋯Cg2i | 0.93 | 2.90 | 3.648 (5) | 139 |
Symmetry code: (i) 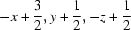 .
.
Acknowledgments
We gratefully acknowledge financial support from the Open Foundation of Jiangsu Province Key Laboratory of Fine Petrochemical Technology (KF1005) and the Analysis Center of Changzhou University.
supplementary crystallographic information
Comment
In the past few years, many novel boron-dipyrromethene (BODIPY) dyes were developed for fluorescence analysis (Weiner et al., 2001; Gabe et al., 2004) and their crystal structures were investigated at the same time (Euler et al., 2002a,b). As part of our ongoing studies of the substituent effect on the solid-state structures of BODIPY derivatives (Cui et al., 2006), we report herein the crystal strcuture of the title compound, 4,4-difluoro- 1,3,5,7-tetramethyl-8-(4'-nitrophenyl)-4-bora-3a,4a-diaza-s-indacene, (I).
The asymmetric unit of the title compound is shown in Fig. 1. There are two independent unique molecules [lablled A and B], in which the dihedral angles between the benzene ring and boron-dipyrromethene mean plane have significantly different values [82.71 (8)° for molecule A, 73.16 (8)° for molecule B]. In the crystal structure, there also exist intermolecular weak edge-to-face C—H···π [C18-H18A···Cg1i (Cg1 = N4, C26, C28, C29, C31): H18A···Cg1, 2.93 Å, C18···Cg1, 3.784 (4) Å, i = x, y, z; and C35-H35A···Cg2ii (Cg2 = N5, C21-C23, C25): H35A···Cg2, 2.90 Å, C35···Cg2, 3.648 (5) Å, i = -x + 3/2, y + 1/2, -z + 1/2 ] interactions, which help to reinforce the packing lattice.
Experimental
Compound (I) was prepared from 2,4-dimethylpyrrole and p-nitrobenzaldehyde in a one-pot reaction (Kollmannsberger et al., 1998). General procedure: 4.5 mmol of 2,4-dimethylpyrrole and 2 mmol of the aldehyde were dissolved in 150 ml of absolute dichloromethane under nitrogen atmosphere. One drop of trifluoroacetic acid was added and the solution was stirred at room temperature until TLC-control showed complete consumption of the aldehyde. At this point, 2 mmol dichlorodicyanobenzoquinone (DDQ) was added, and stirring was continued for 10 min followed by addition of 4 ml of triethylamine and 4 ml of boron trifluoride etherate quickly. After stirring for another 2 h, the reaction mixture was washed with water and dried, and the solvent was evaporated. The residue was chromatographed twice on a silica column (the mixture of dichloromethane and hexane as eluted solvent). Total yield: 48%. Orange crystals. 1H NMR (CDCl3): δ 1.36 (s, 6H, CH3), 2.57 (s, 6H, CH3), 6.02 (s, 2H, CH), 7.55 (d, 2H, CH, J = 21 Hz), 8.40 (d, 2H, CH, J = 22 Hz). MS (ESI), m/z: 368.2 [M—H]-. HRMS: [M—H]- calculated: 368.1496, measured: 368.1472.
Red single crystals suitable for X-ray analysis were obtained by dissolving (I) (0.2 g) in a hexane/dichloromethane (15 ml, v:v: 1:3) mixture and slowly evaporating the solvent at room temperature for a period of about two weeks.
Refinement
All H atoms bound to C atoms were assigned to calculated positions, with C—H = 0.96 Å (methyl) and 0.93 Å (aromatic), and refined using a riding model, with Uiso(H)=1.2Ueq (C).
Figures
Fig. 1.
Molecular structure of the title compound with the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity.
Crystal data
| C19H18BF2N3O2 | F(000) = 3072 |
| Mr = 369.17 | Dx = 1.369 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 9250 reflections |
| a = 30.5729 (6) Å | θ = 2.9–27.5° |
| b = 11.8625 (2) Å | µ = 0.10 mm−1 |
| c = 19.8975 (5) Å | T = 295 K |
| β = 96.732 (1)° | Cuboid, red |
| V = 7166.5 (3) Å3 | 0.60 × 0.31 × 0.12 mm |
| Z = 16 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 6278 independent reflections |
| Radiation source: fine-focus sealed tube | 4790 reflections with I > 2σ(I) |
| graphite | Rint = 0.027 |
| phi and ω scans | θmax = 25.0°, θmin = 1.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2003) | h = −30→36 |
| Tmin = 0.961, Tmax = 0.989 | k = −7→14 |
| 10802 measured reflections | l = −19→23 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.083 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.193 | H-atom parameters constrained |
| S = 1.26 | w = 1/[σ2(Fo2) + (0.0295P)2 + 25.3515P] where P = (Fo2 + 2Fc2)/3 |
| 6278 reflections | (Δ/σ)max = 0.010 |
| 492 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.26 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| F2 | 0.40972 (7) | 0.4438 (2) | 0.09898 (13) | 0.0572 (7) | |
| F3 | 0.70073 (9) | 0.6621 (2) | 0.00916 (13) | 0.0657 (7) | |
| F4 | 0.66392 (7) | 0.7021 (2) | 0.09842 (13) | 0.0587 (7) | |
| F1 | 0.44996 (9) | 0.3905 (2) | 0.01603 (13) | 0.0635 (7) | |
| N1 | 0.48764 (10) | 0.4758 (3) | 0.11631 (17) | 0.0435 (8) | |
| N2 | 0.43918 (10) | 0.5886 (3) | 0.03340 (16) | 0.0409 (7) | |
| N5 | 0.74189 (10) | 0.7350 (3) | 0.11064 (16) | 0.0416 (8) | |
| N3 | 0.60065 (13) | 1.0688 (3) | 0.18716 (19) | 0.0551 (9) | |
| N4 | 0.69020 (10) | 0.8575 (3) | 0.03739 (17) | 0.0456 (8) | |
| C32 | 0.75062 (12) | 0.9378 (3) | 0.11245 (19) | 0.0390 (9) | |
| C12 | 0.46331 (12) | 0.6856 (3) | 0.05529 (19) | 0.0412 (9) | |
| C13 | 0.49862 (11) | 0.6774 (3) | 0.10695 (19) | 0.0375 (8) | |
| O2 | 0.63489 (11) | 1.0849 (3) | 0.16285 (17) | 0.0702 (10) | |
| O1 | 0.58641 (13) | 1.1319 (3) | 0.2283 (2) | 0.0891 (12) | |
| C6 | 0.51053 (12) | 0.5738 (3) | 0.13813 (19) | 0.0404 (9) | |
| C31 | 0.71389 (12) | 0.9519 (3) | 0.06439 (19) | 0.0434 (9) | |
| C33 | 0.77694 (12) | 1.0395 (3) | 0.13595 (19) | 0.0414 (9) | |
| C14 | 0.52499 (12) | 0.7800 (3) | 0.12746 (18) | 0.0387 (9) | |
| N6 | 0.85035 (16) | 1.3323 (3) | 0.1947 (2) | 0.0703 (13) | |
| C18 | 0.59202 (13) | 0.8870 (3) | 0.1283 (2) | 0.0460 (10) | |
| H18A | 0.6203 | 0.8952 | 0.1160 | 0.055* | |
| C21 | 0.80155 (13) | 0.7954 (4) | 0.1816 (2) | 0.0470 (10) | |
| C17 | 0.57416 (13) | 0.9679 (3) | 0.16588 (19) | 0.0421 (9) | |
| C19 | 0.56702 (12) | 0.7928 (3) | 0.1091 (2) | 0.0448 (9) | |
| H19A | 0.5786 | 0.7371 | 0.0835 | 0.054* | |
| C7 | 0.40959 (13) | 0.6181 (4) | −0.0198 (2) | 0.0492 (10) | |
| O4 | 0.88462 (14) | 1.3493 (3) | 0.1696 (2) | 0.0955 (14) | |
| C26 | 0.65831 (13) | 0.8948 (4) | −0.0104 (2) | 0.0539 (11) | |
| C23 | 0.76288 (13) | 0.6440 (4) | 0.1387 (2) | 0.0486 (10) | |
| C38 | 0.81430 (13) | 1.0667 (4) | 0.1053 (2) | 0.0478 (10) | |
| H38A | 0.8230 | 1.0203 | 0.0716 | 0.057* | |
| C25 | 0.76480 (12) | 0.8318 (3) | 0.1355 (2) | 0.0424 (9) | |
| C36 | 0.82497 (14) | 1.2290 (3) | 0.1748 (2) | 0.0503 (10) | |
| C10 | 0.44704 (13) | 0.7764 (4) | 0.0132 (2) | 0.0507 (10) | |
| B1 | 0.44561 (14) | 0.4707 (4) | 0.0653 (2) | 0.0419 (10) | |
| B2 | 0.69829 (14) | 0.7354 (4) | 0.0628 (2) | 0.0437 (11) | |
| C37 | 0.83858 (13) | 1.1622 (4) | 0.1247 (2) | 0.0512 (11) | |
| H37A | 0.8635 | 1.1808 | 0.1044 | 0.061* | |
| C35 | 0.78890 (16) | 1.2029 (4) | 0.2069 (2) | 0.0605 (12) | |
| H35A | 0.7808 | 1.2485 | 0.2414 | 0.073* | |
| C4 | 0.50634 (14) | 0.3885 (4) | 0.1522 (2) | 0.0539 (11) | |
| C28 | 0.66037 (14) | 1.0116 (4) | −0.0143 (2) | 0.0604 (13) | |
| H28A | 0.6417 | 1.0567 | −0.0432 | 0.072* | |
| C2 | 0.54438 (13) | 0.5435 (4) | 0.1908 (2) | 0.0496 (10) | |
| C8 | 0.37869 (15) | 0.5364 (4) | −0.0570 (2) | 0.0629 (13) | |
| H8A | 0.3682 | 0.4846 | −0.0255 | 0.094* | |
| H8B | 0.3938 | 0.4955 | −0.0890 | 0.094* | |
| H8C | 0.3542 | 0.5765 | −0.0806 | 0.094* | |
| C16 | 0.53216 (14) | 0.9590 (4) | 0.1841 (2) | 0.0512 (10) | |
| H16A | 0.5206 | 1.0158 | 0.2090 | 0.061* | |
| C15 | 0.50768 (13) | 0.8643 (4) | 0.1649 (2) | 0.0496 (10) | |
| H15A | 0.4794 | 0.8568 | 0.1770 | 0.060* | |
| C3 | 0.54139 (14) | 0.4284 (4) | 0.1979 (2) | 0.0598 (12) | |
| H3A | 0.5596 | 0.3843 | 0.2281 | 0.072* | |
| C29 | 0.69449 (13) | 1.0504 (4) | 0.0315 (2) | 0.0512 (11) | |
| C20 | 0.83614 (15) | 0.8649 (4) | 0.2222 (2) | 0.0633 (13) | |
| H20A | 0.8532 | 0.8177 | 0.2545 | 0.095* | |
| H20B | 0.8223 | 0.9233 | 0.2455 | 0.095* | |
| H20C | 0.8551 | 0.8982 | 0.1925 | 0.095* | |
| O3 | 0.83572 (15) | 1.3958 (3) | 0.2354 (2) | 0.0983 (14) | |
| C22 | 0.79973 (13) | 0.6791 (4) | 0.1823 (2) | 0.0526 (11) | |
| H22A | 0.8196 | 0.6319 | 0.2076 | 0.063* | |
| C34 | 0.76472 (15) | 1.1072 (4) | 0.1870 (2) | 0.0547 (11) | |
| H34A | 0.7401 | 1.0885 | 0.2081 | 0.066* | |
| C9 | 0.41390 (14) | 0.7324 (4) | −0.0321 (2) | 0.0582 (12) | |
| H9A | 0.3971 | 0.7730 | −0.0658 | 0.070* | |
| C11 | 0.46221 (17) | 0.8973 (4) | 0.0132 (3) | 0.0782 (17) | |
| H11A | 0.4487 | 0.9344 | −0.0268 | 0.117* | |
| H11B | 0.4937 | 0.8995 | 0.0140 | 0.117* | |
| H11C | 0.4540 | 0.9350 | 0.0526 | 0.117* | |
| C27 | 0.62621 (15) | 0.8169 (5) | −0.0502 (2) | 0.0710 (14) | |
| H27A | 0.6330 | 0.7403 | −0.0372 | 0.106* | |
| H27B | 0.6283 | 0.8259 | −0.0976 | 0.106* | |
| H27C | 0.5968 | 0.8346 | −0.0411 | 0.106* | |
| C30 | 0.70618 (16) | 1.1719 (4) | 0.0431 (3) | 0.0757 (16) | |
| H30A | 0.6892 | 1.2172 | 0.0096 | 0.114* | |
| H30B | 0.7370 | 1.1824 | 0.0397 | 0.114* | |
| H30C | 0.6998 | 1.1939 | 0.0873 | 0.114* | |
| C5 | 0.48987 (17) | 0.2703 (4) | 0.1433 (3) | 0.0732 (15) | |
| H5A | 0.4657 | 0.2680 | 0.1079 | 0.110* | |
| H5B | 0.4802 | 0.2443 | 0.1848 | 0.110* | |
| H5C | 0.5132 | 0.2225 | 0.1315 | 0.110* | |
| C24 | 0.74717 (16) | 0.5255 (4) | 0.1245 (3) | 0.0674 (13) | |
| H24A | 0.7167 | 0.5199 | 0.1311 | 0.101* | |
| H24B | 0.7642 | 0.4746 | 0.1547 | 0.101* | |
| H24C | 0.7507 | 0.5062 | 0.0785 | 0.101* | |
| C1 | 0.57686 (15) | 0.6163 (4) | 0.2332 (2) | 0.0654 (13) | |
| H1A | 0.5955 | 0.5698 | 0.2640 | 0.098* | |
| H1B | 0.5614 | 0.6690 | 0.2583 | 0.098* | |
| H1C | 0.5945 | 0.6565 | 0.2044 | 0.098* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| F2 | 0.0406 (12) | 0.0610 (16) | 0.0716 (16) | −0.0079 (11) | 0.0130 (11) | 0.0080 (13) |
| F3 | 0.0670 (16) | 0.0669 (17) | 0.0618 (16) | −0.0016 (14) | 0.0019 (13) | −0.0213 (14) |
| F4 | 0.0402 (13) | 0.0596 (15) | 0.0777 (17) | −0.0075 (11) | 0.0129 (12) | 0.0030 (13) |
| F1 | 0.0705 (17) | 0.0545 (15) | 0.0642 (16) | −0.0017 (13) | 0.0020 (13) | −0.0210 (13) |
| N1 | 0.0387 (17) | 0.0398 (18) | 0.0515 (19) | −0.0023 (15) | 0.0027 (15) | −0.0001 (15) |
| N2 | 0.0385 (17) | 0.0409 (18) | 0.0423 (18) | −0.0067 (14) | 0.0004 (14) | −0.0022 (14) |
| N5 | 0.0382 (17) | 0.0434 (19) | 0.0441 (18) | −0.0041 (15) | 0.0088 (14) | −0.0029 (15) |
| N3 | 0.060 (2) | 0.041 (2) | 0.059 (2) | −0.0081 (18) | −0.0122 (19) | 0.0037 (18) |
| N4 | 0.0351 (17) | 0.055 (2) | 0.0464 (19) | −0.0046 (15) | 0.0038 (15) | 0.0011 (16) |
| C32 | 0.0324 (19) | 0.046 (2) | 0.039 (2) | −0.0021 (17) | 0.0073 (16) | −0.0011 (17) |
| C12 | 0.040 (2) | 0.041 (2) | 0.043 (2) | −0.0040 (17) | 0.0051 (17) | 0.0022 (17) |
| C13 | 0.0322 (19) | 0.041 (2) | 0.040 (2) | −0.0045 (16) | 0.0052 (15) | −0.0044 (17) |
| O2 | 0.065 (2) | 0.069 (2) | 0.075 (2) | −0.0318 (18) | 0.0029 (18) | 0.0075 (18) |
| O1 | 0.088 (3) | 0.061 (2) | 0.116 (3) | −0.009 (2) | 0.005 (2) | −0.041 (2) |
| C6 | 0.036 (2) | 0.042 (2) | 0.044 (2) | −0.0085 (17) | 0.0044 (16) | −0.0043 (17) |
| C31 | 0.037 (2) | 0.050 (2) | 0.044 (2) | −0.0051 (18) | 0.0072 (17) | 0.0024 (19) |
| C33 | 0.040 (2) | 0.040 (2) | 0.044 (2) | 0.0010 (17) | 0.0023 (17) | 0.0013 (18) |
| C14 | 0.040 (2) | 0.040 (2) | 0.035 (2) | −0.0031 (17) | 0.0004 (16) | −0.0011 (17) |
| N6 | 0.076 (3) | 0.047 (2) | 0.078 (3) | −0.006 (2) | −0.034 (2) | 0.003 (2) |
| C18 | 0.036 (2) | 0.049 (2) | 0.053 (2) | −0.0078 (18) | 0.0051 (18) | −0.003 (2) |
| C21 | 0.039 (2) | 0.056 (3) | 0.045 (2) | −0.0044 (19) | 0.0024 (17) | 0.006 (2) |
| C17 | 0.050 (2) | 0.038 (2) | 0.037 (2) | −0.0085 (18) | −0.0024 (17) | −0.0003 (17) |
| C19 | 0.037 (2) | 0.047 (2) | 0.051 (2) | −0.0042 (18) | 0.0086 (17) | −0.0099 (19) |
| C7 | 0.044 (2) | 0.055 (3) | 0.047 (2) | −0.0107 (19) | −0.0028 (18) | 0.001 (2) |
| O4 | 0.085 (3) | 0.080 (3) | 0.116 (3) | −0.035 (2) | −0.015 (3) | 0.012 (2) |
| C26 | 0.040 (2) | 0.076 (3) | 0.045 (2) | −0.003 (2) | −0.0005 (18) | 0.008 (2) |
| C23 | 0.043 (2) | 0.045 (2) | 0.058 (3) | 0.0049 (19) | 0.0096 (19) | 0.005 (2) |
| C38 | 0.042 (2) | 0.052 (3) | 0.050 (2) | −0.0006 (19) | 0.0073 (18) | −0.003 (2) |
| C25 | 0.041 (2) | 0.044 (2) | 0.042 (2) | −0.0020 (18) | 0.0044 (17) | −0.0018 (18) |
| C36 | 0.050 (2) | 0.040 (2) | 0.057 (3) | −0.0040 (19) | −0.013 (2) | 0.001 (2) |
| C10 | 0.045 (2) | 0.048 (2) | 0.058 (3) | −0.0070 (19) | −0.0022 (19) | 0.008 (2) |
| B1 | 0.039 (2) | 0.042 (2) | 0.046 (3) | −0.007 (2) | 0.0058 (19) | −0.005 (2) |
| B2 | 0.037 (2) | 0.047 (3) | 0.048 (3) | −0.005 (2) | 0.008 (2) | −0.011 (2) |
| C37 | 0.039 (2) | 0.057 (3) | 0.057 (3) | −0.004 (2) | −0.0007 (19) | 0.003 (2) |
| C35 | 0.070 (3) | 0.053 (3) | 0.057 (3) | 0.000 (2) | 0.003 (2) | −0.017 (2) |
| C4 | 0.049 (2) | 0.042 (2) | 0.070 (3) | 0.0012 (19) | 0.004 (2) | 0.006 (2) |
| C28 | 0.045 (2) | 0.076 (3) | 0.059 (3) | −0.002 (2) | −0.002 (2) | 0.026 (2) |
| C2 | 0.039 (2) | 0.055 (3) | 0.052 (2) | −0.0047 (19) | −0.0040 (18) | 0.004 (2) |
| C8 | 0.056 (3) | 0.070 (3) | 0.060 (3) | −0.019 (2) | −0.009 (2) | −0.003 (2) |
| C16 | 0.057 (3) | 0.043 (2) | 0.056 (3) | −0.005 (2) | 0.015 (2) | −0.009 (2) |
| C15 | 0.041 (2) | 0.051 (2) | 0.059 (3) | −0.0058 (19) | 0.0182 (19) | −0.004 (2) |
| C3 | 0.050 (3) | 0.054 (3) | 0.071 (3) | 0.003 (2) | −0.007 (2) | 0.014 (2) |
| C29 | 0.039 (2) | 0.059 (3) | 0.055 (3) | −0.002 (2) | 0.0039 (19) | 0.014 (2) |
| C20 | 0.056 (3) | 0.062 (3) | 0.066 (3) | −0.008 (2) | −0.016 (2) | 0.007 (2) |
| O3 | 0.117 (3) | 0.058 (2) | 0.111 (3) | −0.007 (2) | −0.025 (3) | −0.027 (2) |
| C22 | 0.041 (2) | 0.057 (3) | 0.058 (3) | 0.000 (2) | 0.001 (2) | 0.012 (2) |
| C34 | 0.057 (3) | 0.054 (3) | 0.055 (3) | −0.003 (2) | 0.017 (2) | −0.009 (2) |
| C9 | 0.053 (3) | 0.062 (3) | 0.055 (3) | −0.009 (2) | −0.012 (2) | 0.016 (2) |
| C11 | 0.077 (3) | 0.055 (3) | 0.094 (4) | −0.019 (3) | −0.026 (3) | 0.025 (3) |
| C27 | 0.055 (3) | 0.094 (4) | 0.060 (3) | −0.015 (3) | −0.011 (2) | −0.001 (3) |
| C30 | 0.059 (3) | 0.061 (3) | 0.102 (4) | −0.005 (2) | −0.009 (3) | 0.028 (3) |
| C5 | 0.069 (3) | 0.045 (3) | 0.102 (4) | −0.001 (2) | −0.005 (3) | 0.011 (3) |
| C24 | 0.066 (3) | 0.047 (3) | 0.088 (4) | −0.006 (2) | 0.006 (3) | 0.002 (3) |
| C1 | 0.060 (3) | 0.069 (3) | 0.061 (3) | −0.008 (2) | −0.017 (2) | 0.008 (2) |
Geometric parameters (Å, °)
| F2—B1 | 1.389 (5) | C23—C24 | 1.501 (6) |
| F3—B2 | 1.385 (5) | C38—C37 | 1.385 (6) |
| F4—B2 | 1.392 (5) | C38—H38A | 0.9300 |
| F1—B1 | 1.383 (5) | C36—C35 | 1.373 (6) |
| N1—C4 | 1.346 (5) | C36—C37 | 1.375 (6) |
| N1—C6 | 1.400 (5) | C10—C9 | 1.378 (6) |
| N1—B1 | 1.543 (5) | C10—C11 | 1.507 (6) |
| N2—C7 | 1.355 (5) | C37—H37A | 0.9300 |
| N2—C12 | 1.408 (5) | C35—C34 | 1.387 (6) |
| N2—B1 | 1.539 (6) | C35—H35A | 0.9300 |
| N5—C23 | 1.343 (5) | C4—C3 | 1.405 (6) |
| N5—C25 | 1.404 (5) | C4—C5 | 1.494 (6) |
| N5—B2 | 1.544 (5) | C28—C29 | 1.381 (6) |
| N3—O2 | 1.219 (5) | C28—H28A | 0.9300 |
| N3—O1 | 1.226 (5) | C2—C3 | 1.377 (6) |
| N3—C17 | 1.479 (5) | C2—C1 | 1.498 (6) |
| N4—C26 | 1.354 (5) | C8—H8A | 0.9600 |
| N4—C31 | 1.406 (5) | C8—H8B | 0.9600 |
| N4—B2 | 1.545 (6) | C8—H8C | 0.9600 |
| C32—C31 | 1.397 (5) | C16—C15 | 1.379 (6) |
| C32—C25 | 1.391 (5) | C16—H16A | 0.9300 |
| C32—C33 | 1.494 (5) | C15—H15A | 0.9300 |
| C12—C13 | 1.404 (5) | C3—H3A | 0.9300 |
| C12—C10 | 1.419 (5) | C29—C30 | 1.496 (6) |
| C13—C6 | 1.405 (5) | C20—H20A | 0.9600 |
| C13—C14 | 1.490 (5) | C20—H20B | 0.9600 |
| C6—C2 | 1.430 (5) | C20—H20C | 0.9600 |
| C31—C29 | 1.432 (6) | C22—H22A | 0.9300 |
| C33—C34 | 1.380 (6) | C34—H34A | 0.9300 |
| C33—C38 | 1.394 (5) | C9—H9A | 0.9300 |
| C14—C19 | 1.385 (5) | C11—H11A | 0.9600 |
| C14—C15 | 1.388 (5) | C11—H11B | 0.9600 |
| N6—O3 | 1.227 (6) | C11—H11C | 0.9600 |
| N6—O4 | 1.229 (6) | C27—H27A | 0.9600 |
| N6—C36 | 1.479 (6) | C27—H27B | 0.9600 |
| C18—C17 | 1.369 (5) | C27—H27C | 0.9600 |
| C18—C19 | 1.383 (5) | C30—H30A | 0.9600 |
| C18—H18A | 0.9300 | C30—H30B | 0.9600 |
| C21—C22 | 1.381 (6) | C30—H30C | 0.9600 |
| C21—C25 | 1.432 (5) | C5—H5A | 0.9600 |
| C21—C20 | 1.500 (6) | C5—H5B | 0.9600 |
| C17—C16 | 1.379 (6) | C5—H5C | 0.9600 |
| C19—H19A | 0.9300 | C24—H24A | 0.9600 |
| C7—C9 | 1.387 (6) | C24—H24B | 0.9600 |
| C7—C8 | 1.488 (6) | C24—H24C | 0.9600 |
| C26—C28 | 1.390 (7) | C1—H1A | 0.9600 |
| C26—C27 | 1.504 (6) | C1—H1B | 0.9600 |
| C23—C22 | 1.403 (6) | C1—H1C | 0.9600 |
| C4—N1—C6 | 108.0 (3) | C36—C37—C38 | 118.4 (4) |
| C4—N1—B1 | 126.0 (3) | C36—C37—H37A | 120.8 |
| C6—N1—B1 | 125.8 (3) | C38—C37—H37A | 120.8 |
| C7—N2—C12 | 108.0 (3) | C36—C35—C34 | 118.8 (4) |
| C7—N2—B1 | 126.7 (3) | C36—C35—H35A | 120.6 |
| C12—N2—B1 | 125.3 (3) | C34—C35—H35A | 120.6 |
| C23—N5—C25 | 108.4 (3) | N1—C4—C3 | 109.2 (4) |
| C23—N5—B2 | 126.4 (3) | N1—C4—C5 | 122.9 (4) |
| C25—N5—B2 | 125.0 (3) | C3—C4—C5 | 127.9 (4) |
| O2—N3—O1 | 123.9 (4) | C29—C28—C26 | 109.3 (4) |
| O2—N3—C17 | 118.7 (4) | C29—C28—H28A | 125.4 |
| O1—N3—C17 | 117.4 (4) | C26—C28—H28A | 125.4 |
| C26—N4—C31 | 107.7 (4) | C3—C2—C6 | 105.9 (4) |
| C26—N4—B2 | 127.7 (4) | C3—C2—C1 | 124.1 (4) |
| C31—N4—B2 | 124.5 (3) | C6—C2—C1 | 130.0 (4) |
| C31—C32—C25 | 121.9 (4) | C7—C8—H8A | 109.5 |
| C31—C32—C33 | 118.6 (3) | C7—C8—H8B | 109.5 |
| C25—C32—C33 | 119.4 (3) | H8A—C8—H8B | 109.5 |
| N2—C12—C13 | 120.0 (3) | C7—C8—H8C | 109.5 |
| N2—C12—C10 | 107.7 (3) | H8A—C8—H8C | 109.5 |
| C13—C12—C10 | 132.2 (4) | H8B—C8—H8C | 109.5 |
| C6—C13—C12 | 121.4 (3) | C15—C16—C17 | 118.7 (4) |
| C6—C13—C14 | 119.3 (3) | C15—C16—H16A | 120.6 |
| C12—C13—C14 | 119.3 (3) | C17—C16—H16A | 120.6 |
| C13—C6—N1 | 119.7 (3) | C16—C15—C14 | 120.5 (4) |
| C13—C6—C2 | 132.2 (4) | C16—C15—H15A | 119.7 |
| N1—C6—C2 | 108.1 (3) | C14—C15—H15A | 119.7 |
| C32—C31—N4 | 120.2 (4) | C2—C3—C4 | 108.8 (4) |
| C32—C31—C29 | 131.8 (4) | C2—C3—H3A | 125.6 |
| N4—C31—C29 | 107.9 (3) | C4—C3—H3A | 125.6 |
| C34—C33—C38 | 119.6 (4) | C28—C29—C31 | 105.7 (4) |
| C34—C33—C32 | 121.2 (4) | C28—C29—C30 | 124.8 (4) |
| C38—C33—C32 | 119.3 (4) | C31—C29—C30 | 129.5 (4) |
| C19—C14—C15 | 119.1 (4) | C21—C20—H20A | 109.5 |
| C19—C14—C13 | 120.4 (3) | C21—C20—H20B | 109.5 |
| C15—C14—C13 | 120.5 (3) | H20A—C20—H20B | 109.5 |
| O3—N6—O4 | 124.2 (5) | C21—C20—H20C | 109.5 |
| O3—N6—C36 | 117.7 (5) | H20A—C20—H20C | 109.5 |
| O4—N6—C36 | 118.2 (5) | H20B—C20—H20C | 109.5 |
| C17—C18—C19 | 118.4 (4) | C21—C22—C23 | 108.7 (4) |
| C17—C18—H18A | 120.8 | C21—C22—H22A | 125.7 |
| C19—C18—H18A | 120.8 | C23—C22—H22A | 125.7 |
| C22—C21—C25 | 106.2 (4) | C33—C34—C35 | 120.4 (4) |
| C22—C21—C20 | 124.8 (4) | C33—C34—H34A | 119.8 |
| C25—C21—C20 | 129.0 (4) | C35—C34—H34A | 119.8 |
| C18—C17—C16 | 122.2 (4) | C10—C9—C7 | 109.4 (4) |
| C18—C17—N3 | 118.9 (4) | C10—C9—H9A | 125.3 |
| C16—C17—N3 | 118.8 (4) | C7—C9—H9A | 125.3 |
| C18—C19—C14 | 121.1 (4) | C10—C11—H11A | 109.5 |
| C18—C19—H19A | 119.5 | C10—C11—H11B | 109.5 |
| C14—C19—H19A | 119.5 | H11A—C11—H11B | 109.5 |
| N2—C7—C9 | 108.8 (4) | C10—C11—H11C | 109.5 |
| N2—C7—C8 | 123.2 (4) | H11A—C11—H11C | 109.5 |
| C9—C7—C8 | 128.0 (4) | H11B—C11—H11C | 109.5 |
| N4—C26—C28 | 109.4 (4) | C26—C27—H27A | 109.5 |
| N4—C26—C27 | 122.7 (4) | C26—C27—H27B | 109.5 |
| C28—C26—C27 | 127.9 (4) | H27A—C27—H27B | 109.5 |
| N5—C23—C22 | 109.2 (4) | C26—C27—H27C | 109.5 |
| N5—C23—C24 | 123.2 (4) | H27A—C27—H27C | 109.5 |
| C22—C23—C24 | 127.6 (4) | H27B—C27—H27C | 109.5 |
| C37—C38—C33 | 120.5 (4) | C29—C30—H30A | 109.5 |
| C37—C38—H38A | 119.8 | C29—C30—H30B | 109.5 |
| C33—C38—H38A | 119.8 | H30A—C30—H30B | 109.5 |
| C32—C25—N5 | 120.0 (3) | C29—C30—H30C | 109.5 |
| C32—C25—C21 | 132.5 (4) | H30A—C30—H30C | 109.5 |
| N5—C25—C21 | 107.5 (3) | H30B—C30—H30C | 109.5 |
| C35—C36—C37 | 122.3 (4) | C4—C5—H5A | 109.5 |
| C35—C36—N6 | 119.2 (4) | C4—C5—H5B | 109.5 |
| C37—C36—N6 | 118.5 (4) | H5A—C5—H5B | 109.5 |
| C9—C10—C12 | 106.2 (4) | C4—C5—H5C | 109.5 |
| C9—C10—C11 | 124.3 (4) | H5A—C5—H5C | 109.5 |
| C12—C10—C11 | 129.5 (4) | H5B—C5—H5C | 109.5 |
| F2—B1—F1 | 109.4 (3) | C23—C24—H24A | 109.5 |
| F2—B1—N2 | 109.7 (3) | C23—C24—H24B | 109.5 |
| F1—B1—N2 | 110.6 (3) | H24A—C24—H24B | 109.5 |
| F2—B1—N1 | 109.6 (3) | C23—C24—H24C | 109.5 |
| F1—B1—N1 | 110.6 (3) | H24A—C24—H24C | 109.5 |
| N2—B1—N1 | 106.9 (3) | H24B—C24—H24C | 109.5 |
| F3—B2—F4 | 109.1 (3) | C2—C1—H1A | 109.5 |
| F3—B2—N5 | 110.6 (3) | C2—C1—H1B | 109.5 |
| F4—B2—N5 | 109.5 (3) | H1A—C1—H1B | 109.5 |
| F3—B2—N4 | 110.9 (4) | C2—C1—H1C | 109.5 |
| F4—B2—N4 | 109.3 (3) | H1A—C1—H1C | 109.5 |
| N5—B2—N4 | 107.3 (3) | H1B—C1—H1C | 109.5 |
| C7—N2—C12—C13 | −175.3 (4) | O4—N6—C36—C37 | −5.9 (6) |
| B1—N2—C12—C13 | 5.0 (6) | N2—C12—C10—C9 | 0.2 (5) |
| C7—N2—C12—C10 | 0.2 (4) | C13—C12—C10—C9 | 175.0 (4) |
| B1—N2—C12—C10 | −179.4 (4) | N2—C12—C10—C11 | −177.7 (5) |
| N2—C12—C13—C6 | −0.4 (6) | C13—C12—C10—C11 | −2.9 (8) |
| C10—C12—C13—C6 | −174.7 (4) | C7—N2—B1—F2 | −70.0 (5) |
| N2—C12—C13—C14 | 177.4 (3) | C12—N2—B1—F2 | 109.6 (4) |
| C10—C12—C13—C14 | 3.1 (7) | C7—N2—B1—F1 | 50.8 (5) |
| C12—C13—C6—N1 | 1.7 (6) | C12—N2—B1—F1 | −129.7 (4) |
| C14—C13—C6—N1 | −176.1 (3) | C7—N2—B1—N1 | 171.2 (4) |
| C12—C13—C6—C2 | −179.9 (4) | C12—N2—B1—N1 | −9.2 (5) |
| C14—C13—C6—C2 | 2.3 (7) | C4—N1—B1—F2 | 65.3 (5) |
| C4—N1—C6—C13 | 177.7 (4) | C6—N1—B1—F2 | −108.3 (4) |
| B1—N1—C6—C13 | −7.8 (6) | C4—N1—B1—F1 | −55.4 (5) |
| C4—N1—C6—C2 | −1.0 (5) | C6—N1—B1—F1 | 131.1 (4) |
| B1—N1—C6—C2 | 173.5 (4) | C4—N1—B1—N2 | −175.8 (4) |
| C25—C32—C31—N4 | −0.5 (6) | C6—N1—B1—N2 | 10.6 (5) |
| C33—C32—C31—N4 | 175.4 (3) | C23—N5—B2—F3 | −52.3 (5) |
| C25—C32—C31—C29 | −177.0 (4) | C25—N5—B2—F3 | 131.9 (4) |
| C33—C32—C31—C29 | −1.2 (6) | C23—N5—B2—F4 | 67.9 (5) |
| C26—N4—C31—C32 | −176.4 (4) | C25—N5—B2—F4 | −107.8 (4) |
| B2—N4—C31—C32 | 7.4 (6) | C23—N5—B2—N4 | −173.5 (4) |
| C26—N4—C31—C29 | 1.0 (4) | C25—N5—B2—N4 | 10.8 (5) |
| B2—N4—C31—C29 | −175.3 (4) | C26—N4—B2—F3 | 52.0 (5) |
| C31—C32—C33—C34 | 84.0 (5) | C31—N4—B2—F3 | −132.5 (4) |
| C25—C32—C33—C34 | −100.1 (5) | C26—N4—B2—F4 | −68.3 (5) |
| C31—C32—C33—C38 | −95.5 (4) | C31—N4—B2—F4 | 107.2 (4) |
| C25—C32—C33—C38 | 80.4 (5) | C26—N4—B2—N5 | 173.0 (4) |
| C6—C13—C14—C19 | 71.7 (5) | C31—N4—B2—N5 | −11.5 (5) |
| C12—C13—C14—C19 | −106.2 (4) | C35—C36—C37—C38 | 1.4 (6) |
| C6—C13—C14—C15 | −108.7 (4) | N6—C36—C37—C38 | −178.8 (4) |
| C12—C13—C14—C15 | 73.4 (5) | C33—C38—C37—C36 | 0.1 (6) |
| C19—C18—C17—C16 | −0.9 (6) | C37—C36—C35—C34 | −1.6 (7) |
| C19—C18—C17—N3 | 179.6 (4) | N6—C36—C35—C34 | 178.6 (4) |
| O2—N3—C17—C18 | 10.3 (5) | C6—N1—C4—C3 | 0.3 (5) |
| O1—N3—C17—C18 | −170.1 (4) | B1—N1—C4—C3 | −174.1 (4) |
| O2—N3—C17—C16 | −169.2 (4) | C6—N1—C4—C5 | 178.8 (4) |
| O1—N3—C17—C16 | 10.4 (6) | B1—N1—C4—C5 | 4.3 (7) |
| C17—C18—C19—C14 | −0.2 (6) | N4—C26—C28—C29 | 0.5 (6) |
| C15—C14—C19—C18 | 1.0 (6) | C27—C26—C28—C29 | 179.3 (4) |
| C13—C14—C19—C18 | −179.4 (4) | C13—C6—C2—C3 | −177.2 (4) |
| C12—N2—C7—C9 | −0.6 (5) | N1—C6—C2—C3 | 1.3 (5) |
| B1—N2—C7—C9 | 179.1 (4) | C13—C6—C2—C1 | 4.2 (8) |
| C12—N2—C7—C8 | 178.9 (4) | N1—C6—C2—C1 | −177.3 (4) |
| B1—N2—C7—C8 | −1.4 (6) | C18—C17—C16—C15 | 1.2 (6) |
| C31—N4—C26—C28 | −0.9 (5) | N3—C17—C16—C15 | −179.3 (4) |
| B2—N4—C26—C28 | 175.2 (4) | C17—C16—C15—C14 | −0.4 (6) |
| C31—N4—C26—C27 | −179.8 (4) | C19—C14—C15—C16 | −0.7 (6) |
| B2—N4—C26—C27 | −3.7 (7) | C13—C14—C15—C16 | 179.7 (4) |
| C25—N5—C23—C22 | 0.1 (5) | C6—C2—C3—C4 | −1.1 (5) |
| B2—N5—C23—C22 | −176.2 (4) | C1—C2—C3—C4 | 177.6 (4) |
| C25—N5—C23—C24 | 179.0 (4) | N1—C4—C3—C2 | 0.5 (6) |
| B2—N5—C23—C24 | 2.7 (6) | C5—C4—C3—C2 | −177.9 (5) |
| C34—C33—C38—C37 | −1.4 (6) | C26—C28—C29—C31 | 0.1 (5) |
| C32—C33—C38—C37 | 178.1 (4) | C26—C28—C29—C30 | −178.4 (5) |
| C31—C32—C25—N5 | −0.3 (6) | C32—C31—C29—C28 | 176.2 (4) |
| C33—C32—C25—N5 | −176.1 (3) | N4—C31—C29—C28 | −0.7 (5) |
| C31—C32—C25—C21 | 177.4 (4) | C32—C31—C29—C30 | −5.3 (8) |
| C33—C32—C25—C21 | 1.7 (7) | N4—C31—C29—C30 | 177.8 (5) |
| C23—N5—C25—C32 | 177.8 (4) | C25—C21—C22—C23 | −0.6 (5) |
| B2—N5—C25—C32 | −5.8 (6) | C20—C21—C22—C23 | 179.6 (4) |
| C23—N5—C25—C21 | −0.5 (4) | N5—C23—C22—C21 | 0.3 (5) |
| B2—N5—C25—C21 | 175.9 (3) | C24—C23—C22—C21 | −178.6 (4) |
| C22—C21—C25—C32 | −177.4 (4) | C38—C33—C34—C35 | 1.2 (7) |
| C20—C21—C25—C32 | 2.5 (8) | C32—C33—C34—C35 | −178.3 (4) |
| C22—C21—C25—N5 | 0.6 (5) | C36—C35—C34—C33 | 0.3 (7) |
| C20—C21—C25—N5 | −179.5 (4) | C12—C10—C9—C7 | −0.5 (5) |
| O3—N6—C36—C35 | −6.1 (6) | C11—C10—C9—C7 | 177.5 (5) |
| O4—N6—C36—C35 | 173.9 (4) | N2—C7—C9—C10 | 0.7 (5) |
| O3—N6—C36—C37 | 174.1 (4) | C8—C7—C9—C10 | −178.8 (4) |
Hydrogen-bond geometry (Å, °)
| Cg1 and Cg2 are the centroids of the N4/C26/C28/C29/C31 and N5/C21–C23/C25 rings, respectively. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C18—H18A···Cg1 | 0.93 | 2.93 | 3.784 (4) | 154 |
| C35—H35A···Cg2i | 0.93 | 2.90 | 3.648 (5) | 139 |
Symmetry codes: (i) −x+3/2, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2338).
References
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cui, A.-J., Peng, X.-J., Gao, Y.-L. & Fan, J.-L. (2006). Acta Cryst. E62, o4697–o4698.
- Euler, H., Kirfel, A., Freudenthal, S. J. & Müller, C. E. (2002a). Z. Kristallogr. New Cryst. Struct. 217, 541–542.
- Euler, H., Kirfel, A., Freudenthal, S. J. & Müller, C. E. (2002b). Z. Kristallogr. New Cryst. Struct. 217, 543–544.
- Gabe, Y., Urano, Y., Kikuchi, K., Kojima, H. & Nagano, T. (2004). J. Am. Chem. Soc. 126, 3357–3367. [DOI] [PubMed]
- Kollmannsberger, M., Rurack, K., Resch-Genger, U. & Daub, J. (1998). J. Phys. Chem. A, 102, 10211–10220.
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Weiner, A. L., Lewis, A., Ottolenghi, M. & Sheves, M. (2001). J. Am. Chem. Soc. 123, 6612–6616. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811052196/im2338sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811052196/im2338Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report