Abstract
The benzene and phenyl rings in the title compound, C14H9Cl3N2OS, form a dihedral angle of 40.98 (6)°. The molecule exists in the thione form with typical thiourea C—S [1.666 (2) Å] and C—O [1.227 (3) Å] bond lengths as well as shortened C—N bonds [1.345 (3) and 1.386 (2) Å]. An intramolecular N—H⋯O hydrogen bond stabilizes the molecular conformation. In the crystal, pairs of N—H⋯S hydrogen bonds link the molecules into centrosymmetric dimers.
Related literature
For information on thiourea derivatives, see: Patil & Chedekel (1984 ▶); Baily et al. (1996 ▶); Namgun et al. (2001 ▶); Koch (2001 ▶); Wegner et al. (1986 ▶); Krishnamurthy et al. (1999 ▶); Murtaza et al. (2009a
▶,b
▶). For related structures, see: Khawar Rauf et al. (2009a
▶,b
▶). For bond-length data, see: Allen et al. (1987 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶).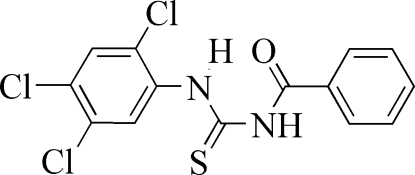
Experimental
Crystal data
C14H9Cl3N2OS
M r = 359.64
Monoclinic,
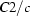
a = 33.111 (8) Å
b = 3.8413 (7) Å
c = 25.220 (6) Å
β = 115.995 (2)°
V = 2883.1 (11) Å3
Z = 8
Mo Kα radiation
μ = 0.78 mm−1
T = 296 K
0.20 × 0.20 × 0.20 mm
Data collection
Rigaku/MSC Mercury CCD diffractometer
Absorption correction: multi-scan (REQAB; Rigaku, 1998 ▶) T min = 0.800, T max = 1.000
11264 measured reflections
3264 independent reflections
2686 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.084
S = 1.06
3264 reflections
190 parameters
H-atom parameters constrained
Δρmax = 0.42 e Å−3
Δρmin = −0.39 e Å−3
Data collection: CrystalClear (Molecular Structure Corporation and Rigaku, 2001 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶) and TEXSAN (Molecular Structure Corporation and Rigaku, 2004 ▶); software used to prepare material for publication: Yadokari-XG_2009 (Kabuto et al., 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811052780/bg2439sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811052780/bg2439Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811052780/bg2439Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1 | 0.86 | 1.89 | 2.586 (2) | 137 |
| N2—H2⋯S1i | 0.86 | 2.83 | 3.6771 (19) | 168 |
Symmetry code: (i) 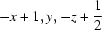 .
.
Acknowledgments
MKR is grateful to the Quaid-i-Azam University, Islamabad, for financial support for a post-doctoral fellowship.
supplementary crystallographic information
Comment
Thiourea derivatives are very useful building blocks for the synthesis of a wide range of aliphatic macromolecular and heterocyclic compounds. Thus, benzothiazoles have been prepared from arylthioureas in the presence of bromine (Patil & Chedekel, 1984), 2-aminothiazoles from the condensation of thiourea with α-halocarbonyl compounds (Baily et al., 1996), and 2-Methyl-aminothiazolines from N-(2-hydroxyethyl)-N'-methylthioureas (Namgun et al., 2001). N, N-dialkyl-N-aroylthioureas have been efficiently used for the extraction of Nickle, Palladium and Platinum metals (Koch, 2001). Aliphatic and acylthioureas are well known for their antimicrobial activities (Wegner et al., 1986). Symmetrical and unsymmetrical thioureas have shown antifungal activity against the plant pathogens (Krishnamurthy et al., 1999). We became interested in the synthesis of these thioureas as intermediates in the synthesis of novel guanidines(Murtaza et al., 2009a ; 2009b) and heterocyclic compounds for the systematic study of bioactivity and Complexation behaviour. Hence, we present here the crystal structure of the title compound, (I), Fig. 1. Comparison with N-benzoyl-N'-phenylthioureas [Cambridge Structural Database (Mogul Version 1.7; Allen, 2002) and (Allen et al., 1987)], show the molecule to exist in the thione form with typical thiourea C—S and C—O bonds, as well as shortened C—N bond lengths. Comparison with N-benzoyl-N'-phenylthioureas (Khawar Rauf et al., 2009a,b) suggests the 2,4,5-trichloro substitution on phenyl ring implies no significant effect on these bond lengths. Compound (I) (Fig. 1) shows the typical Thiourea C═S and C═O double bonds as well as shortened C—N bond lengths. The thiocarbonyl and carbonyl groups are almost coplanar, as reflected by the torsion angles C1—N2—C2—O1 [-5.0 (3)] and N1—C1—N2—C2 [1.7 (3)]. This is associated with the expected typical thiourea intramolecular N—H···O H–bond (Table 1), forming a six-membered ring commonly observed in this class of compounds (Khawar Rauf et al., 2009a,b). The dihedral angles to the N1 C1 S1 N2 C2 O1 plane are 50.97 (4)° for the ring formed by C3 to C8 and 11.44 (7)° for the ring formed by C9 to C14. The crystal packing shows intramolecular N—H···O and intermolecular N—H···S H–bonds (Table 1, Fig. 2). The Cl atoms are not involved in any type of H–bonds.
Experimental
Freshly prepared benzoylisothiocyanate (1.63 g, 10 mmol) was dissolved in acetone (50 ml) and stirred for 45 minutes. Afterwards neat 2,4,5-trichloroaniline(1.96 g, 10 mmol) was added and the resulting mixture was stirred for 1 h. The reaction mixture was then poured into acidified water and stirred well. The solid product was separated and washed with deionized water and purified by recrystallization from methanol/1,1-dichloromethane (1:1 v/v) to give fine crystals of the title compound (I), with an overall yield of 95%. Full spectroscopic and physical characterization will be reported elsewhere.
Refinement
Hydrogen atoms were included in calculated positions and refined as riding on their parent atom with N—H = 0.86 Å and Uiso(H) = 1.2U(Neq), C—H = 0.93 Å and Uiso(H) = 1.2U(Ceq).
Figures
Fig. 1.
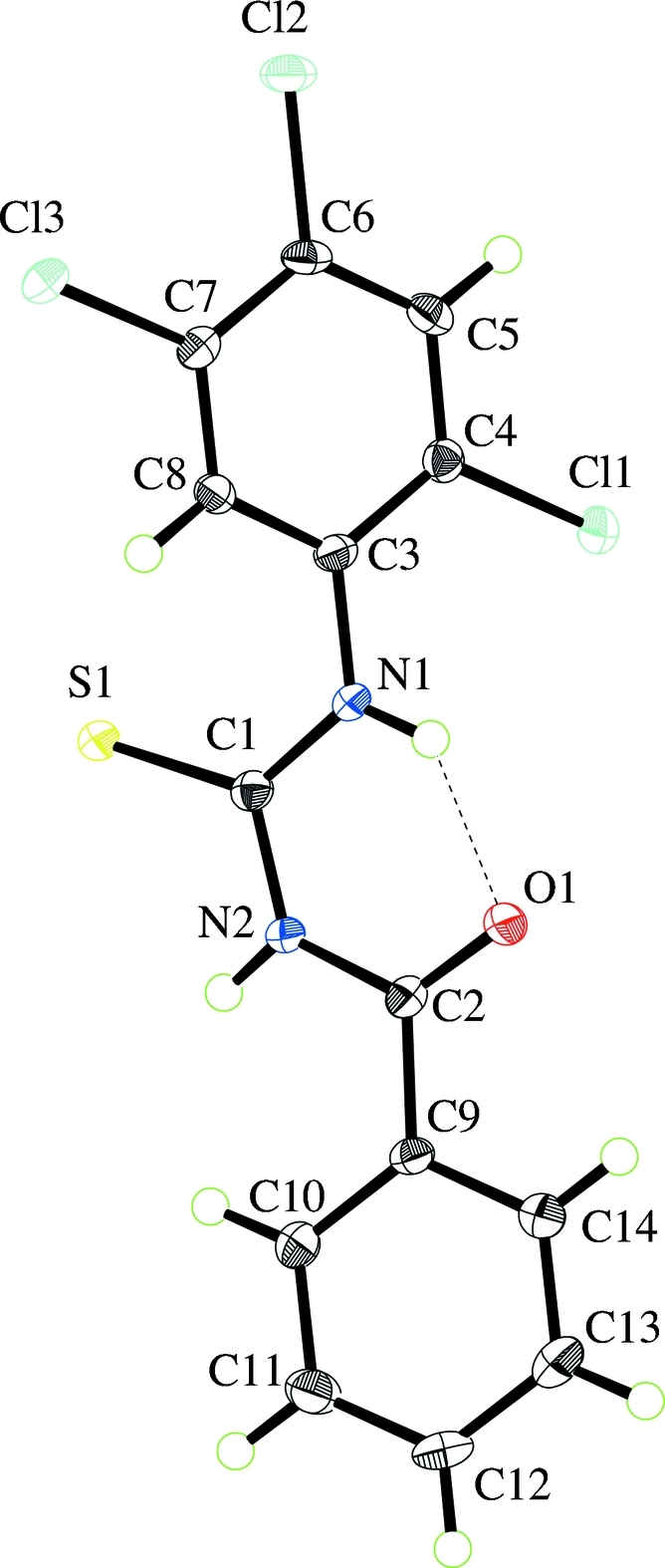
Molecular diagram of (I). Displacement ellipsoids are drawn at the 50% probability level. Hydrogen bonds shown as dashed lines.
Fig. 2.
Packing diagram of (I) viewed along b-axis. Hydrogen bonds shown as dashed lines.
Crystal data
| C14H9Cl3N2OS | F(000) = 1456 |
| Mr = 359.64 | Dx = 1.657 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71070 Å |
| Hall symbol: -C 2yc | Cell parameters from 3588 reflections |
| a = 33.111 (8) Å | θ = 3.4–27.5° |
| b = 3.8413 (7) Å | µ = 0.78 mm−1 |
| c = 25.220 (6) Å | T = 296 K |
| β = 115.995 (2)° | Prism, colorless |
| V = 2883.1 (11) Å3 | 0.20 × 0.20 × 0.20 mm |
| Z = 8 |
Data collection
| Rigaku/MSC Mercury CCD diffractometer | 3264 independent reflections |
| Radiation source: Sealed Tube | 2686 reflections with I > 2σ(I) |
| Graphite Monochromator | Rint = 0.039 |
| Detector resolution: 14.6306 pixels mm-1 | θmax = 27.5°, θmin = 3.2° |
| dtprofit.ref scans | h = −42→31 |
| Absorption correction: multi-scan (REQAB; Rigaku, 1998) | k = −3→4 |
| Tmin = 0.800, Tmax = 1.000 | l = −28→32 |
| 11264 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.084 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0345P)2 + 0.4746P] where P = (Fo2 + 2Fc2)/3 |
| 3264 reflections | (Δ/σ)max = 0.001 |
| 190 parameters | Δρmax = 0.42 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Experimental. ???? |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.43321 (7) | 0.2438 (4) | 0.27137 (9) | 0.0156 (4) | |
| S1 | 0.435250 (18) | 0.44890 (12) | 0.21438 (2) | 0.01721 (13) | |
| N1 | 0.39554 (6) | 0.1658 (4) | 0.27661 (7) | 0.0169 (4) | |
| H1 | 0.3985 | 0.0785 | 0.3095 | 0.020* | |
| N2 | 0.47294 (6) | 0.1480 (4) | 0.31893 (7) | 0.0161 (4) | |
| H2 | 0.4973 | 0.2018 | 0.3166 | 0.019* | |
| C2 | 0.47806 (7) | −0.0243 (5) | 0.36979 (9) | 0.0183 (4) | |
| O1 | 0.44567 (5) | −0.0919 (4) | 0.37941 (7) | 0.0262 (4) | |
| C3 | 0.35153 (7) | 0.2166 (5) | 0.23204 (9) | 0.0159 (4) | |
| C4 | 0.31883 (7) | 0.3736 (5) | 0.24474 (9) | 0.0169 (4) | |
| C5 | 0.27552 (7) | 0.4225 (4) | 0.20171 (10) | 0.0183 (5) | |
| H5 | 0.2542 | 0.5274 | 0.2110 | 0.022* | |
| C6 | 0.26409 (7) | 0.3141 (5) | 0.14447 (9) | 0.0175 (4) | |
| C7 | 0.29612 (7) | 0.1512 (5) | 0.13109 (9) | 0.0170 (4) | |
| C8 | 0.33904 (7) | 0.0996 (4) | 0.17480 (9) | 0.0157 (4) | |
| H8 | 0.3600 | −0.0154 | 0.1658 | 0.019* | |
| Cl1 | 0.332696 (18) | 0.51597 (12) | 0.31608 (2) | 0.02171 (14) | |
| Cl2 | 0.210441 (17) | 0.39364 (12) | 0.09054 (2) | 0.02358 (14) | |
| Cl3 | 0.283000 (19) | 0.00640 (12) | 0.06075 (2) | 0.02230 (14) | |
| C9 | 0.52450 (7) | −0.1244 (4) | 0.41257 (9) | 0.0160 (4) | |
| C10 | 0.56177 (7) | −0.1060 (5) | 0.40132 (10) | 0.0202 (5) | |
| H10 | 0.5589 | −0.0209 | 0.3653 | 0.024* | |
| C11 | 0.60327 (7) | −0.2139 (5) | 0.44354 (10) | 0.0262 (5) | |
| H11 | 0.6282 | −0.2027 | 0.4356 | 0.031* | |
| C12 | 0.60807 (7) | −0.3382 (5) | 0.49742 (10) | 0.0236 (5) | |
| H12 | 0.6361 | −0.4100 | 0.5257 | 0.028* | |
| C13 | 0.57106 (8) | −0.3556 (5) | 0.50921 (10) | 0.0278 (5) | |
| H13 | 0.5741 | −0.4375 | 0.5455 | 0.033* | |
| C14 | 0.52959 (8) | −0.2506 (5) | 0.46687 (10) | 0.0250 (5) | |
| H14 | 0.5047 | −0.2644 | 0.4747 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0159 (11) | 0.0153 (9) | 0.0139 (11) | 0.0006 (7) | 0.0050 (9) | −0.0028 (7) |
| S1 | 0.0155 (3) | 0.0206 (2) | 0.0151 (3) | 0.00181 (18) | 0.0063 (2) | 0.00292 (18) |
| N1 | 0.0143 (9) | 0.0239 (8) | 0.0117 (9) | 0.0017 (6) | 0.0048 (8) | 0.0036 (7) |
| N2 | 0.0117 (9) | 0.0228 (8) | 0.0127 (9) | 0.0001 (6) | 0.0042 (8) | 0.0017 (6) |
| C2 | 0.0181 (12) | 0.0218 (10) | 0.0136 (11) | 0.0013 (8) | 0.0058 (10) | 0.0001 (8) |
| O1 | 0.0157 (9) | 0.0441 (9) | 0.0203 (9) | 0.0027 (6) | 0.0093 (8) | 0.0103 (7) |
| C3 | 0.0135 (11) | 0.0174 (9) | 0.0143 (11) | −0.0006 (7) | 0.0037 (9) | 0.0029 (7) |
| C4 | 0.0176 (12) | 0.0175 (9) | 0.0162 (11) | −0.0011 (7) | 0.0080 (10) | 0.0005 (7) |
| C5 | 0.0143 (11) | 0.0184 (9) | 0.0237 (13) | 0.0005 (7) | 0.0096 (10) | 0.0022 (8) |
| C6 | 0.0108 (11) | 0.0175 (9) | 0.0201 (12) | −0.0010 (7) | 0.0031 (9) | 0.0052 (8) |
| C7 | 0.0180 (11) | 0.0170 (9) | 0.0142 (11) | −0.0017 (7) | 0.0054 (9) | 0.0007 (7) |
| C8 | 0.0143 (11) | 0.0161 (9) | 0.0174 (11) | 0.0009 (7) | 0.0076 (9) | 0.0011 (8) |
| Cl1 | 0.0205 (3) | 0.0287 (3) | 0.0170 (3) | 0.00048 (19) | 0.0092 (2) | −0.00330 (19) |
| Cl2 | 0.0140 (3) | 0.0281 (3) | 0.0231 (3) | 0.00175 (19) | 0.0030 (2) | 0.0041 (2) |
| Cl3 | 0.0202 (3) | 0.0295 (3) | 0.0133 (3) | −0.00027 (19) | 0.0038 (2) | −0.00146 (19) |
| C9 | 0.0144 (11) | 0.0167 (9) | 0.0138 (11) | 0.0013 (7) | 0.0033 (9) | −0.0008 (7) |
| C10 | 0.0217 (12) | 0.0211 (10) | 0.0182 (12) | 0.0011 (8) | 0.0091 (10) | 0.0033 (8) |
| C11 | 0.0178 (12) | 0.0298 (11) | 0.0301 (14) | 0.0022 (9) | 0.0097 (11) | 0.0056 (9) |
| C12 | 0.0176 (12) | 0.0218 (10) | 0.0213 (13) | 0.0016 (8) | −0.0007 (10) | 0.0033 (8) |
| C13 | 0.0291 (14) | 0.0337 (12) | 0.0168 (13) | 0.0025 (9) | 0.0066 (11) | 0.0055 (9) |
| C14 | 0.0193 (13) | 0.0349 (11) | 0.0214 (13) | 0.0032 (9) | 0.0093 (11) | 0.0054 (9) |
Geometric parameters (Å, °)
| C1—N1 | 1.345 (3) | C6—Cl2 | 1.727 (2) |
| C1—N2 | 1.386 (2) | C7—C8 | 1.379 (3) |
| C1—S1 | 1.666 (2) | C7—Cl3 | 1.723 (2) |
| N1—C3 | 1.410 (2) | C8—H8 | 0.9300 |
| N1—H1 | 0.8600 | C9—C10 | 1.384 (3) |
| N2—C2 | 1.386 (3) | C9—C14 | 1.391 (3) |
| N2—H2 | 0.8600 | C10—C11 | 1.382 (3) |
| C2—O1 | 1.227 (3) | C10—H10 | 0.9300 |
| C2—C9 | 1.491 (3) | C11—C12 | 1.382 (3) |
| C3—C8 | 1.391 (3) | C11—H11 | 0.9300 |
| C3—C4 | 1.394 (3) | C12—C13 | 1.383 (3) |
| C4—C5 | 1.380 (3) | C12—H12 | 0.9300 |
| C4—Cl1 | 1.739 (2) | C13—C14 | 1.379 (3) |
| C5—C6 | 1.386 (3) | C13—H13 | 0.9300 |
| C5—H5 | 0.9300 | C14—H14 | 0.9300 |
| C6—C7 | 1.394 (3) | ||
| N1—C1—N2 | 115.16 (18) | C8—C7—C6 | 119.9 (2) |
| N1—C1—S1 | 125.47 (16) | C8—C7—Cl3 | 118.89 (16) |
| N2—C1—S1 | 119.36 (15) | C6—C7—Cl3 | 121.24 (17) |
| C1—N1—C3 | 124.74 (18) | C7—C8—C3 | 121.12 (19) |
| C1—N1—H1 | 117.6 | C7—C8—H8 | 119.4 |
| C3—N1—H1 | 117.6 | C3—C8—H8 | 119.4 |
| C2—N2—C1 | 127.76 (18) | C10—C9—C14 | 119.02 (19) |
| C2—N2—H2 | 116.1 | C10—C9—C2 | 124.6 (2) |
| C1—N2—H2 | 116.1 | C14—C9—C2 | 116.36 (19) |
| O1—C2—N2 | 121.4 (2) | C11—C10—C9 | 120.1 (2) |
| O1—C2—C9 | 121.06 (19) | C11—C10—H10 | 119.9 |
| N2—C2—C9 | 117.51 (18) | C9—C10—H10 | 119.9 |
| C8—C3—C4 | 118.10 (19) | C12—C11—C10 | 120.5 (2) |
| C8—C3—N1 | 121.05 (18) | C12—C11—H11 | 119.7 |
| C4—C3—N1 | 120.81 (19) | C10—C11—H11 | 119.7 |
| C5—C4—C3 | 121.5 (2) | C11—C12—C13 | 119.8 (2) |
| C5—C4—Cl1 | 118.90 (16) | C11—C12—H12 | 120.1 |
| C3—C4—Cl1 | 119.63 (16) | C13—C12—H12 | 120.1 |
| C4—C5—C6 | 119.57 (19) | C14—C13—C12 | 119.6 (2) |
| C4—C5—H5 | 120.2 | C14—C13—H13 | 120.2 |
| C6—C5—H5 | 120.2 | C12—C13—H13 | 120.2 |
| C5—C6—C7 | 119.82 (19) | C13—C14—C9 | 120.9 (2) |
| C5—C6—Cl2 | 118.96 (16) | C13—C14—H14 | 119.5 |
| C7—C6—Cl2 | 121.20 (17) | C9—C14—H14 | 119.5 |
| N2—C1—N1—C3 | −175.25 (16) | C5—C6—C7—Cl3 | 178.95 (14) |
| S1—C1—N1—C3 | 6.3 (3) | Cl2—C6—C7—Cl3 | −2.6 (2) |
| N1—C1—N2—C2 | 1.7 (3) | C6—C7—C8—C3 | −1.9 (3) |
| S1—C1—N2—C2 | −179.68 (15) | Cl3—C7—C8—C3 | 178.94 (14) |
| C1—N2—C2—O1 | −5.0 (3) | C4—C3—C8—C7 | 2.9 (3) |
| C1—N2—C2—C9 | 175.03 (17) | N1—C3—C8—C7 | −179.55 (16) |
| C1—N1—C3—C8 | 49.5 (3) | O1—C2—C9—C10 | 169.75 (18) |
| C1—N1—C3—C4 | −133.0 (2) | N2—C2—C9—C10 | −10.3 (3) |
| C8—C3—C4—C5 | −1.9 (3) | O1—C2—C9—C14 | −9.0 (3) |
| N1—C3—C4—C5 | −179.45 (17) | N2—C2—C9—C14 | 170.96 (17) |
| C8—C3—C4—Cl1 | 178.74 (14) | C14—C9—C10—C11 | 0.4 (3) |
| N1—C3—C4—Cl1 | 1.2 (2) | C2—C9—C10—C11 | −178.33 (18) |
| C3—C4—C5—C6 | −0.1 (3) | C9—C10—C11—C12 | −0.5 (3) |
| Cl1—C4—C5—C6 | 179.25 (14) | C10—C11—C12—C13 | 0.1 (3) |
| C4—C5—C6—C7 | 1.2 (3) | C11—C12—C13—C14 | 0.4 (3) |
| C4—C5—C6—Cl2 | −177.30 (14) | C12—C13—C14—C9 | −0.6 (3) |
| C5—C6—C7—C8 | −0.2 (3) | C10—C9—C14—C13 | 0.2 (3) |
| Cl2—C6—C7—C8 | 178.26 (14) | C2—C9—C14—C13 | 178.98 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1 | 0.86 | 1.89 | 2.586 (2) | 137. |
| N2—H2···S1i | 0.86 | 2.83 | 3.6771 (19) | 168. |
Symmetry codes: (i) −x+1, y, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BG2439).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, 115–119.
- Baily, N., Dean, A. W., Judd, D. B., Middlemiss, D., Storer, R. & Watson, S. P. (1996). Bioorg. Med. Chem. Lett. 6, 1409–1413.
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Kabuto, C., Akine, S., Nemoto, T. & Kwon, E. (2009). J. Crystallogr. Soc. Jpn, 51, 218–224.
- Khawar Rauf, M., Bolte, M. & Badshah, A. (2009a). Acta Cryst. E65, o177. [DOI] [PMC free article] [PubMed]
- Khawar Rauf, M., Bolte, M. & Badshah, A. (2009b). Acta Cryst. E65, o240. [DOI] [PMC free article] [PubMed]
- Koch, K. R. (2001). Coord. Chem. Rev. 216–217, 473–488.
- Krishnamurthy, R., Govindaraghavan, S. & Narayanasamy, J. (1999). J. Pestic. Sci. 52, 145–151.
- Molecular Structure Corporation and Rigaku (2001). CrystalClear MSC, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Molecular Structure Corporation and Rigaku (2004). TEXSAN MSC, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Murtaza, G., Ebihara, M., Said, M., Khawar Rauf, M. & Anwar, S. (2009a). Acta Cryst. E65, o2297–o2298. [DOI] [PMC free article] [PubMed]
- Murtaza, G., Hanif-Ur-Rehman, Khawar Rauf, M., Ebihara, M. & Badshah, A. (2009b). Acta Cryst. E65, o343. [DOI] [PMC free article] [PubMed]
- Namgun, L., Mi-Hyun, C. & Taek, H. K. (2001). J. Korean Chem. Soc. 45, 96–99.
- Patil, D. G. & Chedekel, M. R. (1984). J. Org. Chem. 49, 997–1000.
- Rigaku (1998). REQAB Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wegner, P., Hans, R., Frank, H. & Joppien, H. (1986). Eur. Patent No. 190611.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811052780/bg2439sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811052780/bg2439Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811052780/bg2439Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



