Summary
Introspection makes it clear that we do not see the visual motion generated by the approximately three saccadic eye movements we make each second. We refer to the lack of awareness of the motion across the retina that is generated by a saccade as saccadic omission [1]: the visual stimulus generated by the saccade is omitted from our subjective awareness. In the laboratory, saccadic omission is often studied by investigating saccadic suppression- the reduction in visual sensitivity prior to and during a saccade (see Ross et al.[2] and Wurtz [3] for reviews). We investigated whether perceptual stability requires that a mechanism like saccadic suppression removes peri-saccadic stimuli from visual processing to prevent their presumed harmful effect on perceptual stability [4,5]. Our results show that a stimulus that undergoes saccadic suppression and is unseen can nevertheless generate a shape contrast illusion. The shape contrast illusion can be generated when the inducer and test stimulus are separated in space and is therefore thought to be generated at a later stage of visual processing [6]. This shows that perceptual stability is attained without removing stimuli from processing and suggests a conceptually new view of perceptual stability in which peri-saccadic stimuli are processed by the early visual system, but these signals are prevented from reaching awareness at a later stage of processing.
Results
To determine the fate of a saccadically omitted stimulus, we used a visual shape illusion in which the presentation of a line distorts the perceived shape of a subsequently presented ellipse [6] (figure 1). Our goal was to present the line within 75ms preceding a saccade – such that the observer would not be aware of it on some trials– and then determine whether such a saccadically omitted stimulus nevertheless retained the ability to change the subsequent perceived shape of an ellipse. The observers first reported whether or not they saw the line which could be horizontal or vertical and was physically present on only 50% of trials. They then reported the shape of the ellipse that was presented once the eyes had landed. This allowed us to determine the percentage of trials in which they perceived the ellipse to be horizontally elongated. We refer to this as %PHE (Perceived Horizontal Elongation). To quantify the size of the illusion we presented ellipse stimuli with a physical horizontal or vertical elongation of 10%, 7.5%, 5%, or 2.5% of the diameter (3 degrees of visual angle). A separate cumulative Gaussian was fitted to the %PHE in the horizontal and vertical line conditions. The subjective point of circularity was taken as the physical elongation at which the fitted function reached 50%PHE and the strength of the illusion was defined as the difference between the points of subjective circularity induced by presentation of the horizontal and vertical lines. Subjective circularity is measured in units of percentage of elongation of the diameter of the test circle. We first confirmed that the shape illusion occurs at fixation for briefly flashed stimuli (Figure 2, Supplementary Materials, Experiment 1) and also when an eye movement intervenes in the 250ms between the presentation of the line and the ellipse (Supplementary Materials, Experiment 2). We then analyzed separately the saccade trials in which the subject reported no subjective awareness of the inducing line (saccadic omission was achieved) and those trials in which subjects reported awareness of the inducing line (saccadic omission was not achieved).
Figure 1.
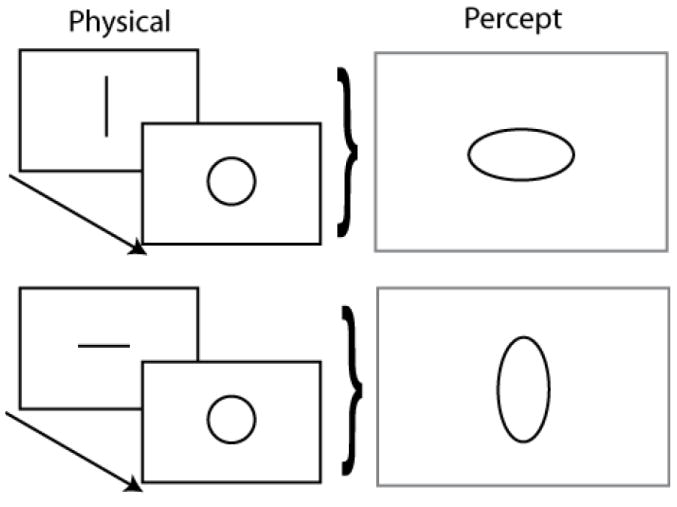
The shape contrast illusion. A circle presented after a line appears elongated orthogonal to the line direction.
Figure 2.
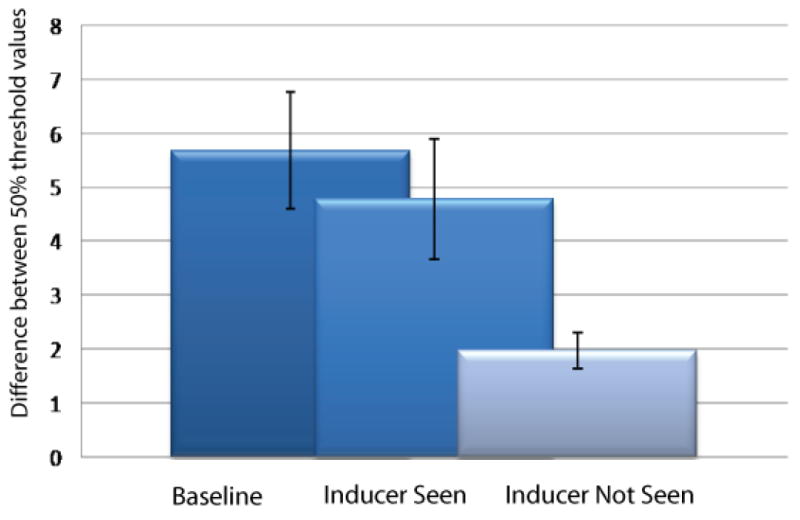
Average strength of the shape contrast illusion. Illusion strength is measured as the difference in point of subjective circularity of test stimuli following presentation of a horizontal compared to vertical line (see main text). This calculation was carried out separately during fixation (dark bars), when the inducer was seen during an eye movement (mid bars) and was not seen during an eye movement (light bars). Error bars represent 95% confidence intervals.
Figure 2 shows our critical finding that the participants had a significant shape illusion even without awareness of the inducer (t4=2.8125, P<0.05 (see supplementary analysis for further details)). Hence, even when saccadic omission was complete, the omitted stimulus affected subsequent perception. This implies that the line was processed at least by those higher visual areas responsible for this shape illusion (See Discussion) and shows that perceptual stability does not require perisaccadic visual stimuli to be removed from processing.
The data in Figure 2 also show that the shape illusion was reduced for those lines that were reported not to be seen compared to lines that were reported to be seen. This shows that the influence a stimulus exerts on visual processing is diminished most when it is not seen. Our data show however, that this suppression is not enough to explain the omission because a stimulus that is only partially suppressed nevertheless can be fully omitted.
Our interpretation of the data relies on taking seriously the subjective report and its binary nature (seen/not seen). We believe that this is appropriate and in fact essential in this context because under most everyday circumstances, normal observers do not perceive any retinal motion during an eye movement and the absolute nature of this phenomenon requires an explanation. This notwithstanding, there could be a concern that – in the critical condition of complete omission with incomplete suppression– observers may have experienced the stimulus despite answering to the contrary. In the supplementary materials we analyzed this possibility and conclude that to generate an effect of the appropriate size the observers would have to be wrong about their perceptual experience in more than 25% of trials. We believe this to be an unlikely explanation of our findings. Moreover, questioning upon completion of the experiments also reassured us that participants were reporting not seeing the stimulus when they were convinced that no stimulus existed; i.e. when they were experiencing saccadic omission.
Discussion
Our data show that a stimulus that is successfully removed from awareness by saccadic omission is nevertheless processed by the visual system. This shows that perceptual stability can be attained without removing a peri-saccadic stimulus from visual processing. We discuss this finding in the light of a number of debates surrounding perceptual stability and saccadic suppression.
Early versus late
The shape contrast illusion we used here has been shown to survive when the inducing and test stimuli are separated in space, indicating that the illusion involves an area whose shape-selective neurons have large receptive fields such as STS or IT [6]. Our data therefore suggest that saccadically omitted stimuli are nevertheless processed by such higher visual areas. The fact that the illusion is reduced in size during omission suggests that there may be a (possibly early) stage at which the efficacy of a stimulus is reduced [4]. A modulation – including suppression- of the visual responses of single cells has been shown as early as the lateral geniculate nucleus [7].
Retinal versus extraretinal
Two main mechanisms of saccadic suppression have been proposed. The first relies on an extra retinal signal that prepares the visual system to discount the upcoming high velocity peri-saccadic retinal motion. The second is a form of masking whereby the perisaccadic stimulus is ‘wiped out’ by the stable, fixated stimuli before or after the saccade. Both of these mechanisms are thought to occur early in the visual processing hierarchy to stabilize the visual world by removing the intrusion of the peri-saccadic stimulus into the stable representation of fixated scenes [3]. The extra retinal component of saccadic suppression suppresses mainly low spatial frequency, luminance defined stimuli [4]which are considered the domain of the magnocellular visual pathway and does not appear to affect judgments involving isoluminant chromatically defined stimuli which are processed mainly by the parvocellular pathway [4].
In our experiments the inducing line was presented just before the eye movement; a time at which the extra-retinal mechanism of suppression is known to operate. Moreover, the luminance modulated line was flashed briefly and would likely activate the magnocellular pathway. Masking mechanisms will also be activated by the background luminance of the CRT screen and by presentation of the ellipse after the eye movement. Hence, our experiments do not allow us to distinguish between extra retinal and masking mechanisms of suppression, but it is likely that both mechanisms were operating and therefore both were only partially effective in removing the stimulus from processing even when omission was complete.
Dorsal versus ventral
The shape contrast illusion reported here presumably arises from interactions in the ventral stream. Hence one could hypothesize that complete omission with incomplete suppression is a phenomenon that is unique to the ventral stream. Or, stated differently, that the dorsal stream undergoes suppression and omission, while the ventral stream only undergoes omission. Such a distinction is consistent with behavioral data that show that saccadic suppression mainly targets stimuli that drive the magnocellular stream, which, in turn, mainly projects to the dorsal stream. The neural evidence, however, suggests that saccadic reductions in firing rate can be found in both magnocellular and parvocellular cells of the LGN [7] and in both dorsal [8][9] and ventral [10] cortical areas. Hence it seems unlikely that ventral areas undergo no suppression. Conversely, it is also unlikely that suppression is complete in dorsal areas; the perisaccadic reversal of direction preference reported by Thiele et al. [8]for instance, suggests that saccadic suppression is more complex than simply not responding to perisaccadic stimuli.
Our data, together with the extensive literature on perisaccadic changes in neural response properties lead us to rethink the purpose of saccadic suppression. We speculate that peri-saccadic signals are useful; the visual motion signals generated by the eye movement, for instance, are excellent indicators of the size and speed of the eye movement. These signals could be used to improve perceptual stability as long as they do not directly enter visual awareness. This suggests the view that peri-saccadic processing leading to saccadic omission consists of three conceptually different components; a (possibly early-visual) reduction in sensitivity (saccadic suppression), and a component that processes peri-saccadic signals to extract information useful for visual stability (saccadic information extraction). Additionally, perceptual stability requires a conceptually separate component that prevents the peri-saccadic signals from reaching awareness (saccadic omission).
Most research whose purported goal is to investigate the neural mechanisms of saccadic omission has only looked for signatures of the first component. Even though some studies did indeed find neural correlates of a reduced peri-saccadic sensitivity [11-15], others have reported complex changes in peri-saccadic activity that are difficult to reconcile with a mere suppression of visual processing [7-9,15-17](see [3] for a review). Within our framework, however, complex changes in activity are expected as the visual system tries to extract information from the peri-saccadic visual inputs, or because the system processes the information while keeping it hidden from awareness. Importantly, our data show that even if a neural correlate of the change in sensitivity could be found in early visual areas, then we would still not understand the neural basis of saccadic omission and how it leads to perceptual stability. Our conceptualization therefore provides a useful framework to guide further research, and interpret findings about the neural basis of perceptual stability.
Materials and methods
Subjects
All conditions were completed by four naïve participants and one experimenter. The four naive participants received remuneration and all had normal or corrected to normal vision.
Visual Stimuli
The stimuli consisted of a bar and a ring. Both were presented with half cosine profile luminance graduated edges against a background luminance of 45 cd/m2 and a peak luminance of 47 cd/m2. The inducing line was 7×1 degrees of visual angle while the circle had a diameter of 3 degrees of visual angle and an outline line width of 0.8 degrees of visual angle. The bar was present on the screen for 16ms while the ring was presented for 100ms. The ring was always presented 250ms after onset of the inducing line.
Apparatus
Stimuli were presented on a Sony FD Trinitron (GDM-C520) CRT monitor with a resolution of 1024 × 768 pixels at a refresh rate of 120Hz. Stimuli were generated using Neurostim (neurostim.sourceforge.net). Eye movements were measured with a head mounted Eyelink II eye tracker (SR Research, Mississauga, Canada). The pupil of the left eye was tracked at a sample rate of 500Hz and a spatial resolution of 0.1°. Participants were seated in a darkened room at 57cm distance from the display. Head movements were restricted via individually molded bite bars.
Procedure
Experiment 1
To test the basic shape contrast illusion with stimuli optimized for saccadic suppression experiment one was carried out without eye movements and all stimuli were presented at the center of the screen. The inducing bar could be either horizontal or vertical but was only presented on half of all trials while the test stimuli were presented with a horizontal or vertical elongation of 10%, 7.5%, 5%, and 2.5% of the diameter. All possible pairings of bar and ring orientation were presented in a randomized order and 40 trials were collected for each making a total of 640 trials.
Participants were made aware that only half the trials contained a line stimulus and were asked to report the orientation of the elongation of the ring stimulus via a key press once the stimulus had appeared.
Experiment 2
Stimuli were as described in experiment one with the exception of the bar being presented 5 deg above the midpoint of the monitor and the ellipse being presented 5 deg below the midpoint of the monitor.
In this experiment, if at any stage fixation was not achieved or maintained as required, the trial was terminated. The terminated trial was repeated at a later stage within the same test session. Participants were first shown a 0.2 × 0.2 degree white fixation point five degrees above the center of the screen. They were required to fixate this until it disappeared. 1000ms after fixation was achieved a 0.3 × 0.3 deg white saccade target appeared 5 degrees below the center of the screen. The cue to move the eyes (disappearance of the current fixation point) was given between 1900 to 2200 ms after initial fixation. At 2200ms after initial fixation was achieved the inducing bar appeared around the location of the now invisible upper fixation point. This timing aimed to present the inducing bar to the participant within the window of saccadic suppression but before the eye had started the saccade. The ring stimulus was then presented at the location of the saccade target 250ms after presentation of the inducing bar. Participants were required to have achieved fixation of this target at least 100ms prior to presentation of the ring stimulus. This fixation requirement ensured that the ring stimulus always landed on a stationary retina.
Participants reported whether they saw the inducing bar and the axis of elongation of the ring. Both tasks were carried out via a key press after the completion of the eye movement.
An additional set of trials were carried out with the same procedure however the inducing line was presented 2050ms after initial fixation was achieved. This ensured that the inducing line was presented well before the saccadic suppression window and provided an estimate of participants’ miss rate for the inducing line.
Data Analysis
Experiment 1 & 2
A separate cumulative Gaussian distribution was fit to the reported perceived axis of elongation of the ellipse during trials with a vertical inducer, a horizontal inducer, and the trials without an inducer. The Psignifit toolbox for Matlab[18,19] was used for this analysis. The threshold at 50% was considered the point of subjective circularity under each different inducing condition. As a test of significance, we used the bootstrapping methods of the Psignifit toolbox to test the null hypothesis that the cumulative Gaussians for vertical and horizontal bars could be generated by the same underlying distribution.
Experiment 2
Trials during which appropriate fixation was not achieved were automatically discarded. Additionally, we selected only those trials in which the test stimulus was presented during the 75 ms before an eye movement was initiated. Additional to the separation of trials according to orientation of the inducing bar, trials were also grouped according to participants’ responses about its visibility.
Supplementary Material
Acknowledgments
The authors are grateful to Kai Schreiber and Adam Morris for comments on the manuscript and thank the Human Frontiers Science Program (TLW) and the Pew Charitable Trusts (BK) for financial support.
Reference List
- 1.Campbell FW, Wurtz RH. Saccadic omission: why we do not see a grey-out during a saccadic eye movement. Vision Res. 1978;10:1297–303. doi: 10.1016/0042-6989(78)90219-5. [DOI] [PubMed] [Google Scholar]
- 2.Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;2:113–21. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- 3.Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;20:2070–89. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994;6497:511–3. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- 5.Holt EB. Eye movements and central anaesthesia. Psychological Review. 1903:3–45. [Google Scholar]
- 6.Suzuki S, Cavanagh P. A shape-contrast effect for briefly presented stimuli. J Exp Psychol Hum Percept Perform. 1998;5:1315–1341. doi: 10.1037//0096-1523.24.5.1315. [DOI] [PubMed] [Google Scholar]
- 7.Reppas JB, Usrey WM, Reid RC. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron. 2002;5:961–74. doi: 10.1016/s0896-6273(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 8.Thiele A, Henning P, Kubischik M, Hoffmann KP. Neural mechanisms of saccadic suppression. Science. 2002;5564:2460–2. doi: 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- 9.Ibbotson MR, Crowder NA, Cloherty SL, Price NS, Mustari MJ. Saccadic modulation of neural responses: possible roles in saccadic suppression, enhancement, and time compression. J Neurosci. 2008;43:10952–10960. doi: 10.1523/JNEUROSCI.3950-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron. 2001;3:757–67. doi: 10.1016/s0896-6273(01)00250-1. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett JR, Doty RW, L BB, Sr, Sakakura H. Influence of saccadic eye movements on geniculostriate excitability in normal monkeys. Exp Brain Res. 1976:487–509. doi: 10.1007/BF00239783. [DOI] [PubMed] [Google Scholar]
- 12.Duffy FH, Burchfiel JL. Eye movement-related inhibition of primate visual neurons. Brain Res. 1975;1:121–32. doi: 10.1016/0006-8993(75)90139-0. [DOI] [PubMed] [Google Scholar]
- 13.Sylvester R, Haynes JD, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr Biol. 2005;1:37–41. doi: 10.1016/j.cub.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 14.Vallines I, Greenlee MW. Saccadic suppression of retinotopically localized blood oxygen level-dependent responses in human primary visual area V1. J Neurosci. 2006;22:5965–5969. doi: 10.1523/JNEUROSCI.0817-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price NS, Ibbotson MR, Ono S, Mustari MJ. Rapid Processing of Retinal Slip During Saccades in Macaque Area MT. J Neurophysiol. 2005:235–246. doi: 10.1152/jn.00041.2005. [DOI] [PubMed] [Google Scholar]
- 16.Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr Biol. 2004;5:386–90. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester R, Rees G. Extraretinal saccadic signals in human LGN and early retinotopic cortex. Neuroimage. 2006;1:214–9. doi: 10.1016/j.neuroimage.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001;8:1314–29. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- 19.Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;8:1293–313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


