Abstract
B cell behaviour is fine-tuned by internal regulatory mechanisms and external cues such as cytokines and chemokines. SOCS3 is a key regulator of STAT3-dependent cytokine responses in many cell types, and has been reported to inhibit CXCL12-induced retention of immature B cells in the bone marrow. Using mice with SOCS3 exclusively deleted in the B cell lineage (Socs3Δ/Δmb1cre+), we analysed the role of SOCS3 in the response of these cells to CXCL12 and the STAT3-inducing cytokines IL-6 and IL-21. Our findings refute a B cell-intrinsic role for SOCS3 in B cell development, as SOCS3 deletion in the B lineage did not affect B cell populations in naïve mice. SOCS3 was strongly induced in B cells stimulated with IL-21 and in plasma cells exposed to IL-6. Its deletion permitted excessive and prolonged STAT3 signaling following IL-6 stimulation of plasma cells, and in a T cell-dependent immunization model, reduced the number of GC B cells formed and altered the production of antigen-specific IgM and IgE. These data demonstrate a novel regulatory signal transduction circuit in plasma cells, providing the first evidence of how these long-lived, sessile cells respond to the external signals that mediate their longevity.
Introduction
SOCS3 is a negative feedback inhibitor of cytokine signaling through the JAK-STAT pathway. Signal attenuation is achieved via inhibition of signaling intermediates, either by direct or competitive binding, or by engaging ubiquitination machinery and targeting activated JAKs, STATs or cytokine receptors for proteasomal degradation (1). SOCS3 is a transcriptional target of STAT3, and in turn promotes the proteasomal degradation of phosphorylated STAT3 in a negative feedback loop (2–5). As IL-6 and IL-21 signal via STAT3, and have been described as important determinants of B cell and plasma cell survival and proliferation (6–8), we sought to determine the consequences of SOCS3 deletion in the B cell lineage, both on the development of B cells and plasma cells in the steady state and in response to challenge with antigen.
SOCS3 has been ascribed a role in early B cell development, specifically in reducing CXCL12-induced FAK phosphorylation and adhesion to VCAM-1 in the bone marrow (BM) (9). Using MMTV-cre-mediated SOCS3 deletion, these authors showed a 2-fold increase in the number of immature B cells in the BM in the absence of SOCS3. The expression of cre recombinase under control of the MMTV promoter, however, causes deletion of floxed genes not only in B and T cells, but also in some epithelial and secretory cell types as well as megakaryocytes and erythroid cells (10). B cell-related effects observed in this study may therefore be secondary consequences of deletion of SOCS3 in cells other than B cells. An unequivocal B cell-intrinsic role for SOCS3 in B cell development has not yet been determined.
SOCS3 may also influence B cell activation and differentiation in response to antigenic stimulation. Typical B cell responses to T cell-dependent antigens usually occur within the GC (11). The GC reaction is a coordinated process in which activated B cells migrate between the dark zone and the light zone. The dark zone is a site of intense B cell proliferation where the predominant chemokine is CXCL12 (12). Following bursts of proliferation and somatic hyper-mutation, B cells become responsive to CXCL13, which directs them out of the dark zone and towards the light zone (12, 13). Here, follicular dendritic cells and T follicular helper (Tfh) cells promote the selection and survival of B cell clones (termed centrocytes) with high affinity for antigen (13). Tfh cells secrete IL-21 (14, 15), which has direct effects on B cells (16, 17). IL-21 activates STAT3 in B cells to drive their expression of Bcl-6 (18), which occurs also in GC B cells, halting GC dissolution while B cells in the GC undergo affinity maturation (16, 17). Loss of IL-21, Bcl-6 or STAT3 impairs B cell responses to T cell-dependent antigens (7, 16–19). Thus, the responsiveness of GC B cells to chemokines and cytokines is essential for balancing proliferation and differentiation, as it is for B cell preservation.
To examine the cell-intrinsic roles of SOCS3 in regulating B cell and plasma cell development and behaviour, we generated mice with SOCS3 deleted exclusively in the B cell lineage by crossing mice with floxed alleles of SOCS3 (20) with mice expressing cre recombinase from the mb1 locus (21). The mb1 gene encodes CD79α, an intracellular component of the B cell receptor, which is expressed exclusively in B cells beginning at the pro-B cell stage (21). Our data refutes the finding that SOCS3 expression in immature B cells is a prerequisite for their timely exit from the bone marrow (BM) (9), and shows that SOCS3 regulates both the maintenance of the GC and IgE affinity maturation during a T cell-dependent immune response.
Materials and Methods
Mice
Mice with floxed (20) or null (22) alleles of Socs3 were crossed with mice expressing cre recombinase under the mb1 promoter (21) or the MMTV promoter (10). All mice were on a C57BL/6 background, and were maintained in a conventional animal facility. All procedures were performed in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Melbourne Health Animal Ethics Committee.
Antibodies and flow cytometry for identification of B cell subsets
Single cell suspensions from BM or spleen were treated to lyse red blood cells and stained with antibody conjugates for flow cytometry, including αCD19 (clone ID3),αCD21 (clone 7G6), αCD23 (clone B3B4), αB220 (clone RA3-6B2), αCD5 (clone 53–7.3) and αSynd-1 (clone 281; CD138), all purified and conjugated in our laboratory or purchased from BD Pharmingen. Avidin-Cy5 was obtained from Southern Biotechnology Associates, Inc. B cell subsets were identified based on surface marker expression: in BM, pre/pro-B cells were identified as B220+ IgM− IgD− (a population which may also contain some macrophage precursors, DCs and NK cells), immature B cells as B220lo IgM+ IgD− and recirculating B cells as B220+ IgM+ IgD+. In spleen, transitional-1 (T1) B cells were identified as B220+ IgM+ IgD− CD21−CD23−, transitional-2 (T2) B cells as B220+ IgMhi IgD+ CD21hi CD23+, marginal zone (MZ) B cells as B220+ IgMhi IgDlo CD21+ CD23−, follicular B cells as B220+ IgM+ IgD+ CD21+ CD23+. Plasma cells were identified as B220−/int Synd-1+.
Flow cytometry was performed on an LSR II or FACSCalibur (Beckton Dickinson) cytometer and data on at least 104 viable cells, determined by propidium iodide exclusion, were collected. Live cells were sorted on the basis of propidium iodide exclusion on a FACSDiVa (BD Instruments) or MoFlo (DaKoCytomation) cytometer.
Quantification of gene expression
To measure cytokine induction of SOCS3, IL-21 receptor (IL-21R), IL-6 receptor (IL-6R α) or Bcl-6 mRNA expression, cells were FACS-sorted as above, then stimulated with either 10 ng/mL recombinant mouse IL-21 (a gift from Zymogenetics) or 10 ng/mL IL-6 [supernatant from transfected hybridoma cell line, optimal concentration determined by cell culture (23)]. Total RNA was isolated from sorted B cells or plasma cells using an RNeasy Mini Kit (Qiagen), and quantified by spectrophotometer at 260 nm absorbance. Using 100–600 ng total RNA (with equal amounts for comparable samples and the calibrator sample), cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The calibrator sample for expression of SOCS3 and IL-6Rα was total RNA isolated from murine ES cells stimulated in vitro with LIF for 30 minutes. The calibrator sample for expression of Bcl-6 was total RNA isolated from wildtype skeletal muscle. The calibrator sample for expression of IL-21R was FACS-sorted wildtype unstimulated B cells (B220+ Synd-1−). The negative control was the calibrator sample reaction without reverse transcriptase.
Real-time quantitative PCR (QPCR) was performed in triplicate or quadruplicate using TaqMan Gene Expression Assays (Applied Biosystems) for SOCS3 (Assay ID: Mm01249143_g1), IL-21R (Assay ID: Mm00600319_m1), IL-6Rα(Assay ID: Mm00439653_m1), Bcl-6 (Assay ID: Mm00477633_m1) or the endogenous control HPRT1 (Assay ID: Mm01318743_m1), using an Applied Biosystems 7900HT sequence detection system. Data were analysed using SDS 2.2 software (Applied Biosystems) and the Relative Quantification (ΔΔCt) method. Data are shown as mean relative expression of SOCS3, normalized for HPRT1 expression, compared with the calibrator sample.
Detection of intracellular phosphorylated STAT3
Detection of phosphorylated STAT3 by flow cytometry was achieved using the STAT3 (pY705)-AlexaFluor647 antibody and Phosflow reagents (BD Pharmingen) according to the manufacturer’s instructions. Flow cytometry was performed on an LSR II or FACSCalibur (Beckton Dickinson) cytometer and data was collected on at least 104 viable cells, determined by propidium iodide exclusion.
Transwell migration assays
Sorted cells were resuspended in RPMI + 5% FCS at a known cell concentration which varied between cell populations depending on sort efficiency. This cell suspension (600 μL) was pipetted into the top wells of a 4 μm pore transwell plate (Corning Life Sciences) that were then placed into the lower wells containing 100 μL of either medium alone or 0.4 μg/mL CXCL12 (PeproTech). In some cases, cells were concurrently stimulated with 10 μg/mL F(ab′)2 goat anti-mouse IgM (Jackson ImmunoResearch). The plates were incubated at 37 °C with 10% CO2 for 4h. The top wells were then removed and a known number of BD Calibrite™ beads (BD Biosciences) added to each lower well. This allowed the number of cells that had migrated through the pores to the lower wells to be accurately measured by flow cytometry.
Immunization
Mice were challenged with the model T cell-dependent antigen NP-KLH (Cambridge Research Biochemicals, UK), precipitated in alum. The antigen was injected (100 μL containing 100 μg of antigen) i.p. and mice were kept on a heat pad and monitored for 24h (24). Immunized mice were then bled retro-orbitally or mandibularly each week and immune serum was isolated and tested for the presence and affinity of NP-specific antibody by ELISA as described below.
Detection of NP-specific GC and memory B cells in the spleen was achieved essentially as described (16). Briefly, granulocytes, plasma cells and naïve unswitched B cells were excluded from analysis using a dump channel. B220+ cells were then examined for their expression of surface IgG1 and their ability to bind NP directly conjugated to PE. Cells with both these characteristics were then further subdivided based on CD38 expression into GC (CD38−) or memory (CD38+) subsets.
ELISA and ELISPOT assays
For ELISA, serum was isolated from non-heparin treated blood by allowing blood to clot for 4 hours at 4°C, then centrifuging at 13,500 rpm for 7 min. The supernatant serum was removed and stored at −20°C. Serum Ig concentration was measured using ELISA and Ig-secreting cells were counted by ELISPOT as described (25).
For capture of antigen-specific IgG1, NP coupled to BSA at either a high (NP13-BSA) or low (NP2-BSA) haptenation rate was used as a plate coat for ELISAs or ELISPOTs, as described (24). Detection of anti-NP antibodies of different isotypes was achieved using isotype-specific secondary antibodies (anti-IgM, anti-IgG1 or anti-IgE) directly coupled to horseradish peroxidase (SouthernBiotechnology Associates, Inc.).
Cell culture
Sorted spleen B cells (CD19+) were cultured in triplicate at 5×104 or 3×104 cells in 200 μL of RPMI + 5% FCS + 100 μM 2-ME with an optimal concentration of baculovirus-derived CD40L (determined by cell culture), IL-4 (100 U; Peprotech), and IL-21 (10 ng/mL) for four days at 37°C with 10% CO2. Following this culture period, a known number of CaliBRITE beads (BD Biosciences) was added to each well and the live cell number was determined on the basis of propidium iodide exclusion by flow cytometry.
Cell cycle analysis
Between 20,000 and 50,000 GC B cells were sorted per mouse, washed, and resuspended in 100 μL of PI buffer (0.1% sodium acetate, 0.2% Triton X-100, 10 μg/mL RNaseA and 50 μg/mL propidium iodide). Cells were vortexed twice for 10 sec then incubated at room temperature for 20 min in the dark before analysis on a Canto II cytometer (BD Instruments). At least 10,000 events were recorded and data were analysed using FlowJo version 8.8.2 cell cycle analysis software (Tree Star, Inc.).
Statistical analyses
Unpaired, two-tailed t-tests were performed using Microsoft Excel software. Differences were deemed significant where p < 0.05.
Results
SOCS3 expression is developmentally regulated in B cells and plasma cells and is further induced by IL-6 and IL-21
Given that SOCS3 expression has been reported to increase as a B cell progresses through developmental stages in the BM (9), we assessed the consequences of B cell-specific SOCS3 deletion on B cell development and differentiation. B cells of different subsets were sorted from BM and spleen and SOCS3 mRNA expression was measured by QPCR (Fig. 1A). Pre/pro-B cells and immature B cells in the BM contained a low level of SOCS3 mRNA, which was increased 3-fold (compared to pre/pro-B cells) in recirculating mature B cells in the BM, 6-fold in follicular B cells and 4-fold in plasma cells in the spleen (Fig. 1A).
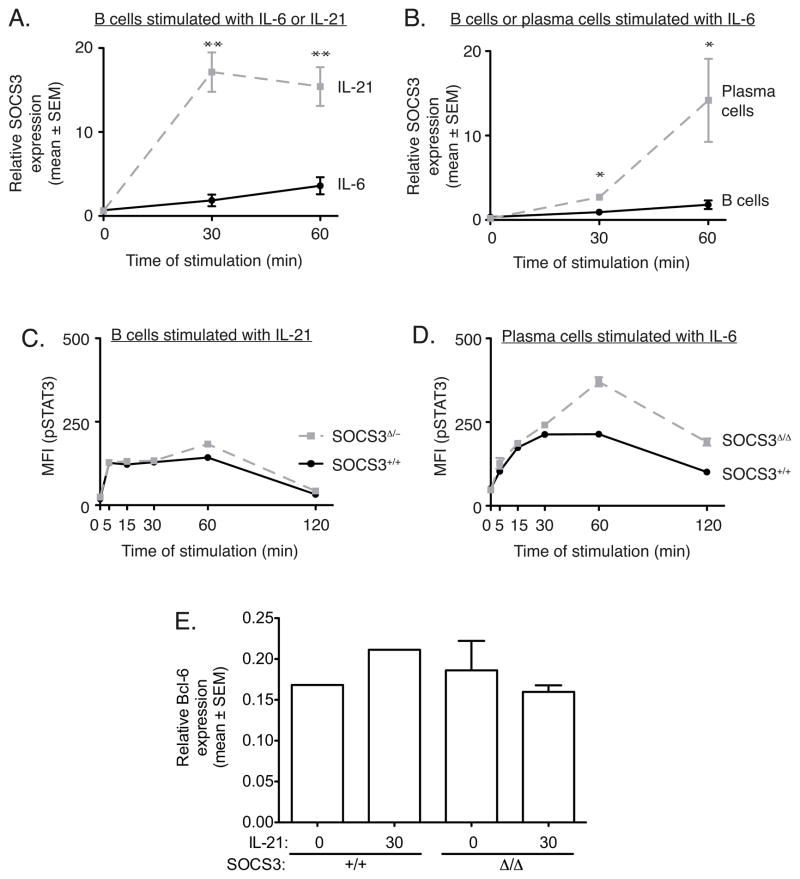
Figure 1. SOCS3 is expressed in B cells and plasma cells and is further induced by cytokines.
Data in each panel are expressed as mean relative expression of SOCS3, IL-21R or IL-6Rα, normalized for HPRT1 expression, compared with the calibrator sample, as described in Materials and Methods. In panels A and B, bars represent mean ± SEM for cells from 3–5 mice.
(A) SOCS3 mRNA expression in FACS-sorted wildtype B cell populations: pre/pro-B (BM, B220+ IgM− IgD−), immature (BM, B220lo IgM+ IgD−), recirculating (BM, B220+ IgM+ IgD+), follicular (spleen, B220+ IgM+ IgD+ CD21+ CD23+) and plasma cells (spleen, B220−/int Synd-1+). (B) FACS-sorted naïve B cells (B220+ Synd-1−) from Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice were stimulated with 10 ng/mL IL-21 for the indicated times in minutes, and SOCS3 mRNA expression measured by QPCR (** p < 0.01 vs unstimulated SOCS3+/+). (C) FACS-sorted naïve B cells (B220+ Synd-1−) from Socs3fl/flmb1cre− (SOCS3+/+; n=1) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ; n=3) mice were stimulated with 10 ng/mL IL-21 for the indicated times in minutes, and IL-21R mRNA expression measured by QPCR. (D) FACS-sorted naïve B cells (B220+ Synd-1−) from Socs3fl/flmb1cre− (SOCS3+/+; n=1) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ; n=3) mice were stimulated with 10 ng/mL IL-21 for the indicated times in minutes, and IL-6Rα mRNA expression measured by QPCR.
SOCS3 mRNA expression was dramatically induced by IL-21 in wildtype splenic B cells, increasing 25-fold after 30 mins relative to B cells at rest (Fig. 1B). This effect was not observed in Socs3fl/flmb1cre+ splenic B cells, demonstrating efficient mb1-cre-mediated deletion of the loxP-flanked Socs3 locus in these cells (Fig. 1B). The lack of SOCS3 mRNA induction with IL-21 stimulation was not due to downregulation of IL-21R, as expression of IL-21R and IL-6Rα were both comparable in SOCS3+/+ and SOCS3Δ/Δ B cells, with or without IL-21 stimulation (Fig. 1C and 1D).
IL-21 was a much stronger inducer of SOCS3 mRNA expression than IL-6 in wildtype splenic B cells (Fig. 2A). Stimulation of plasma cells with IL-6 caused considerable induction of SOCS3 expression, the magnitude of which was greater than that of B cells stimulated with IL-6 (Fig. 2B).
Figure 2. SOCS3 deficiency alters the STAT3 response of plasma cells to IL-6.
(A) FACS-sorted naïve B cells (B220+ Synd-1−) from wildtype mice were stimulated with IL-21 or IL-6 for the indicated times in minutes, and SOCS3 mRNA expression measured by QPCR (** p < 0.01 vs IL-6 stimulation). (B) FACS-sorted naïve B cells (B220+ Synd-1−) or plasma cells (B220−/int Synd-1+) from wildtype mice were stimulated with IL-6 for the indicated times in minutes, and SOCS3 mRNA expression measured by QPCR (* p < 0.05 vs 0 min). In panels (A) and (B), bars represent mean ± SEM for cells from 3–5 mice. (C) Whole spleen cell suspensions from wildtype mice (Socs3+/+mb1cre+; SOCS3+/+) or mice heterozygous for Socs3 in all other cells types but lacking both alleles of Socs3 in B cells (Socs3fl/−mb1cre+; SOCS3Δ/−) were stimulated with IL-21 for the indicated times in minutes and then fixed, permeabilized and stained for intracellular pSTAT3 levels and surface markers. The pSTAT3 response of B cells (B220+ Synd-1−) is shown. (D) Whole spleen cell suspensions from Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice were stimulated with IL-6 for the indicated times in minutes and then fixed, permeabilized and stained for intracellular pSTAT3 levels and surface markers. The pSTAT3 response of plasma cells (B220− Synd-1+) is shown. In panels (C) and (D), the responses of cells from 1–3 mice are shown and the data are representative of at least 3 independent experiments. (E) FACS-sorted naïve B cells (B220+ Synd-1−) from Socs3fl/flmb1cre− (SOCS3+/+; n=1) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ; n=3) mice were stimulated with 10 ng/mL IL-21 for the indicated times in minutes, and Bcl-6 mRNA expression measured by QPCR.
SOCS3 limits the intensity and duration of IL-6 mediated STAT3 signaling in plasma cells
Despite the robust induction of SOCS3 mRNA expression upon stimulation with IL-21 (Fig. 1B and 2A), SOCS3 deficiency did not affect the magnitude or duration of STAT3 phosphorylation in B cells in response to IL-21 (Fig. 2C). Consistent with this finding, expression of the IL-21 target gene Bcl-6 was not affected by SOCS3 deletion (Fig. 2E). This indicates the involvement of alternative regulatory mechanisms in limiting IL-21-induced STAT3 activation and downstream target gene effects in B cells.
In plasma cells, interaction with BM stromal cells induces expression of IL-6, which may act to enhance plasma cell survival (26). As IL-6 signaling through STAT3 is regulated by SOCS3 in macrophages (2, 3, 5), we examined whether SOCS3 regulation of STAT3 phosphorylation occurred in plasma cells. SOCS3 deficiency in plasma cells resulted in elevated and sustained levels of phosphorylated STAT3 following their stimulation with IL-6 (Fig. 2D). These data are the first to demonstrate that in plasma cells, as in macrophages, IL-6 induces phosphorylation of STAT3, causing expression of SOCS3, and that SOCS3 then attenuates signaling through this pathway by truncating STAT3 activity.
Deletion of SOCS3 does not impair B cell development or response to CXCL12
Using MMTV-cre-mediated deletion, SOCS3 deficiency has been reported to cause a 2-fold accumulation of immature B cells in the BM (9), although this finding cannot be attributed to the loss of SOCS3 only in B cells. To isolate B cell-intrinsic effects of SOCS3 deficiency from those due to perturbations in the BM stromal environment, to which developing B cells are highly sensitive, B cell development was examined in Socs3fl/+mb1cre+ (SOCS3Δ/+) and Socs3fl/−mb1cre+ (SOCS3Δ/−) mice. In these mice, no differences were observed compared to wildtype in the number or proportion of immature B cells or any other subset in the BM, and nor were any differences detected in the sizes of splenic B cell populations (Fig. 3A). Moreover, in our hands there were no changes detected in the number or proportion of immature B cells in the BM of Socs3fl/flMMTVcre+ (SOCS3Δ/Δ) mice (Fig. 3B), in contrast to what has previously been reported (9). Thus, our data do not support a role for B cell-expressed SOCS3 in regulating immature B cell development.
Figure 3. SOCS3 deficiency does not alter B cell development or migration.
B cells in the BM were identified as pre/pro-B (B220+ IgM− IgD−), immature (B220lo IgM+ IgD−), recirculating (B220+ IgM+ IgD+) or plasma cells (B220−/int Synd-1+), and B cells in the spleen were identified as T1 (B220+ IgM+ IgD− CD21− CD23−), T2 (B220+ IgMhi IgD+ CD21hi CD23+), MZ (B220+ IgMhi IgDlo CD21+ CD23−), follicular (B220+ IgM+ IgD+ CD21+ CD23+) or plasma cells (B220−/int Synd-1+).
(A) Frequencies of B cell populations in the BM and spleen were determined by FACS in wildtype mice (Socs3+/+mb1cre+; SOCS3+/+), mice lacking one allele of Socs3 in B cells (Socs3fl/+mb1cre+; SOCS3Δ+) or mice heterozygous for Socs3 in all other cells types but lacking both alleles of Socs3 in B cells (Socs3fl/−mb1cre+; SOCS3Δ/−). (B) BM B cell populations were determined by FACS in wildtype mice (Socs3+/+MMTVcre+; SOCS3+/+) or mice in which Socs3 was deleted using MMTV-cre (Socs3fl/flMMTVcre+; SOCS3Δ/Δ). (C) B cell populations were sorted by FACS from the BM of Socs3fl/+mb1cre+ (SOCS3Δ/+) or Socs3fl/−mb1cre+ (SOCS3Δ/−) mice and subjected to transwell migration assay where specific migration towards CXCL12 was determined over a 4-hour period. There were no statistically significant differences in migration towards CXCL12 in SOCS3Δ/− compared to SOCS3Δ/+ for any of the B cell subsets tested. In each panel, bars represent mean ± SEM for cells from 3–5 mice.
As well as its roles in polarising the GC reaction and in directing plasma cells to the BM, the chemokine CXCL12 has been shown to retain developing B cells in the BM until they reach the immature stage, a process reported to be subject to SOCS3 regulation (9). To examine whether SOCS3 deletion only in B cells alters their responsiveness to CXCL12, BM B cell populations were sorted from Socs3fl/+mb1cre+ (SOCS3Δ/+) and Socs3fl/−mb1cre+ (SOCS3Δ/−) mice and a transwell assay performed to assess specific migration towards CXCL12 (Fig. 3C). The rate of migration of immature B cells towards CXCL12 was lower than that of pre/pro-B cells or recirculating mature B cells, indicating that a reduced response to CXCL12 may indeed be associated with BM egress of immature B cells (Fig. 3C). Loss of SOCS3, however, did not change the extent of migration of any B cell subset towards CXCL12, arguing against a role for B cell-expressed SOCS3 in regulating this response. Furthermore, the frequency of plasma cells in the BM, which are dependent on CXCL12 for their initial BM homing and subsequent persistence, was unchanged in the absence of SOCS3 (Fig. 3A). It appears, therefore, that SOCS3 produced by the B lineage is not a critical regulator of B cell responsiveness to CXCL12 migration or survival signals.
SOCS3 deficiency does not affect steady-state plasma cell formation or survival
To assess the cellular effects of IL-6 hyper-responsiveness in SOCS3 deficient plasma cells, we measured the number of plasma cells in the BM and spleen of Socs3fl/+mb1cre+ (SOCS3Δ/+) and Socs3fl/−mb1cre+ (SOCS3Δ/−) mice as well as their isotype profile and Ig secretion. Despite our finding that SOCS3 regulates IL-6-induced STAT3 activation (Fig. 2D), deletion of SOCS3 did not alter the number or proportion of plasma cells in the BM and spleen of naïve animals (Fig. 3A). The absence of SOCS3 and resultant increase in STAT3 signaling did not change the amount of IgG in the serum (Fig. 4).
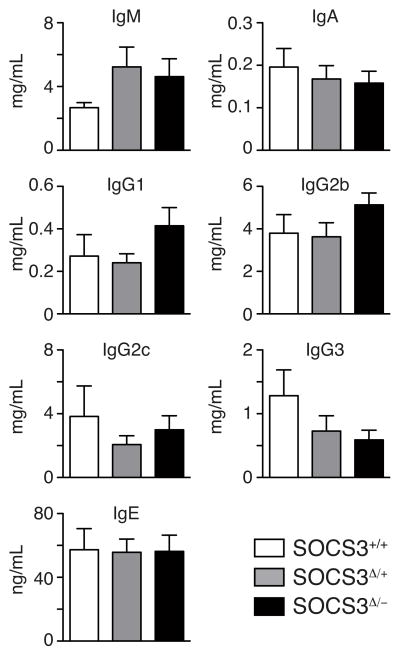
Figure 4. Absence of SOCS3 does not alter steady-state antibody production.
Serum was extracted from non-immunized Socs3+/+mb1cre+ (SOCS3+/+), Socs3fl/+mb1cre+ (SOCS3Δ/+) or Socs3fl/−mb1cre+ (SOCS3Δ/−) mice and antibody levels determined by ELISA. Bars represent mean plus SEM of serum from 6–9 mice.
Humans with STAT3 deficiency develop hyper-IgE syndrome (27) and deficiency of IL-21, which signals through STAT3, is also associated with hyper-secretion of IgE (16, 28, 29). These observations suggest that alterations to STAT3 signaling in the absence of SOCS3 might perturb serum IgE levels. However, no differences were detected in IgE serum levels when SOCS3 was deleted in the B lineage, and nor were there any other significant differences in the serum levels of other antibody isotypes (Fig. 4).
SOCS3 in B cells sustains the GC reaction in response to T cell-dependent immunization
There is significant interest in the roles of IL-21 and STAT3 signaling in maintaining the GC reaction and promoting appropriately timed plasma cell differentiation (7, 16, 17). As our data showed that SOCS3 is induced upon IL-21 stimulation of B cells, we examined the effect of SOCS3 deficiency on the humoral response to immunization with the model GC-inducing antigen, NP-KLH.
In a GC, T cells provide co-stimulatory factors including CD40L, IL-4 and IL-21 to B cells, and these factors sustain GC B cell survival and expansion (11, 16, 17). Stimulation of splenic B cells with the Tfh cell co-stimulatory factors CD40L plus IL- 4 and IL-21 in vitro resulted in fewer B cells after 4 days when SOCS3 was absent (Fig. 5A). To determine whether this translated to an in vivo deficiency in GC B cell numbers, we examined the effect of SOCS3 deletion on the number of GC B cells produced after immunization with NP-KLH. GC B cells were enumerated by flow cytometry by identifying (IgM, IgD, Synd-1, Gr-1)-negative, B220+ B cells that had switched to IgG1, possessed the ability to bind NP, and had not yet restored expression of CD38 (Fig. 5B).
Figure 5. SOCS3 deletion impairs GC B cell maintenance.
(A) Sorted naïve B cells (B220+ Synd-1−;) from spleens of Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice were stimulated with CD40L, IL-4 and IL-21 for 4 days and cell numbers determined by FACS. Bars represent mean ± SEM from cells derived from 2 mice per genotype, and results are representative of 2 independent experiments. (B) Gating strategy for FACS analysis of GC and memory B cells. The dump channel (IgM, IgD, Synd-1, Gr-1) excluded naïve, unswitched B cells, plasma cells and granulocytes. Switched B cells (dump− B220+) were then interrogated for their expression of IgG1 and capacity to bind NP. Antigen-specific IgG1+ B cells were further divided according to CD38 expression into GC (CD38−) and memory B cell (CD38+) subsets. (C) Using this strategy, GC B cells were sorted from spleens of Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice immunized with NP-KLH 8 days prior. GC B cells were fixed, permeabilized and stained with propidium iodide to determine their cell cycle status. (D) The frequency of GC B cells in Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice was determined at time points following immunization with NP-KLH. Bars represent means plus SEM for at least 4 mice, and data are representative of 4 independent experiments (* p < 0.05).
The proportions of GC B cells in the G2 and S phases of the cell cycle at day 8 of the response were not altered by SOCS3 deficiency (Fig. 5C), showing that SOCS3 does not affect early proliferation of GC B cells. Supporting this, the number of SOCS3-deficient GC B cells was equal to wildtype on day 10 after immunization (Fig. 5D). Later in the response, however, GC B cells lacking SOCS3 were not sustained, and their numbers had diminished in comparison to wildtype by day 28 (Fig. 5D).
GC output includes both memory B cells and plasma cells that secrete isotype-switched, high affinity antibody against the challenging antigen. No role was found for B cell-expressed SOCS3 in the production of IgG1 memory B cells during the early or late phases of the response (Fig. 6A), and the numbers of IgG1 plasma cells in BM and spleen were not significantly altered by SOCS3 deletion (Fig. 6B). In the BM and spleen, SOCS3 deletion had no significant effect on the numbers of anti-NP IgG1 plasma cells at day 28 (Fig. 6B), and this was reflected in the serum levels of anti-NP IgG1 being equivalent to wildtype at the same time point (Fig. 7B). Affinity maturation of NP-specific IgG1-secreting plasma cells in BM and spleen was not affected by SOCS3 deficiency, as shown by the normal affinity maturation of anti-NP IgG1 plasma cells that developed in the absence of SOCS3 (Fig. 6C). Together, these data show that the defect in SOCS3-deficient GC B cell maintenance does not translate into a defective IgG1 response.
Figure 6. GC IgG1+ output is unaffected by deletion of SOCS3.
(A) IgG1+ memory B cells in the spleens of Socs3+/+mb1cre+ (SOCS3+/+) or Socs3fl/flmb1cre+ (SOCS3Δ/Δ) mice, identified by the FACS gating strategy detailed in Figure 5, were measured 10 and 28 days following immunization with NP-KLH. (B) The formation of antigen-specific, IgG1-switched plasma cells in the spleen and their homing to the BM was measured by ELISPOT assay 28 days after immunization. NP13 denotes all plasma cells whose antibody binds NP, regardless of affinity. NP2 refers to plasma cells that express a high affinity anti-NP antibody. (C) Affinity maturation was calculated by comparing the frequency of high affinity (NP2) plasma cells to the total anti-NP plasma cell population (NP13). Bars represent mean plus SEM of n = 3 mice per genotype (days 0 and 10) or n = 10 mice per genotype (day 28).
Figure 7. Alterations in the antibody response to TD antigen in the absence of SOCS3.
The appearance of NP-specific antibodies of the (A) IgM, (B) IgG1 and (C) IgE isotypes were measured by ELISA following immunization with NP-KLH. Total anti-NP antibody was captured using an ELISA plate coat containing NP conjugated to BSA at a high conjugation ratio (NP13-BSA). The proportion of this total anti-NP antibody that binds NP with high affinity was determined using a separate ELISA plate coat containing NP conjugated to BSA at a low conjugation ratio (NP2-BSA). The extent of affinity maturation was determined by measuring the ratio of these two titres. It was not appropriate to determine the affinity maturation of IgM due to its general exclusion from SHM and selection, and also its pentameric structure that increases the avidity with which it can bind antigen. Bars represent mean plus SEM of serum derived from 3–10 mice (* p < 0.05, ** p < 0.001).
In parallel with GC formation, NP-KLH normally induces extra-follicular proliferation of immature plasma cells that produce a peak of NP-specific IgM around 10 days after immunization (30, 31). This early antigen-specific IgM production was significantly diminished by SOCS3 deletion (Fig. 7A), indicating that prolonged STAT3 phosphorylation may skew the response away from the extra-follicular IgM course. No bias was detected, however, towards antigen-specific IgG1 production by SOCS3-deficient plasma cells during the early phase of the response (Fig. 7B).
An additional consequence of immunization with NP-KLH in alum is the further switching of a small number of antigen-specific B cells to IgE, a process that appears to be regulated by IL-21 signaling in mice (28). The appearance of anti-NP IgE in serum of mice lacking SOCS3 in the B cell lineage initially occurred to the same extent as wildtype, but surpassed the wildtype level as the response progressed (Fig. 7C). This was particularly apparent when the analysis was restricted to anti-NP IgE of high affinity, which continued to increase in the SOCS3-deleted animals after reaching a plateau in wildtype mice (Fig. 7C).
Together, these data reveal a B cell-intrinsic role for SOCS3 in maintaining GC B cell numbers during the late phase of a T cell-dependent antibody response. We have also shown that SOCS3 modulates the production of antigen-specific IgM and IgE in response to immunization with a T cell-dependent antigen, but its deletion has no significant effects on the IgG1 response.
Discussion
Fine regulation of the formation and behaviour of B cells and their responsiveness to various immune environments are achieved through the activity of external factors including cytokines and chemokines. Several of these factors act via a STAT3-dependent signal transduction pathway. Since SOCS3 is a key feedback inhibitor of STAT3 signaling, particularly in response to IL-6 stimulation of macrophages (2, 3, 5), we sought to determine whether this inhibitory system extended to the B cell lineage and whether deletion of SOCS3 would impact facets of B cell behaviour that are influenced by STAT3-inducing stimuli.
SOCS3 expression was low in pre/pro-B cells and immature B cells, and increased as the cells matured, supporting previously published data (9). Using MMTV-cre-mediated SOCS3 deletion, Le and colleagues found that SOCS3 inhibited adhesion responses of developing B cells in the BM and allowed their timely egress to the periphery (9). SOCS3 deletion was reported to cause a 2-fold increase in the number of immature B cells retained in the BM (9). The use of MMTV-driven cre recombinase expression, however, will result in SOCS3 deletion in a number of haemopoietic cell types, including B and T cells, megakaryocytes and erythroid cells (10). Although this level of deletion avoids the embryonic lethality that occurs in mice lacking SOCS3 in all tissues (22, 32), Socs3fl/flMMTVcre+ mice show evidence of disrupted immune function due to altered chemokine secretion and neutrophil responses (33, 34). We have shown that when SOCS3 is deleted exclusively in B cells using mb1-cre, there is no accumulation of immature B cells in the BM. In addition, in our colonies of Socs3fl/flMMTVcre+ mice, numbers of immature B cell populations were not different from wildtype (Fig. 3B). Therefore, evidence presented here is not consistent with the finding that SOCS3 deficiency in immature B cells prevents them from leaving the BM (9), and points to a role for SOCS3 in other cell types as the explanation for this phenomenon.
In mature B cells, the level of SOCS3 mRNA expression was around half that produced by ES cells stimulated with LIF. Upon exposure to IL-6, SOCS3 expression by B cells increased 5-fold over 60 minutes, whereas IL-21 raised SOCS3 transcript levels 25-fold within 30 minutes. A role for IL-21 has recently been described in maintaining B cells as they progress through the GC reaction (16, 17). GC B cells are subject to major assault on the integrity of genes at the Ig loci during the process of somatic hyper-mutation and class switch recombination (CSR), and while this process occurs and the cell cycle is halted, IL-21 signaling prevents apoptosis of GC B cells (16, 17). This is thought to occur mainly via the induction of Bcl-6 by IL-21-stimulated STAT3 activation (18, 35, 36), although STAT3 deficiency in B cells does not fully recapitulate the phenotype of mice with impaired IL-21 signaling (7, 16, 17).
Our data shows that SOCS3 deficiency does not alter STAT3 phosphorylation or Bcl-6 expression following IL-21 stimulation of mature B cells, indicating that alternative mechanisms can modulate signaling in this pathway when SOCS3 is absent. This is not the case for the IL-6 pathway in plasma cells, where we found that SOCS3 was required for the regulation of STAT3 phosphorylation – a phenomenon that is also observed in macrophages (2, 3, 5).
Our data reveals previously unknown roles for IL-21-induced SOCS3 in promoting B cell proliferation and negatively regulating IgE switching. In vitro stimulation of B cells with the T cell co-stimulatory factors CD40L, IL-4 and IL-21 produced fewer B cells after four days when SOCS3 was absent. While this indicated that early GC B cell proliferation might be impaired in the absence of SOCS3, there was no effect of SOCS3 deletion on either the cell cycle status of ex vivo GC B cells on day 8 of the response, or on in vivo GC B cell numbers on day 10.
Complete deletion of IL-21 in mice increases the levels of both total and antigen-specific IgE in serum (16, 28, 37), and STAT3 deficiency in humans causes hyper-IgE syndrome (27). Since deletion of SOCS3 in B cells also increases the production of antigen-specific IgE, all three molecules appear to be negative regulators of IgE switching. CSR to Igε is division-linked (38), therefore the effects of IL-21, STAT3 and SOCS3 on IgE production may be via limiting the small fraction of B cells that continue to proliferate and switch to IgE.
IL-21 reduces B cell proliferation and blocks excess IgE production in response to a T cell-dependent antigen (16, 28, 37). In the absence of SOCS3, GC B cells may be more sensitive to IL-21 signals, causing an increase in IgE plasma cell production and a reduction in cell proliferation at the later stages of the GC reaction. Enhanced STAT3 signaling, which is associated with IgG1 switching (7), might cause a concomitant increase in the proportion of GC B cells switching to IgG1. On balance, these two factors could result in an apparently normal number of IgG1+ memory B cells and plasma cells exiting the GC in the absence of SOCS3.
Post-GC plasma cell formation requires STAT3 (7), which we have shown to be activated when plasma cells are exposed to IL-6. SOCS3 has a non-redundant role in regulating this pathway as its loss increases the duration of STAT3 phosphorylation. In addition, it has been shown that excess IL-6 signaling promotes a surplus of plasma cells (39–41). Alterations at the biochemical level, however, do not translate to cellular changes in SOCS3-deficient plasma cells in the steady state. The number of plasma cells in the spleen and BM was not affected by SOCS3 deficiency, and nor was the general IgG1 plasma cell response to NP-KLH immunization substantially altered by the loss of SOCS3. The production of antigen-specific IgM, however, was significantly lower when SOCS3 was absent. Whether this can be attributed to a role for SOCS3 in sustaining the proliferation of B cells destined for extra-follicular plasmablast responses, or in inhibiting switching away from IgM, is unclear.
By deleting SOCS3 exclusively in B cells, we have demonstrated the cell-intrinsic roles of SOCS3 in B cell and plasma cell development and behaviour. While SOCS3 was induced in B cells and plasma cells with IL-21 and IL-6 stimulation respectively, its deletion did not have major cellular consequences in the steady state. Upon immunization with a T cell-dependent antigen, however, the maintenance of SOCS3-deficient GC B cells was impaired. A novel role for SOCS3 was found in the formation of antigen-specific IgM early in the GC reaction, and in inhibiting the production of IgE in the later stages of the response. These findings may have implications for the treatment of allergies in which IgE production is thought to be pathophysiologically significant.
Acknowledgments
Many thanks to Ms Luarna French and Ms Emma Lanera for technical assistance, Prof Geoff Lindeman for providing the MMTV-cre mice, Dr Kristy Boyle for providing LIF-stimulated ES cells and Dr Ben Croker for helpful discussions.
Grant support
This work was supported by a Program Grant (461219), Fellowships (L.R., W.S.A., D.M.T.) and an Independent Research Institutes Infrastructure Support Scheme Grant (361646) from the National Health and Medical Research Council of Australia, the Australian Cancer Research Fund, a Victorian State Government Operational Infrastructure Support Grant and the National Institutes of Health USA (CA022556). S.A.J. was supported by an Australian Postgraduate Award and C.A.W. by an NHMRC Peter Doherty Training Fellowship (461284).
Abbreviations
- BM
bone marrow
- ES
embryonic stem
- GC
germinal center
- KLH
keyhole limpet haemocyanin
- MMTV
mouse mammary tumour virus
- NP
(4-hydroxy-3-nitrophenyl)acetyl
- SOCS3
suppressor of cytokine signaling 3
References
- 1.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Ann Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 2.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 3.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 4.Babon JJ, Sabo JK, Soetopo A, Yao S, Bailey MF, Zhang JG, Nicola NA, Norton RS. The SOCS box domain of SOCS3: structure and interaction with the elonginBC-cullin5 ubiquitin ligase. J Mol Biol. 2008;381:928–940. doi: 10.1016/j.jmb.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 6.Chou WC, Levy DE, Lee CK. STAT3 positively regulates an early step in B-cell development. Blood. 2006;108:3005–3011. doi: 10.1182/blood-2006-05-024430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- 11.Tarlinton D. B-cell memory: are subsets necessary? Nat Rev Immunol. 2006;6:785–790. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 12.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 13.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M, Tokuhisa T. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006;18:1079–1089. doi: 10.1093/intimm/dxl041. [DOI] [PubMed] [Google Scholar]
- 19.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 20.Kiu H, Greenhalgh CJ, Thaus A, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. Regulation of multiple cytokine signalling pathways by SOCS3 is independent of SOCS2. Growth Factors. 2009;27:384–393. doi: 10.3109/08977190903210954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS. Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci USA. 2001;98:9324–9329. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka Y, Rolink AG, Suematsu S, Kishimoto T, Melchers F. An interleukin-6 transgene expressed in B lymphocyte lineage cells overcomes the T cell-dependent establishment of normal levels of switched immunoglobulin isotypes. Eur J Immunol. 1995;25:1332–1337. doi: 10.1002/eji.1830250530. [DOI] [PubMed] [Google Scholar]
- 24.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oracki SA, Tsantikos E, Quilici C, Light A, Schmidt T, Lew AM, Martin JE, Smith KG, Hibbs ML, Tarlinton DM. CTLA4Ig alters the course of autoimmune disease development in Lyn−/− mice. J Immunol. 2010;184:757–763. doi: 10.4049/jimmunol.0804349. [DOI] [PubMed] [Google Scholar]
- 26.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 27.Tangye SG, Cook MC, Fulcher DA. Insights into the role of STAT3 in human lymphocyte differentiation as revealed by the hyper-IgE syndrome. J Immunol. 2009;182:21–28. doi: 10.4049/jimmunol.182.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 29.Avery DT, Ma CS, Bryant VL, Santner-Nanan B, Nanan R, Wong M, Fulcher DA, Cook MC, Tangye SG. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 2008;112:1784–1793. doi: 10.1182/blood-2008-02-142745. [DOI] [PubMed] [Google Scholar]
- 30.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 31.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–627. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- 33.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhu BM, Ishida Y, Robinson GW, Pacher-Zavisin M, Yoshimura A, Murphy PM, Hennighausen L. SOCS3 negatively regulates the gp130-STAT3 pathway in mouse skin wound healing. J Invest Dermatol. 2008;128:1821–1829. doi: 10.1038/sj.jid.5701224. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 36.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, Ozato K, Levy DE, Nutt SL, Calame K, Leonard WJ. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang XZ, Ma KY, Radewonuk J, Li J, Song XY, Griswold DE, Emmell E, Li L. IgE isotype switch and IgE production are enhanced in IL-21-deficient but not IFN-gamma-deficient mice in a Th2-biased response. Cell Immunol. 2006;241:66–74. doi: 10.1016/j.cellimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Hasbold J, Lyons AB, Kehry MR, Hodgkin PD. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur J Immunol. 1998;28:1040–1051. doi: 10.1002/(SICI)1521-4141(199803)28:03<1040::AID-IMMU1040>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC, 3rd, Kishimoto T, Potter M, Janz S. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci USA. 2002;99:1509–1514. doi: 10.1073/pnas.022643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dedera DA, Urashima M, Chauhan D, LeBrun DP, Bronson RT, Anderson KC. Interleukin-6 is required for pristane-induced plasma cell hyperplasia in mice. Brit J Haematol. 1996;94:53–61. doi: 10.1046/j.1365-2141.1996.6282074.x. [DOI] [PubMed] [Google Scholar]