Abstract
Leaf hydraulic conductance (Kleaf) is a major determinant of photosynthetic rate in well-watered and drought-stressed plants. Previous work assessed the decline of Kleaf with decreasing leaf water potential (Ψleaf), most typically using rehydration kinetics methods, and found that species varied in the shape of their vulnerability curve, and that hydraulic vulnerability correlated with other leaf functional traits and with drought sensitivity. These findings were tested and extended, using a new steady-state evaporative flux method under high irradiance, and the function for the vulnerability curve of each species was determined individually using maximum likelihood for 10 species varying strongly in drought tolerance. Additionally, the ability of excised leaves to recover in Kleaf with rehydration was assessed, and a new theoretical framework was developed to estimate how rehydration of measured leaves may affect estimation of hydraulic parameters. As hypothesized, species differed in their vulnerability function. Drought-tolerant species showed shallow linear declines and more negative Ψleaf at 80% loss of Kleaf (P80), whereas drought-sensitive species showed steeper, non-linear declines, and less negative P80. Across species, the maximum Kleaf was independent of hydraulic vulnerability. Recovery of Kleaf after 1 h rehydration of leaves dehydrated below their turgor loss point occurred only for four of 10 species. Across species without recovery, a more negative P80 correlated with the ability to maintain Kleaf through both dehydration and rehydration. These findings indicate that resistance to Kleaf decline is important not only in maintaining open stomata during the onset of drought, but also in enabling sustained function during drought recovery.
Keywords: Cavitation, dehydration, EFM, Kleaf, rehydration, refilling, safety margins, turgor loss point, vulnerability curves
Introduction
In dicotyledons, the leaf hydraulic conductance strongly constrains gas exchange and growth (Sack et al., 2003; Sack and Holbrook, 2006). The resistance of open stomata to vapour diffusion out of the leaf is typically far greater than the hydraulic resistance to bulk flow of the liquid through the plant, and transpiration rates are thus dictated by this diffusion process, which in turn depends on the stomatal and boundary layer conductances and the difference in vapour pressure between the intercellular air spaces of the leaf and the atmosphere (Cowan, 1972; Sack and Tyree, 2005; Sack and Holbrook, 2006). However, the maintenance of open stomata depends on the leaf being well hydrated; that is, having a high leaf water potential (Ψleaf), which, in turn, depends on the plant hydraulic conductance being sufficiently high. Because in dicotyledons, the leaf accounts for on average 30% of the plant hydraulic resistance (Sack et al., 2003; Sack and Holbrook, 2006), the leaf hydraulic conductance (Kleaf=flow rate/water potential driving force, i.e. 1/leaf hydraulic resistance) is thus a critical variable. Water enters the petiole, moves through several vein orders of diminishing size, then exits into the bundle sheath and moves through or around cells before evaporating into the intercellular airspace and being transpired from the stomata. The Kleaf declines with ψleaf during drought, due to losses of conductance resulting from cavitation and/or collapse of xylem conduits, and/or to decline in the permeability of extra-xylem tissues, and this response drives stomatal closure to prevent leaf desiccation (e.g. Salleo et al., 2000; Brodribb and Holbrook, 2004a; Sack and Holbrook, 2006; Scoffoni et al., 2008; Brodribb and Cochard, 2009; Brodribb et al., 2010; Scoffoni et al., 2011). Understanding species variation in hydraulic vulnerability is thus critical, and several techniques have been applied, especially the rehydration kinetics method (RKM; Supplementary Table S1 available at JXB online; Brodribb and Holbrook, 2003a). The aim of this study was to quantify this response using an independent, steady-state method, for species varying strongly in drought tolerance, and to determine the ability of dehydrated leaves to recover in Kleaf after rehydration.
Previous studies using the RKM found species to vary strongly in leaf hydraulic vulnerability, quantified as the Ψleaf at 50% loss of Kleaf (P50; e.g. Hao et al., 2008; Blackman et al., 2009; Chen et al., 2009; Johnson et al., 2009a; Saha et al., 2009). Additionally, species with a low P50 also had low osmotic potential at the turgor loss point (Blackman et al., 2010), and could thus maintain stomata open as leaves dehydrate. Further, these studies tested the classic trade-off between hydraulic efficiency and safety, previously found for stems, and showed this to be absent in leaves: the maximum Kleaf for hydrated leaves (Kmax) was independent of P50 (Sack and Holbrook, 2006; Blackman et al., 2010).
Notably, the various methods for measuring Kleaf all have value but can raise potential concerns (Sack and Tyree, 2005). There was thus a need to test leaf hydraulic vulnerability with a method independent of the RKM. The typically used RKM measures Kleaf from water uptake into the mesophyll of a dehydrated leaf for a known time, and involves some uncertainty because uptake to leaf cells continues even after leaf collection for Ψleaf determination, though a recently modified version of the RKM (‘dynamic RKM’) has overcome this limitation (Brodribb and Cochard, 2009; Blackman and Brodribb, 2011; Brodribb, Blackman, and PrometheusWiki contributors, 2011). Additionally, in the RKM, water uptake into mesophyll cells might not always mimic the complete pathways of natural transpiration (Scoffoni et al., 2008). Furthermore, the RKM may give low resolution of Kleaf declines in the well-hydrated range of the vulnerability curve if such leaves rehydrate completely during measurement. The evaporative flux method (EFM) has the advantage of allowing Kleaf measurement during steady-state transpiration and, further, using the EFM, leaves can be acclimated to high irradiance, which influences Kleaf for many species (Sack et al., 2002; Nardini et al., 2005; Tyree et al., 2005; Cochard et al., 2007; Sellin and Kupper, 2007; Scoffoni et al., 2008; Sellin et al., 2008). One previous study applied a variant of the EFM to generate vulnerability curves (the heat-flux method, ‘Heat-FM’; Brodribb and Holbrook, 2006) which involved some complexity. A heat gun was used on the leaf to drive a transiently high transpiration rate, after which the stomata closed, establishing a lower flow rate. The leaf was removed, and Kleaf was determined as the steady-state flow rate divided by the final Ψleaf (Ψfinal), and the vulnerability curve was determined as Kleaf plotted against Ψfinal. However, that method could not determine the lowest Ψleaf induced in the leaf during the high transpiration rates driven by the hot air (Ψlowest), which may have triggered the Kleaf decline. In this study, the EFM was modified to allow measurement of both Ψlowest and Ψfinal, such that Kleaf could be plotted against both.
A second aim of this study was to refine the statistical analysis of the Kleaf decline with dehydration for improved accuracy and mechanistic insight. Typically, studies have fitted the same function for all species, chosen for approximate fit to the data; polynomial (including linear), sigmoidal, and logistic functions have all been used (Supplementary Table S1 at JXB online). However, species may differ in the shape of their vulnerability curve, and choosing the appropriate function is important both for accuracy and also to allow interpretation of the underlying processes (Brodribb and Holbrook, 2006, 2007). Notably few studies have directly discussed the underlying basis for different shapes of vulnerability curves, probably due to the lack of an approach to select the appropriate function objectively, but the literature has pointed to several potential mechanisms for differently shaped curves (reviewed in Table 1). As a next step, a rigorous analysis is needed to resolve species differences in the shape of the function. Thus, for 10 diverse species, the maximum likelihood function was selected for each species. Drought-tolerant species were hypothesized to show shallower, linear declines, whereas drought-sensitive species were expected to show stronger initial Kleaf declines due to greater sensitivity in one or more components of the water transport system. Tests were made of the impact on estimated hydraulic vulnerability parameters of using different functions as in previous studies (Supplementary Table S1), and the degree to which it matters how vulnerability curves are plotted, namely whether unbinned data for Kleaf are plotted against Ψlowest or Ψfinal, or whether data are binned by Ψleaf intervals.
Table 1.
Mechanisms that would theoretically influence the shape of the response of leaf hydraulic conductance (Kleaf) to dehydration (i.e. decreasing leaf water potential, Ψleaf) and thus the function that best fitted to the data. A linear decline implies no threshold Ψleaf before which Kleaf declines (i.e. Kleaf declines immediately as Ψleaf declines), and also a proportional decline of Kleaf with Ψleaf. A non-linear decline of Kleaf with Ψleaf can include a threshold Ψleaf before the decline begins and/or a disproportionate decline of Kleaf as Ψleaf declines. For these possibilities three types of mechanisms were included—those relating to air seeding causing cavitation in the xylem conduits (and analogous effects would occur given collapse of xylem conduit walls), those arising from venation architecture, and those arising in the pathways outside the xylem. References are provided to studies of these potential mechanisms per se and/or on their influence on the shape of stem or leaf vulnerability curves.
| Shape of Kleaf decline | Air seeding | Venation architecture | Pathways outside the xylem |
| Linear decline: | |||
| No threshold before decline | If air seeding begins at high Ψleaf because of large pit membrane pore size (Neufeld et al., 1992; Pammenter and Vander Willigen, 1998) | If a loss of membrane permeability (e.g. due to aquaporin activity or loss of cell turgor) begins immediately as Ψleaf declines (Brodribb and Holbrook, 2006) | |
| Proportional decline of Kleaf with declining Ψleaf | If conduits of different sizes all have a wide range in maximum pit membrane pore size such that cavitation occurs equally across conduit sizes (Pammenter and Vander Willigen, 1998; Choat et al., 2005) | If higher major vein length/area (=vein density) confers hydraulic redundancy, such that first embolisms of the vein xylem conduits do not cause a dramatic decline (Scoffoni et al., 2011) | If membrane permeability declines linearly as the average cell tugor declines with Ψleaf (Kubiske and Abrams, 1990; Brodribb and Holbrook, 2006). If the Kleaf declines due to loss of water-filled pathways through cell walls (Pieruschka et al., 2010) |
| Non-linear decline (logistic, sigmoidal, exponential): | |||
| Threshold before decline | If a threshold for air seeding determined by the largest pit membrane pore size leads to a retention of Kleaf until a Ψleaf threshold (Neufeld et al., 1992; Pammenter and Vander Willigen, 1998; Domec et al., 2006) | If there is a threshold Ψleaf below which aquaporins are deactivated and membrane permability declines (North and Nobel, 2000; Miyazawa et al., 2008). If the Kleaf is insensitive to turgor or turgor is maintained by osmotic adjustment until a cavitation threshold is reached (Brodribb and Holbrook, 2006) |
|
| Disproportionate decline of Kleaf with declining Ψleaf | If larger conduits conferring the bulk of the vein xylem conductivity have larger pit membrane pores or greater pore numbers, and cavitate first, followed by smaller conduits that have decreasing impact on Kleaf (Neufeld et al., 1992; Pammenter and Vander Willigen, 1998; Tyree and Zimmermann, 2002) | If leaves with lower major vein density suffer strong decline in Kleaf with first embolism of xylem conduits in the low-order veins (Scoffoni et al., 2011) | If strong declines due to aquaporin deactivation occur at high Ψleaf (Johansson et al., 1998; Kim and Steudle, 2007; Scoffoni et al., 2008). If a greater loss of turgor in cells with relatively weak solute potential (e.g. bundle sheath cells) during leaf dehydration lead to especially rapid decline in Kleaf (Nonami and Schulze 1989; Koroleva et al., 1997) |
A third aim in this study was to quantify the recovery of Kleaf with rehydration, a related, essential process that has received little attention. One previous study found that excised and dehydrated sunflower leaves recovered rapidly in Kleaf when rehydrated with petioles under water (Trifilo et al., 2003a). Species differences in this ability were tested for. Species with the greatest hydraulic vulnerability were hypothesized to show the greatest recovery, as they would derive most benefit. Further, all studies of vulnerability have involved leaf rehydration during measurement, but none has accounted for this in interpretation; tests were developed to determine how the measurements might be affected. The main benefit of a low hydraulic vulnerability has typically been framed as the ability to keep stomata open without dehydrating the mesophyll. It was hypothesized that a low hydraulic vulnerability would also confer the ability to maintain Kleaf through both dehydration and rehydration.
Materials and methods
Plant material
This study was conducted alongside a study of the importance of venation architecture and leaf size in determining species variation in hydraulic vulnerability (Scoffoni et al., 2011). Ten species were selected across nine families and spanning a wide range of drought sensitivity; five species were native to dry habitats (mainly California chaparral) and five species to moist habitats (Table 2). Study species included mature trees and shrubs in and around the campus of University of California, Los Angeles and Will Rogers State Park, Los Angeles, California, and sunflower Helianthus annuus var. Sunspot grown from seeds (Botanical Interests; Broomfield, Colorado, USA) in 3.6 l pots in a greenhouse (average minimum, mean, and maximum values for temperature. 21.1, 23.2, and 26.0 °C; for humidity, 44, 51, and 59%). Sunflowers were irrigated every 2 d, with 200–250 ppm of 20:20:20 N:P:K; the irradiance measured at mid-day on a sunny day was up to 550 μmol photon m−2 s−1, and on average 300 μmol photon m−2 s−1 (LI-250 light meter; LI-COR Biosciences, Lincoln, NE, USA).
Table 2.
Study species, family, native range, and mean values ±SE for pressure–volume curve parameters and leaf hydraulic vulnerability parameters, i.e. leaf hydraulic conductance at full hydration (Kmax), leaf water potential at 50% and 80% decline of leaf hydraulic conductance (P50 and P80), calculated from the maximum likelihood function for the ‘Ψlowest unbinned’ plot, and results of t-tests on species’ means (for hydraulics parameters) or of analyses of variance for the difference between moist and dry area species, and among species nested within those categories (for pressure–volume parameters). Data are from Scoffoni et al. (2011).
| Species | Family | Native rangea | Kmax (mmol m−2 s−1 MPa−1) | P50 (–MPa) | P80 (–MPa) | Turgor loss point (–MPa) | Osmotic potential (–MPa) | Modulus of elasticity (MPa) | Saturated water content (g g−1) |
| Dry habitat species | |||||||||
| Cercocarpus betuloides | Rosaceae | California. Mexico | 4.36 | 2.76 | 5.25 | 2.59±0.03 | 1.64±0.04 | 10.1±0.701 | 0.79±0.02 |
| Comarostaphylis diversifolia | Ericaceae | California. Mexico | 2.96 | 2.85 | 4.56 | 3.45±0.34 | 2.51±0.34 | 17.3±2.23 | 0.70±0.01 |
| Hedera canariensis | Araliacaeae | Canary Islands | 5.73 | 0.64 | 1.18 | 1.98±0.09 | 1.49±0.07 | 17.9±1.28 | 2.81±0.09 |
| Heteromeles arbutifolia | Rosaceae | California. Mexico | 20.7 | 2.57 | 4.12 | 2.53±0.10 | 2.08±0.10 | 16.4±0.486 | 1.38±0.07 |
| Quercus agrifolia | Fagaceae | California. Mexico | 3.96 | 2.40 | 3.83 | 3.00±0.12 | 2.31±0.12 | 12.8±0.787 | 0.93±0.01 |
| Moist habitat species | |||||||||
| Camellia sasanqua | Theaceae | Japan | 5.99 | 1.78 | 2.84 | 2.12±0.18 | 1.61±0.04 | 7.98±1.11 | 1.74±0.03 |
| Helianthus annuus | Asteraceae | Across N. America | 6.45 | 0.83 | 1.16 | 1.09±0.12 | 0.875±0.10 | 13.3±1.31 | 11.2±0.79 |
| Lantana camara | Verbenaceae | Pantropical | 11.4 | 0.80 | 1.41 | 1.37±0.04 | 1.10±0.04 | 9.14±0.525 | 2.73±0.15 |
| Magnolia grandiflora | Magnoliaceae | Southern USA | 5.24 | 0.42 | 2.06 | 2.06±0.05 | 1.43±0.34 | 5.49±0.792 | 1.50±0.07 |
| Platanus racemosa | Platanaceae | California, Mexico | 34.1 | 0.09 | 0.35 | 2.03±0.06 | 1.54±0.12 | 4.85±0.331 | 1.34±0.03 |
| Average ±SE | Dry habitat species | 7.55±3.32 | 2.24±0.41 | 3.79±0.69 | 2.71±0.14 | 2.01±0.19 | 14.9±1.49 | 1.32 ±0.04 | |
| Moist habitat species | 12.6±5.48 | 0.78±0.28 | 1.56±0.42 | 1.74±0.09 | 1.31±0.14 | 8.16±1.51 | 3.71 ±0.21 | ||
| ANOVA or t-test | Dry/moist species | NS | * | * | ****** | ****** | ****** | ****** |
Croat (1978); Kitamura and Murata (1979); eFloras (2008).
NS, P >0.05; *P <0.025;***P <0.001.
Experiments were conducted in May–September 2008. On the day prior to measurements, for 3–10 plants per species, exposed branches with mature, healthy leaves were collected into plastic bags with moist paper towel; for sunflowers, whole shoots were collected. Each shoot was re-cut by at least two nodes in the laboratory under ultrapure water (MilliPore, 0.22 μm Thornton 200CR, Molshem, France) and rehydrated overnight at laboratory temperature (20–25 °C), covered with dark plastic bags.
Measuring the dehydration response of Kleaf with the evaporative flux method
Using the EFM, Kleaf is determined as the ratio of steady-state transpirational flow rate (E, mmol m−2 s−1) to the water potential driving force (ΔΨleaf, MPa; Sack et al., 2002). Notably, in this system, the overall driving force for flow through the whole leaf is the water potential gradient between the outside air and the water entering the petiole, but the important component of that driving force is the vapour pressure gradient between the outside air and leaf air spaces; this vapour pressure driving force, and stomatal conductance, determine the transpiration rate (see Introduction). However, for the liquid phase part of flow (i.e. the hydraulic system), the driving force at steady state is the water potential gradient between the leaf mesophyll where water evaporates (estimated as the Ψleaf measured at the end of the measurement, i.e. the Ψfinal) and the water entering the petiole at atmospheric pressure (i.e. 0 MPa relative pressure).
In this study, the focus was on the dehydration response of the whole-leaf hydraulic system, including the petiole. The leaf was cut from the shoot with a fresh razor blade under ultrapure water that was used as flow solution (0.22 μm Thornton 200 CR; MilliPore), degassed for at least 8 h with a vacuum pump (Gast, Benton Harbor, MI, USA), and refiltered (0.2 μm; Syringe filter, Cole-Parmer, Vernon Hills, IL.USA). The petiole was then rapidly connected to silicon tubing (Cole-Parmer) under ultrapure water to prevent air entering the system. The tubing connected the leaf to a cylinder on a balance (models XS205 and AB265, ±10 μg sensitivity; Mettler Toledo, Columbus, OH, USA) that logged data every 30 s to a computer for the calculation of flow rate through the leaf (E). Leaves were held adaxial surface upwards in wooden frames strung with fishing line above a large box fan (Lakewood Engineering & Manufacturing Company, Chicago, IL, USA). Leaves were illuminated with >1000 mmol m−2 s−1 photosynthetically active radiation at the leaf surface by floodlights (model 73828 1000 W, ‘UV filter’; Sears, Roebuck, Hoffman Estates, IL, USA) suspended above a Pyrex container (Corning Incorporated, Corning, NY, USA) filled with water to absorb the heat of the lamp. Leaf temperature was determined using a thermocouple (Cole-Parmer) and maintained between 23 °C and 28 °C.
Leaves were allowed to transpire on the apparatus for at least 30 min and until the flow rate stabilized, with no upward or downward trend, and with a coefficient of variation <5% for at least five measurements made at 30 s flow intervals. When the flow rate was very low (<8 μg s−1), stability was determined with the same criterion, but using the running averages of the last five 30 s intervals. Previous studies found these criteria to be sufficient for stabilization of E, Ψleaf, and Kleaf; tests with longer measurement periods after stable flow was established showed no relationship of Kleaf to measurement time for seven species of a wide range of leaf capacitance (Scoffoni et al., 2008; Pasquet-Kok et al., 2010). The minimum 30 min flow period was chosen to ensure that leaves had sufficient time to acclimate to high irradiance, which has been found to enhance Kleaf by up to 8-fold depending on species, apparently due to the expression and/or activation of aquaporins (Sack et al., 2002; Nardini et al., 2005; Tyree et al., 2005; Cochard et al., 2007; Scoffoni et al., 2008; Voicu et al., 2008). Measurements were discarded if the flow suddenly changed, due either to apparent leakage from the seal or to blockage in the system by particles or air bubbles. Following the stabilization of the flow rate, leaf temperature was recorded with a thermocouple and the final five flow rate measurements were averaged. The leaf was quickly removed from the tubing, the petiole was dabbed dry, and the leaf was placed into a sealable bag (Whirl-Pak; Nasco, Fort Atkinson, WI, USA), which had been previously exhaled in, to halt transpiration. Following at least 30 min equilibration, the final leaf water potential (Ψfinal) was measured with a pressure chamber (Plant Moisture Stress, Model 1000, Albany, OR, USA). Kleaf was calculated as E/–ΔΨleaf (where ΔΨleaf=Ψfinal–0 MPa) and further normalized by leaf area measured with a LI-COR 3100 leaf area meter. To correct for changes in Kleaf induced by the temperature dependence of water viscosity, Kleaf values were standardized to 25 °C (Weast, 1974; Yang and Tyree, 1993; Sack et al., 2002).
To determine the stomatal conductance of leaves measured with the EFM, the final E was divided by the mole fraction vapour pressure deficit (VPD), derived from temperature and relative humidity (RH) measurements in the lab from a weather station that logged measurements every 5 min (HOBO Micro Station with Smart Sensors; Onset, Bourne, MA, USA), where mole fraction VPD=[1–(RH×VPsat)]/101.3 kPa, and VPsat is the saturation vapour pressure determined using the Arden Buck equation (Buck, 1981).
The EFM was modified to allow determination of Kleaf for dehydrated leaves. Shoots were cut into segments with at least three leaves under ultrapure water and then dehydrated with a fan for different periods of time to a range of Ψleaf values. The bench drying of shoots to achieve a leaf vulnerability curve has been used in studies using the RKM (e.g. Brodribb and Holbrook, 2003a; Blackman et al., 2009), and previous studies found similar vulnerability curves when constructed from bench-drying shoots as from leaves on plants progressively droughted (Brodribb and Holbrook, 2004a; Blackman et al., 2009; Pasquet-Kok et al., 2010). In the present study, shoots were allowed to equilibrate for at least 30 min before two leaves were excised and measured for initial Ψleaf (Ψo) using a pressure chamber. If the difference in the Ψleaf of those two leaves was >0.1 MPa, the shoot was discarded; for very dehydrated shoots, this range was extended to 0.3 MPa. The third leaf (typically the middle leaf) was used to determine Kleaf with the EFM. When dehydrated leaves are measured with the EFM, the stomata open (see Results); before steady-state flow is achieved, the leaf may rehydrate such that Ψfinal is less negative than Ψo, or, alternatively, the leaf may further dehydrate such that Ψfinal is more negative than Ψo. For each species, at least six Kleaf values were obtained for each 0.5 MPa interval from full hydration to strong dehydration. Outlier tests were conducted for each 0.5 MPa interval (Dixon test; Sokal and Rohlf, 1995); 0–4 outliers were removed over the whole curve for given species (representing 0–8% of the 26–74 data points per curve).
To test the importance of the method for constructing vulnerability curves, these were determined in three ways previously applied (Supplementary Table S1 at JXB online). First, Kleaf was plotted against whichever was lowest, Ψo or Ψfinal (=‘Ψlowest’); that is, the Ψleaf associated with the strongest dehydration experienced during the experiment, and each leaf was considered as a data point (‘unbinned Ψlowest’). Additionally, Kleaf was plotted against Ψlowest with data averaged in 0.5 MPa bins (‘binned Ψlowest’), with the exception of H. annuus averaged in 0.2 MPa bins because of its distinctively narrower Kleaf response, with negligible values below –1.5 MPa. Finally, Kleaf was plotted against Ψfinal rather than Ψlowest (‘Ψfinal’), with each leaf considered as a data point. Determination of these alternative versions of the vulnerability curve also allowed interpretation of the recovery of Kleaf during the measurement (see section below).
In the above-described methods, as in previous studies of Kleaf, the pressure chamber balance pressure was taken as Ψleaf. In actuality, the balance pressure for an equilibrated leaf gives the xylem pressure potential (Px), and –Px is less negative than the bulk Ψleaf by the amount of the vein xylem solute potential (πx; Tyree and Zimmermann, 2002). Notably, previous studies on a range of species have measured πx values of approximately –0.05 MPa, a difference that would not affect the present findings significantly (Boyer, 1967). Tests were carried out to verify such low πx for C. sasanqua, H. arbutifolia, and L. camara. Shoots of four leaves were rehydrated overnight and dehydrated to a range of Ψleaf (–0.04 MPa to –1.5 MPa). Two leaves were excised for initial Ψleaf measurement, a third was bagged for determination of initial πx, and the fourth was placed in the EFM apparatus until a steady-state flow rate was achieved. Leaf vein πx was determined using vapour pressure osmometry (Vapro 5520, Wescor Inc., Logan, UT, USA). The leaf margin was excised to open the tips of the midrib and second-order veins, and the leaf was pressurized in the pressure chamber and xylem sap exuded from the petiole was collected onto a filter paper, while moist paper towels surrounded the chamber and petiole to minimize evaporation. The filter paper was transported to the osmometer in a weighing bottle filled with moist paper towel. All πx values were less negative than the least negative measurable value with this instrument, –0.05 MPa, and thus indistinguishable from pure water in the instrument, indicating that the present findings would not be significantly impacted by πx.
Model testing and estimation of parameters for the decline of Kleaf with dehydration
Maximum likelihood was used to select the function for each species’ Kleaf vulnerability response (Burnham and Anderson, 2002), using the optim function in R 2.9.2 (http://www.r-project.org; Burnham and Anderson, 2004; Sack et al., 2006; the scripts are available on request). A linear function (Kleaf=aΨleaf+yo), was tested, in addition to sigmoidal 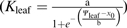 and logistic functions
and logistic functions 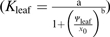 , as used previously in the literature on leaf vulnerability (Supplementary Table S1 at JXB online), and an exponential function (), as previously used for whole-plant vulnerability (Iovi et al., 2009). The maximum likelihood parameters were determined by the Simulated Annealing procedure for global optimization, followed by the Nelder–Mead simplex procedure for local optimization; standard errors for parameters were generated from the Hessian matrix. For each data set, functions were compared using the Akaike Information Criterion (AIC), corrected for low n. The function with the lowest AIC value was chosen as the best fit function for that data set, with differences >2 considered as meaningful (Burnham and Anderson, 2002, 2004).
, as used previously in the literature on leaf vulnerability (Supplementary Table S1 at JXB online), and an exponential function (), as previously used for whole-plant vulnerability (Iovi et al., 2009). The maximum likelihood parameters were determined by the Simulated Annealing procedure for global optimization, followed by the Nelder–Mead simplex procedure for local optimization; standard errors for parameters were generated from the Hessian matrix. For each data set, functions were compared using the Akaike Information Criterion (AIC), corrected for low n. The function with the lowest AIC value was chosen as the best fit function for that data set, with differences >2 considered as meaningful (Burnham and Anderson, 2002, 2004).
To compare species in their hydraulic parameters, and to determine correlations between hydraulic parameters and other leaf traits, values for the maximum Kleaf at full hydration (Kmax) and the Ψleaf at which Kleaf had declined by 50% and 80% (P50 and P80) were determined from the vulnerability curves. For these parameters, each species’ maximum likelihood function was used [i.e. that with lowest AIC and highest r2 determined from the unbinned data plots (‘unbinned Ψlowest’ and ‘Ψfinal’)]. The steepness of the vulnerability curve was also determined, as the first derivative of the maximum likelihood function at Ψleaf= –0.5 MPa, where the steepest declines were observed. As an additional method for determining Kmax, the average Kleaf for points above –0.5 MPa was calculated for each species; this was the method used in most previous leaf hydraulics studies that measured only Kleaf for hydrated leaves, and not its vulnerability to dehydration (e.g. Sack et al., 2002; Brodribb and Holbrook, 2003b; Nardini et al., 2005).
To determine the degree to which the choice of function and data set matters, tests were made of the sensitivity of vulnerability curve parameters (Kmax, P50, and P80) to the choice of function, and, for each function, of plotting Kleaf against ‘unbinned Ψlowest’, ‘binned Ψlowest’, or ‘Ψfinal’.
Hydraulic safety margins were calculated as the difference between the Ψleaf at which the leaves of a given species lose turgor (πTLP; data from Scoffoni et al., 2011) and those at which hydraulic function was substantially lost (P50 or P80). Positive numbers indicate a safety margin, whereas negative numbers indicate a loss of hydraulic function even above the turgor loss point.
Testing the recovery of leaf hydraulic conductance after dehydration
Experiments were performed to test the recovery of Kleaf for leaves rehydrated after dehydration (method after Trifilo et al., 2003a). For the 10 species, shoots were dehydrated with a fan to a known Ψleaf below their respective turgor loss points (determined as described in the following section). Leaves from each shoot were excised in air using a fresh razor blade and measured for Ψleaf (Ψdehydration), and other leaves were excised under ultrapure water, and rehydrated for 1 h with their petioles under water in a beaker, covered with a dark plastic bag. Following rehydration, leaves were equilibrated in a plastic bag for at least 10 min and either had their petioles cut in air and were measured for Ψleaf (Ψrehydration), or had their petioles re-cut under ultrapure water and were immediately connected to the EFM to determine Kleaf (n= 4–12 per species). The percentage recovery of Kleaf was determined as the Kleaf after rehydration divided by the Kleaf at Ψdehydration, which was estimated from the species’ maximum likelihood vulnerability curve ×100%. The recovery was considered significant if the Kleaf after 1 h rehydration was greater than the Kleaf at Ψdehydration (t-test; Minitab Release 15). The recovery was determined as complete if Kleaf after 1 h rehydration was not significantly lower than the Kleaf at Ψrehydration which was estimated from the species’ maximum likelihood vulnerability curve.
Testing for the recovery of leaf hydraulic conductance during EFM measurement
As in other methods for determining leaf hydraulic vulnerability (i.e. RKM and Heat-FM; see Introduction), the EFM partially rehydrates the dehydrated leaf, as the petiole is connected to water at atmospheric pressure. Two analyses were developed to test for the potential recovery of Kleaf during the EFM measurement. The first analysis was a test of residual variation. If Kleaf recovered completely during the EFM measurement, one would expect no influence of the dehydration treatment prior to measurement on the final Kleaf value; rather, the measured Kleaf would simply relate to Ψfinal (i.e. the leaf water potential during the final steady-state flow). Thus, for each species, from the maximum likelihood vulnerability curve for the ‘Ψfinal’ plot, the residuals of Kleaf against Ψfinal were calculated. These residuals represented the variation in Kleaf unrelated to Ψfinal. A test was made for correlation of these residuals with Ψlowest values (Minitab Release 15). If the residual Kleaf variation was negatively correlated with Ψlowest, there was a persistent impact of Ψlowest on Kleaf, independently of Ψfinal. In other words, the effect of the dehydration treatment persisted even at the end of the EFM measurement, and, thus, the Kleaf had not recovered completely during the measurement. The second analysis was the calculation of an index of the recoverability of Kleaf during the EFM. For each species, a sample of the vulnerability data was selected that was analogous to the 1 h rehydration experiment (see previous section). Data were selected for leaves that had been dehydrated to a Ψleaf below the turgor loss point but that had rehydrated during the EFM measurement to Ψleaf values similar to those for leaves measured by the EFM after the 1 h rehydration experiment (n=4–7 for each species). The percentage recovery of Kleaf during EFM was determined as the average measured Kleaf for this leaf sample divided by the Kleaf at Ψdehydration, which was estimated from the species’ maximum likelihood vulnerability curve ×100%. The significance of the recovery of Kleaf was tested as for leaves in the 1 h rehydration experiment.
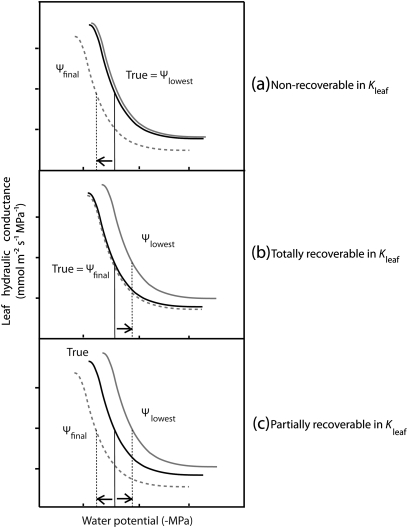
Given that some species showed a partial recovery of Kleaf with rehydration during the EFM (see Results), a theoretical consideration was made of how Kleaf recovery during measurement should influence the calculation of vulnerability parameters. Based on the diversity of tissues in the leaf hydraulic pathway, the vulnerability of Kleaf is expected to involve several components, some of which might be recoverable on a short time scale, while others might be reversible after a longer time scale under low tension (cf. Brodribb and Holbrook, 2006; Scoffoni et al., 2008). The most appropriate vulnerability plot would depend on the degree to which leaves are recoverable in the short term (Fig. 1). Bounding cases were considered in which (a) leaves were non-recoverable in Kleaf during the measurement; (b) leaves were totally recoverable; and (c) leaves were partially recoverable. In case (a) in which Kleaf is non-recoverable, an accurate vulnerability curve would be obtained by plotting Kleaf against Ψlowest, as only the minimum Ψleaf during the whole experiment is important for influencing Kleaf. In case (a), plotting Kleaf against Ψfinal would overestimate the leaf’s vulnerability. In contrast, in case (b) in which Kleaf recovers completely during measurement, an accurate vulnerability curve would be determined by plotting Kleaf against Ψfinal, because only the Ψleaf during steady state at the end of the measurement is important for influencing Kleaf. In case (b), plotting Kleaf against Ψlowest would underestimate the leaf’s vulnerability. Finally, in case (c), in which Kleaf is partially recoverable, the accurate vulnerability curve would be intermediate between the plots of Kleaf against Ψfinal and against Ψlowest. Additional scenarios were not considered, for example if leaves recover in Kleaf differently depending on their degree of dehydration; notably, such scenarios should fall within the bounding cases considered. Tests were conducted to determine whether the estimation of vulnerability parameters Kmax, P50, and P80 was improved by using for each species the plot appropriate to its Kleaf recovery. Thus, for the species that showed no Kleaf recovery during EFM measurement, parameters were re-calculated from the maximum likelihood function for the ‘Ψlowest unbinned’ plot and, for the species with partial recovery, parameters were averaged from those determined from the ‘Ψfinal’ and ‘Ψlowest unbinned’ plots. These re-calculated parameters were compared with those determined using the ‘Ψlowest unbinned’ for all species, as has been the most typical procedure in previous studies (Supplementary Table S1 at JXB online).
Fig. 1.
A theoretical framework for the construction of vulnerability curves according to the degree that leaves recover in leaf hydraulic conductance (Kleaf) with rehydration. The black line is the ‘true’ vulnerability curve, the grey line is the vulnerability curve plotting Kleaf against ‘Ψlowest’, and the grey dotted line is the vulnerability curve plotting Kleaf against ‘Ψfinal’. Bounding cases were considered: (a) leaves were non-recoverable in their Kleaf during the measurement; (b) leaves were totally recoverable in their Kleaf; and (c) leaves were partially recoverable in their Kleaf (see the Materials and methods).
Statistical analysis of differences among species and trait correlations across species
Trait differences between moist and dry habitat species were tested using analyses of variance (ANOVAs) with species nested within habitat type, or by using t-tests on species means (Table 1; Minitab Release 15). All data were log-transformed to improve normality and heteroscedasticity (Sokal and Rohlf, 1995). Correlations among traits were considered significant only if P <0.05 for both Spearman and Pearson coefficients (rs and rp, respectively); when relationships were non-linear, correlations for log-transformed data were determined. Standard major axes were fitted when determining slopes of relationships between traits, to account for error in both x and y variables (using SMATR; Sokal and Rohlf, 1995; Warton et al., 2006).
Results
Vulnerability curves: species differences in the response of Kleaf to dehydration
The EFM was effective for determining vulnerability curves for leaves dehydrated from near full turgor to beyond the turgor loss point (Fig. 2). Leaves that had been previously dehydrated opened their stomata and established steady-state transpiration during the EFM measurement, as indicated by even the lowest transpiration rates observed representing stomatal conductance values 2.2- to 7.3-fold higher than cuticular conductance for these species (Supplementary Table S2 at JXB online).
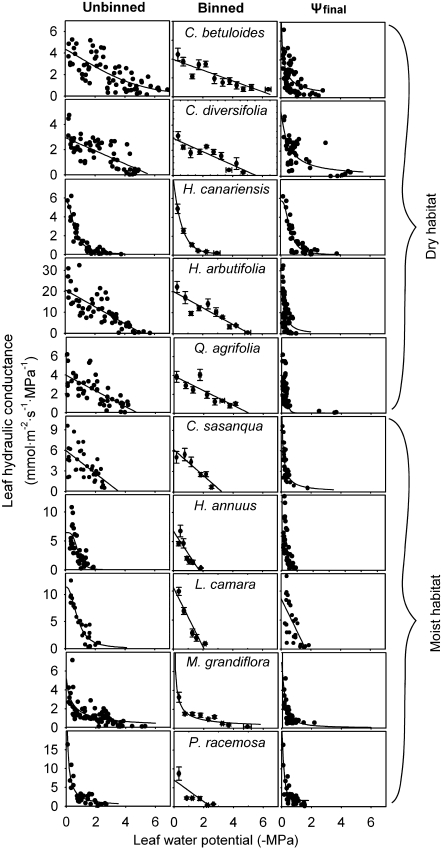
Fig. 2.
Vulnerability curves for leaf hydraulic conductance (Kleaf) for 10 species varying widely in drought tolerance, determined using the evaporative flux method using three different plots (‘Ψlowest unbinned’, ‘Ψlowest binned’, and ‘Ψfinal’). For the ‘Ψlowest unbinned’ and ‘Ψfinal’ panels, each point represents a different leaf measured. Standard errors are represented for each bin point in the ‘Ψlowest binned’ plot. The lines plotted are the maximum likelihood functions using each plot for each species (Supplementary Table S3 at JXB online).
Species differed significantly in the shape of the leaf hydraulic vulnerability curves. For four species the linear function was selected by maximum likelihood for Kleaf plotted against ‘Ψlowest unbinned’, and for six species a non-linear function was selected (Fig. 2; Supplementary Table S3 at JXB online). The logistic function was selected for five species and the sigmoidal for C. betuloides. Species from dry habitats had a greater tendency to show a linear decline in Kleaf as one of their selected functions, that is, within an AIC of 2 of the maximum likelihood function (4/5 species versus 1/5 for moist habitats; P=0.018; proportion test). The slope of the vulnerability curve at Ψleaf= –0.5 MPa varied from –10 mmol m−2 s−1 MPa−2 to –0.5 mmol m−2 s−1 MPa−2, and drought-sensitive species had on average 3-fold steeper slopes than drought-tolerant species (–6.5 mmol m−2 s−1 MPa−2 versus –1.6 mmol m−2 s−1 MPa−2, respectively; t-test; P=0.009, n=5).
Vulnerability curves: sensitivity of derived parameters to the choice of function and plot
The use of maximum likelihood to select the vulnerability function for each species based on plots of Kleaf against ‘Ψlowest unbinned’ was considered to be the most appropriate practice, and was the one used for interpretation and comparison among species. However, because many previous studies have applied a single function and plot to all species’ data, the sensitivity of the derived vulnerability parameters to the choice of function and plot and whether such choices affected the resolution of species ranking in vulnerability was tested. Notably, the functions selected by maximum likelihood with AIC values within 2 of the minimum depended on the choice of plot, and multiple functions were often selected for given species (Fig. 2, Supplementary Table S3 at JXB online). Thus, when using the ‘Ψlowest binned’ plot, the linear function was selected for 8/10 species, the logistic for two, and the exponential for one species. In contrast, when using the ‘Ψlowest unbinned’ plot, the logistic function was selected for eight species, the sigmoidal for six, the linear for five, and the exponential for four. When using the ‘Ψfinal’ plot, the logistic was selected for nine species, the exponential for eight, the sigmoidal for five, and the linear for two. The best fit function selected using the ‘Ψlowest unbinned’ plot was one of those selected when using the ‘Ψlowest binned’ data set for 5/10 species, and when using the ‘Ψfinal’ plot for only 3/10 species.
The estimation of vulnerability parameters Kmax, P50, and P80, was sensitive to the function and the plot used, but typically the values determined in different ways were correlated across species (Fig. 2, data in Supplementary Table S4 at JXB online). When using the ‘Ψlowest unbinned’ plot, the Kmax, P50, and P80 values generated by the four different functions, averaged across species, varied by 12–27%, 0.21–0.76 MPa, and 0.12–0.74 MPa, respectively, and correlated across species in 15/18 comparisons (rp=0.81–0.99; P <0.05). The use of the three plots produced Kmax values from the four given functions that varied on average by 3–40%, and correlated across species in 11/12 comparisons (rp=0.64–0.99; P <0.05; Fig. 2). Notably, for a species such as Platanus, with a steep initial hydraulic decline, determining Kmax from a ‘Ψlowest unbinned’ plot was critical to resolve its high Kmax. For P50 and P80, the use of the ‘Ψlowest unbinned’ and ‘Ψlowest binned’ plots produced values for given functions that differed on average by 0.08–0.6 MPa, and correlated across species in 7/8 comparisons (rp=0.56–0.99; P <0.05). In contrast, the use of the ‘Ψfinal’ plot produced P50 and P80 values 0.8–2 MPa less negative than when using the other plots (Fig. 2; paired t-test; P <0.05), and values were not correlated across species (rp= –0.40 to 0.48; P=0.11–0.81). The values of Kmax determined using the function selected using the ‘Ψlowest unbinned’ or ‘Ψlowest binned’ plots did not differ on average across species from those determined by taking the mean of Kleaf values at Ψleaf of 0 to –0.5 MPa (data in Supplementray Table S5 at JXB online; P=0.10–0.15; paired t-test). However, Kmax determined using the ‘Ψfinal’ plot was on average 44% higher than Kmax determined by taking the mean of Kleaf values at Ψleaf of 0 to –0.5 MPa (P=0.02), but again the species’ values with the two methods were correlated (rp=0.74; P <0.01).
Species variation in maximum Kleaf and vulnerability, and lack of an efficiency–safety trade-off
Species were compared in the parameters determined from their maximum likelihood functions using the ‘Ψlowest unbinned’ plot (Table 2). Species differed by >11-fold in Kmax, with no average differences between species from moist and dry habitats, though species differences were significant (considering Kmax as the mean of Kleaf values at Ψleaf of 0 to –0.5 MPa; ANOVA; P <0.001). Species also differed greatly in their vulnerabilities, varying 32-fold in P50 and 15-fold in P80, from the most vulnerable species (H. annuus and P. racemosa) with values less than –1 MPa to the least vulnerable species, C. diversifolia, with P50 and P80 values of –3.54 MPa and –5.25 MPa, respectively (Fig. 2). Species’ P50 and P80 values were strongly correlated (rp and rs=0.88–0.96, P <0.01). Species with greater vulnerability (i.e. with less negative P50 and P80 values) had steeper vulnerability curve slopes (rp and rs= –0.72 to –0.83, P <0.01; data in Supplementary Table S5 at JXB online). On average, species from dry habitats had 2.4- to 2.9-fold more negative P50 and P80 than species from moist habitats.
Species with lower vulnerability had greater hydraulic safety margins. Thus, safety margins based on P50 were negatively correlated with P50, and safety margins based on P80 were negatively correlated with both P50 and P80 (rp and rs= –0.70 to –0.95; P <0.05; data in Supplementary Table S5 at JXB online). Safety margins based on P50 ranged from –1.9 MPa to 0.17 MPa and were positive for two species (C. diversifolia and H. arbutifolia); thus, most species lost leaf turgor at lower Ψleaf than P50 as determined using the steady-state method. However, safety margins calculated from P80 ranged from –1.7 MPa to 2.7 MPa, and seven species had positive safety margins. Safety margins did not differ between habitat types (t-test, P <0.05).
Both P50 and P80 were independent of Kmax across species (|rp| and |rs|=0.37–0.62, P >0.05).
Recovery of Kleaf with leaf rehydration and a new importance for leaf hydraulic vulnerability
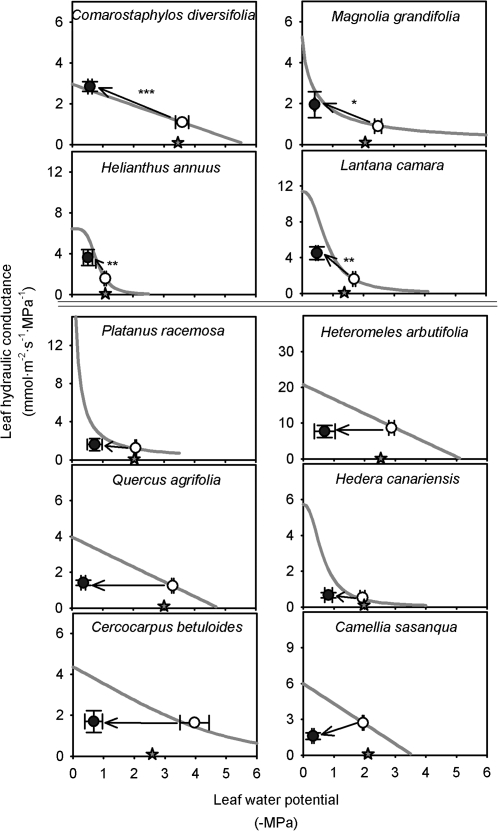
Species varied strongly in the ability to recover in Kleaf after dehydration below their turgor loss point (such that Kleaf declined by 57–97% depending on species) followed by 1 h rehydration with their petioles under water (Fig. 3). For four species (C. diversifolia, H. annuus, L. camara, and M. grandiflora), Kleaf increased 2.2- to 2.8-fold (Fig. 3; P <0.05); C. diversifolia and M. grandiflora recovered fully in Kleaf to their expected values. Three of these species were moist habitat species (L. camara, H. annuus, and M. grandiflora) and one was a dry habitat species (C. diversifolia). The six other species showed no significant recovery. The percentage recovery of Kleaf after rehydration did not correlate with Kmax, P50, or P80 (P >0.05; data in Supplementary Table S5 at JXB online).
Fig. 3.
Recovery of leaf hydraulic conductance (Kleaf) after 1 h rehydration with their petioles under water, for 10 species varying widely in drought tolerance. The grey curves are the best-fit functions of the species’ response to dehydration from Fig. 2; open and filled symbols represent the predicted Kleaf at the dehydrated leaf water potential, and Kleaf after 1h rehydration respectively; stars on the x-axis represent the turgor loss point. Species depicted in the upper four panels showed significant recovery in Kleaf (*P=0.04; **P=0.001; ***P <0.001); only C. diversifolia and M. grandiflora showed total recovery. Species depicted in the lower panels showed no significant recovery in Kleaf.
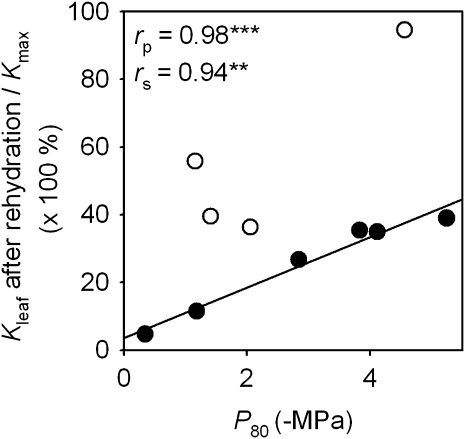
For the six species that did not recover in Kleaf with 1 h rehydration, a nearly perfect correlation was found of the ability to maintain Kleaf after dehydration and rehydration episodes and low P50 and P80 (rs and rp= –0.94 to –0.98; P <0.005; Fig. 4). Thus, among the species that did not recover in Kleaf with rehydration, a low vulnerability predicted the ability to retain hydraulic capacity despite strong, short-term dynamics in water status.
Fig. 4.
The ability of hydraulic vulnerability to predict the degree that leaf hydraulic conductance (Kleaf) was maintained after strong dehydration and rehydration for 1 h with petiole in water, calculated as Kleaf after rehydration divided by maximum Kleaf (Kmax). Filled circles represent species without recovery of Kleaf and open circles species that did show recovery of Kleaf. The line was fitted only for species without recovery of Kleaf (**P=0.005; ***P <0.001).
Testing Kleaf recovery during EFM, and its impact on the estimation of hydraulic parameters
No species showed full recovery in Kleaf of dehydrated leaves during EFM measurements; for all species there was a persistent impact of dehydration. When the residuals of Kleaf against Ψfinal were plotted against Ψlowest (see the Materials and methods), this correlation was significant for seven species (rp= –0.49 to –0.79; n=25–74; P <0.05; Table 3). For the other three species (C. sasanqua, H. annuus, and H. canariensis) the lack of significant correlation of residuals with Ψlowest did not imply a complete recovery of Kleaf during EFM measurement. In the case of H. annuus and H. canariensis, the Ψlowest values were typically the Ψfinal values because the leaves dehydrated further during measurement, rather than recovering in Ψleaf, and, in the case of C. sasanqua, because the Ψlowest correlated with Ψfinal (rp=0.53; P <0.001) there may not have been sufficient residual variation for a powerful test. There were broadly consistent results in the second analysis of the recovery of Kleaf during the EFM measurement (i.e. the calculation of the percentage recovery of Kleaf for leaves that rehydrated over the same Ψleaf interval as the 1 h rehydration experiment). Again there was no evidence for total recovery of Kleaf. There was a significant partial recovery of Kleaf in 3/10 species (P <0.007; Table 3), with Kleaf increasing by 158–178%. Across species, the recovery of Kleaf during the EFM was positively correlated with that observed after 1 h rehydration (rp and rs=0.83–0.84; P <0.05). The percentage recovery of Kleaf during the EFM was 13% lower on average than that after 1 h rehydration, consistent with the leaf rehydrating for a shorter period of time, under subatmospheric pressure (paired t-test; P=0.04).
Table 3.
Results from the tests of the recovery of leaf hydraulic conductance (Kleaf) during the evaporative flux method (EFM), and during 1 h rehydration in the dark. In the residual test for recovery during the EFM, significance indicates that Kleaf did not fully recover. For the indices of Kleaf recovery during the EFM, and during the 1 h rehydration experiments, significance before the comma indicates some degree of significant recovery, and significance after the comma indicates that Kleaf did not recover fully (see the Materials and methods).
| Species | Residual test for recovery during EFM, R2 (n) | Index of recovery in Kleaf during EFM (% increase) | Index of recovery in Kleaf after 1 h rehydration (% increase) |
| Camellia sasanqua | 0.029NS (41) | 114NS, ** | 58.9NS, *** |
| Cercocarpus betuloides | 0.48*** (70) | 119NS, ** | 119NS, ** |
| Comarostaphylos diversifolia | 0.33*** (57) | 178**, * | 259***, NS |
| Hedera canariensis | 0.036NS (41) | 159**, ** | 150NS, *** |
| Helianthus annuus | 0.017NS (36) | 124NS, * | 230**, * |
| Heteromeles arbutifolia | 0.62*** (58) | 66.4NS, * | 79.3NS, * |
| Lantana camara | 0.61*** (25) | 161NS, ** | 284**, ** |
| Magnolia grandiflora | 0.24* (74) | 158**, *** | 218*, NS |
| Platanus racemosa | 0.35*** (38) | 104NS, * | 130NS, * |
| Quercus agrifolia | 0.38*** (46) | 72.2NS, ** | 113NS, *** |
*P <0.05; **P <0.01; ***P <0.001. NS, non-significant
Given that three species indicated partial Kleaf recovery during EFM measurement, an analysis was made of its potential influence on derived vulnerability parameters (see the Materials and methods). Re-calculating these species’ Kmax, P50, and P80 values while considering the partial Kleaf recovery produced values that were correlated with those determined using both the ‘Ψlowest unbinned’ and ‘Ψlowest binned’ plots (rs and rp=0.57–0.99; P <0.001–0.09), indicating that species comparisons using those vulnerability plots are robust even despite partial Kleaf recovery. However, the re-calculated parameters accounting for partial recovery did not correlate with those determined using the ‘Ψfinal’ plot (rs and rp=0.08–0.30; P=0.16–0.83).
Discussion
The new steady-state EFM developed for determining the hydraulic vulnerability of leaves acclimated to high irradiance allowed an independent confirmation and extension of key relationships first shown using rehydration methods. Additionally, refined statistical methods for analysing vulnerability data allowed fitting of the appropriate function for each species and considering the effect of recovery during the measurement. These approaches showed novel variation among species in leaf vulnerability, and relationships with species’ habitat. Further, rehydration experiments quantifying the rapid recovery of Kleaf after dehydration indicated novel species variation, and a new role for leaf vulnerability in determining function after episodes of dehydration and rehydration. This work provided new insights into the vulnerability response, and will additionally enable higher resolution in future work investigating the underlying mechanisms for leaf hydraulic vulnerability.
Species’ differences in Kleaf decline and potential mechanisms
Species differed strikingly in their vulnerability parameters P50 and P80, and in the shape of their vulnerability curves. Notably, because species varied strongly in initial Kleaf values (Kmax) and in the steepness of their decline in Kleaf, P80 was useful to allow comparison of species’ vulnerabilities at a similar stage of their trajectory, namely after the steepest decline phase (Fig. 2), whereas P50 values often occurred in the middle of the steepest decline, which for some species occurred at very high Ψleaf. For such species the P50 may not be an effective index of drought resistance. Further, it is noted that several species (e.g. P. racemosa) had very high Kmax, with substantial Kleaf decline before Ψleaf reached –0.5 MPa. Though part of the true range of leaf hydraulic behaviour in such species, such very high Kleaf values are outside of the range found in nature, as they would not occur for leaves transpiring in vivo, in which the soil and plant hydraulic resistance would cause a further Ψleaf drop not experienced by leaves in the EFM. Species with such steep, non-linear decline were typical of moist habitat species, whereas species with shallow, linear declines were associated with dry habitats.
The Kleaf decline during dehydration arises due to loss of hydraulic conductance in the petiole and/or vein xylem, and/or the extra-xylem pathways (Table 1). The importance of (i) cavitation due to air seeding in major veins leading to subsequent embolism was supported by studies showing ultra-acoustic emissions that may reflect cavitation events (Kikuta et al., 1997; Salleo et al., 2000; Johnson et al., 2009a), as well as dye and cryo-scanning electron microscope studies showing embolism in vein xylem (Salleo et al., 2001; Nardini et al., 2003, 2008; Johnson et al., 2009a), measurement of relatively low air seeding pressures in the leaf petiole and midrib (Choat et al., 2005), and a correlation across species of hydraulic vulnerability with low major vein length per leaf area, as such leaves have less xylem redundancy to protect from the impact of embolism (Scoffoni et al., 2011). Another mechanism may be (ii) the collapse of xylem conduits in the leaf veins; indeed xylem cell collapse has been found for tracheids in the vein of pine needles and in the transfusion tissue of a tropical conifer, at Ψleaf values as high as –1.5 MPa, in advance of cavitation (Cochard et al., 2004; Brodribb and Holbrook, 2005). Indeed, xylem cell collapse has been hypothesized to occur in the minor vein xylem in angiosperms too, but has not yet been visualized directly (Blackman et al., 2010). Additionally, Kleaf decline might relate to (iii) the loss of turgor in living cells in the extra-xylem flow pathways (Brodribb and Holbrook, 2006), in particular the cells of the bundle sheath, mesophyll, and epidermis, which may shrink with walls retracting, and/or may undergo plasmolysis. Tissues with low solute potential, such as bundle sheath, might lose turgor in advance of the mesophyll (Giles et al., 1974; Palta and Leestadelmann, 1983; Nonami and Schulze, 1989; Canny and Huang, 2006). Such changes in cell volume and turgor may alter the flow pathways, and additionally reduce membrane permeability, for example via deactivation of aquaporins (Kim and Steudle, 2007). A final mechanism for the Kleaf decline especially in well-hydrated leaves is (iv) the evaporation of liquid water in the cells walls during transpiration, leaving walls moist but with empty pores and thus lower permeability (Kim and Steudle, 2007; Lee et al., 2009; Voicu et al., 2009).
The shapes of functions fitted to Kleaf data from the present study using maximum likelihood provide several key insights and hypotheses for the action of these mechanisms and point to a diversity in specific impacts across species. Given that embolism or collapse of vein xylem conduits is a principal driver of the Kleaf decline, the linear decline observed for four species implies that air seeding or collapse begins at high Ψleaf for these species (see references in Table 1). The linear decline also implies that conduits of different sizes tend to have approximately equal distributions of air seeding pressures and tendencies to collapse, and/or that a high major vein density provides redundancy that protects the leaf from a disproportionate effect of cavitation of the major vein xylem. A linear decline of Kleaf would also be consistent with a direct role for loss in mesophyll, epidermis, or bundle sheath cell volume or turgor, or the number of water pathways through cell walls declining approximately linearly with Ψleaf above the turgor loss point (Table 1). The logistic decline observed in five species and sigmoidal decline in C. betuloides indicate a qualitative difference. Given that xylem cavitation and/or collapse play a principal role, for these species the steep decline at high Ψleaf that slows with ongoing dehydration is consistent with an unequal distribution of air seeding pressures, for example the larger vessels that confer the bulk of vein xylem conductivity cavitating and/or collapsing first, and smaller vessels having lower air seeding pressures or wall strength and losing function at lower Ψleaf (Table 1). A disproportionate decline at high Ψleaf could also relate to species having low major vein densities; and thus, embolism occurring early in these veins, would result in substantial declines in Kleaf (Table 1). If losses in cell permeability are important, the disproportionate decline at high Ψleaf could relate to a strong sensitivity of Kleaf to losses in volume in particular cells, with low solute potential, for example bundle sheath cells, that may shrink at high Ψleaf and/or undergo aquaporin deactivation (Table 1). If losses of cell wall pathways contribute to the loss of Kleaf, a disproportionate decline at high Ψleaf would be consistent with the cell walls behaving as observed for other porous media that show non-linear declines in conductivity with declining water potential, for example soil (Laio et al., 2001). The species variation in vulnerability curves points to the critical importance of research to disentangle the specific mechanisms of Kleaf decline for given species. Notably, previous work has shown species variation in partitioning of hydraulic resistance between petiole and lamina, and among vein orders, and between the vein xylem and extra-xylem pathways (Trifilo et al., 2003b; Sack et al., 2004, 2005). These species differences would also result in variation in the important mechanisms underlying sensitivity to hydraulic decline because Kleaf would be most sensitive to declines in conductance in the component that accounted for the greatest part of the leaf resistance (Scoffoni et al., 2011).
In this study the focus was on on the response of Kleaf to dehydration under high irradiance. It is noted that many species show an increase of Kleaf under high irradiance, and this response may interact with the response to dehydration (Kim and Steudle, 2007; Lee et al., 2009; Voicu et al., 2009). The decline in conductance under low irradiance occurs in the extra-xylem tissues (Nardini et al., 2005); thus, under low irradiance, the extra-xylem tissues would account for a greater proportion of leaf resistance, and cavitation or collapse of vein xylem would have a lesser impact on Kleaf, and any reduction in the permeability of extra-xylem tissues due to dehydration would have a stronger impact (Nardini et al., 2005; Scoffoni et al., 2008; Voicu et al., 2008). The interaction of the light and dehydration responses of Kleaf is an important area for future investigation.
Quantifying the vulnerability of Kleaf: importance and limitations of the steady-state method
Since it is not yet possible to determine Kleaf directly across a full range of Ψleaf in vivo, hydraulic methods have been applied to excised leaves. The EFM is the latest of several approaches to measuring Kleaf vulnerability on excised leaves. These methods have advantages over indirect methods, such as the audio method, which registers amplified ultrasonic acoustic emissions (UAEs) within drying plant tissue, hypothesized to arise from cavitation (Milburn and Johnson, 1966; Tyree and Dixon, 1983, 1986; Kikuta et al., 1997; Johnson et al., 2009a), or visual methods using dye or cryo-scanning electron microscopy that directly demonstrate embolism in dehydrated leaves (Salleo et al., 2000, 2001), and collapse of conduits in dehydrated conifer leaves (Cochard et al., 2004; Brodribb and Holbrook, 2005), because these methods do not provide information of possible extra-xylem decline, or directly measure hydraulic vulnerability. Hydraulic methods applied to excised leaves include, in addition to the EFM, the high pressure flowmeter (Nardini et al., 2001), the vacuum pump method (Lo Gullo et al., 2003), and the RKM, most frequently used for determining leaf hydraulic vulnerability, which estimates Kleaf from the uptake of water during rehydration by analogy to the charging of a capacitor in series with a resistor (Brodribb and Holbrook, 2003a, 2004a, b, 2006; Woodruff et al., 2007, 2008; Hao et al., 2008; Blackman et al., 2009; Brodribb and Cochard, 2009; Johnson et al., 2009a, b; Saha et al., 2009; Blackman and Brodribb, 2011). As described in the Introduction, these methods all have merits and disadvantages. The steady-state EFM is independent of the RKM, and here it confirmed and extended key findings.
Several limitations of the EFM applied to excised leaves apply equally to the other methods for leaf vulnerability. These methods cannot assess the decline of Kleaf and Ψleaf that occurs in vivo, when xylem water is under tension, and leaf cells are equilibrated at very low water potentials; the xylem cells may be collapsed, leaf cells shrunken, and aquaporins inactivated (Cochard et al., 2002, 2004; Brodribb and Holbrook, 2005, 2006; Canny and Huang, 2006). Excising the leaf under water relieves the tension, and some of these effects might be reversed rapidly. Discovery of such effects would require new in vivo methods for measuring Kleaf decline. In the meantime, vulnerability measured on excised leaves must be considered as conservative, because these methods measure only the Kleaf decline that is not instantly recoverable, for example embolism in veins, which may require many minutes to hours of low tension and active processes to recover (Tyree and Zimmermann, 2002; Bucci et al., 2003; Trifilo et al., 2003a), and persistent effects on living tissues. Further, all the methods may be affected by recovery of Kleaf with rehydration during the measurement itself, but the analysis in this study showed that comparative estimates of hydraulic vulnerability remained robust despite such recovery.
Linkage of vulnerability with drought sensitivity
Species of dry habitats had lower vulnerability (i.e. lower P50 and P80) than species of moist habitat. This finding was consistent with that of a study of Australian species using the RKM (Blackman et al., 2010), here extended with the steady-state method to a set of species very diverse in drought tolerance. This study also confirmed no trade-off across species between Kmax and hydraulic vulnerability, as previously reported using RKM (Blackman et al., 2010) and in a meta-analysis combining data collected with different methods (Sack and Holbrook, 2006), a relationship frequently found for stems (Tyree and Zimmermann, 2002; Maherali et al., 2004; Meinzer et al., 2010). Notably, species from dry and moist habitats did not differ on average in their Kmax. This finding is consistent with multiple types of adaptation to drought. Some drought-tolerant species use water sparingly via low maximum rates of gas exchange, consistent with low Kmax, while others conduct rapid gas exchange when water is available, consistent with high Kmax, and then ‘gear-down’ during shortage, (Maximov, 1931; Grubb, 1998), as illustrated by species such as H. arbutifolia (maximum photosynthetic rate of 14 μmol CO2 m−2 s−1; Valladares and Pearcy, 1997).
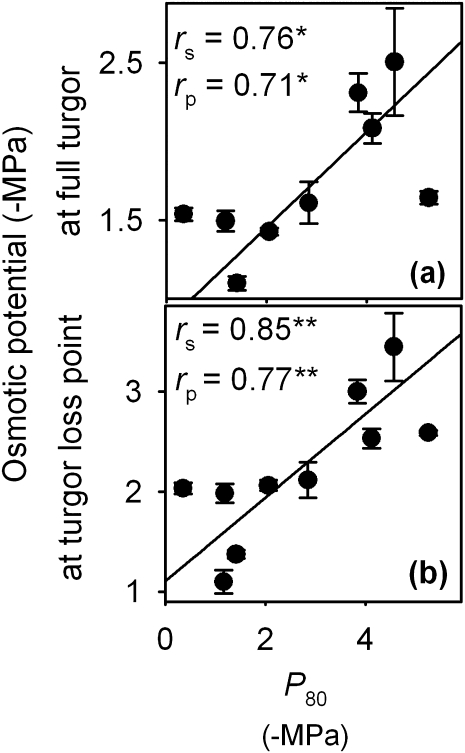
It was also found that across species P50 and P80 were strongly correlated with the bulk leaf turgor loss point (πTLP) and osmotic potential (Scoffoni et al., 2011; Fig. 5). This finding confirmed and extended the correlation previously reported between P50 and πTLP for 19 species using the RKM (Blackman et al., 2010). A low πTLP might confer resistance to Kleaf decline directly, if it allows cells to preserve their structural integrity at lower bulk Ψleaf (Blackman et al., 2010). Previous work has demonstrated the heterogeneity of solute potential and across lamina locations and tissues (Slavik, 1959; Nonami and Schulze, 1989; Koroleva et al., 1997, 2002), and the correlation with vulnerability might be even stronger with the turgor loss point of individual tissues important in the water flow pathways, for example the bundle sheath, rather than for the bulk leaf.
Fig. 5.
Correlation of the leaf water potential at 80% loss of leaf hydraulic conductance (P80) with osmotic potentials (a) at full turgor (πo) and (b) at turgor loss point (πTLP), for 10 species of a wide range of drought tolerance. Fitted standard major axes: (a) πo=0.30×P80+0.85; (b) πTLP=0.42×P80+1.1. Data for πo and πTLP are from Scoffoni et al. (2011).
One consequence of the correlation of vulnerability and πTLP is a mechanism for inducing protective stomatal closure in drought-sensitive species (Brodribb and Cochard, 2009; Hao et al., 2010). The narrow safety margins found in this study were consistent with past studies showing angiosperms often operating at close to cavitation thresholds (Lo Gullo et al., 2003; Brodribb and Holbrook, 2004a, b) in contrast to conifers and ferns which can have wide safety margins (Brodribb and Holbrook, 2004b). Declines in Kleaf accelerate further declines in Ψleaf at a given transpiration rate, and guard cells lose turgor against the background of epidermal cell pressure (Franks and Farquhar, 1999; Damour et al., 2010). After that point, cuticular water loss would lead to slower declines of Ψleaf and of Kleaf (Pasquet-Kok et al., 2010). In contrast, in species with low hydraulic vulnerability, the maintenance of Kleaf would allow stomata to remain open without desiccating the mesophyll during diurnal water stress or soil drought (Brodribb and Holbrook, 2003a). This contribution of Kleaf sensitivity to stomatal control is important in whole-plant drought tolerance (Brodribb and Cochard, 2009; Blackman et al., 2010).
Species differences in Kleaf recovery and a new importance of resistance to Kleaf decline
A strong, novel variation across species was found in the ability of dehydrated leaves to recover rapidly in Kleaf with 1 h of rehydration. Six species showed no recovery and four increased in Kleaf by 2.5- to 2.8-fold. This study thus partially confirmed one previous report of a complete recovery for sunflower (Trifilo et al., 2003a). Typically Kleaf did not fully recover after 1 h rehydration, indicating a partial irreversibility consistent with embolisms that require refilling, or losses of cell permeability that might require energy transduction for recovery (Bucci et al., 2003).
The present data on vulnerability and recovery highlighted a new importance for leaf hydraulics in determining performance with changing plant water status. A recent meta-analysis of data for 31 species found that at minimum daily Ψleaf, species varied greatly in their Kleaf decline, with roughly half the species being below P50 (Johnson et al., 2009b). Our study showed that among species that did not recover rapidly in Kleaf with rehydration, a low hydraulic vulnerability conferred the ability to maintain Kleaf at a high value through both dehydration and rehydration. Species resistant to hydraulic decline could thus maintain Kleaf at high levels despite transient but severe dynamics in Ψleaf, and gain a benefit in maintaining performance during diurnal water stress or soil drought. These findings are consistent with the correlation of low leaf hydraulic vulnerability and the ability of severly droughted plants to recover in transpiration after rewatering (Blackman et al., 2009; Brodribb and Cochard, 2009). Tests are needed of the degree that rapid leaf hydraulic recovery, as shown in this study, contributes to whole-plant hydraulic recovery and tolerance of dynamic water regimes.
Supplementary data
Supplementary data are available at JXB online.
Table S1. A summary of previous studies of leaf hydraulic vulnerability on whole leaves, indicating the various methods used, the different functions fitted to the data, and whether the data were binned or not before line fitting.
Table S2. Minimum and maximum transpirational flow rates (E) for each species measured with the evaporative flux method and corresponding estimated stomatal conductances (g), and cuticular conductances for these species.
Table S3. Parameters for the decline of leaf hydraulic conductance (Kleaf) with declining leaf water potential for 10 species, fitted with four different functions, R2 for observed values plotted against predicted values from the fitted function, and values for the Akaike Information Criterion (AIC). For each function, three plots were tested for Kleaf against leaf water potential: (1) ‘Ψlowest unbinned’; (2) ‘Ψlowest binned’; (3) and ‘Ψfinal’ (see the Materials and methods for additional information).
Table S4. Parameters of leaf hydraulic vulnerability curves (Kmax, P50, and P80) determined by fitting four functions to the data for each species (linear, sigmoidal, logistic, and exponential) and using three kinds of plots ‘Ψowest unbinned’, ‘Ψlowest binned’, and’ Ψfinal’).
Table S5. Species means ±SEs for leaf hydraulic vulnerability parameters and pressure–volume parameters for 10 species ranging widely in drought tolerance.
Acknowledgments
We thank M. Bartlett, W. Dang, A. Gibson, P. Rundel, and Y. Taniguchi for logistical assistance, and T. Brodribb for discussion. This research was supported by UCLA Department of Ecology and Evolutionary Biology, a UCLA Vavra Research Fellowship and by National Science Foundation Grant #0546784.
References
- Blackman CJ, Brodribb TJ. Two measures of leaf capacitance: insights into the water transport pathway and hydraulic conductance in leaves. Functional Plant Biology. 2011;38:118–126. doi: 10.1071/FP10183. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell and Environment. 2009;32:1584–1595. doi: 10.1111/j.1365-3040.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist. 2010;188:1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Leaf water potentials measured with a pressure chamber. Plant Physiology. 1967;42:133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Blackman CJ. Prometheus Wiki contributors. PROTOCOL: non-steady state rehydration to determine leaf hydraulic conductance, vulnerability and capacitance. PrometheusWiki. Retrieved 02June2011, from http://www.publish.csiro.au/prometheuswiki/tiki-pagehistory.php?page=PROTOCOL Non-steady state rehydration to determine leaf hydraulic conductance, vulnerability and capacitance&preview= [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Sack L. Viewing leaf structure and evolution from a hydraulic perspective. Functional Plant Biology. 2010;37:488–498. [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology. 2003a;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist. 2003b;158:295–303. [Google Scholar]
- Brodribb TJ, Holbrook NM. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant, Cell and Environment. 2004a;27:820–827. [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytologist. 2004b;162:663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology. 2005;137:1139–1146. doi: 10.1104/pp.104.058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell and Environment. 2006;29:2205–2215. doi: 10.1111/j.1365-3040.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Forced depression of leaf hydraulic conductance in situ: effects on the leaf gas exchange of forest trees. Functional Ecology. 2007;21:705–712. [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg LDL. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant, Cell and Environment. 2003;26:1633–1645. [Google Scholar]
- Buck AL. New equations for computing vapor-pressure and enhancement factor. Journal of Applied Meteorology. 1981;20:1527–1532. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference. 2nd edn. New York: Springer; 2002. [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference—understanding AIC and BIC in model selection. Sociological Methods and Research. 2004;33:261–304. [Google Scholar]
- Canny MJ, Huang CX. Leaf water content and palisade cell size. New Phytologist. 2006;170:75–85. doi: 10.1111/j.1469-8137.2005.01633.x. [DOI] [PubMed] [Google Scholar]
- Chen JW, Zhang Q, Li XS, Cao KF. Independence of stem and leaf hydraulic traits in six Euphorbiaceae tree species with contrasting leaf phenology. Planta. 2009;230:459–468. doi: 10.1007/s00425-009-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Lahr EC, Melcher P, Zwieniecki MA, Holbrook NM. The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant, Cell and Environment. 2005;28:1082–1089. [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology. 2002;128:282–290. [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Froux F, Mayr FFS, Coutand C. Xylem wall collapse in water-stressed pine needles. Plant Physiology. 2004;134:401–408. doi: 10.1104/pp.103.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology. 2007;143:122–133. doi: 10.1104/pp.106.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan IR. Electrical analog of evaporation from, and flow of water in plants. Planta. 1972;106:221–226. doi: 10.1007/BF00388099. [DOI] [PubMed] [Google Scholar]
- Croat TB. Flora of Barro Colorado Island. California: Stanford University Press; 1978. [Google Scholar]
- Damour G, Simonneau T, Cochard H, Urban L. An overview of models of stomatal conductance at the leaf level. Plant, Cell and Environment. 2010;33:1419–1438. doi: 10.1111/j.1365-3040.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- Domec JC, Lachenbruch B, Meinzer FC. Bordered pit structure and function determine spatial patterns of air-seeding thresholds in xylem of Douglas-fir (Pseudotsuga menziesii; Pinaceae) trees. American Journal of Botany. 2006;93:1588–1600. doi: 10.3732/ajb.93.11.1588. [DOI] [PubMed] [Google Scholar]
- eFloras. Flora of North America. Missouri Botanical Garden & Harvard University Herbaria. 2008;Vol. 2010 St. Louis, MO and Cambridge, MA http://www.efloras.org/ [Google Scholar]
- Franks PJ, Farquhar GD. A relationship between humidity response, growth form and photosynthetic operating point in C-3 plants. Plant, Cell and Environment. 1999;22:1337–1349. [Google Scholar]
- Giles KL, Beardsel MF, Cohen D. Cellular and ultrastructural changes in mesophyll and bundle sheath-cells of maize in response to water stress. Plant Physiology. 1974;54:208–212. doi: 10.1104/pp.54.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb PJ. A reassessment of the strategies of plants which cope with shortages of resources. Perspectives in Plant Ecology Evolution and Systematics. 1998;1:3–31. [Google Scholar]
- Hao GY, Hoffmann WA, Scholz FG, Bucci SJ, Meinzer FC, Franco AC, Cao KF, Goldstein G. Stem and leaf hydraulics of congeneric tree species from adjacent tropical savanna and forest ecosystems. Oecologia. 2008;155:405–415. doi: 10.1007/s00442-007-0918-5. [DOI] [PubMed] [Google Scholar]
- Hao GY, Sack L, Wang AY, Cao KF, Goldstein G. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Functional Ecology. 2010;24:731–740. [Google Scholar]
- Iovi K, Kolovou C, Kyparissis A. An ecophysiological approach of hydraulic performance for nine Mediterranean species. Tree Physiology. 2009;29:889–900. doi: 10.1093/treephys/tpp032. [DOI] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. The Plant Cell. 1998;10:451–459. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Meinzer FC, Woodruff DR, McCulloh KA. Leaf xylem embolism, detected acoustically and by cryo-SEM, corresponds to decreases in leaf hydraulic conductance in four evergreen species. Plant, Cell and Environment. 2009a;32:828–836. doi: 10.1111/j.1365-3040.2009.01961.x. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology. 2009b;29:879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- Kikuta SB, LoGullo MA, Nardini A, Richter H, Salleo S. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant, Cell and Environment. 1997;20:1381–1390. [Google Scholar]
- Kim YX, Steudle E. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. Journal of Experimental Botany. 2007;58:4119–4129. doi: 10.1093/jxb/erm270. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Murata G. Colored illustrations of woody plants of Japan. 1979 Revised editions, Vol. II. Osaka: Hoikusha, 158–159. [Google Scholar]
- Koroleva OA, Farrar JF, Tomos AD, Pollock CJ. Patterns of solute in individual mesophyll, bundle sheath and epidermal cells of barley leaves induced to accumulate carbohydrate. New Phytologist. 1997;136:97–104. [Google Scholar]
- Koroleva OA, Tomos AD, Farrar J, Pollock CJ. Changes in osmotic and turgor pressure in response to sugar accumulation in barley source leaves. Planta. 2002;215:210–219. doi: 10.1007/s00425-002-0744-2. [DOI] [PubMed] [Google Scholar]
- Kubiske ME, Abrams MD. Pressure–volume relationships in non-rehydrated tissue at various water deficits. Plant, Cell and Environment. 1990;13:995–1000. [Google Scholar]
- Laio F, Porporato A, Ridolfi L, Rodriguez-Iturbe I. Plants in water-controlled ecosystems: active role in hydrologic processes and response to water stress—II. Probabilistic soil moisture dynamics. Advances in Water Resources. 2001;24:707–723. [Google Scholar]
- Lee SH, Chung GC, Zwiazek JJ. Effects of irradiance on cell water relations in leaf bundle sheath cells of wild-type and transgenic tobacco (Nicotiana tabacum) plants overexpressing aquaporins. Plant Science. 2009;176:248–255. [Google Scholar]
- Lo Gullo MA, Nardini A, Trifilo P, Salleo S. Changes in leaf hydraulics and stomatal conductance following drought stress and irrigation in Ceratonia siliqua (Carob tree) Physiologia Plantarum. 2003;117:186–194. [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Maximov NA. The physiological significance of the xeromorphic structure of plants. Journal of Ecology. 1931;19:273–282. [Google Scholar]
- Meinzer FC, McCulloh KA, Lachenbruch B, Woodruff DR, Johnson DM. The blind men and the elephant: the impact of context and scale in evaluating conflicts between plant hydraulic safety and efficiency. Oecologia. 2010;164:287–296. doi: 10.1007/s00442-010-1734-x. [DOI] [PubMed] [Google Scholar]
- Milburn JA, Johnson RPC. Conductance of sap. 2. Detection of vibrations produced by sap cavitation in Ricinus xylem. Planta. 1966;69:43–52. doi: 10.1007/BF00380209. [DOI] [PubMed] [Google Scholar]
- Miyazawa S, Yoshimura S, Shinzaki Y, Maeshima M, Miyake C. Deactivation of aquaporins decreases internal conductance to CO2 diffusion in tobacco leaves grown under long-term drought. Functional Plant Biology. 2008;35:553–564. doi: 10.1071/FP08117. [DOI] [PubMed] [Google Scholar]
- Nardini A, Ramani M, Gortan E, Salleo S. Vein recovery from embolism occurs under negative pressure in leaves of sunflower (Helianthus annuus) Physiologia Plantarum. 2008;133:755–764. doi: 10.1111/j.1399-3054.2008.01087.x. [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Andri S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot) Plant, Cell and Environment. 2005;28:750–759. [Google Scholar]
- Nardini A, Salleo S, Raimondo F. Changes in leaf hydraulic conductance correlate with leaf vein embolism in Cercis siliquastrum L. Trees-Structure and Function. 2003;17:529–534. [Google Scholar]
- Nardini A, Tyree MT, Salleo S. Xylem cavitation in the leaf of Prunus laurocerasus and its impact on leaf hydraulics. Plant Physiology. 2001;125:1700–1709. doi: 10.1104/pp.125.4.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld HS, Grantz DA, Meinzer FC, Goldstein G, Crisosto GM, Crisosto C. Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiology. 1992;100:1020–1028. doi: 10.1104/pp.100.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H, Schulze ED. Cell water potential, osmotic potential, and turgor in the epidermis and mesophyll of transpiring leaves. Combined measurements with the cell pressure probe and nanoliter osmometer. Planta. 1989;177:35–46. doi: 10.1007/BF00392152. [DOI] [PubMed] [Google Scholar]
- North GB, Nobel PS. Heterogeneity in water availability alters cellular development and hydraulic conductivity along roots of a desert succulent. Annals of Botany. 2000;85:247–255. [Google Scholar]
- Palta JP, Leestadelmann OY. Vacuolated plant-cells as ideal osmometer—reversibility and limits of plasmolysis, and estimation of protoplasm volume in control and water-stress tolerant cells. Plant, Cell and Environment. 1983;6:601–610. [Google Scholar]
- Pammenter NW, Vander Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Pasquet-Kok J, Creese C, Sack L. Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa. Plant, Cell and Environment. 2010;33:2084–2100. doi: 10.1111/j.1365-3040.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- Pieruschka R, Huber G, Berry JA. Control of transpiration by radiation. Proceedings of the National Academy of Sciences, USA. 2010;107:13372–13377. doi: 10.1073/pnas.0913177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Liu WH, Middleton E, Pardee T. How strong is intracanopy leaf plasticity in temperate deciduous trees? American Journal of Botany. 2006;93:829–839. doi: 10.3732/ajb.93.6.829. [DOI] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany. 2002;53:2177–2184. doi: 10.1093/jxb/erf069. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Tyree MT. Leaf hydraulics and its implications in plant structure and function. In: Holbrook NM, Zweiniecki MA, editors. Vascular transport in plants. Oxford: Elsevier/Academic Press; 2005. pp. 93–114. [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist. 2005;167:403–413. doi: 10.1111/j.1469-8137.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Holbrook NM, Montti L, Goldstein G, Cardinot GK. Water relations of Chusquea ramosissima and Merostachys claussenii in Iguazu National Park, Argentina. Plant Physiology. 2009;149:1992–1999. doi: 10.1104/pp.108.129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell and Environment. 2001;24:851–859. [Google Scholar]
- Salleo S, Nardini A, Pitt F, Lo Gullo MA. Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.) Plant, Cell and Environment. 2000;23:71–79. [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell and Environment. 2008;31:1803–1812. doi: 10.1111/j.1365-3040.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Rawls M, McKown A, Cochard H, Sack L. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiology. 2011;156:832–843. doi: 10.1104/pp.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin A, Kupper P. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology. 2007;27:679–688. doi: 10.1093/treephys/27.5.679. [DOI] [PubMed] [Google Scholar]
- Sellin A, Ounapuu E, Kupper P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula) Physiologia Plantarum. 2008;134:412–420. doi: 10.1111/j.1399-3054.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Slavik B. Gradients of osmotic pressure of cell sap in the area of one leaf blade. Biologia Plantarum. 1959;1:39–47. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- Trifilo P, Gasco A, Raimondo F, Nardini A, Salleo S. Kinetics of recovery of leaf hydraulic conductance and vein functionality from cavitation-induced embolism in sunflower. Journal of Experimental Botany. 2003a;54:2323–2330. doi: 10.1093/jxb/erg259. [DOI] [PubMed] [Google Scholar]
- Trifilo P, Nardini A. Lo Gullo MA, Salleo S. Vein cavitation and stomatal behaviour of sunflower (Helianthus annuus) leaves under water limitation. Physiologia Plantarum. 2003b;119:409–417. [Google Scholar]
- Tyree MT, Dixon MA. Cavitation events in Thuja occidentalis L. Ultrasonic acoustic emissions from the sapwood can be measured. Plant Physiology. 1983;72:1094–1099. doi: 10.1104/pp.72.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Dixon MA. Water-stress induced cavitation and embolism in some woody plants. Physiologia Plantarum. 1986;66:397–405. [Google Scholar]
- Tyree MT, Nardini A, Salleo S, Sack L, El Omari B. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? Journal of Experimental Botany. 2005;56:737–744. doi: 10.1093/jxb/eri045. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer; 2002. [Google Scholar]
- Valladares F, Pearcy RW. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll. Heteromeles arbutifolia. Plant, Cell and Environment. 1997;20:25–36. [Google Scholar]
- Voicu MC, Cooke JEK, Zwiazek JJ. Aquaporin gene expression and apoplastic water flow in bur oak (Quercus macrocarpa) leaves in relation to the light response of leaf hydraulic conductance. Journal of Experimental Botany. 2009;60:4063–4075. doi: 10.1093/jxb/erp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voicu MC, Zwiazek JJ, Tyree MT. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiology. 2008;28:1007–1015. doi: 10.1093/treephys/28.7.1007. [DOI] [PubMed] [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biological Reviews. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Weast RC, editor. Handbook of chemistry and physics. 54th edn. Cleveland, OH: CRC Press; 1974. [Google Scholar]
- Woodruff DR, McCulloh KA, Warren JM, Meinzer FC, Lachenbruch B. Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas-fir. Plant, Cell and Environment. 2007;30:559–569. doi: 10.1111/j.1365-3040.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- Woodruff DR, Meinzer FC, Lachenbruch B. Height-related trends in leaf xylem anatomy and shoot hydraulic characteristics in a tall conifer: safety versus efficiency in water transport. New Phytologist. 2008;180:90–99. doi: 10.1111/j.1469-8137.2008.02551.x. [DOI] [PubMed] [Google Scholar]
- Yang SD, Tyree MT. Hydraulic resistance in Acer saccharum shoots and its influence on leaf water potential and transpiration. Tree Physiology. 1993;12:231–242. doi: 10.1093/treephys/12.3.231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.