Abstract
The diameter of vascular conduits increases towards the stem base. It has been suggested that this profile is an efficient anatomical feature for reducing the hydraulic resistance when trees grow taller. However, the mechanism that controls the cell diameter along the plant is not fully understood. The timing of cell differentiation along the stem was investigated. Cambial activity and cell differentiation were investigated in a Picea abies tree (11.5 m in height) collecting microsamples at nine different heights (from 1 to 9 m) along the stem with a 4 d time interval. Wood sections (8–12 μm thick) were stained and observed under a light microscope with polarized light to differentiate the developing xylem cells. Cell wall lignification was detected using cresyl violet acetate. The first enlarging cells appeared almost simultaneously along the tree axis indicating that cambium activation is not height-dependent. A significant increase in the duration of the cell expansion phase was observed towards the tree base: at 9 m from the ground, xylem cells expanded for 7 d, at 6 m for 14 d, and at 3 m for 19 d. The duration of the expansion phase is positively correlated with the lumen area of the tracheids (r2=0.68, P < 0.01) at the same height. By contrast, thickness of the cell wall of the earlywood did not show any trend with height. The lumen area of the conduits down the stem appeared linearly dependent on time during which differentiating cells remained in the expansion phase. However, the inductive signal of such long-distance patterned differentiation remains to be identified.
Keywords: Auxin, cambium, cell differentiation, conduit tapering, Picea abies polar pattern growth
Introduction
In the xylem, water transport occurs through a complex network of very narrow conduits. There are still a number of unanswered questions about the way in which the three-dimensional organization of xylem conduits (in the order of micrometres) can guarantee an efficient delivery of water to the leaves over very long distances (up to 100 m in the world’s tallest trees) so that trees can be correctly defined as masters of microfluidics (Holbrook and Zwieniecki, 2008). According to physical laws (Hagen–Poiseuille), the amount of water passing through a cylindrical conduit in a unit of time under a given pressure (hydraulic conductivity, Kh) is strongly affected by the length and diameter of xylem conduits (Tyree and Ewers, 1991):
| (1) |
where ρ is the density and η the dynamic viscosity of water, and l and d are length and diameter of the conduit, respectively. For increased conduit length, like the increase in root-to-leaf distance in real trees during ontogenesis, equation 1 states that Kh decreases accordingly unless the conduit width increases basipetally (see the hydraulic limitation hypothesis, Ryan and Yoder, 1997): for high rates of conduit enlargement towards the base (i.e. conduit tapering), the negative effect of path length on Kh is markedly reduced (Becker et al., 2000; Enquist, 2003; Petit and Anfodillo, 2009), because Kh per unit length (hydraulic conductivity, k) increases from the stem apex downwards (Yang and Tyree, 1993; Petit et al., 2008).
The longitudinal variation in xylem conduit diameter has been extensively studied and the pattern of conduit enlargement towards the base (i.e. the so-called conduit tapering) was similar among plants of different size and species (Leitch, 2001; Anfodillo et al., 2006; Weitz et al., 2006; Coomes et al., 2007; Petit et al., 2008, 2009, 2010). Typically, the variation is less than linear, with conduit width increasing from the stem apex to the base following a power trajectory of the form:
| (2) |
where d is the conduit diameter of the xylem conduits, L the distance from the stem apex (path length), a the allometric constant, and b the scaling exponent. In tall trees, the scaling exponent b approaches the value of 0.20 which is considered the minimum degree of tapering to overcome the negative effect of increased hydraulic resistance when trees grow taller (Petit et al., 2008, 2010).
Given the importance of the size of xylem conduits in the transport system, a question naturally arises: how do plants control the size of the xylem conduits along the pathway of the interconnected elements that move water from roots to leaves? This question appears particularly intriguing because the hydraulic system has to be completed before the whole network is functioning. In fact, the effective hydraulic resistance of the entire conduit path is tested after cell plasmolysis occurs, when the inner protoplasts are destroyed, xylem cells are biologically dead, and no other change in the vascular structure is possible. In trees, the question is even more fascinating because this control mechanism has to be applied in leaves, stems, and roots over distances that, in the tallest individuals, can exceed 100 m.
A pivotal role in controlling the differentiation of the vascular tissues would seemed to be played by auxin, namely, indole-3-acetic acid (IAA) (Aloni, 2004). Although the understanding of how auxin regulates secondary xylem development at molecular level is still rudimentary (Nilsson et al., 2008), there is evidence that (i) the major sources of auxin are the developing buds and young shoots (Uggla et al., 1998; Scarpella and Meijer, 2004); (ii) auxin moves in a polar fashion from the apex to the stem base (Aloni, 2001; Muday and DeLong, 2001; Lovisolo et al., 2002; Friml, 2003) according to an asymmetric disposition within the cells of efflux-facilitating transmembrane proteins of the PIN family (Berleth et al., 2007); (iii) the major path of the auxin flow in trees is through the vascular cambium (Sundberg et al., 2000); (iv) there is a general decrease in the auxin concentration downwards in the stem (Lovisolo et al., 2002).
In their model, Aloni and Zimmermann (1983) proposed that high auxin concentrations would accelerate cell differentiation, thus reducing the period available for cell enlargement. So, according to the different auxin concentration along the stem of a tree, conduit elements should be narrower at the top and attain the greatest sizes at the base, because of the longer duration of the expansion phase. This simple explanation has often been criticized because of the observed radial gradient in auxin concentration and the unclear mechanism of influence of this hormone on cell expansion (Uggla et al., 1998; Lev-Yadun, 2000). Indeed, recent analyses showed that the role of auxin in determining cell dimension seems to be more complex than thought, because auxin responsive genes are more expressed (i.e. cells would become more sensitive to auxin) even if the level of auxin decreased, as occurs away from the cambial zone (Nilsson et al., 2008).
Whatever the control and variations in cell responsiveness, the xylogenetic processes along the stem are largely unknown and detailed information on the relationship between timings of differentiation and cell size is still lacking. The aim of this paper is to answer the following two questions: (i) does the duration of cell expansion differ along the stem? (ii) is the duration of cell expansion correlated with xylem conduit diameter?
Monitoring of xylem formation at high temporal resolution was carried out on one Picea abies tree to provide the basic anatomical and developmental information for clarifying the possible role of inducing signals (e.g. auxin concentration and/or responsiveness) in regulating structure and size of the conductive pathways in plants.
Materials and methods
Sample collection and preparation
One 30-year-old Norway spruce (Picea abies) was selected in a mixed forest in the Dolomites (North-Eastern Italy) (46°26′ N, 12°13′ E, 1000 m a.s.l.). The tree height and diameter at breast height were 11.5 m and 25 cm, respectively, with good vegetative conditions (annual longitudinal increment about 0.5 m) and living branches until 1.5 m above ground. An 8-m-tall trestle was erected to enclose the tree and collect samples along and around the stem. Sampling was carried out in 2003, from 16April (day of the year [DOY] 105) to 13November (DOY 316), every 3–5 d until July, when the greatest cambial divisions occur (Rossi et al., 2006) and weekly from July to November. During sampling, microcores were collected along the stem at 1 m from the ground and at intervals of 1 m on the same longitudinal line. The microcores (2.5×20 mm) were collected with a surgical needle (Trapsystem®; Rossi et al., 2006) from extraction points at least 5 cm apart. Overall, 40 samples for each position were extracted, leading to a total of 360 microcores.
Immediately after sampling, the microcores were placed in Eppendorf microtubes with an ethanol solution (50% in water) and stored as soon as possible at 5 °C in order to avoid tissue deterioration. Each sample was oriented by marking the transverse side with a pencil under a stereo-microscope at 10–20 magnifications. The microcores were dehydrated with successive immersions in ethanol and D-limonene, embedded in paraffin according to Rossi et al. (2006b) and transverse sections of 8–12 μm thickness were cut with a rotary microtome. Sections were stained with cresyl violet acetate (0.16% in water) and observed within 20 min with visible and polarized light under a light microscope at 400–500 magnifications to distinguish the developing cells.
Dynamic of cell differentiation
For each sample, the radial number of cells in the cambial zone, radial expanding phase, cell wall thickening and lignification phase, and mature cells were counted along three rows (Deslauriers et al., 2003; Rossi et al., 2006a). Cells in the cambial zone and in radial enlargement showed only primary wall that, unlike secondary wall, did not shine under polarized light. The radially expanding cells were wider in diameter and nearly isometric in shape compared with cambial cells and could be quite easily detected (Rossi et al., 2006b). The transition from the expanding tracheids to those forming a secondary wall was determined by the presence of birefringence under polarized light (Abe et al., 1997), because of the arrangement of the cellulose microfibrils. Once their final size had been reached, the cells began maturing through cell wall thickening and lignification, which was detected by staining the sections with cresyl violet acetate reacting with the lignin (Deslauriers et al., 2003). Lignification was shown by a colour change from violet (unlignified secondary cell walls) to blue (lignified cell walls). The blue colour over the whole cell wall indicated the end of lignification and the attainment of the mature stage for the tracheid.
The duration of tracheid expansion was calculated as the difference between the onset of xylem differentiation and the date when cell wall formation began. Similarly, the duration of cell wall formation was found as the difference between the onset of secondary cell wall formation and the date when the first mature tracheids were observed (Deslauriers et al., 2009). Therefore, our analysis of duration of cell differentiation processes deals essentially with the first formed cells of earlywood, which are the most important in determining the efficiency of the water transport system.
Anatomy of xylem
At the end of the growing season, the lumen area of conduits and cell wall thickness were measured with WinCELL™ (Régent Instruments Inc., Quebec, Canada), using digitalized images taken by a Nikon camera mounted on a microscope at 400 magnifications. Three cell rows from earlywood to latewood of the tree ring formed during 2003 were analysed and measured. Cell diameter was also calculated considering the lumen to be circular.
In order to compare the estimated durations of each differentiation phase and the degree of tapering of xylem conduits all the cells with a diameter of more than half the diameter of the largest one (James et al., 2003) were used. Only the selected cells were then included in the calculation of the weighted average of hydraulic diameters (Dh) as proposed by Mencuccini et al., (1997) according to the following equation of
| (3) |
where dn is the diameter of the n cell (Sperry et al., 1994) which weights the hydraulic diameters of single cells according to hydraulic conductance.
Meteorological and physiological measurements
A standard meteorological station was installed close to the sampled tree. By using very thin thermocouples (copper-constantan), temperatures of needles (two probes, north and south facing), stem (two probes, north and south facing), and soil (one probe, 10 cm depth) were recorded every minute and averaged every 15 min. In an adjacent tree of the same dimensions as the sampled one, sap flow density was measured using the thermal dissipation method (Granier, 1985) for monitoring tree water transport throughout the year. All data were averaged and stored in a datalogger (Campbell CR10X).
In parallel with the sampling of the cambial activity, phenological observations on vegetative organs (timing of budburst) were recorded on two branches at two different tree heights (4 m and 7 m) and with north and south exposures.
Results
Xylem and budburst phenology
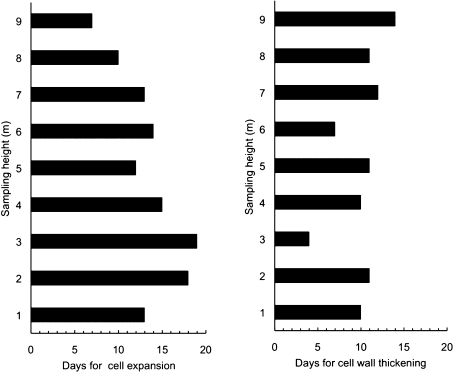
The number of cells in the cambial zone changed throughout the year: in mid-April, cambium was composed of 5–6 rows of cells at the base and 8–9 at the top of the tree, indicating that no division was occurring and meristems were inactive. During the growing season (May–September), the number of cambial cells was 13–14, irrespective of tree height (data not shown). The first expanding cells were observed in late April, within a 5 day interval, irrespective of tree height (Table 1), which revealed a lack of temporal trend in the post-cambial growth along the stem. By contrast, cell wall thickening and lignification started earlier at the top of the tree, showing an evident height-dependent trend. Cell expansion lasted 7 d at 9 m while a longer duration (19 d) was observed at 3 m. At 1 m, the duration of cell expansion decreased again to 14 d (Fig. 1). The duration of cell wall thickening and lignification for the first-formed tracheid lasted between 7 d and 17 d at all sampling heights, except at 3 m where the duration was estimated as 4 d. So, no evident temporal pattern was observed along the stem for this differentiation phase (Fig. 1).
Table 1.
Dynamics of cell differentiation at different tree heights
| Sampling height(m) | Cambial reactivation (DOY)* | First cell with secondarywall appearing (DOY)a | First mature cell appearing (DOY)a |
| 1 | 122 | 135 | 145 |
| 2 | 120 | 138 | 149 |
| 3 | 122 | 141 | 145 |
| 4 | 122 | 137 | 147 |
| 5 | 122 | 134 | 145 |
| 6 | 123 | 137 | 144 |
| 7 | 118 | 131 | 143 |
| 8 | 121 | 131 | 142 |
| 9 | 120 | 127 | 141 |
Day of the year.
Fig. 1.
Duration of expansion phase (left) and cell wall thickening and lignification (right) of the first earlywood cells at different sampling stem heights along the stem, from 1 to 9 m from the ground.
Notably, cambial activity appeared to be uncoupled from the phenology of vegetative organs: budburst generally occurred 15 d after the reactivation of the cambial cells. In south-facing branches, buds were activated on DOY 136, with no difference among tree heights. In north-facing branches, budburst occurred on DOY 140 at 7 m height, while the latest opening shoots were recorded on the bottom north-facing branches on DOY 143.
Xylem anatomy
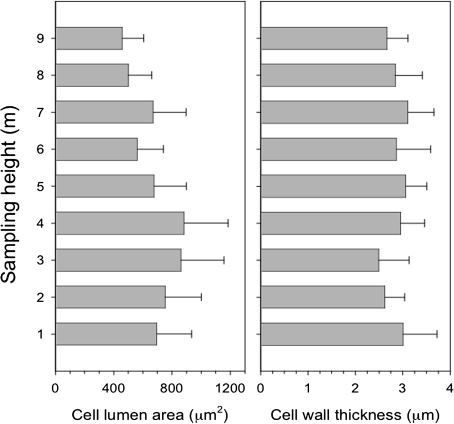
The lumen area of the cells varied along the stem with the smallest values (460 μm2) observed at 9 m (Fig. 2). Increasing values were measured towards the base up to 3-4 m, where lumen area exceeded 860 μm2. At heights of 1–2 m from the ground, lumen area decreased again slightly with mean values varying between 690 and 750 μm2. The differences between sampling heights were statistically significant (F=5.26, P <0.05). Cell wall thickness of the first formed cells was 2.5–3 μm, with no statistical difference between sampling heights (ANOVA, F=0.74, P >0.05) and no clear trend along the stem (Fig. 2).
Fig. 2.
Variation of tracheid lumen area (μm2) (left) and cell wall thickness (μm) at different sampling heights (right). Values are expressed as mean and standard deviation.
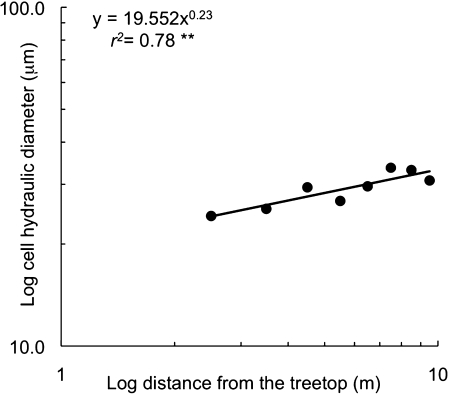
A significant relationship was found (r2=0.78 P=0.003) between the weighted average of the hydraulic diameters and the distance from the tree top, which allowed the degree of conduits tapering to be estimated (Fig. 3) (i.e. the exponent of the regression which equals 0.23).
Fig. 3.
Variation of the cell diameter (μm) versus the distance from the treetop (m) expressed in a log–log scale. Because of the well-known phenomenon of decreasing lumen area near the root collar due to mechanical stresses (Spicer and Gartner, 2001), the sampling position at 1 m was excluded from the analysis. ** P <0.01.
Relationship between anatomy and differentiation phases
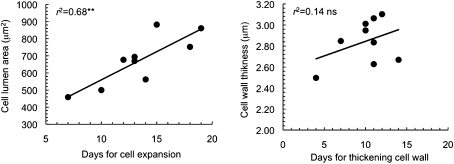
A close relationship was found between the cell lumen area (μm2) of the measured cells and duration of the expansion phase (r2=0.73, P <0.01). The significant and positive regression showed that larger cells required longer duration of cell expansion, indicating that the period in which a cell remains in post-cambial growth plays an essential role in determining its final size (Fig. 4). The estimated rate of cell expansion is c. 32 μm2 d−1: this means that an expanding cell with lumen of 300 μm2 would need about 10 d more to reach 600 μm2. However, no significant relationship was found between cell wall thickness and duration of cell wall thickening and lignification phase (r2=0.14, P >0.05) (Fig. 4).
Fig. 4.
Relationship between cell lumen area (μm2) and duration of expansion phase (d) (left) and between cell wall thickness (μm) and duration of cell wall thickening and lignification phase (d) (right). **P <0.01, ns, P >0.05.
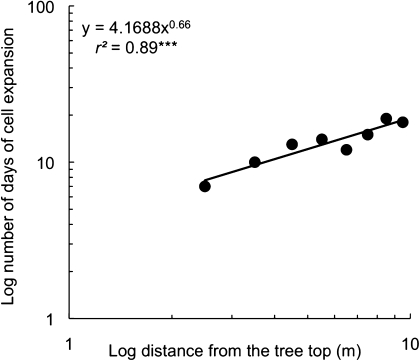
Due to the relationship between cell diameter and distance from the tree top, a strong correlation appeared even when the duration of expansion phase was plotted versus the distance from the tree top (Fig. 5) (r2=0.88, P <0.001).
Fig. 5.
Relationship between the duration of cell expansion phase (d) and the distance from the tree top (m) of the different sampling points expressed in a log–log scale. Similarly to Fig. 1 the sampling position at 1 m was excluded. ***P <0.001.
Stem temperature and sap flux density
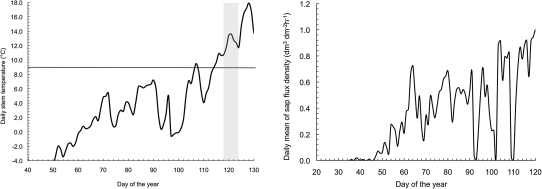
Although above-zero daily temperatures occurred in the stem steadily at the beginning of March (Fig. 6), conditions of water thawing in the xylem during the day can occur from mid-February. Sap flow density, which is correlated with leaf gas exchange, was about 50% of the annual maximum during mid-March (Fig. 6), much earlier than the observed onset of cambial activity.
Fig. 6.
Variation of the daily mean stem temperature during March–May 2003 (left). The grey window highlights the time span of the onset of cambial activity along the stem axis and the line at 9 °C shows the thermal threshold for the onset of xylogenesis (Rossi et al., 2007). Variation of the daily average of sap flow density (dm3 dm−2 h−1) during the beginning of the growing season (right).
Discussion
Xylem phenology
The onset of cambial activity did not appear to be synchronized either with bud reactivation or with onset of photosynthesis. In the middle crown, budburst occurred more than 2 weeks before the onset of cambial activity. Different relationships are found between bud development and cambial activity and these seem species dependent (Rossi et al., 2009). The independence of onset of cambial activity from leaf expansion is a phenomenon often reported in ring-porous species, in which cambium can reactivate up to 5–6 weeks before leaf expansion and, as discussed below, it is believed to be the main cause of the formation of very large vessels (up to 500 μm in diameter) (Aloni, 2004).
However, in broadleaves such as Fagus sylvatica, leaf unfolding and cambium reactivation at the stem base occurred almost at the same time (Čufar et al., 2008). At the top of hybrid poplars, the onset of cell differentiation occurred a week earlier than or at the same time as complete leaf opening, showing high variability depending on the position in the tree (Deslauriers et al., 2009).
Moreover, the behaviour of the sap flow density confirmed that cambial reactivation was not correlated with the beginning of photosynthetic activity after the winter season (Fig. 6). Indeed, in conifers, relevant photosynthetic rates are commonly observed in early spring, if soil is not frozen and the xylem water is above the freezing threshold during the thermally favourable periods (Wieser, 1997; Goodine et al., 2008).
Our data supported the hypothesis that wood formation is a process controlled by specific thermal thresholds (Rossi et al., 2007). Although daily temperatures below 4−5 °C are still favourable for photosynthesis, thermal conditions even above these values could inhibit the allocation of assimilated carbon to structural investment, i.e. xylem growth (Rossi et al., 2007). In high altitude Picea abies trees, the thermal threshold that induces a probability of 0.5 of cambial reactivation, considered as the daily mean of stem temperature, is between 7.2 °C and 9 °C (Rossi et al., 2007). This threshold also seems rather to be predictive for low-altitude spruce trees since the onset of xylogenesis occurred just after mean stem temperature reached 9.5 °C (Fig. 6).
The strict dependence of cambial activity on temperature would imply that the onset of wood formation is well synchronized in all sampling heights, given that no significant variations of daily mean stem temperature might be expected at different tree height in a closed forest. Indeed, no clear trend was found in the onset of cambial activity along the stem: cambium reactivated within a week at all sampling heights. These results for Picea abies are in contrast with those found in young hybrid poplars, where the onset of vessel enlargement proceeded down the stem at about 0.5 m d−1 (Deslauriers et al., 2009), which suggests the occurrence of species-specific mechanisms of cambial resumption or phenology.
Tapering and duration of expansion phase
Despite the synchronous starting of cambial activity, the duration of the expansion phase was highly variable and well correlated with the position in the stem: at the tree top, cell expansion was significantly shorter than at the bottom, where the first cell completed expansion more than 10 d later. This led to a tapered chain of conduit elements (along the direction of the sap flow into the xylem) that efficiently compensates for the possible increase in total hydraulic resistance within the transport system during the growth in height of trees (Petit et al., 2010). The so-called degree of tapering, represented by the exponent of the power function relating tracheid diameter with the distance from the tree top, was relatively large (0.23), as expected in young and fast height-growing individuals (Anfodillo et al., 2006). The different duration of cell expansion seems to be one key step in controlling the rate of progressive lumen widening from the stem apex to base, which is the typical axial conduit pattern commonly referred to as conduit tapering.
This pattern might be consistent with the simple mechanisms proposed by Aloni (1989, 2004) in which a key role is played by the longitudinal gradient of auxin in the cambial zone: the lower concentration of auxin in the bottom part of the plant (farther away from the most active production sites) would induce a longer expansion phase due to the fact that cell differentiation is slowed down. The linear relationship between cell lumen area and number of days in which the expansion phase occurs would suggest that the rate of cell expansion per se (i.e. variation per day) is not affected by the level of auxin. Moreover, the power relationship between number of days of the expansion phase at different distances from the tree top allows it to be supposed that the concentration of auxin down the stem should follow a similar power law. Thus, auxin would act as a morphogen-like substance with a dose-dependent effect only on the ‘duration’ of the cell expansion phase, but not on the expansion ‘rate’ which should be driven by the cell turgor and cell wall exstensibility.
Is this simple differentiation behaviour consistent with the available observations? Critical points are the effective longitudinal auxin gradient in trees and cell responsiveness to auxin signal. It is agreed that auxin is distributed differentially within plant tissues (Vanneste and Friml, 2009), but the pattern of longitudinal gradients of auxin in large trees has rarely been measured. Uggla et al. (1998) reported the auxin concentration in three points along the stem in Pinus sylvestris. They found a relevant gradient of auxin concentration in fast-growing trees but not in slow-growing individuals. However, looking at the reported data of total auxin, in spite of the fact that fitting only three points might be considered speculative, the behaviour of the gradient in relation to distance from the tree top seemed to follow a power function pattern in three out of six trees measured, including a slow-growing tree. When auxin gradients were generated by experimental manipulation, coherent results were found, showing that vessel lumen dimensions were smaller where auxin accumulation was higher (i.e. in downward oriented shoots) (Lovisolo et al., 2002).
A different pattern of duration of the differentiation phases along the stem was reported for hybrid poplars: the time of vessel formation was estimated at 10–14 d for both top and bottom and, as vessel formation started earlier at the top, mature vessels were observed earlier at the top of the stem (Deslauriers et al., 2009), suggesting a possible difference (or control) between broadleaves with indefinite growth (e.g. Populus spp.) and conifers, which are characterized by a pre-determinate growth.
Overall, it could be speculated that the role of other possibly involved processes, namely variations in turgor pressure within the cell and/or in metabolic rate of cell wall formation should not be highly relevant in determining the final cell size. This leads, in addition, to the duration of the expansion phase and distance from the tree top being related to a power function (Fig. 5).
Our knowledge is still fragmentary about how a tree can perceive and regulate the concentration along the stem given that the active polar movement of auxin is too slow (10 mm per hour) for suitably detecting the signal successfully, especially in large trees (Friml, 2003). Probably a multiple and redundant mechanism such as biosynthesis, conjugation, deconjugation, degradation, and intercellular transport act in regulating the gradients within the whole plant (Vanneste and Friml, 2009).
A simple passive response based on a specific physiological response (e.g. cell enlargement) related to a given concentration of auxin was recently questioned when important results on cell responsiveness to similar auxin concentration became available. Nilsson et al. (2008) clearly demonstrated, for example, that, in the older cells experiencing lower auxin concentrations than those found in the developing zone (Uggla et al., 1996), a large proportion of auxin-responsive genes were expressed at higher levels than in cambium, thus proving that all physiological processes promoted by auxin might not simply be related to auxin concentration but to the specific sensitivity of the cells.
Moreover, after having transgenically altered auxin metabolism in some hybrid aspen plants, Nilsson et al. (2008) reported that the less sensitive plants to auxin signals showed smaller xylem conduits compared to wild type, thus demonstrating that cell expansion appears to be a process that is sensitive to alteration in auxin responsiveness.
Conclusions
The dynamics of xylem formation assessed along the stem of P. abies at a very short time-scale have demonstrated that (i) the phase of the cell expansion differs downwards in the stem, thus it could be one key factor in controlling the rate of progressive lumen widening from the stem apex to the base, which is the typical axial conduit pattern commonly referred to as ‘conduit tapering’; (ii) the rate of cell expansion proceeds steadily, thus leading to a strict relationship between the final size of the first earlywood cells and the time spent in expansion.
The data presented here did not test the physiological role of auxin in regulating plant development and vascular differentiation. However, our findings revealed the existence of a pattern of cell expansion along the stem axis that supports the simple hypothesis of an auxin concentration gradient along the stem and could clarify the mechanisms used by trees to control their tapered vascular structure (Aloni and Zimmermann, 1983).
Acknowledgments
The authors wish to thank Benedetto Ruperti for the helpful comments on the manuscript and Benedetto Fausto Fontanella for the technical support in collecting microcores. Giai Petit received financial support by the University of Padova (CPDR081920).
References
- Abe H, Funada R, Ohtani J, Fukazawa K. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids. Trees. 1997;11:328–332. [Google Scholar]
- Aloni R. Control of xylogenesis within the whole tree. Annals of Forest Science. 1989;46s:267s–272s. [Google Scholar]
- Aloni R, Zimmermann MH. The control of vessel size and density along the plant axis—a new hypothesis. Differentiation. 1983;24:203–208. [Google Scholar]
- Aloni R. Foliar and axial aspects of vascular differentiation - hypotheses and evidence. Journal of Plant Growth Regulation. 2001;20:22–34. [Google Scholar]
- Aloni R. The induction of vascular tissues by auxin. In: Davies PJ, editor. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Kluwer Academic Publishers; 2004. pp. 471–492. [Google Scholar]
- Anfodillo T, Carraro V, Carrer M, Fior C, Rossi S. Convergent tapering of xylem conduits in different woody species. New Phytologist. 2006;169:279–290. doi: 10.1111/j.1469-8137.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Becker P, Gribben RJ, Lim CM. Tapered conduits can buffer hydraulic conductance from path-length effects. Tree Physiology. 2000;20:965–967. doi: 10.1093/treephys/20.14.965. [DOI] [PubMed] [Google Scholar]
- Berleth T, Scarpella E, Prusinkiewicz P. Towards the systems biology of auxin-transport-mediated patterning. Trends in Plant Science. 2007;12:151–159. doi: 10.1016/j.tplants.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Coomes DA, Jenkins KL, Cole LES. Scaling of tree vascular transport system along gradients of nutrient supply and altitude. Biology Letters. 2007;3:86–89. doi: 10.1098/rsbl.2006.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čufar K, Prislan P, de Luis M, Gričar J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees. 2008;22:749–758. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Canadian Journal of Forest Research. 2003;33:190–200. [Google Scholar]
- Deslauriers A, Giovannelli A, Rossi S, Castro G, Fragnelli G, Traversi L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiology. 2009;29:1223–1235. doi: 10.1093/treephys/tpp061. [DOI] [PubMed] [Google Scholar]
- Enquist BJ. Cope's Rule and the evolution of long-distance transport in vascular plants: allometric scaling, biomass partitioning and optimization. Plant, Cell and Environment. 2003;26:151–161. [Google Scholar]
- Friml J. Auxin transport: shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Goodine GK, Lavigne MB, Krasowski MJ. Springtime resumption of photosynthesis in balsam fir (Abies balsamea) Tree Physiology. 2008;28:1069–1076. doi: 10.1093/treephys/28.7.1069. [DOI] [PubMed] [Google Scholar]
- Granier A. Une nouvelle methode pour la mesure de flux de seve brute dans le tronc des arbres. Annals of Forest Science. 1985;42:193–200. [Google Scholar]
- Holbrook NM, Zwieniecki MA. Transporting water to the tops of trees. Physics Today. 2008;61:76–77. [Google Scholar]
- James SA, Meinzer FC, Goldstein G, Woodruff D, Jones T, Restom T, Mejia M, Clearwater M, Campanello P. Axial and radial water transport and internal water storage in tropical forest canopy trees. Oecologia. 2003;134:37–45. doi: 10.1007/s00442-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Leitch MA. Vessel-element dimensions and frequency within the most current growth increment along the lenght of Eucalyptus globulus stems. Trees. 2001;15:353–357. [Google Scholar]
- Lev-Yadun S. Cellular patterns in dicotyledonous woods: their regulation. In: Savidge RA, Barnett JR, Napir R, editors. Cell and molecular biology of wood formation. Oxford: BIOS Scientific Publishers Ltd.; 2000. pp. 315–324. [Google Scholar]
- Lovisolo C, Schubert A, Sorce C. Are xylem radial development and hydraulic conductivity in downwardly-growing grapevine shoots influenced by perturbed auxin metabolism? New Phytologist. 2002;156:65–74. doi: 10.1046/j.1469-8137.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Grace J, Fioravanti M. Biomechanical and hydraulic determinants of the tree structure in Scots pine: anatomical characteristics. Tree Physiology. 1997;17:105–113. doi: 10.1093/treephys/17.2.105. [DOI] [PubMed] [Google Scholar]
- Muday GK, DeLong A. Polar auxin transport: controlling where and how much. Trends in Plant Science. 2001;6:535–542. doi: 10.1016/s1360-1385(01)02101-x. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Karlberg A, Antti H, Lopez-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. The Plant Cell. 2008;20:843–855. doi: 10.1105/tpc.107.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G, Anfodillo T, De Zan C. Degree of tapering of xylem conduits in stems and roots of small Pinus cembra and Larix decidua trees. Canadian Journal of Botany. 2009;87:501–508. [Google Scholar]
- Petit G, Anfodillo T, Mencuccini M. Tapering of xylem conduits and hydraulic limitation in sycamore (Acer pseudoplatanus) trees. New Phytologist. 2008;177:653–664. doi: 10.1111/j.1469-8137.2007.02291.x. [DOI] [PubMed] [Google Scholar]
- Petit G, Anfodillo T. Plant physiology in theory and practice: an analysis of the WBE model for vascular plants. Journal of Theoretical Biology. 2009;259:1–4. doi: 10.1016/j.jtbi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Petit G, Pfautsch S, Anfodillo T, Adams M. The challenge of tree height in Eucalyptus regnans: when xylem tapering overcomes hydraulic resistance. New Phytologist. 2010;187:1146–1153. doi: 10.1111/j.1469-8137.2010.03304.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA Journal. 2006a;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA Journal. 2006b;27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152:1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Rathgeber CBK, Deslauriers A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Annals of Forest Science. 2009;66:206–214. [Google Scholar]
- Ryan MG, Yoder BJ. Hydraulic limits to tree height and tree growth. BioScience. 1997;47:235–242. [Google Scholar]
- Scarpella E, Meijer AH. Pattern formation in the vascular system of monocot and dicot plant species. New Phytologist. 2004;164:209–242. doi: 10.1111/j.1469-8137.2004.01191.x. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JE, Eastlack M. Xylem embolism in ring porous, diffuse porous and coniferous trees of northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Spicer R, Gartner BL. The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii ) sapwood. Trees. 2001;15:222–229. [Google Scholar]
- Sundberg B, Uggla C, Tuominen H. Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napir R, editors. Cell and molecular biology of wood formation. Oxford: BIOS Scientific Publishers Ltd.; 2000. pp. 169–188. [Google Scholar]
- Tyree MT, Ewers FW. The hydraulic architecture of trees and other woody plants. New Phytologist. 1991;119:345–360. [Google Scholar]
- Uggla C, Mellerowicz EJ, Sunderberg B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiology. 1998;117:133–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences, USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Weitz JS, Ogle K, Horn HS. Ontogenetically stable hydraulic design in woody plants. Functional Ecology. 2006;20:191–199. [Google Scholar]
- Wieser G. Carbon dioxide gas exchange of cembran pine (Pinus cembra) at the alpine timberline during winter. Tree Physiology. 1997;17:47–477. doi: 10.1093/treephys/17.7.473. [DOI] [PubMed] [Google Scholar]
- Yang S, Tyree MT. Hydraulic resistance in Acer saccharum shoots and its influence on leaf water potential and transpiration. Tree Physiology. 1993;12:231–242. doi: 10.1093/treephys/12.3.231. [DOI] [PubMed] [Google Scholar]