Abstract
Iron is a critical cofactor for a number of metalloenzymes involved in respiration and photosynthesis, but plants often suffer from iron deficiency due to limited supplies of soluble iron in the soil. Iron deficiency induces a series of adaptive responses in various plant species, but the mechanisms by which they are triggered remain largely unknown. Using pH imaging and hormone localization techniques, it has been demonstrated here that root Fe(III) reductase activity and proton extrusion upon iron deficiency are up-regulated by systemic auxin signalling in a Fe-efficient woody plant, Malus xiaojinensis. Split-root experiments demonstrated that Fe-deprivation in a portion of the root system induced a dramatic increase in Fe(III) reductase activity and proton extrusion in the Fe-supplied portion, suggesting that the iron deficiency responses were mediated by a systemic signalling. Reciprocal grafting experiments of M. xiaojinensis with Malus baccata, a plant with no capability to produce the corresponding responses, indicate that the initiation of the systemic signalling is likely to be determined by roots rather than shoots. Iron deficiency induced a substantial increase in the IAA content in the shoot apex and supplying exogenous IAA analogues (NAA) to the shoot apex could mimic the iron deficiency to trigger the corresponding responses. Conversely, preventing IAA transport from shoot to roots blocked the iron deficiency responses. These results strongly indicate that the iron deficiency-induced physiological responses are mediated by systemic auxin signalling.
Keywords: IAA signal, iron deficiency responses, root Fe(lll) reductase activity, proton extrusion, Malus xiaojinensis
Introduction
Iron is an essential element required for plant growth and development. Despite the usually high abundance of Fe in soils, plants often suffer from Fe-deficiency because Fe is generally present in a form of insoluble ferric hydroxide complexes in aerobic environments at neutral or basic pH (Guerinot, 1994; Connolly et al., 2003). Plants use two basic strategies to cope with iron limitation (Briat et al., 1995; Mori, 1999). In strategy I plants (dicots and non-grass monocot plants), Fe is acquired in a three-step process, comprising mobilization of the insoluble ferric hydroxide complexes by plasmalemma H+-ATPase-mediated proton extrusion, reduction of ferric chelates by a plasmalemma-localized reductase system, and uptake of the ferrous ion by metal transporters (Chaney et al., 1972; Buckhout et al., 1989; Grusak et al., 1989; Grusak and Pezeshgi, 1996; Brüggemann et al., 1990). Hence, the regulation of root Fe(III) reductase and proton extrusion activity are thought to be crucial events controlling iron acquisition.
In response to Fe-deficiency, plants have evolved mechanisms for mobilizing the poorly soluble iron compounds to facilitate iron uptake. It is known that Fe-deficiency induces a significant increase in root Fe(III) reductase activity (Kochian and Lucas, 1991). An early study with potato (Solanum tuberosum L.) suggested that iron-deficiency-induced up-regulation of Fe(III) reductase activity may be controlled by roots (Bienfait et al., 1987). By contrast, a study with pea suggests that the Fe(III) reductase activity is regulated through a long-distance signal that presumably conveys information relevant to the shoot's Fe status (Grusak and Pezeshgi, 1996). Other studies also suggest that the involvement of shoot-to-root communication may be critical in the regulation of root Fe(III) reductase activity (Landsberg, 1984; Maas et al., 1988; Bienfait, 1989). By using reciprocal grafting between a Fe-hyper-accumulating mutant (dgf) of pea (Pisum sativum) and its parenta1 genotype DGV, Grusak and Pezeshgi (1996) reported that shoots of dgf generated a transmissible signal that enhanced Fe(III) reductase activity in roots. Iron deficiency also induced the up-regulation of the expression of the ferric chelate reductase gene (NtFRO1), apparently via a long-distance signal (Enomoto et al., 2007). In addition to the up-regulation of Fe(III) reductase activity, Fe-deficiency induced an increase in root proton extrusion, consequently decreasing plant rhizoshere pH (Dell'Orto et al., 2000; Schmidt et al., 2003). Removal of the shoot apex prevented Fe deficiency-induced proton extrusion from the roots of bean and sunflower plants (Landsberg, 1981a). In split-root experiments with tomato plants, it was found that iron deficiency in one portion of the roots induced increase in proton extrusion in the iron-sufficient portion of the root system (Schmidt et al., 2003). These findings suggest that proton extrusion is also induced by iron deficiency through a systemic signal.
Plant hormones have been implicated in iron deficiency-induced adaptive responses (Landsberg, 1981a, b, c; Romheld and Marschner, 1986). ABA application inhibited Fe-deficient-induced rhizosphere acidification (Landsberg, 1981a, b, c, 1986). Fe deficiency increased ethylene production in roots (Romera and Alcantara, 1994; Romera et al., 1999). Exogenous application of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) mimicked iron deficiency to induce morphological responses in pea and tomato plants and therefore it was proposed that ethylene may be essential for transducing the iron deficiency signal to induce adaptive changes in root morphology (Schmidt et al., 2000; Schmidt and Schikora, 2001). Römheld and Marschner (1986) reported that Fe-deficient sunflower roots produced higher levels of auxin than the Fe-sufficient ones. Furthermore, exogenous application of the auxin analogue 2,4-dichlorophenoxyacetic acid (2,4-D) to the shoot apex mimicked iron-deficiency-induced root morphological changes(Römheld and Marschner, 1986). By contrast, Schmidt (1994) found that exogenous application of 2,4-D did not significantly induce root ferric reductase activity in Plantago plants. Similarly, other studies also indicated that the exogenous application of 2,4-D had no effect on the regulation of root ferric reductase activity (Schmidt et al., 2000, 2003; Schikora and Schmidt, 2001). Thus the role of auxin in the regulation of iron deficiency responses needs to be investigated further.
Long-distance signalling, also known as ‘systemic signalling’, enables an organism to make adaptive responses to a local stimulus quickly. In plants, systemic signalling may have embodied a more advanced behaviour to cope with environmental stresses. As mentioned above, many lines of evidence suggest systemic signalling in iron-deficient responses, and hormones have been implicated in the regulation of these responses. However, there is no conclusive evidence that plant hormones act as systemic signals in the regulation of iron deficiency responses. In addition, all of the previous studies were performed with herbaceous plants. The mechanism underlying iron deficiency responses in woody plants is unknown. In this study, iron deficiency-triggered systemic signalling was investigated in Malus xiaojinensis, an iron- efficient woody plant. Our findings have provided strong evidence that the primary perception of the iron-deficiency signal is determined by roots themselves. Importantly auxin acts as a systemic signal for long-distance transmission of the primary signal to mediate iron deficiency-induced up-regulations of root Fe(III) reductase activity and proton extrusion.
Materials and methods
Plant materials and growth conditions
Seeds of M. xiaojinensis and M. baccata were germinated at 4 °C in the dark and then were sown in wet sterile roseite after germination. At the stage of two true leaves, the seedlings were transferred to an aerated, half-strength, standard nutrition solution, for 1 week. The solution was then switched to a standard nutrition solution (Han et al., 1994), with the pH was adjusted to 6.0 using 1 M NaOH. The nutrition solution was replaced every week. Plants were grown for 180 d in a growth room at 25±2 °C day/17±2 °C night with a 16 h photoperiod at a light intensity of 250 μmol·m−2·s−1.
The split-root experiments were performed as described by Gansel et al. (2001). (i) Both portions were supplied with normal Fe (40 μM Fe); (ii) one portion of the root system was transferred to the Fe-deprivation (0 μM Fe) solution, with the other portion remaining supplied with normal Fe as before; (iii) one portion was supplied with normal Fe, roots of the Fe-deficient portion were cut off at different times; (iv) one portion was supplied with normal Fe, roots of the Fe-deficient portion had the normal Fe supplement resumed at different times. At this stage, special care was taken to avoid cross-contamination between compartments.
Reciprocal grafting was performed between M. xiaojinensis and M. baccata. The resulting plants were randomly divided into two groups in every vessel. The first group was grown in a solution containing a normal iron concentration as before and the second group was grown in the absence of iron.
The shoot-tip removal experiment had three treatments: (i) supplying with normal Fe; (ii) supplying with 0 μM Fe; (iii) applying NAA with Fe deficiency.
Plants in the girdling experiment received one of the following two Fe treatments: (i) normal Fe (40 μM) or (ii) 0 μM Fe.
Measurement of Fe3+ reductase activity and rhizosphere pH
Solution pH was always adjusted to 6.3 initially. Thereafter, the pH of solutions was measured daily between 10.30–11.30 h.
Root Fe3+ reductase activity was determined as described by Li et al. (2000). Briefly, the plants were transferred to the nutrient solution described above, with added Fe-EDTA (100 μM) and 2,2-bipyridyl (400 μM), pH 5.0, for 2 h. The environmental conditions during the measurement were the same as for growth. The reducing capacity was determined by measuring the concentration of the Fe2+-dipyidyl complex formed at A520 in a spectrophotometer (Unico UV-2012). Each experiment was repeated at least three times.
Measurement of IAA in roots
Approximately 0.3 g new (white) root was taken and frozen in liquid nitrogen, then stored at –80 °C. The roots were then ground into a powder, extracted using cold 80% methanol, centrifuged, filtered, and then vacuum concentrated at 40 °C. The pH of the concentrate fluid was then adjusted to 2.8 and stored at 4 °C overnight. The fluid was then extracted three times using 1:1 petroleum ether and 1:1 ethyl acetate, respectively. The organic phase was evaporated to dryness in a rotary evaporator at 40 °C and the residue was dissolved in 2 ml ethanol. Finally, the solution was purified using a C-18 column and filtered using 0.45 μm membrane filter and then 20 μl was injected for HLPC analysis. The HPLC system was equipped as follows: Waters 600 pump, Waters 2487 ultraviolet detector, Spherisorb C-18 column. The mobile phase consisted of methanol and water (6:4 v/v, pH adjusted to 3.5 with acetic acid). The wavelength was set to 254 nm and the flow rate was 0.8 ml·min−1.
Measurement of apoplastic pH using SNARF
After plants were grown in standard nutrient solution for 2 weeks, they were then transferred into normal Fe or Fe-deficient nutrient solution. The lateral roots were cut and equilibrated for 1 h, then placed in distilled water under the following conditions: temperature 22 °C, humidity 80%, PPFD of 300 μmol ·m– 2 s−1. After equilibration, the roots were transferred into vials containing 2 ml pH indicator at a concentration of 150 μg·ml−1. For SNARF, the excitation wavelength was 514 nm, and the fluorescence emission window was, respectively, set to 580 nm and 640 nm at power intensities of 5%. The sample pH was determined using a confocal microscope system (Nikon A1, Japan). Fluorescence images were acquired using a ×10 objective. Spectral resolution checking proved SNARF feature spectrum.
IAA immunocytochemical localization
Excised tissue samples were immediately prefixed in 3% (w/v) aqueous solution of EDAC (Sigma) and post-fixed in FAA (50% ethyl alcohol, 10% glacial acetic acid, 10% formaldehyde; 8:1:1, by vol.) at 4 °C for 16 h, dehydrated with a graded ethanol series, embedded in paraffin, and sectioned to 10 μm slides. Sections were affixed onto slides (Probe-On Plus, Thermo Scientific, USA). After overnight drying at 60 °C, sections were deparaffinized with xylene and hydrated in an ethanol–water series. Slides were incubated in a blocking solution containing 10 mm phosphate-buffered saline (PBS; 2.68 mM KCl, 0.15 M Na2HPO4, and 0.086 M KH2PO4), 0.1% (v/v) Tween 20, 1.5% (v/v) Gly, and 5% (w/v) BSA, for 45 min at 22 °C, then rinsed in a regular salt rinse solution [10 mm PBS, 0.88% (w/v) NaCl, 0.1% (v/v) Tween 20, and 0.8% (w/v) BSA], and washed briefly with 10 mm PBS and 0.8% (w/v) BSA solution to remove the Tween 20. One hundred microlitres of 1:80 (w/v) anti-IAA antibody (1 mg·ml−1) (Agdia, USA) were placed on each slide, covered with a cover slip, and incubated overnight in a humidity chamber at 4 °C. Two 10 min vigorous washes with high-salt rinse solution, 10 mm PBS, 2.9% (w/v) NaCl, 0.1% (v/v) Tween 20, and 0.1% (v/v) BSA were followed by a 10 min wash with a regular salt rinse and a brief rinse with 10 mm PBS and 0.8% (v/v) BSA. One hundred microlitres of anti-mouse IgG-HRP conjugate working solution (DAKO, Denmark) were added to each slide, which was covered with a cover slip and incubated for 30 min in a humidity chamber at 22 °C. Two 15 min washes with a regular salt rinse were followed by a 15 min wash with water. Two hundred microlitres of ready-to-use AEC (Zymed, USA) were added to each slide; each slide was then covered with a coverslip and incubated in the dark for 10 min. When a red colour was observed, slides were rinsed with water and then dehydrated in a graded water–ethanol series, ethanol–xylene, xylene. Slides were mounted with buffered glycerol, and observed under an inverted microscope (Nikon-TI200, Japan). Photomicrographs were taken by CCD (Nikon, Japan) attached to the microscope and processed with the NIS software. In the negative controls primary antibodies was omitted from the procedure.
Gene-expression analysis by RT-PCR
Root samples were frozen in liquid nitrogen immediately after collection and stored at –80 °C. About 100 mg of tissue was ground in liquid nitrogen and total RNA was extracted by CTAB, and then the first-strand cDNA was synthesized with the total RNA by PrimeScript RT reagent kit (Takara, Japan). All RNA samples were checked for DNA contamination before cDNA synthesis.
Real-time RT-PCR was performed using the power SYBR Master Mix (Applied Biosystems, Foster City, CA) in a 20 μl total volume. The primers used to amplify MxFRO2-Like were F: 5′-TTTGTTACATCATCTACTGGGCTGC-3′, R: 5′-ATGCGAGGGATGGTAGTTGCC-3′ to amplify 139 bp from the MxFRO2-Like transcript. The RT-PCR analysis was performed with ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) with the following reaction conditions: 3 min at 94 °C, 30 s at 95 °C, 30 s at 60 °C, 30 s at 72 °C for 28 cycles which was the optimized linear range for MxFRO2-Like amplification. Each cDNA sample was run at least twice. Amplification of PCR products were monitored by agarose gel electrophoresis via intercalation of EB.
Results
Root Fe(III) reductase activity and proton extrusion in relation to iron high efficiency in M. xiaojinensis
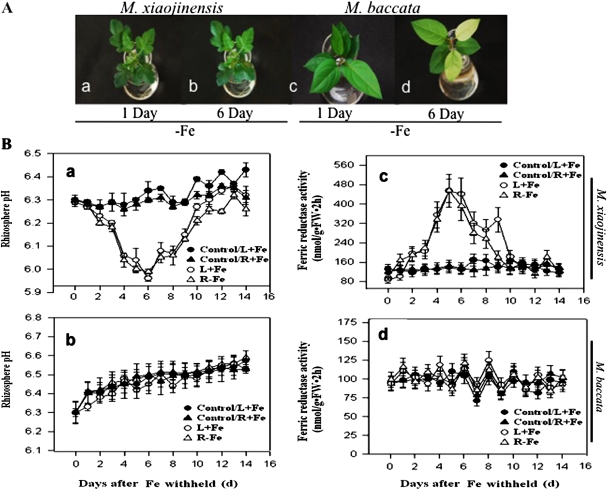
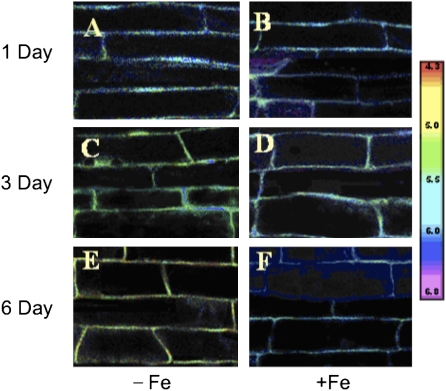
Malus xiaojinensis is an important woody plant used as a stock for apple trees. M. xiaojinensis has been characterized by its iron uptake with high efficiency (Han et al., 1994, 1998, 2005). However, compared with that in M. xiaojinensis, the iron uptake efficiency in M. baccata is much lower. As shown in Fig. 1A, 6 d after low iron treatment, chlorosis, a typical iron-deficiency symptom, was quite obvious in M. baccata but not in M. xiaojinensis, indicating a major difference in the ability to cope with iron limitation between the two plant species. As a first step in understanding the mechanism behind the distinct iron deficiency responses, two crucial events closely associated with the iron uptake were determined, i.e. the regulation of the root proton extrusion and Fe(III) reductase activity in response to iron deficiency. Iron deficiency induced a substantial increase in the proton extrusion activity in M. xiaojinensis, suggested by a decrease in the nutrient solution pH (Fig. 1B, a), but not in M. baccata (Fig. 1B, b). Similarly, iron deficiency induced a significant increase in the root Fe(III) reductase activity in M. xiaojinensis (Fig. 1B, c), but not in M. baccata (Fig. 1B, d). To assess whether pH changes in the nutrient solution were due to alterations of the proton extrusion activity, a pH imaging technique was developed involving loading a pH indicator into the root apoplast, by which the proton extrusion activity can be directly detected in the roots at the subcellular level. Consistent with the observations in the medium solution pH in response to iron deficiency, a significant decrease in the apoplastic pH occurred in the root tips of M. xiaojinensis (Fig. 2A, C, E) whereas no alterations in the apoplastic pH could be observed in the iron sufficient plants (Fig. 2B, D, F). Therefore, the different capabilities to regulate root proton extrusion and Fe(III) reductase activity can explain the difference in coping with iron limitation between M. xiaojinensis and M. baccata.
Fig. 1.
Comparison of iron-deficiency responses between M. xiaojinensis and M. baccata. (A) Observed iron-deficiency symptoms in M. xiaojinensis and M. baccata. (a) M. xiaojinensis supplied with 4 μM Fe after 1 d; (b) M. xiaojinensis supplied with 4 μM Fe after 6 d; (c) M. baccata supplied with 4 μM Fe after 1 d; (d) M. baccata supplied with 4 μM Fe after 6 d. (B) Rhizosphere pH and root Fe (III) reductase activity measured in split-root systems of M. xiaojinensis and M. baccata. One portion of the root system were transferred to the Fe-deprivation (0 μM Fe) solution, with the other portion remaining supplied with normal Fe. (a) Rhizosphere pH measured in split-root systems of M. xiaojinensis. (b) Rhizosphere pH measured in split-root systems of M. baccata. (c) Root Fe (III) reductase activity measured in split-root systems of M. xiaojinensis. (d) Root Fe (III) reductase activity measured in split-root systems of M. baccata. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
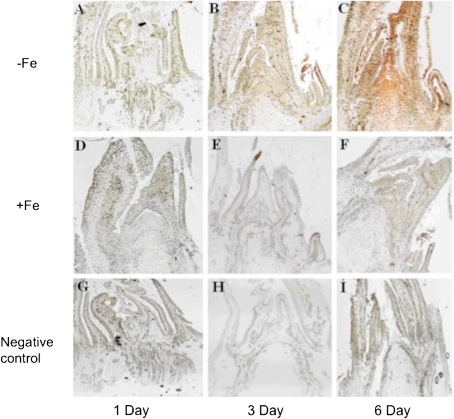
Fig. 6.
Endogenous IAA content of shoot apex response-marked iron deficiency by using an IAA localization technique. (A–C) Shoot apex of plants which were treated with 0 μM Fe for 1, 3, or 6 d, respectively. (D–F) Shoot apex of plants which were treated with normal Fe for 1, 3, or 6 d, respectively. (G–I) Negative control labelled with mouse IgG instead of the IAA mono-antibody.
Fig. 2.
Calibration of the pH indicator SNARF in the root cap apoplast. Ratio image of SNARF fluorescence in the root tips M. xiaojinensis. The image was pseudocolour-coded according to the scale (pH 4.3–6.8) on the right. (A, C, E) Plants were treated with normal Fe for 1, 3, or 6 d, respectively. (B, D, F) Plants were treated with 0 μM Fe for 1, 3, or 6 d, respectively.
Up-regulation of root Fe(III) reductase activity and proton extrusion is associated with systemic signalling
An attempt was made to assess whether the iron deficiency-induced alterations in root Fe(III) reductase activity and proton extrusion are mediated by systemic signalling. Split-root experiments were conducted, in which the root system was divided into two portions: one transferred to the Fe-deprived (0 μM Fe) solution and the other supplied with normal Fe. The results showed that Fe deprivation in one portion of the root system caused a significant increase in both root Fe(III) reductase activity (Fig. 1B, c) and proton extrusion (Fig. 1B, a) in the Fe-sufficient portion, indicting that the iron deficiency-induced up-regulation of both Fe(III) reductase activity and proton extrusion was mediated by systemic signalling.
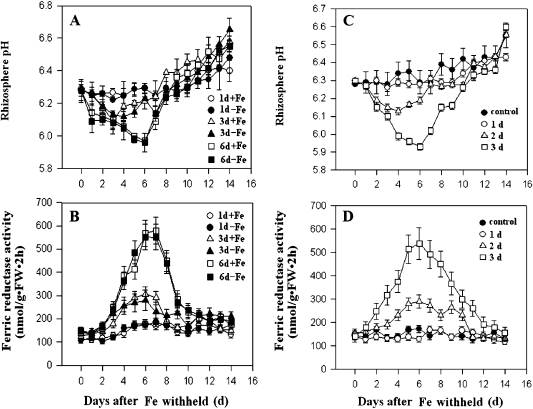
Two possible mechanisms for the adaptive responses are considered. The adaptive responses may require a sustained emission of signals from the Fe-deficient portion of the root system. Alternatively, the Fe-deficient roots only function to trigger a primary response, which could subsequently induce a sustained signalling that activates the iron-deficiency responses. In this scenario, the initial iron-deficiency trigger will not be required once the sustained signalling is activated. To distinguish between these two possible mechanisms, the Fe-deficient treatment was removed at different times after the treatment was started by adding Fe to the Fe-deficient media. Root Fe(III) reductase activity and proton extrusion were followed. As shown in Fig. 3, the intensity of two adaptive responses was dependent on the duration of the iron deficiency applied, i.e. the increase in the Fe(III) reductase and proton extrusion activities was correlated with the duration of the iron deficiency stress (Fig. 3A, B). The minimum length of time for the iron deficiency treatment to cause significant responses was about 3 d, and the responses continued for several days after Fe was supplemented on day 3. Six-day treatments produced an identical change in Fe(III) reductase and proton extrusion activities as continuous treatments (compare Fig. 1B with Fig. 3A, B). Furthermore, in split-root experiments, the removal of the iron deficiency-treated portion of the root system did not reduce the iron-deficient responses in the untreated portion if the treatment lasted 3 d or longer (Fig. 3C, D). Taken together, these results suggest that the iron-deficient responses do not require a sustained presence of the primary iron-deficiency trigger, but involves the production of a systemic signal that is sufficient to activate the iron-deficiency responses in untreated roots.
Fig. 3.
Duration of Fe-deficiency treatments required for the activation of Fe-deficiency responses in the M. xiaojinensis split-root system. (A, B) One portion of the root system was supplied with normal Fe (+Fe), and the other with the medium lacking Fe (–Fe). The Fe-deficient portion was then supplemented with Fe on the first, third, and sixth days. Rhizosphere pH (A) and root Fe (III) reductase activity (B) were measured in both Fe- sufficient (+Fe) and Fe-deficient (–Fe) portions from day 1 to day 14. (C, D) Rhizosphere pH and root Fe (III) reductase activity were measured in Fe-sufficiency-treated roots on various days after Fe-deficient-treated roots were removed. In the controls, one portion was supplied with normal Fe, roots of the Fe-deficient portion were removed at 0 d. (C) Rhizosphere pH. (D). Root Fe (III) reductase activity. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
Integration of roots and shoots in relation to the triggering of iron-deficient responses
As described above, M. xiaojinensis and M. baccata are related plants but with a rather different capabilities to cope with iron deficiency. Given their distinct characteristics in iron-deficiency responses, reciprocal grafting experiments were performed in order to reveal the roles of roots and shoots in triggering the iron-deficient responses. As shown in Fig. 4, iron deficiency induced up-regulation of root proton extrusion (Fig. 4A) and Fe(III) reductase activity (Fig. 4B) only when M. xiaojinensis was used as the stock. In other words, with M. xiaojinensis as the stock, iron deficiency was capable of inducing up-regulation of both proton or Fe(III) reductase activity in the roots. This result demonstrates that it is roots not shoots that determine the initial trigger of the iron-deficiency responses.
Fig. 4.
Rhizosphere pH and root Fe (III) reductase activity in various grafting combinations of M. xiaojinensis with M. baccata. Various grafting combinations from M. xiaojinensis and M. baccata, respectively, are grafted as shown, and grafted plants are treated with Fe-deficient and Fe-sufficient media. (A) Rhizosphere pH. (B) Root Fe (III) reductase activity. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
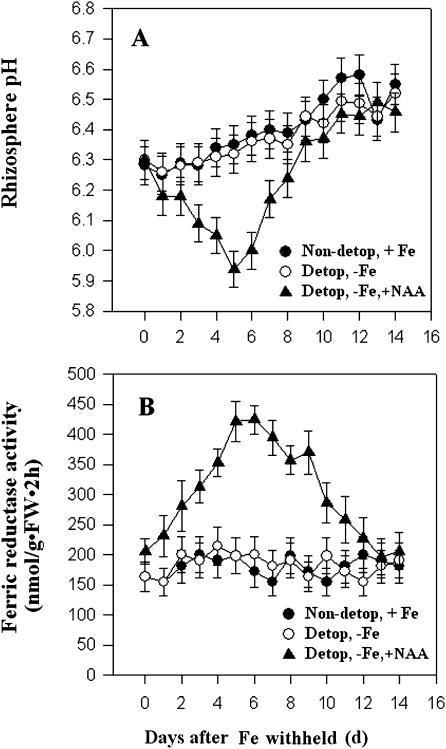
It was next investigated whether shoots are involved in the transmission of the initial root-origin signal that triggers the iron-deficient responses by removing the shoot apex of M. xiaojinensis just prior to iron deprivation. As shown in Fig. 5, removing the shoot apex completely blocked up-regulation of both proton extrusion (Fig. 5A) and Fe(III) reductase activity (Fig. 5B) in roots induced by iron deficiency, suggesting a critical role for the shoot apex in the iron deficiency responses. Interestingly, applying NAA to the de-topped shoot could recover the iron-deficiency-induced up-regulation of proton extrusion and Fe(III) reductase activity (Fig. 5), indicating that IAA is most likely to be involved in the transmission of the initial signal that triggers the iron-deficiency responses.
Fig. 5.
Removing shoot apex and applying NAA effect on Fe-deficient responses. Rhizosphere pH (A) and root Fe (III) reductase activity (B) of M. xiaojinensis in response to shoot tip removal and NAA application under normal Fe or 0 mM Fe supply. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
IAA functions to mediate the iron deficiency triggered-systemic signalling
Given the finding that exogenous application of NAA to the shoot apex induced iron-deficient responses in de-topped plants, the spatio-temporal alteration of endogenous IAA content upon iron deficiency was investigated using an IAA localization technique. IAA localization in the shoot apex was monitored by immunochemical staining with a mono-antibody against IAA (Fig. 6). On the first day after iron deprivation began, no immuno-staining signal was detected in either the iron-deficient (Fig. 6A) or the iron-sufficient plants (Fig. 6D). On the third day, a clear immuno-staining signal was observed in the iron-deficient plants, particularly in the apical meristematic tissue but not in iron-sufficient plants (Fig. 6B). On the sixth day, while the whole apex was strongly stained in the iron-deficient plants (Fig. 6C), no immuno-staining signal was detected in the iron-sufficient plants (Fig. 6F). As a control of the immuno-staining, the apex tissues were labelled with mouse IgG instead of the IAA mono-antibody and, as shown in Fig. 6G–I, in all cases no immuno-staining signal was detected, supporting the reliability of the IAA localization. These results demonstrate that iron deficiency is indeed capable of causing a significant accumulation of endogenous IAA in the shoot apex.
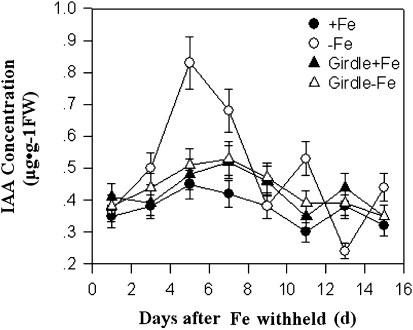
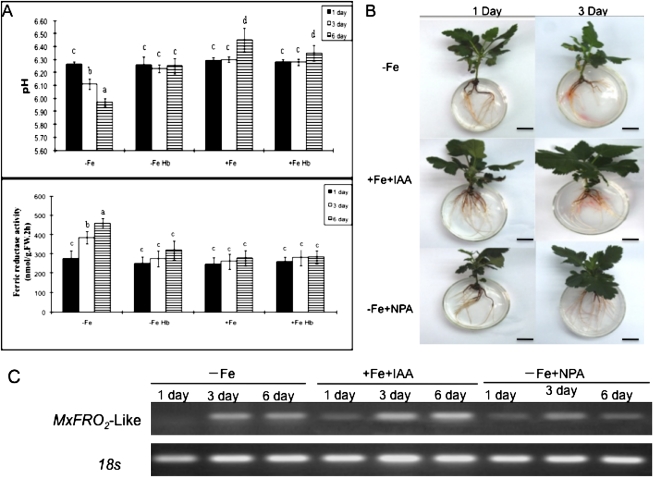
The hypothesis that the iron- deficiency-triggered systemic signalling is mediated by IAA transport from shoot to roots was then tested. The effect of stem girdling on the alterations in root IAA content was analysed by using an HLPC analysis method. Stem girdling is expected to prevent the shoot to root transport. As shown in Fig. 7, iron-deficiency induced a significant increase in root IAA contents. As expected, stem girdling arrested the increase in the iron deficiency-induced increase in root IAA levels, suggesting that the increase in root IAA upon iron deficiency may be a result of IAA transport from the shoot to the roots. Next, it was determined whether auxin transport inhibitors and stem girdling blocked the iron-deficiency responses (Fig. 8). As shown in Fig. 8, both stem girdling and auxin transport inhibitors compromised the iron-deficiency responses. Stem girdling blocked the up-regulation of both proton extrusion and Fe(III) reductase activity in roots induced by iron deficiency (Fig. 8A). The addition of IAA to the medium induced an increase in reductase activity. By contrast, NPA application strongly inhibited the iron-deficiency-induced increase in reductase activity (Fig. 8B). Furthermore, it was found that the Fe-deficiency-induced increase in root IAA levels was correlated with the increased expression of MxFRO2-Like. Exogenous application of auxin to plants significantly enhanced MxFRO2 –Like expression, but these responses were markedly repressed by exposure to the auxin transport inhibitor, NPA (Fig. 8C), implying that a certain endogenous auxin level is necessary for the Fe deficiency-based induction of root MxFRO2-Like expression. Taken together, these results demonstrate that IAA plays a critical role in the iron-deficiency-induced systemic signalling.
Fig. 7.
Stem girdling blocked endogenous IAA accumulation in roots induced by iron deficiency. In the controls IAA concentration in roots of normal plant without girdling was measured. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
Fig. 8.
Both stem girdling and the application of auxin transport inhibitors blocked the Fe-deficiency responses. (A) Rhizosphere pH and root Fe (III) reductase activity of M. xiaojinensis in response to stem girdling. (B) Root Fe (III) reductase activity of M. xiaojinensis in response to treatment with –Fe, exogenous IAA +Fe, and NPA –Fe. (C) Expression of MxFRO2 genes in roots of M. xiaojinensis treated with –Fe, exogenous IAA +Fe, and NPA –Fe. Values are means based on at least ten plants; bars represent SD. FW, fresh weight. Means ±SD followed by different letters indicate a statistical difference at P <0.05. Bar=2 cm.
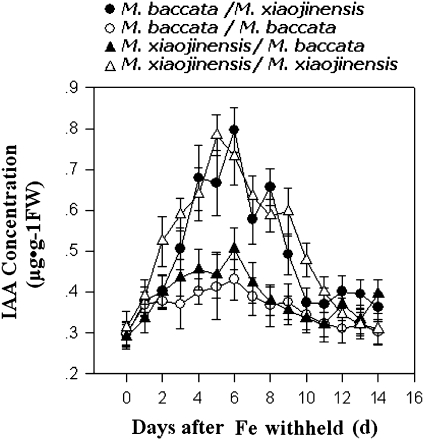
Given the findings that the iron-deficiency response is determined by a trigger from roots rather than shoots, and that IAA production in the shoot apex is crucial for the iron-deficiency-triggered systemic signalling, the mechanisms for the triggering of IAA production upon iron deficiency were investigated further with the reciprocal grafting technique. As shown in Fig. 9, regardless of the species of scions, iron deficiency was capable of triggering IAA production in shoots only if M. xiaojinensis was used as the stock in the grafting combination and, conversely, iron deficiency was not capable of triggering IAA production if M. baccata was used as the stock. This result strongly suggests that IAA production in the shoot upon iron deficiency is most likely triggered by a signal coming from the roots.
Fig. 9.
Iron deficiency was capable of triggering IAA production in the shoots only if M. xiaojinensis was used as the stock in the grafting combination. Changes of endogenous IAA concentrations in the roots of the same species and combinations of species seedlings with Fe deficiency. Values are means based on at least ten plants; bars represent SD. FW, fresh weight.
Discussion
Iron deficiency has long been known to induce a wide range of adaptive responses, but how iron deficiency induces these responses remained unclear, particularly in woody plants. By taking advantage of the distinct capabilities of two related woody species (M. xiaojinensis and M. baccata) to cope with iron deficiency, compelling evidence that the sensing of the initial iron-deficiency signal occurs in the root and that this sensing leads to the production of a systemic IAA signal that activates iron-deficiency responses, including increases in Fe(III) reductase activity and proton extrusion, has been provided. Our data suggest that the systemic auxin signal accumulates in the shoot apex and is transported to the root to co-ordinate the iron-deficiency responses. Our studies provide the first mechanistic insights into iron-deficiency responses in woody species as well as the most comprehensive picture of the signalling mechanism underlying adaptive iron deficiency responses in a single plant system to date.
Iron deficiency induces both morphological responses, such as the development of subapical root hairs and transfer cells, and the physiological responses, such as enhancement of root proton extrusion and ferric reductase activity. A fundamental issue regarding the mechanisms underlying these diverse responses has focused on the roles of both roots and shoots in the sensing and transmission of the iron deficiency signal. Some early studies support an absolute requirement for roots in the regulation of iron-deficient responses (Bienfait et al., 1987), but how roots participate in that regulation remained unknown until our current work. Our findings clearly show that roots are involved in the initial sensing of iron deficiency. With reciprocal grafting experiments, the present study has provided direct evidence that iron deficiency is able to trigger physiological responses that are determined by roots rather than by shoots because root replacement has totally changed the characteristics of M. xiaojinensis and M. baccata plants in their responses to iron deficiency (Fig. 4). Our study showed that the replacement of M. xiaojinensis roots by M. baccata roots completely prevented the occurrence of the corresponding responses, suggesting that leaf iron status should not be a major player in the iron-deficiency responses in M. xiaojinensis. However, this did not exclude the possibility that leaves could play a role in iron sensing in other plant species. The addition of Fe-EDTA to leaves of Phaseolus vulgaris L. could decrease proton extrusion and ferric chelate reduction (Maas et al., 1988). Similarly, a recent study showed that the addition of Fe(II)-EDTA to the iron-deficient leaves of tobacco plants significantly reduced the expressions of NtIRT1 and NtFRO1, two genes encoding an iron-regulated transporter and Fe(III) reductase, respectively (Enomoto et al., 2007). Vert et al. (2003) have presented two models, in which the shoot iron status has been proposed either positively or negatively to control the expression of FOR2 and IRT1 in Arabidopsis. Nonetheless, further studies are needed to determine whether iron sensing indeed takes place in the leaves.
Previous studies have also suggested an important role for the shoot in the regulation of the iron-deficiency responses (Romera et al., 1992; Landsberg, 1981a, b, c, 1984; Schmidt and Schikora, 2001; Schmidt et al., 2003). Two types of experiments, i.e. the shoot decapitation (Landsberg, 1981a, b, c, 1984) and split-root experiment (Romera et al., 1992; Schikora and Schmidt, 2001; Schmidt et al., 2003), have been used to assess the role of the shoot. These split-root experiments demonstrate that iron-deficiency responses can occur in the iron-sufficient half portion of the root system only if the other portion is stimulated by iron deprivation, implying that a signal must travel through the shoot to activate the Fe-deficiency responses in the Fe-sufficient part of the roots. Our split root experiments also support the existence of systemic signalling in woody plants, Importantly, our shoot apex decapitation experiments (Fig. 5) strongly suggest an absolute requirement of the shoot apex in the induction of the iron-deficiency responses. Together these results demonstrate that an integrated function of the roots and shoot is necessary for iron deficiency to induce the physiological responses. Based on these findings, it is proposed that the iron-deficiency signal is initially sensed in the root, which leads to the production of a systemic signal that is dependent on the shoot (Fig. 10).
Fig. 10.
Schematic model of IAA-mediated systemic signalling under iron deficiency. The roots should function to perceive iron deficiency and trigger primary reactions leading to the production of the initial signals; the root-borne signals move to the shoot to enhance the biosynthesis of IAA; the long-distance transport of IAA from shoot to roots functions to mediate the corresponding responses in the roots. Dashed arrows denote transduction pathways.
Plant hormones have been implicated in modulating a wide range of adaptive responses to iron deficiency and have been speculated to mediate the systemic iron- deficiency responses. A role for ethylene and auxin in the regulation of iron-deficiency responses has been suggested from the pharmacological experiments as well as the use of some plant mutants. Application of ethylene precursor or auxin mimicked iron deficiency in inducing the morphological responses (Romera and Alcantara, 1994; Romera et al., 1999; Schmidt et al., 2000; Schikora and Schmidt, 2002; Schmidt et al., 2003). Furthermore, some ethylene and IAA mutants are impaired in the iron-deficiency-induced morphological responses (Romera and Alcantara, 1994; Schmidt, 1994). It was proposed that both auxin and ethylene play crucial roles in iron deficiency induced morphological responses (Romera et al., 2006). Application of auxin to the shoot apex induced the morphological changes in the root that are reminiscent of those induced by iron deficiency, which implies that auxin may mediate systemic signalling to the morphological responses. It was shown that the iron-deficiency-induced increase in ferric reductase activity was impaired in the mutant aux1-7, implicating a potential role for IAA in the regulation of iron-deficiency-induced physiological responses in Arabidopsis (Chen et al., 2010). Until our current work, however, no reports had conclusively demonstrated a definite role for auxin action in triggering the physiological responses (Schikora and Schmidt, 2002; Schmidt et al., 2003). The present study, for the first time, has provided a series of evidence demonstrating the involvement of auxin in the stimulation of root proton and Fe(III) reductase activity in response to iron deficiency. First, the IAA localization technique, in combination with the IAA quantitative analysis, showed that iron deficiency markedly stimulated an IAA accumulation in the shoot apex of M. xiaojinensis plants (Figs 6, 7). Second, stem girdling totally prevented the iron deficiency-induced IAA accumulation in roots and concomitantly arrested the up-regulations of the root proton and Fe(III) reductase activity (Figs 7, 8). Third, decapitation of the shoot apex prevented the up-regulations of the root proton and Fe(III) reductase activity, but addition of IAA to the decapitated shoot recovered the two physiological responses. Last, but not the least, treatments with auxin transport inhibitor NPA compromised iron-deficiency-induced responses (Fig. 8). These results strongly suggest that IAA functions to mediate the iron deficiency-induced physiological responses in M. xiaojinensis plants. Importantly, they provide the first comprehensive set of evidence indicating a role for auxin as a systemic signal in regulating Fe-deficiency responses.
Based on the data presented in this study, a model is proposed for the systemic signalling of the iron-deficiency responses in M. xiaojinensis (Fig. 10). Our results suggest that the roots first function to perceive the iron deficiency, which triggers a primary reaction leading to the production of an initial long-distance signal(s). This unknown root-borne signal(s) moves to the shoot to enhance the accumulation of IAA in the shoot apex. It is well known that auxin is synthesized in the shoot apex and then redistributed to other parts of plants including roots via auxin polar transport. Our data suggest that the long-distance transport of IAA from the shoot to roots functions to mediate the iron-deficiency responses in the roots (Fig. 10). To our knowledge, this is the first comprehensive model for systemic signalling in the regulation of iron-deficiency responses that is strongly supported by experimental data. Our model also provides an important framework for the future investigation of the detailed mechanisms underpinning the control of iron-deficiency responses. Key future questions that need to be addressed include but not limited to (i) what the nature of the iron deficiency sensor in the root is, (ii) how the sensor triggers the production of the primary long distance signal, (iii) what this initial signal is, (iv) how this signal regulates the accumulation of auxin in the shoot apex, (v) how plants distinguish the iron deficiency-triggered auxin signal from other auxin signals involved in the normal growth and development of plants, and, finally, whether plant responses to the deficiency of other important metal ions follow a similar model to that described here for iron deficiency. Undoubtedly the work described in this report will trigger a series of exciting future research projects to elucidate the mechanisms governing plant adaptation to the deficiency in important metal ions.
Acknowledgments
We are grateful to the Functional Genomics Platform of CAU for providing the Microscopic observation System. We are indebted to Technical Engineers (Nikon Company, Beijing) for their help with our calibration of the pH indicator experiments. This work was supported by the 973 project (2011CB100600), the National genetically modified major projects (2009ZX08009-122B), and the National Natural Science Foundation of China (No. 30971982).
References
- Bienfait B. Prevention of stress in iron metabolism of plants. Acta Botanica Neerlandica. 1989;38:105–129. [Google Scholar]
- Bienfait HF, de Weger LA, Kramer D. Control of the development of iron-efficiency reactions in potato as a response to iron deficiency is located in the roots. Plant Physiology. 1987;83:244–247. doi: 10.1104/pp.83.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J, Fobis-Loisy I, Grignon N, Lobraux S, Pascal N, Savino G, Thoiron S, von Wirn N, Van Wuytswinkel O. Cellular and molecular aspects of iron metabolism in plants. Biology of the Cell. 1995;84:69–81. [Google Scholar]
- Brüggemann W, Moog PR, Nakagawa H, Janiesch P, Kuiper PJC. Plasma membrane-bound NADH: Fe3+-EDTA reductase and iron deficiency in tomato (Lycopersicon esculentum). Is there a Turbo reductase? Physiologia Plantarum. 1990;79:339–346. [Google Scholar]
- Buckhout TJ, Bell PF, Luster DG, Chaney RL. Iron-stress induced redox activity in tomato (Lycopersicum esculentum Mill.) is localized on the plasma membrane. Plant Physiology. 1989;90:151–156. doi: 10.1104/pp.90.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiology. 2010;154:810–819. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney RL, Brown JC, Tiffin LO. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiology. 1972;50:208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiology. 2003;133:1102–1110. doi: 10.1104/pp.103.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orto M, Santi S, De Nisi P, Cesco S, Varanini Z, Zocchi G, Pinton R. Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: involvement of plasma membrane H+-ATPase activity. Journal of Experimental Botany. 2000;51:695–701. [PubMed] [Google Scholar]
- Enomoto Y, Hodoshima H, Shimada H, Shoji K, Yoshihara T, Goto F. Long-distance signals positively regulate the expression of iron uptake genes in tobacco roots. Planta. 2007;227:81–89. doi: 10.1007/s00425-007-0596-x. [DOI] [PubMed] [Google Scholar]
- Gansel X, Munos S, Tillard P, Gojon A. Differential regulation of the NO3− and NH4+ transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. The Plant Journal. 2001;26:143–155. doi: 10.1046/j.1365-313x.2001.01016.x. [DOI] [PubMed] [Google Scholar]
- Grusak MA, Pezeshgi S. Shoot-to-root signal transmission regulates root Fe(III) reductase activity in the dgl mutant of pea. Plant Physiology. 1996;110:329–334. doi: 10.1104/pp.110.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak MA, Welch RM, Kochian LV. A transport mutant for the study of plant root iron absorption. In: Dainty J, De Michelis MI, Marre E, Rasi-Caldogno F, editors. Plant membrane transport: the current position. Amsterdam, New York, Oxford: Elsevier Science Publishers; 1989. pp. 61–66. [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiology. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZH, Han C, Xu X, Wang Q. Relationship between iron deficiency stress and endogenous hormones in iron-efficient versus inefficient apple genotypes. Journal of Plant Nutrition. 2005;28:1887–1895. [Google Scholar]
- Han ZH, Shen T, Korcak RF, Baligar VC. Screening for iron-efficient species in the genus Malus. Journal of Plant Nutrition. 1994;17:579–592. [Google Scholar]
- Han ZH, Shen T, Korcak RF, Baligar VC. Iron absorption by iron-efficient and -inefficient species of apples. Journal of Plant Nutrition. 1998;21:181–190. [Google Scholar]
- Kochian U, Lucas WJ. Do plasmalemma oxidoreductases play a role in plant mineral ion transport? In: Crane FU, Morre DJ, Low HE, editors. Oxidoreduction at the plasma membrane: relation to growth and transport. Madison, Wiconsin, USA: Soil Science Society of America; 1991. pp. 189–205. [Google Scholar]
- Landsberg EC. Energy driven H+ efflux pump in sunflower roots: activated by Fe-deficiency stress. Plant Physiology. 1981a;67(Supplement):702. [Google Scholar]
- Landsberg EC. Fe stress induced transfer cell formation: regulated by auxin? Plant Physiology. 1981b;67(Supplement):563. [Google Scholar]
- Landsberg EC. Organic acid synthesis and release of hydrogen ions in response to Fe deficiency stress of mono- and dicotyledonous plant species. Journal of Plant Nutrition. 1981c;3:579–591. [Google Scholar]
- Landsberg EC. Regulation of iron-stress response by whole-plant activity. Journal of Plant Nutrition. 1984;7:609–621. [Google Scholar]
- Landsberg EC. Function of rhizodermal transfer cells in the Fe stress response mechanism of Capsicum annuum L. Plant Physiology. 1986;82:511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu X, Zhang F. Role of shoot in regulation of iron deficiency responses in cucumber and bean plants. Journal of Plant Nutrition. 2000;23:1809–1818. [Google Scholar]
- Maas FM, van de Wetering DA, van Beusichem ML, Bienfait HF. Characterization of phloem iron and its possible role in the regulation of Fe-efficiency reactions. Plant Physiology. 1988;87:167–171. doi: 10.1104/pp.87.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Iron acquisition by plants. Current Opinion in Plant Biology. 1999;2:250–253. doi: 10.1016/S1369-5266(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Römheld H, Marschner V. Mobilization of iron in the rhizosphere of different plant species. Advances in Plant Nutrition. 1986;2:155–204. [Google Scholar]
- Romera FJ, Alcantara E. Iron-deficiency stress responses in cucumber (Cucumis sativus L.) roots (a possible role for ethylene?) Plant Physiology. 1994;105:1133–1138. doi: 10.1104/pp.105.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E, De La Guardia MD. Role of roots and shoots in the regulation of the Fe efficiency responses in sunflower and cucumber. Physiologia Plantarum. 1992;85:141–146. [Google Scholar]
- Romera FJ, Alcantara E, De La Guardia MD. Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by Strategy 1 plants. Annals of Botany. 1999;83:51–55. [Google Scholar]
- Romera FJ, Lucena C, Alcàntara E. Plant hormones influencing iron uptake in plants. In: Barton LL, Abadia J, editors. Iron nutrition in plants and rhizospheric microorganisms. Dordrecht, The Netherlands: Springer; 2006. pp. 251–278. [Google Scholar]
- Schikora A, Schmidt W. Acclimative changes in root epidermal cell fate in response to Fe and P deficiency: a specific role for auxin? Protoplasma. 2001;218:67–75. doi: 10.1007/BF01288362. [DOI] [PubMed] [Google Scholar]
- Schikora A, Schmidt W. Formation of transfer cells and H+-ATPase expression in tomato roots under P and Fe deficiency. Planta. 2002;215:304–311. doi: 10.1007/s00425-002-0738-0. [DOI] [PubMed] [Google Scholar]
- Schmidt W. Root-mediated ferric reduction-responses to iron deficiency, exogenously induced changes in hormonal balance and inhibition of protein synthesis. Journal of Experimental Botany. 1994;45:725–731. [Google Scholar]
- Schmidt W, Michalke W, Schikora A. Proton pumping by tomato roots. Effect of Fe deficiency and hormones on the activity and distribution of plasma membrane H+-ATPase in rhizodermal cells. Plant, Cell and Environment. 2003;26:361–370. [Google Scholar]
- Schmidt W, Schikora A. Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiology. 2001;125:2078–2084. doi: 10.1104/pp.125.4.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiology. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert GA, Briat JF, Curie C. Dual regulation of the Arabidopsis high-affinity root iron uptake system by local and long-distance signals. Plant Physiology. 2003;132:796–804. doi: 10.1104/pp.102.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]