Abstract
In the present study, to investigate the mechanisms regulating carotenoid accumulation in citrus, a culture system was set up in vitro with juice sacs of three citrus varieties, Satsuma mandarin (Citrus unshiu Marc.), Valencia orange (Citrus sinensis Osbeck), and Lisbon lemon (Citrus limon Burm.f.). The juice sacs of all the three varieties enlarged gradually with carotenoid accumulation. The changing patterns of carotenoid content and the expression of carotenoid metabolic genes in juice sacs in vitro were similar to those ripening on trees in the three varieties. Using this system, the changes in the carotenoid content and the expression of carotenoid metabolic genes in response to environmental stimuli were investigated. The results showed that carotenoid accumulation was induced by blue light treatment, but was not affected by red light treatment in the three varieties. Different regulation of CitPSY expression, which was up-regulated by blue light while unaffected by red light, led to different changes in carotenoid content in response to these two treatments in Satsuma mandarin and Valencia orange. In all three varieties, increases in carotenoid content were observed with sucrose and mannitol treatments. However, the accumulation of carotenoid in the two treatments was regulated by distinct mechanisms at the transcriptional level. With abscisic acid (ABA) treatment, the expression of the genes investigated in this study was up-regulated in Satsuma mandarin and Lisbon lemon, indicating that ABA induced its own biosynthesis at the transcriptional level. This feedback regulation of ABA led to decreases in carotenoid content. With gibberellin (GA) treatment, carotenoid content was significantly decreased in the three varieties. Changes in the expression of genes related to carotenoid metabolism varied among the three varieties in response to GA treatment. These results provided insights into improving carotenoid content and composition in citrus during fruit maturation.
Keywords: Carotenoid, citrus, in vitro, juice sacs, regulatory mechanism
Introduction
Carotenoids, which are important natural isoprenoid pigments, fulfil a variety of critical functions in plants, such as the stabilization of lipid membranes, light harvesting for photosynthesis, as well as protecting the photosystem from photo-oxidation (Havaux, 1998; Ledford and Niyogi, 2005). In addition, carotenoids are also precursors of the plant hormone abscisic acid (ABA), and are exploited as colouring agents in flowers and fruits to attract pollinators (Schwartz et al., 1997; Cunningham and Gantt, 1998). Carotenoids are important not only to the plants that produce them, but also to animals and humans. Some carotenoids containing β-ring moieties are the precursors of vitamin A, and have been proved to prevent the onset of certain chronic diseases and cancers (Giovannucci, 1999; Krinsky et al., 2003). In citrus, carotenoids are the pigments responsible for the external and internal coloration of the fruits, and their contents and compositions are important indexes for the commercial and nutritional quality of the fruits. Carotenoid content and composition are influenced by growing conditions, geographical origins, and fruit maturity; therefore, they vary greatly among citrus varieties. In Satsuma mandarin (Citrus unshiu Marc.), β-cryptoxanthin (β-cry) is accumulated predominantly in juice sacs (Goodner et al., 2001; Kato et al., 2004). In contrast, Valencia orange (Citrus sinensis Osbeck) mainly accumulates violaxanthin (vio) isomers, with 9-cis-violaxanthin (c-vio) as the principal carotenoid (Molnár and Szabolcs, 1980; Lee and Castle, 2001). Lisbon lemon (Citrus limon Burm.f.) also accumulates β-cry as the principal carotenoid, but it accumulates a much lower level of carotenoid than Satsuma mandarin and Valencia orange. These citrus varieties, which exhibit different carotenoid profiles, are useful for investigating the mechanism of carotenoid accumulation. In previous studies, the relationships between carotenoid accumulation and expression of genes related to carotenoid metabolism in different citrus varieties were investigated during natural ripening (Kato et al., 2004, 2006; Alquézar et al., 2009).
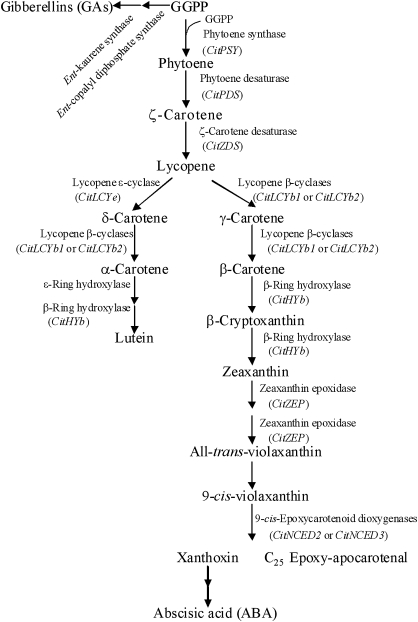
Carotenoid metabolism has been well documented in various plant species, including Arabidopsis (Park et al., 2002), tomato (Isaacson et al., 2002), pepper (Bouvier et al., 1998), tobacco (Busch et al., 2002), alga (Steinbrenner and Linden, 2001), citrus (Kato et al., 2004; Rodrigo et al., 2004; Rodrigo and Zacarías, 2007), and apricot (Marty et al., 2005; Kita et al., 2007). As shown in Fig. 1, the pathway of carotenoid metabolism in plants is a series of desaturation, cyclization, hydroxylation, and epoxidation steps (Cunningham and Gantt, 1998; Kato et al., 2004). The conversion of geranylgeranyl diphosphate (GGPP) to phytoene by phytoene synthase (PSY) is the first and rate-limiting step in the pathway. Two functionally similar enzymes, phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS), convert phytoene to lycopene via phytofluene, ζ-carotene, and neuroporene. The cyclization of lycopene catalysed by lycopene cyclase (LCY) is a key branch point in the pathway in citrus fruits, yielding α-carotene (α-car) and β-carotene (β-car). The genes of lycopene β-cyclase (LCYb) and lycopene ϵ-cyclase (LCYe) have been identified in citrus (Kato et al., 2004). α-Car is converted to lutein (lut), a major xanthophyll, by ϵ-ring hydroxylase (HYe) and β-ring hydroxylase (HYb). β-Car is converted to zeaxanthin (zea) via β-cry by a two-step hydroxylation, which is catalysed by HYb, then zea is converted to vio by zea expoxidase (ZEP). In addition, carotenoid metabolism is closely related to the biosynthesis of the plant hormones ABA and gibberellin (GA). In higher plants, ABA is biosynthesized by the oxidative cleavage of certain xanthophylls. 9-Cis-epoxycarotenoid dioxygenases (NCEDs) catalyse the cleavage of c-vio or 9'-cis-neoxanthin (c-neo) to form C25 epoxy-apocarotenal and xanthoxin (C15), from which the latter is the direct precursor of ABA. Similar to ABA, GA is also closely associated with the biosynthesis of carotenoids. As in the initial reaction of carotenoid biosynthesis, GGPP is also the substrate for ent-copalyl diphosphate synthase, which, together with ent-kaurene synthase, leads to the metabolic flux into the biosynthesis of GA.
Fig. 1.
Carotenoid metabolic pathway in citrus. GGPP, geranylgeranyl diphosphate. The expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, CitZEP, CitNCED2, and CitNCED3 was analysed by real-time PCR.
Recently, genes encoding enzymes for the main steps of carotenoid metabolism have been isolated and their expression has been characterized in plants (Kato et al., 2004, 2006; Kita et al., 2007; Alquézar et al., 2009). During fruit ripening, transcriptional regulation of carotenoid genes appears to be a major mechanism by which biosynthesis and accumulation of specific carotenoids are regulated. In tomato, increases in the expression of PSY and PDS, and decreases in the expression of LCYb and LCYe led to the accumulation of lycopene during fruit ripening (Pecker et al., 1996; Ronen et al., 1999). In previous studies, it was found that as fruit maturation progressed, a simultaneous increase in the expression of genes (CitPSY, CitPDS, CitZDS, CitLCYb, CitHYb, and CitZEP) led to massive β,β-xanthophll (β-cry, zea, and vio) accumulation in the flavedo and juice sacs of Satsuma mandarin and Valencia orange (Kato et al., 2004). Meanwhile, the expression of CitNCED2 and CitNCED3 in Satsuma mandarin and the expression of CitNCED2 in Lisbon lemon were primarily responsible for the accumulation of ABA in juice sacs, while in Valencia orange the extremely low level of CitNCED2 was primarily responsible for the low level of ABA (Kato et al., 2006).
Carotenoid metabolism is a complicated process, which is regulated throughout the life cycle of a plant with dynamic changes in content and composition in response to environmental stimuli (Cazzonelli and Pogson, 2010). Light and sugar have been reported to be important environmental factors regulating carotenoid metabolism in plants (Huff, 1983, 1984; Alba et al., 2000; Domingo et al., 2001; Schofield and Paliyath, 2005; Wu et al., 2007; Liu et al., 2009). Additionally, the plant hormones ABA and GA, which are closely involved in carotenoid metabolism, also play a crucial role in adjusting carotenoid content and composition in plants (Wan and Li, 2006; Rodrigo and Zacarías, 2007). To date, however, although significant advances have been made in understanding the accumulation of carotenoids and the expression of carotenoid metabolic genes during the maturation of citrus fruits, information about the changes in carotenoid metabolism in response to various environmental stimuli in citrus fruits is still limited (Rodrigo and Zacarías, 2007; Matsumoto et al., 2009). The tissue culture technique is one of the key tools to study plant growth and development, by which undefined variables were minimized and medium compositions and environmental factors were carefully controlled. So far, several attempts have been made to culture citrus in vitro using different plant tissues (Mukai et al., 2000; Harada et al., 2001; Khan et al., 2009). In the present study, to investigate further how carotenoid accumulation is regulated in response to different environmental factors in citrus, a culture system was set up with juice sacs of three different citrus varieties, Satsuma mandarin, Valencia orange, and Lisbon lemon. The juice sacs of the three varieties grew with carotenoid accumulation, and no callus formed throughout the experimental period. Using this system, the effects of environmental conditions [blue and red LED (light-emitting diode) lights, sucrose, and mannitol] and plant hormones (ABA and GA) on carotenoid content and composition, and the expression of genes related to carotenoid biosynthesis and catabolism were investigated in vitro in the three varieties, Satsuma mandarin, Valencia orange, and Lisbon lemon. This study provided more information on how carotenoid accumulation is regulated, which might produce new strategies to enhance carotenoid production in citrus.
Materials and methods
Plant materials
Satsuma mandarin (C. unshiu Marc.), Valencia orange (C. sinensis Osbeck), and Lisbon lemon (C. limon Burm.f.) cultivated at the National Institute of Fruit Tree Science, Department of Citrus Research, Okitsu (Shizuoka, Japan) were used as materials.
In vitro culture system
The fruits were surface-sterilized by a 10 min soak in 70% ethanol and a 30 min soak in 1% (w/v) NaOCl, and were then rinsed in sterile water. Juice sacs were excised from the equatorial region of the fruit, placed on 10 ml of agar medium in culture tubes (22×120 mm), and incubated in the dark at 25 °C. The explants were placed with the endocarp side up, so that the juice sacs were not in contact with the Murashige and Skoog (MS) medium supplemented with 10% (w/v) sucrose and 1% (w/v) agar. The pH of the MS medium was adjusted to 5.7 and autoclaved. The explants were taken out of their tubes and the carotenoid content was determined every 2 weeks. The juice sacs were immediately frozen in liquid nitrogen, and kept at –80 °C until use.
Extraction and determination of ascorbic acid
The ascorbic acid content was assayed by high-performance liquid chromatography (HPLC). Each frozen sample was homogenized using a mortar and pestle in 10 vols of extractant solution (3% metaphosphoric acid and 8% acetic acid). The homogenate was centrifuged at 14 000 g for 20 min, and then the supernatant was filtered through Miracloth (Calbiochem). The pH of the filtrate was adjusted by adding an equal volume of 0.2 M potassium phosphate buffer (pH 7.5). The total ascorbic acid was assayed by adding 0.5 ml of 6 mM dithiothreitol (DTT) to 0.1 ml of aliquot of filtrate and incubated in the dark at 30 °C for 15 min. After the sample was filtered through a 0.22 μm cellulose acetate filter (Advantec), a 20 μl aliquot was injected onto a J’sphere ODS-M80 column (YMC) attached to an LC-10AD pump (Shimadzu). The column kept at 20 °C was eluted with 1.5% ammonium dihydrogen phosphate (pH 3.8) at a flow rate of 1.0 ml min−1. The ascorbic acid content was monitored at 245 nm (retention time 2.6 min) using an SPD-10A spectrophotometric detector (Shimadzu) attached to a chart recorder (C-R6A, Shimadzu). Peaks were converted to concentrations by using the dilution of stock ascorbic acid to construct a standard curve.
Treatments
The juice sacs were cultured for 2 weeks under the same conditions as described above, and then irradiated with blue (peak wavelength, 470 nm) and red (peak wavelength, 660 nm) LED lights at an intensity of 50 μmol m−2 s−1 for 2 weeks. For the sucrose and mannitol treatments, the explants with the endocarp side up were placed on MS medium supplemented with 15% (w/v) sucrose or 6% (w/v) mannitol for 4 weeks. For the ABA and GA treatments, the explants with the endocarp side up were placed on MS medium supplemented with ABA (10 μM) and GA3 (10 μM) for 4 weeks. Juice sacs cultured in the dark for 4 weeks were used as the control. After each treatment, the juice sacs were immediately frozen in liquid nitrogen, and kept at –80 °C until use.
Extraction and determination of carotenoids
The identification, extraction, and quantification of carotenoid in citrus have been described previously (Kato et al., 2004). β-Car, β-cry, all-trans-violaxanthin (t-vio), c-vio, and lut were quantified in the juice sacs of Satsuma mandarin, Valencia orange, and Lisbon lemon during the experimental period. The contents of carotenoids were expressed as μg g−1 fresh weight. Carotenoid quantification was performed in three replicates.
Total RNA extraction and real-time quantitative reverse transcription-PCR (RT-PCR)
Total RNA was extracted from the juice sacs of Satsuma mandarin, Valencia orange, and Lisbon lemon fruits at different stages according to the method described by Ikoma et al. (1996). The total RNA was cleaned up with the RNeasy Mini Kit (Qiagen, Hilden, Germany) with on-column DNase digestion. The reverse transcription reactions were performed with 2 μg of purified RNA and a random hexamer at 37 °C for 60 min using TaqMan Reverse Transcription Reagents (Applied Biosystems).
TaqMan MGB probes and sets of primers for CitPSY, CitPDS, CitZDS, CitLCYb, CitHYb, CitZEP, CitNCED2, and CitNCED3 were designed on the basis of the common sequences among the three varieties for each gene with the Primer Express software (Applied Biosystems; Kato et al., 2007; Alquézar et al., 2009). For the endogenous control, the TaqMan Ribosomal RNA Control Reagents VIC Probe (Applied Biosystems) was used. TaqMan real-time PCR was carried out with the TaqMan Universal PCR Master Mix (Applied Biosystems) using ABI PRISM 7300 (Applied Biosystems) according to the manufacturer’s instructions. Each reaction contained 900 nM primers, a 250 nM TaqMan MGB Probe, and template cDNA. The thermal cycling conditions were 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. The levels of gene expression were analysed with ABI PRISM 7300 Sequence Detection System Software (Applied Biosystems) and normalized with the results of 18S rRNA. Real-time quantitative RT-PCR was performed in three replicates for each sample.
Statistical analysis
All values are shown as the mean±SE for three replicates. The data were analysed, and Tukey’s honestly significant difference (HSD) test was used to compare the means at P <0.05.
Results
Tissue culture of citrus juice sacs in vitro
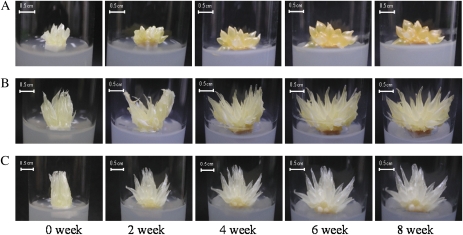
The juice sacs of Satsuma mandarin, Valencia orange, and Lisbon lemon were cultured in vitro for 8 weeks. As shown in Fig. 2, the juice sacs of all three varieties enlarged rapidly without the formation of callus throughout the experimental period. In Satsuma mandarin and Valencia orange the juice sacs turned yellow gradually, while in Lisbon lemon the changes in the colour of juice sacs were less obvious during the experimental period (Fig. 2).
Fig. 2.
Changes in the appearance of juice sacs in the three citrus varieties during culture in vitro. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon.
Changes in the carotenoid content and the expression of genes related to carotenoid metabolism
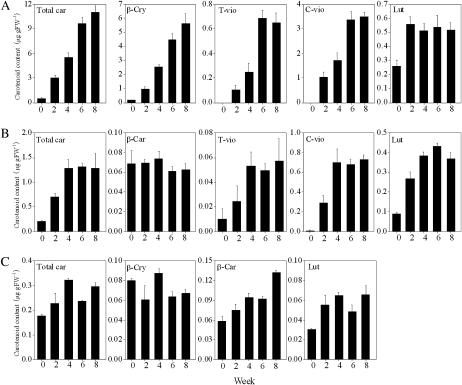
Changes in the content and composition of carotenoids were examined every 2 weeks. Massive accumulation of carotenoids, especially β,β-xanthophylls, occurred in Satsuma mandarin and Valencia orange during the experimental period (Fig. 3A, B). In Satsuma mandarin, the contents of β-cry, t-vio, and c-vio increased rapidly along with total carotenoid accumulation throughout the experimental period. In Valencia orange, the contents of t-vio and c-vio increased significantly in the first 4 weeks. In Lisbon lemon, the total carotenoid content remained extremely low, although β-cry accumulated gradually throughout the experimental period (Fig. 3C). The content of lut, a major β,ϵ-carotenoid, increased clearly in the first 2 weeks and then remained constant in the three varieties.
Fig. 3.
Changes in the carotenoid content in juice sacs of the three citrus varieties during culture in vitro. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. Total car, total carotenoids. β-Car, β-carotene; β-cry, β-cryptoxanthin; T-vio, all-trans-violaxanthin; C-vio, 9-cis-violaxanthin; Lut, lutein. The value for total carotenoids was the sum of identified carotenoids. Columns and bars represent the means and SE (n=3), respectively.
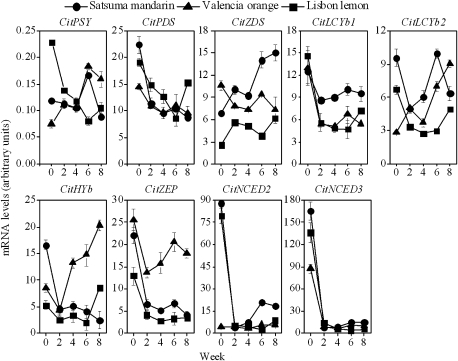
The expression of a set of genes involved in the production of β,β-xanthophylls (CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, and CitZEP) increased from the second week after being cultured in vitro in Satsuma mandarin and Valencia orange. Moreover, the expression levels of CitZDS, CitLCYb1, and CitLCYb2 were higher in Satsuma mandarin than in Valencia orange. In contrast, the expression levels of CitHYb and CitZEP were much higher in Valencia orange than in Satsuma mandarin. In Lisbon lemon, the expression of the set of genes that produce β,β-xanthophylls increased slightly from the sixth week after being cultured in vitro. However, the expression levels of CitPSY, CitZDS, CitLCYb2, CitHYb, and CitZEP were much lower than those in Satsuma mandarin and Valencia orange (Fig. 4).
Fig. 4.
Changes in the expression of carotenoid metabolism-related genes in juice sacs of the three citrus varieties. The results shown are the mean±SE for triplicate samples.
Effects of blue and red LED lights on carotenoid content and the expression of genes related to carotenoid metabolism
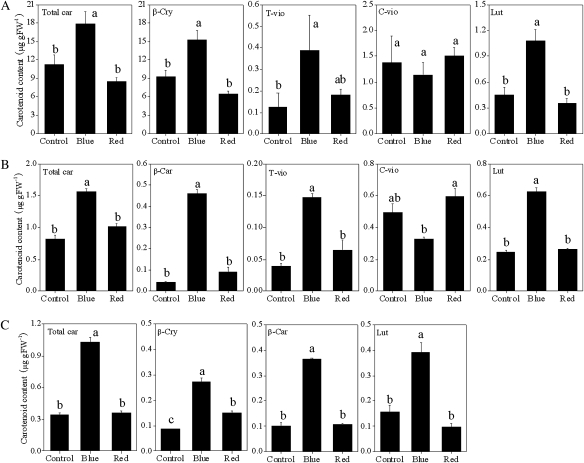
With the blue light treatment, the accumulation of β-cry, t-vio, and lut was induced significantly along with an increase in the total carotenoid content in Satsuma mandarin (Fig. 5A). In Valencia orange, the contents of β-car, t-vio, and lut were increased by treatment with blue light, and as a result the total carotenoid content was much higher than in the control (Fig. 5B). In Lisbon lemon, the three carotenoids detected in this study (β-car, β-cry, and lut) were clearly increased by the blue light treatment (Fig. 5C). In contrast to blue light treatment, red light treatment had no obvious effects on the contents of the carotenoids investigated in the present study in Satsuma mandarin and Valencia orange. In Lisbon lemon, the red light treatment induced a slight increase in β-cry, while it did not affect the contents of other carotenoids.
Fig. 5.
Effect of blue and red LED lights on the carotenoid content in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. β-Car, β-carotene; β-cry, β-cryptoxanthin; T-vio, all-trans-violaxanthin; C-vio, 9-cis-violaxanthin; Lut, lutein. The value for total carotenoids was the sum of identified carotenoids. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, blue light, and red light treatments at the 5% level by Tukey’s HSD test.
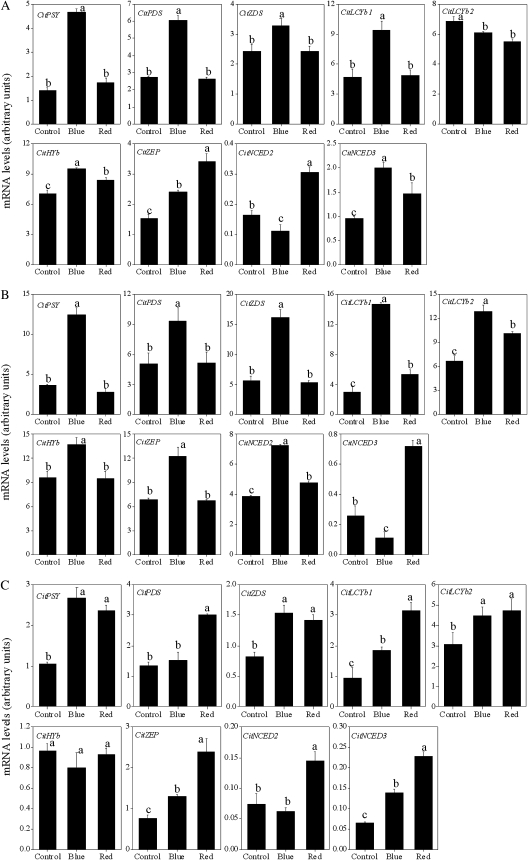
As shown in Fig. 6, in Satsuma mandarin, the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitHYb, CitZEP, and CitNCED3 was up-regulated simultaneously by blue light (Fig. 6A). In Valencia orange, the expression of the genes investigated in the present study was up-regulated by blue light, except for CitNCED3 (Fig. 6B). In Lisbon lemon, the induction of CitPSY, CitZDS, CitLCYb1, CitLCYb2, CitZEP, and CitNCED3 was observed in the blue light treatment (Fig. 6C).
Fig. 6.
Effect of blue and red LED lights on the expression of carotenoid metabolism-related genes in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, blue light, and red light treatments at the 5% level by Tukey’s HSD test.
Red light treatment did not affect the expression of CitPSY, CitPDS, CitZDS, or CitLCYb1, while it slightly increased the expression of CitHYb, CitZEP, CitNCED2, and CitNCED3 in Satsuma mandarin (Fig. 6A). Similar to Satsuma mandarin, in Valencia orange, no noticeable increase in the expression of CitPSY, CitPDS, and CitZDS was observed in the red light treatment. The expression of CitLCYb1, CitLCYb2, CitNCED2, and CitNCED3 was up-regulated in red light-treated Valencia orange (Fig. 6B). In Lisbon lemon, the expression of the genes investigated in the present study was up-regulated by the red light treatment, except for that of CitHYb (Fig. 6C).
Effects of sucrose and mannitol on carotenoid content and expression of genes related to carotenoid metabolism
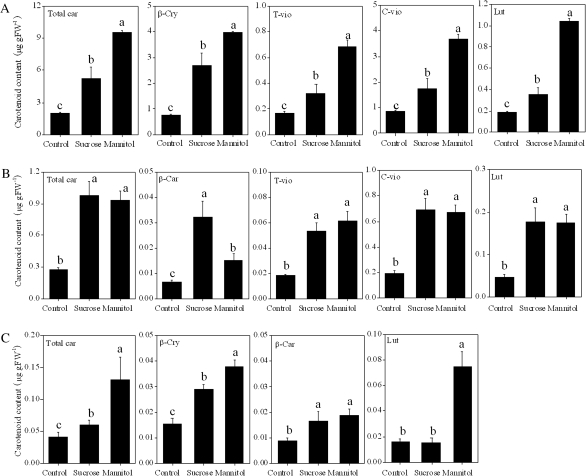
The treatments with sucrose and mannitol induced the accumulation of carotenoids in the juice sacs of Satsuma mandarin, Valencia orange, and Lisbon lemon (Fig. 7). In Satsuma mandarin and Valencia orange, the contents of carotenoids investigated in the present study were simultaneously increased by the treatments with sucrose and mannitol. In Lisbon lemon, β-car and β-cry contents were increased by the treatments with sucrose and mannitol. The content of lut was increased by mannitol treatment, while it was not significantly affected by sucrose treatment in Lisbon lemon.
Fig. 7.
Effect of sucrose and mannitol on the carotenoid content in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. β-Car, β-carotene; β-cry, β-cryptoxanthin; T-vio, all-trans-violaxanthin; C-vio, 9-cis-violaxanthin; Lut, lutein. The value for total carotenoids was the sum of identified carotenoids. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, sucrose, and mannitol treatments at the 5% level by Tukey’s HSD test.
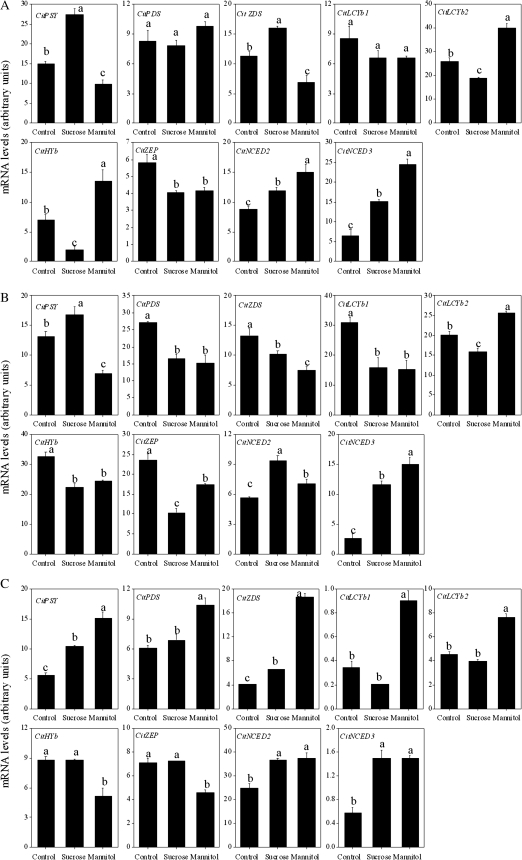
In Satsuma mandarin, the expression of CitPSY, CitZDS, CitNCED2, and CitNCED3 was up-regulated, while the expression of CitLCYb2, CitHYb, and CitZEP was down-regulated by treatment with sucrose. In Valencia orange, the expression of CitPSY, CitNCED2, and CitNCED3 was up-regulated, while the expression of CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, and CitZEP was down-regulated by treatment with sucrose. In Lisbon lemon, the expression of CitPSY, CitZDS, CitNCED2, and CitNCED3 was up-regulated by sucrose treatment.
With mannitol treatment, the expression of CitPSY, CitZDS, and CitZEP was slightly down-regulated, while the expression of CitLCYb2, CitHYb, CitNCED2, and CitNCED3 was up-regulated in Satsuma mandarin (Fig. 8A). In Valencia orange, the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitHYb, and CitZEP was down-regulated, while the expression of CitLCYb2, CitNCED2, and CitNCED3 was up-regulated by mannitol treatment (Fig. 8B). In Lisbon lemon, the up-regulation of expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitNCED2, and CitNCED3, and the down-regulation of expression of CitHYb and CitZEP was observed with mannitol treatment (Fig. 8C).
Fig. 8.
Effect of sucrose and mannitol on the expression of carotenoid metabolism-related genes in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, sucrose, and mannitol treatments at the 5% level by Tukey’s HSD test.
Effects of ABA and GA on carotenoid content and expression of genes related to carotenoid metabolism
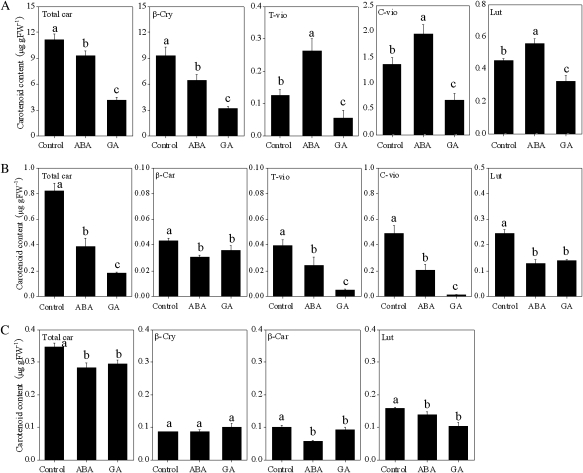
In Satsuma mandarin, the contents of t-vio, c-vio, and lut were increased slightly by the treatment with ABA. The content of β-cry, the prominent carotenoid accumulated in Satsuma mandarin, was decreased significantly along with a decrease in the total carotenoid content by ABA treatment. With GA treatment, the contents of β-cry, t-vio, c-vio, and lut were simultaneously decreased, and as a result the total carotenoid content was much lower than that of the control (Fig. 9A). In Valencia orange, the contents of β-car, t-vio, c-vio, and lut were decreased significantly by the treatments with ABA and GA (Fig. 9B). In Lisbon lemon, the contents of total carotenoid, β-car, and lut were decreased by ABA and GA treatments, while the β-cry content was not affected by the two treatments (Fig. 9C).
Fig. 9.
Effect of ABA and GA on the carotenoid content in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. Total car, total carotenoids. β-Car, β-carotene; β-cry, β-cryptoxanthin; T-vio, all-trans-violaxanthin; C-vio, 9-cis-violaxanthin; Lut, lutein. The value for total carotenoids was the sum of identified carotenoids. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, ABA, and GA treatments at the 5% level by Tukey’s HSD test.
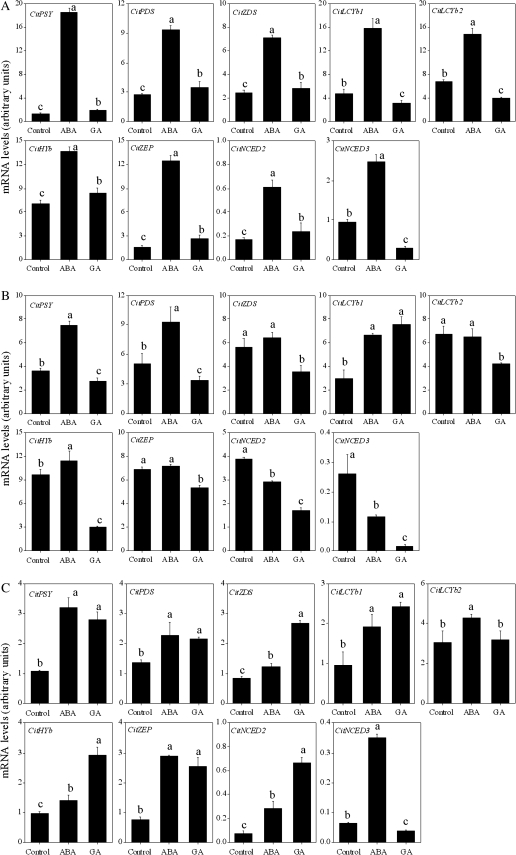
In Satsuma mandarin and Lisbon lemon, the expression of genes detected in the present study (CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, CitZEP, CitNCED2, and CitNCED3) was simultaneously up-regulated by the treatment with ABA (Fig. 10A, C). In Valencia orange, the expression of CitPSY, CitPDS, CitLCYb1, and CitHYb was up-regulated, while the expression of CitNCED2 and CitNCED3 was down-regulated by the treatment with ABA (Fig. 10B).
Fig. 10.
Effect of ABA and GA on the expression of carotenoid metabolism-related genes in juice sacs of the three citrus varieties. (A) Satsuma mandarin; (B) Valencia orange; (C) Lisbon lemon. Columns and bars represent the means and SE (n=3), respectively. The letters a, b, and c indicate significant differences among the control, ABA, and GA treatments at the 5% level by Tukey’s HSD test.
With GA treatment, the expression of CitPSY, CitPDS, CitZDS, CitHYb, CitZEP, and CitNCED2 was up-regulated, while the expression of CitLCYb1, CitLCYb2, and CitNCED3 was down-regulated in Satsuma mandarin. In Valencia orange, the expression of the genes investigated in this study was simultaneously down-regulated by the treatment with GA, except for CitLCYb1. In Lisbon lemon, the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitHYb, CitZEP, and CitNCED2 was up-regulated, while the expression of CitLCYb2 was not affected by the treatment with GA (Fig. 10).
Discussion
Carotenoid accumulation in vitro
Carotenoid metabolism is a complicated process in plants, which is affected by developmental requirements and environmental stimuli (Cazzonelli and Pogson, 2010). It is difficult to evaluate the effects of environmental stimuli on carotenoid metabolism in fruit ripening on trees, as the growing conditions on trees are not uniform and are hard to control. In the present study, to investigate further the regulation of carotenoid metabolism in citrus, first an in vitro system was developed in which undefined variables were minimized and medium compositions and environmental factors were carefully controlled. In this system, the juice sacs enlarged gradually with carotenoid accumulation and no callus formed throughout the experimental period in the three citrus varieties, Satsuma mandarin, Valencia orange, and Lisbon lemon. In a previous study, sugar accumulation in the juice sacs has been reported using the same culture system (Mukai et al., 2000). This study showed that sugar contents gradually increased until 2 months, and then increased rapidly. The pattern of sugar accumulation was similar to that of juice sacs in field-grown fruits. After 8 weeks, the sugar content reached 4.01% in the juice sacs cultured in vitro, which is similar to that in intact fruits (3.91%). In the present study, the changes in carotenoid contents were detected in the juice sacs of the three varieties cultured in vitro. After 8 weeks, in Satsuma mandarin and Lisbon lemon, the content of β-cry, which is the predominantly accumulated carotenoid, reached 5.5 μg g−1 and 0.13 μg g−1, respectively. In Valencia orange, c-vio was abundant, and increased significantly in the first 4 weeks, reaching 0.8 μg g−1 after 8 weeks. It has been reported that a change from β,ϵ-carotenoid accumulation to β,β-xanthophyll accumulation occurred in the flavedo and juice sacs of citrus fruits during the ripening process (Kato et al., 2004; Alquézar et al., 2008). In this study, the accumulation of β,β-xanthophylls was observed in the three citrus varieties during the experimental period, whereas the content of lut, which is a major β,ϵ-carotenoid in citrus, clearly increased in the first 2 weeks and then remained constant. The changes in the carotenoid content and composition in the three citrus varieties cultured in vitro were similar to those in citrus fruits ripening on trees (Kato et al., 2004; Alquézar et al., 2008). In addition, changes in ascorbic acid in the juice sacs cultured in vitro were also detected. During the experimental period, the ascorbic acid content remained constant at a lower level in Satsuma mandarin, while it decreased significantly in Valencia orange and Lisbon lemon (Supplementary Table S1 available at JXB online). The changes in the ascorbic acid content in the juice sacs cultured in vitro were similar to those in the intact fruits.
Transcriptional regulation of carotenoid genes is a major mechanism by which the biosynthesis and accumulation of specific carotenoids are regulated during fruit ripening (Kato et al., 2004, 2006; Kita et al., 2007; Alquézar et al., 2009). In the present study, simultaneous increases in the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, and CitZEP were observed in Satsuma mandarin and Valencia orange. Compared with Satsuma mandarin and Valencia orange, the expression of CitPSY, CitZDS, CitLCYb1, CitHYb, and CitZEP was much lower in Lisbon lemon. Additionally, the mRNA levels of CitZDS, CitLCYb1, and CitLCYb2 were higher in Satsuma mandarin than those in Valencia orange. In contrast, the mRNA levels of CitHYb and CitZEP were higher in Valencia orange than those in Satsuma mandarin. The differences in gene expression led to the differences in the β,β-xanthophyll composition between Satsuma mandarin and Valencia orange. The changing patterns of gene expression in the three citrus varieties in vitro were similar to those in the citrus fruits during the natural ripening process (Kato et al., 2004). Therefore, in the present study, a culture system of citrus juice sacs in vitro, in which carotenoid metabolism in the juice sacs was similar to that in the intact fruits, was successfully set up. This system was useful to investigate further the regulation of carotenoid metabolism by different environmental factors in citrus fruits in vitro.
Effects of blue and red LED lights on carotenoid metabolism
In higher plants, sensing of light is carried out by various light photoreceptors (Briggs, 2001). Thus, plants exhibit different responses to various lights. In the present study, the results showed that the total carotenoid content was increased by treatment with blue light (peak wavelength, 470 nm) in Satsuma mandarin, Valencia orange, and Lisbon lemon. Wu et al. (2007) reported that the β-car content was much higher in the red light-treated group than in the blue light-treated group in leaves and stems of pea seedlings. In tomatoes, the accumulation of lycopene along with an increase in total carotenoid content was also observed in response to red light treatment (Schofield and Paliyath, 2005; Liu et al., 2009). However, the present results showed that irradiation with red light (peak wavelength, 660 nm) did not affect the content or composition of carotenoids in Satsuma mandarin and Valencia orange. In Lisbon lemon, red light slightly increased the content of β-cry, while the total carotenoid content was not significantly affected (Fig. 5). These results indicated that the regulatory effects of the blue and red lights on carotenoid accumulation were cultivar dependent, and in citrus blue light treatment was more effective in inducing carotenoid accumulation than red light.
PSY, which is a rate-limiting enzyme for carotenoid biosynthesis, is regulated by light through a phytochrome-mediated process (von Lintig et al., 1997; Alba et al., 2000). Bohne and Linden (2002) found that in Chlamydomonas reinhardtii blue light was effective in up-regulating the expression of the PSY gene, whereas illumination with red light had no effects on the expression of this gene. In the present study, it was found that the expression of CitPSY was up-regulated by the treatment with blue light in Satsuma mandarin, Valencia orange, and Lisbon lemon. The elevated expression of CitPSY was consistent with the accumulation of carotenoids in the three varieties treated with blue light. In contrast to blue light, red light did not have significant effects on the expression of CitPSY in Satsuma mandarin and Valencia orange. Welsch et al. (2003) reported that the cis-acting elements in response to blue and red lights were separated and located in different positions on the PSY promoter. In Satsuma mandarin and Valencia orange, the difference in the regulation of CitPSY in response to blue and red light might be related to the different cis-acting elements for the two treatments in the CitPSY promoter.
Effects of sucrose and mannitol on carotenoid metabolism
In citrus fruits, sugar treatment promoted the accumulation of carotenoids and advanced the rate of colour break, in which the colour of the citrus peel changed from green to orange (Huff, 1983, 1984; Domingo et al., 2001). In a previous study, it was found that sucrose (5, 10, and 15%) and mannitol (0, 3, and 6%) concentration dependently induced the carotenoid accumulation in the juice sacs of citrus (data not shown). In recent years, sugars have been reported to act as primary messengers in signal transduction processes that trigger gene expression in plants and regulate many important processes (Foyer et al., 1997; Loreti et al., 2005). To date, however, it is still unknown how carotenoid metabolism is regulated by sugar at the transcriptional level in citrus fruits. In the present study, the results showed that the expression of CitPSY simultaneously increased with sucrose treatment in Satsuma mandarin, Valencia orange, and Lisbon lemon. The higher expression level of CitPSY contributed to the increases in the carotenoid contents in the sucrose-treated samples of the three citrus varieties. In tomato fruit, an increase in the expression of PSY was also observed with sucrose treatment (Telef et al., 2006). With mannitol treatment, a simultaneous increase in the expression of CitLCYb2 was observed in all three varieties. LCYb2 is a key gene for the regulation of the flux of carotenes into the β,β-branch of the pathway to lead to the increase of xanthophylls in citrus (Alquézar et al., 2009). With mannitol treatment, the up-regulation of CitLCYb2 contributed to the accumulation of carotenoids in the three varieties. These results suggested that the sucrose- and mannitol-induced carotenoid accumulation was mediated by regulating different steps of the carotenoid biosynthetic pathway in citrus fruits.
In addition, the two carotenoid catabolic genes, CitNCED2 and CitNCED3, were up-regulated simultaneously by the sucrose and mannitol treatments in the three citrus varieties. The expression of NCED, the rate-limiting enzyme for ABA biosynthesis, is highly activated by stress conditions. Iuchi et al. (2000) found that the induction of VuNCED1 was mainly responsible for ABA biosynthesis under water stress in cowpea. Increases in NCED gene expression in response to drought stress were also observed in Arabidopsis, maize, and tomato (Burbidge et al., 1997; Schwartz et al., 1997; Qin and Zeevaart, 1999). Sucrose and mannitol not only provide the common sources of carbon in tissue cultures, but might also induce osmotic stress. Therefore, the up-regulation of CitNCED2 and CitNCED3 in the three citrus varieties in vitro might be attributed to the osmotic stress caused by sucrose and mannitol.
Effects of plant hormones on carotenoid metabolism
In higher plants, the biosynthesis of ABA, which is formed by the oxidative cleavage of c-vio and c-neo, is involved in the carotenoid biosynthesis pathway (Fig. 1). As ABA and carotenoids share some steps in their biosynthesis pathways, the ABA level is closely related to the carotenoid content (Rodrigo et al., 2003; Sarmad et al., 2007). In a previous study, it was found that ABA accumulation in Satsuma mandarin, Valencia orange, and Lisbon lemon exhibited different changing patterns during fruit maturation, which indicated that the physiological role of ABA accumulation may be involved in the formation of different profiles of carotenoids in the three citrus varieties (Kato et al., 2006). In this study, the content of total carotenoid was clearly decreased by ABA treatment in Satsuma mandarin, Valencia orange, and Lisbon lemon (Fig. 9). The expression of the genes investigated in the present study (CitPSY, CitPDS, CitZDS, CitLCYb1, CitLCYb2, CitHYb, CitZEP, CitNCED2, and CitNCED3) was simultaneously up-regulated by the treatment with ABA in Satsuma mandarin and Lisbon lemon, which indicated that ABA treatment induced its own biosynthesis at the transcriptional level in the two citrus varieties (Fig. 10). This positive feedback regulation of ABA led to decreases in the carotenoid content in Satsuma mandarin and Lisbon lemon. In Valencia orange, the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, and CitHYb was up-regulated, while the expression of CitNCED2 and CitNCED3 was down-regulated by treatment with ABA. The ABA content of Valencia orange was much lower than that of Satsuma mandarin and Lisbon lemon (Kato et al., 2006). The extremely low level of ABA was closely related to the low mRNA level of CitNCED2 in the juice sacs of Valencia orange. The differences in the regulation of NCED gene expression between the Valencia orange and the other two citrus varieties in response to ABA treatment might be attributed to the differences in the metabolism of ABA between Valencia orange and the other two varieties.
Similar to ABA, GA is also closely related to the biosynthesis of carotenoids (Fig. 1). It has been shown that treatment with GA has an important effect on carotenoid metabolism by modification of the early steps of the carotenoid biosynthetic pathway (Zhou et al., 1996; Iglesias et al., 2001; Rodrigo and Zacarías, 2007). The results herein showed that the total carotenoid content was decreased by treatment with GA in Satsuma mandarin, Valencia orange, and Lisbon lemon (Fig. 9). However, changes in gene expression varied among the three varieties in response to GA treatment (Fig. 10). In the GA-treated Valencia orange, the expression of CitPSY, CitPDS, CitZDS, CitLCYb2, CitHYb, and CitZEP was simultaneously down-regulated, which was consistent with the decrease in the carotenoid content. In Satsuma mandarin, the down-regulation of expression of CitLCYb1 and CitLCYb2, which are the key genes related to the biosynthesis of xanthophylls, led to a decrease in the content of β-cry, t-vio, and c-vio upon treatment with GA. In Lisbon lemon, the expression of CitPSY, CitPDS, CitZDS, CitLCYb1, CitHYb, and CitZEP was up-regulated by treatment with GA, which was not consistent with the decrease in the carotenoid content in GA-treated Lisbon lemon. Other regulatory mechanism, such as post-transcriptional factors and other genes in the methyl erythritol phosphate pathway (MEP), may also be involved in regulation of the carotenoid content in Lisbon lemon in response to GA treatment.
In conclusion, carotenoid metabolism was investigated in response to different environmental conditions (blue and red LED lights, sucrose, and mannitol) and plant hormones (ABA and GA) in three citrus varieties, Satsuma mandarin, Valencia orange, and Lisbon lemon, in vitro. The results showed that carotenoid accumulation was induced by blue light, sucrose, and mannitol treatment, while it was suppressed by ABA and GA treatment in the three citrus varieties. The carotenoid metabolism in the three citrus varieties was not sensitive to red light treatment, and thus the total carotenoid content was not significantly affected. In addition, gene expression results showed that carotenoid metabolism in response to these treatments was highly regulated at the transcriptional level in Satsuma mandarin, Valencia orange, and Lisbon lemon. The results presented here provide more insights into the regulatory mechanism of carotenoid metabolism in citrus, which might facilitate the improvement of carotenoid content and composition in citrus.
Supplementary data
Supplementary data are available at JXB online.
Table S1. The changes in ascorbic acid content in the juice sacs cultured in vitro and in vivo.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (22780020) and a JSPS Postdoctoral Fellowships for Research Abroad. We would like to thank Dr Susanne Baldermann for her critical reading and revision of the manuscript.
Glossary
Abbreviations
- ABA
abscisic acid
- α-car
α-carotene
- β-car
β-carotene
- C-neo
9′-cis-neoxanthin
- β-Cry
β-cryptoxanthin
- C-vio
9-cis-violaxanthin
- GA
gibberellin
- GGPP
geranylgeranyl diphosphate
- HYb
β-ring hydroxylase
- HYe
ϵ-ring hydroxylase
- LCY
lycopene cyclase
- LCYb
lycopene β-cyclase
- LCYe
lycopene ϵ-cyclase; lut, lutein
- NCED
9-cis-epoxycarotenoid dioxygenase
- PDS
phytoene desaturase
- PSY
phytoene by phytoene synthase; vio, violaxanthin; zea, zeaxanthin
- ZDS
ζ-carotene desaturase
References
- Alba R, Cordonnier-Pratt MM, Pratt LH. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiology. 2000;123:363–370. doi: 10.1104/pp.123.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquézar B, Rodrigo MJ, Zacarías L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry. 2008;69:1997–2007. doi: 10.1016/j.phytochem.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Alquézar B, Zacarías L, Rodrigo MJ. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. Journal of Experimental Botany. 2009;60:1783–1797. doi: 10.1093/jxb/erp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne F, Linden H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochimica et Biophysica Acta. 2002;1579:26–34. doi: 10.1016/s0167-4781(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Bouvier F, Backhaus RA, Camara B. Induction and control of chromoplast-specific carotenoid genes by oxidative stress. Journal of Biological Chemistry. 1998;273:30651–30659. doi: 10.1074/jbc.273.46.30651. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, et al. The phototropin family of photoreceptors. The Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, Taylor I. Structure and expression of a cDNA encoding a putative neoxanthin cleavage enzyme (NCE) isolated from a wilt related tomato (Lycopersiconesculentum Mill.) library. Journal of Experimental Botany. 1997;47:2111–2112. [Google Scholar]
- Busch M, Seuter A, Hain R. Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiology. 2002;128:439–453. doi: 10.1104/pp.010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends in Plant Science. 2010;15:266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Domingo JI, Francisco RT, Francisco L, Edwardo PM, Manuel T. In vivo sucrose stimulation of colour change in citrus fruit epicarps; interaction between nutritional and hormonal signals. Physiologia Plantarum. 2001;112:244–250. doi: 10.1034/j.1399-3054.2001.1120213.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiologia Plantarum. 1997;100:241–254. [Google Scholar]
- Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. Journal of the National Cancer Institute. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- Goodner KL, Rouseff RL, Hofsommer HJ. Orange, mandarin, and hybrid classification using multivariate statistics based on carotenoid profiles. Journal of Agricultural and Food Chemistry. 2001;49:1146–1150. doi: 10.1021/jf000866o. [DOI] [PubMed] [Google Scholar]
- Harada H, Mukai H, Takagi T. Effects of explant age, growth regulators and carbohydrates on sugar accumulation in Citrus juice vesicles cultured. in vitro. Scientia Horticulturae. 2001;90:109–119. [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends in Plant Science. 1998;3:147–151. [Google Scholar]
- Huff A. Nutritional control of regreening and degreening in citrus peel segments. Plant Physiology. 1983;73:243–249. doi: 10.1104/pp.73.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff A. Sugar regulation of plastic interconversions in epicarp of citrus fruits. Plant Physiology. 1984;73:307–312. doi: 10.1104/pp.76.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias DJ, Tadeo FR, Legaz F, Primo-Millo E, Talon M. In vivo sucrose stimulation of color change in citrus fruit epicarps: interactions between nutritional and hormonal signals. Physiologia Plantarum. 2001;112:244–250. doi: 10.1034/j.1399-3054.2001.1120213.x. [DOI] [PubMed] [Google Scholar]
- Ikoma Y, Yano M, Ogawa K, Yoshioka T, Xu ZC, Hisada S, Omura M, Moriguchi T. Isolation and evaluation of RNA from polysaccharide-rich tissues in fruit for quality by cDNA library construction and RT-PCR. Journal of the Japanese Society for Horticultural Science. 1996;64:809–814. [Google Scholar]
- Isaacson T, Ronen G, Zamir D, Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. The Plant Cell. 2002;14:333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. A stress-inducible gene for 9-cis-epoxycartenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought tolerant cowpea. Plant Physiology. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, Yano M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiology. 2004;134:824–837. doi: 10.1104/pp.103.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Matsumoto H, Ikoma Y, Kuniga T, Nakajima N, Yoshida T, Yano M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes and carotenoid cleavage dioxygenase genes during fruit maturation in the juice sacs of ‘Tamami’, ‘Kiyomi’ tangor, and ‘Wilking’ mandarin. Journal of the Japanese Society for Horticultural Science. 2007;76:103–111. [Google Scholar]
- Kato M, Matsumoto H, Ikoma Y, Okuda H, Yano M. The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit. Journal of Experimental Botany. 2006;57:2153–2164. doi: 10.1093/jxb/erj172. [DOI] [PubMed] [Google Scholar]
- Khan EU, Fu XZ, Wang J, Fan QJ, Huang XS, Zhang GN, Shi J, Liu JH. Regeneration and characterization of plants derived from leaf in vitro culture of two sweet orange (Citrus sinensis (L) Osbeck) cultivars. Scientia Horticulturae. 2009;120:70–76. [Google Scholar]
- Kita M, Kato M, Ban Y, Honda C, Yaegaki H, Ikoma Y, Moriguchi T. Carotenoid accumulation in Japanese Apricot (Prunus mume Siebold & Zucc.): molecular analysis of carotenogenic gene expression and ethylene regulation. Journal of Agricultural and Food Chemistry. 2007;55:3414–3420. doi: 10.1021/jf063552v. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Review of Nutrition. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- Ledford HK, Niyogi KK. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant, Cell and Environment. 2005;28:1037–1045. [Google Scholar]
- Lee HS, Castle WS. Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold, and Budd Blood orange juices. Journal of Agricultural and Food Chemistry. 2001;49:877–882. doi: 10.1021/jf000654r. [DOI] [PubMed] [Google Scholar]
- Liu LH, Zabaras D, Bennett LE, Aguas P, Woonton BW. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chemistry. 2009;115:495–500. [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiology. 2005;137:1130–1138. doi: 10.1104/pp.104.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty I, Bureau S, Sarkissian G, Gouble B, Audergon JM, Albagnac G. Ethylene regulation of carotenoid accumulation and carotenogenic gene expression in colour-contrasted apricot varieties (Prunus armeniaca) Journal of Experimental Botany. 2005;56:1877–1886. doi: 10.1093/jxb/eri177. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ikoma Y, Kato M, Nakajima N, Hasegawa Y. Effect of postharvest temperature and ethylene on carotenoid accumulation in the flavedo and juice sacs of Satsuma mandarin (Citrus unshiu Marc.) fruit. Journal of Agricultural and Food Chemistry. 2009;57:4724–4732. doi: 10.1021/jf9005998. [DOI] [PubMed] [Google Scholar]
- Molnár P, Szabolcs J. β-Citraurin epoxide, a new carotenoid from Valencia orange peel. Phytochemistry. 1980;19:633–637. [Google Scholar]
- Mukai H, Takagi T, Harada H, Murai Y. Sugar accumulation by in vitro cultured juice vesicles of Satsuma mandarin. Journal of the Japanese Society for Horticultural Science. 2000;69:57–59. [Google Scholar]
- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ. Identification of carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. The Plant Cell. 2002;14:321–332. doi: 10.1105/tpc.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecker I, Gabbay R, Cunningham FX Jr, Hirschberg J. Cloning and characterization of the cDNA for lycopene beta-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Molecular Biology. 1996;30:807–819. doi: 10.1007/BF00019013. [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JA. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proceedings of the National Academy of Sciences, USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Zacarías L. Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. Journal of Agricultural and Food Chemistry. 2004;52:6724–6731. doi: 10.1021/jf049607f. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Zacarías L. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biology and Technology. 2007;43:14–22. [Google Scholar]
- Ronen G, Cohen M, Zamir D, Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. The Plant Journal. 1999;17:341–351. doi: 10.1046/j.1365-313x.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Sarmad J, Shariati M, Madadkar Haghjou M. Relationship between endogenous abscisic acid and β-carotene synthesis in the unicellular green alga Dunaliella. American-Eurasian Journal of Agricultural and Environmental Science. 2007;2:559–564. [Google Scholar]
- Schofield A, Paliyath G. Modulation of carotenoid biosynthesis during tomato fruit ripening through phytochrome regulation of phyoene synthase activity. Plant Physiology and Biochemistry. 2005;43:1052–1060. doi: 10.1016/j.plaphy.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Steinbrenner J, Linden H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiology. 2001;125:810–817. doi: 10.1104/pp.125.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telef N, Stammitti-Bert L, Mortain-Bertrand A, Maucourt M, Carde JP, Rolin D, Gallusci P. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Molecular Biology. 2006;62:453–469. doi: 10.1007/s11103-006-9033-y. [DOI] [PubMed] [Google Scholar]
- von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. The Plant Journal. 1997;12:625–634. doi: 10.1046/j.1365-313x.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- Wan XR, Li L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochemical and Biophysical Research Communications. 2006;347:1030–1038. doi: 10.1016/j.bbrc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Welsch R, Medina J, Giuliano G, Beyer P, Von Lintig J. Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta. 2003;216:523–534. doi: 10.1007/s00425-002-0885-3. [DOI] [PubMed] [Google Scholar]
- Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chemistry. 2007;101:1753–1758. [Google Scholar]
- Zhou YC, Tang YL, Tan XJ, Guo JR. Effects of exogenous ABA, GA3 and cell-wall-degrading enzyme activity, carotenoid content in ripening mango fruit. Acta Phytophysiologica Sinica. 1996;22:421–426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.