Abstract
Fruit ripening in response to treatments with propylene, 1-methycyclopropene (1-MCP), and low temperature was characterized in ‘Sanuki Gold’ kiwifruit, Actinidia chinensis Planch. Propylene treatment immediately induced rapid fruit softening, increased AC-PG (polygalacturonase) and AC-EXP (expansin) mRNA accumulation, and stimulated an increase in the soluble solid concentration (SSC) and a decrease in titratable acidity (TA). After 3 d exposure to propylene, ethylene production and AC-PL (pectate lyase) mRNA accumulation were observed. 1-MCP treatment after 24 h exposure to propylene eliminated AC-PG mRNA accumulation and suppressed continued changes in SSC and TA. Application of 1-MCP at the start of the treatment, followed by continuous propylene exposure, markedly delayed fruit softening, and the expression of the cell wall-modifying genes, and changes in the SSC and TA, indicating that kiwifruit become insensitive to ethylene at least for 3 d following 1-MCP exposure. Surprisingly, significant fruit softening, mRNA accumulation of AC-PG, AC-PL, and AC-EXP, and decreased TA were observed without ethylene production in intact fruit stored at low temperature for 1 month, but not in fruit stored at room temperature. Repeated 1-MCP treatments (twice a week) failed to inhibit the changes that occurred in low temperature storage. These observations indicate that low temperature modulates the ripening of kiwifruit in an ethylene-independent manner, suggesting that kiwifruit ripening is inducible by either ethylene or low temperature signals.
Keywords: Ethylene, fruit ripening, kiwifruit, low-temperature, 1-MCP
Introduction
Fruit ripening is genetically programmed and involves physiological, biochemical, and structural changes, such as cell wall hydrolysis, pigment degradation and synthesis, carbohydrate metabolism, and generation of secondary metabolism compounds which influence fruit appearance, texture, flavour, and aroma (Seymour et al., 1993). Based on ripening regulation, fruit are largely divided into two groups: climacteric fruit that ripen with ethylene and non-climacteric fruit that ripen independently of ethylene. In climacteric fruit, ethylene is critical for the induction of fruit ripening since most ripening-related events are regulated or accelerated by ethylene (Saltveit, 1999). In tomatoes and melons, suppression of ethylene biosynthesis or the transcription factor for ethylene signalling by transgenic engineering resulted in the inhibition or significant delay of most of ripening-related events (Hamilton et al., 1990; Murray et al., 1993; Ayub et al., 1996; Guis et al., 1997; Yokotani et al., 2009).
In various climacteric fruit including kiwifruit, pre-climacteric application of 1-methylcyclopropene (1-MCP), a potent inhibitor of ethylene perception due to its largely irreversible binding to ethylene receptors, has been reported to delay ripening and senescence significantly, and consequently to lead to a prolonged storage life and/or shelf life (Watkins, 2006). Using 1-MCP, it was demonstrated that peel de-greening and aroma volatile development in bananas are mediated by ethylene, while sugar accumulation is not always dependent on ethylene (Golding et al., 1998). Previously, it was demonstrated that after the onset of ripening, 1-MCP application interrupted fruit softening in pears (Hiwasa et al., 2003) and melons (Nishiyama et al., 2007), and reduced the ripening-regulated expression of cell wall-related genes such as polygalacturonase (PG) and expansin (EXP). These results suggested that the ethylene signal is essential not only for the onset of fruit ripening, but also for its completion, and that fruit softening is totally dependent on ethylene.
Expression of the PG gene during ripening has been reported in kiwifruit as well as in other fleshy fruit species, and has been primarily shown to be responsible for depolymerization and solubilization of the pectic backbone of the cell wall polysaccharides (Wang et al., 2000) and to correlate positively with the softening of fruit flesh tissues during ripening in various climacteric fruit. Pectate lyase (PL) is known also to target cell wall pectins; it cleaves the de-esterified pectins through a β-elimination reaction, in contrast to PG, which hydrolytically cleaves α-1,4-galacturonosyl linkages in unesterified pectins (Wong, 1995). EXPs have no apparent hydrolytic enzymatic activity but apparently link accessibility of wall polymers to enzyme action, and thereby accelerate wall hydrolysis (Rose et al., 1997; Cosgrove, 2000). In transgenic tomatoes overexpressing recombinant EXP1, extensive hemicellulose depolymerization and considerable softening occurred in the absence of polyuronide depolymerization in mature green fruit (Brummell et al., 1999).
Kiwifruit have been classified as a climacteric fruit (Pratt and Reid, 1974) due to their high sensitivity to ethylene. Kiwifruit softening is inducible upon exposure to extremely low concentrations of ethylene (as low as 0.01 μl l−1; Arpaia et al., 1987; Mitchell, 1990). However, Yano and Hasegawa (1993) have suggested that kiwifruit do not initiate ethylene production without development of disease such as ripe rot or exposure to exogenous ethylene. In response to exogenous ethylene, kiwifruit initiates fruit ripening followed by high levels of ethylene evolution, >100 nl g−1 h−1 at the climacteric peak (Mworia et al., 2010). Ethylene biosynthesis genes encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase and ACC oxidase, which have been shown to be associated with system II ethylene biosynthesis, are regulated by a positive feedback system during ripening in kiwifruit (Whittaker et al., 1997; Xu et al., 1998); they are induced by exogenous ethylene and suppressed by 1-MCP (Mworia et al., 2010). However, unlike in other climacteric fruit, ethylene production in kiwifruit starts well after the fruit have significantly softened in response to ethylene supplied from exogenous sources (Sfakiotakis et al., 1997).
During low temperature storage and subsequent room temperature ripening, the role of ethylene in healthy intact fruit that are not exposed to exogenous ethylene has elicited great interest since kiwifruit can soften extensively in the absence of any measurable increase in ethylene production (i.e. <10 N; Kim et al., 1999; Yin et al., 2009). In a previous study, it was shown that ‘Sanuki Gold’ kiwifruit softened to ∼20% of firmness at harvest after 1 month of storage at 4 °C under ambient conditions without any detectable ethylene production (Mworia et al., 2011). Similar observations have been reported in ‘Hayward’ kiwifruit (Manolopoulou et al., 1997). Thus far, kiwifruit softening during low temperature storage has been attributed to an increased sensitivity to ethylene during storage, on the assumption that the basal ethylene levels (system 1, <0.1 nl g−1 h−1) play a role (Kim et al., 1999). Whether the gradual softening in low temperature storage is caused by basal ethylene, exogenous ethylene, and/or high sensitivity of the fruit to ethylene via the ethylene signal, or because of another mechanism, is still not yet clear. Exogenous ethylene arising from diseased fruit is likely to induce softening and ethylene production in intact neighbouring fruit (Yano and Hasegawa, 1993). Therefore, careful handling to eliminate the effects of diseased fruit is crucial in studies of the mechanism of fruit ripening in kiwifruit under either low or room temperature conditions.
This study characterized ‘Sanuki Gold’ kiwifruit ripening in response to exogenous propylene, an ‘ethylene analogue’, and determined the duration of ethylene insensitivity after 1-MCP treatment. Using repeated 1-MCP treatments in fruit stored at 25 °C or 4 °C, this study demonstrated that low temperature modulates kiwifruit ripening independently of ethylene. The objective of this study was to demonstrate that kiwifruit can ripen in both an ethylene-dependent and an independent manner.
Materials and methods
Plant materials and treatments
‘Sanuki Gold’ kiwifruit, Actinidia chinensis Planch., were obtained from a commercial orchard in Kagawa, Japan. After flowering in the middle of May, individual fruit were bagged in paper fruit bags immediately after fruit set, and careful spraying regimes were carried out during the growing season. After harvesting at commercial maturity stage (154 days after pollination), careful selection was conducted to exclude fruit with physical injury, disease, or incidences of pests. To monitor for quiescent infections, ethylene production of all fruit was measured individually and a few fruit out of 400 were found to be producing ethylene. The fruit were set aside and they developed disease symptoms within days. Therefore, they were excluded from the experiments.
Experiment 1: ethylene-dependent fruit ripening
Fruit were categorized into four treatment groups. The first category contained non-treated fruit (Control). The second category was treated with propylene (Propylene) where fruit were held in 15.0 l gas-tight plastic containers and treated with 5000 μl l−1 propylene, a well-known ‘ethylene analogue’ (Burg and Burg, 1967; McMurchie et al., 1972; Yang, 1985). The containers enclosing the fruit were opened at 12 h intervals in a fume chamber for 1 h to replace the air inside and then the containers were re-injected with propylene until day 3 when ethylene production was detected. The third group was exposed to 5000 μl l−1 propylene for 24 h, followed by a single exposure to 5 μl l−1 1-MCP treatment [P(24h)+MCP]. 1-MCP (Rohm and Hass, Philadelphia, PA, USA) was generated from SmartFresh™ powder (active ingredient 0.14%), injected into 15.0 l plastic gas-tight containers enclosing the fruit, and incubated for 12 h as previously reported (Mworia et al., 2010). The fourth group of fruit was treated with a single exposure to 1-MCP treatment at harvest followed by a 5000 μl l−1 propylene treatment until ethylene production was detected (MCP+P). Reapplication of propylene was done as explained above. All treatments were carried out at 25 °C. Soda lime was placed in plastic containers with fruit during propylene and 1-MCP treatments to reduce CO2 accumulation.
Experiment 2: low-temperature-modulated fruit ripening
Fruit were stored either at 25 °C (room temperature) or at 4 °C (low temperature). At room temperature, fruit were stored as non-treated (RT-Cont) or repeatedly treated with 1-MCP (RT-MCP). Similarly at low temperature (LT), fruit were stored as non-treated (LT-Cont) or repeatedly treated with 1-MCP (LT-MCP). 1-MCP was applied for 12 h twice a week throughout the storage period based on the observations that 1-MCP-treated ‘Sanuki Gold’ kiwifruit remains completely insensitive to ethylene for at least 3 d after treatment. Ethylene production of individual fruit was monitored at weekly intervals throughout the storage period. Fruit producing ethylene were set aside for observation, within days developed disease symptom, and were excluded. The excluded fruit were <10% of total by the end of experiment.
Evaluation of ethylene production and fruit quality indices
Ethylene production was determined by incubating individual fruit in a 440 ml container for 2 h, after which 1 ml of headspace gas was withdrawn and injected into a gas chromatograph (Model-GC4 CMPF, Shimadzu, Kyoto, Japan), equipped with a flame ionization detector and an activated alumina column. This procedure has a minimum ethylene production detection capacity of 0.01 nl g−1 h−1. Flesh firmness was measured at four equatorial regions of the peeled flesh using a penetrometer (model SMT-T-50, Toyo Baldwin, Tokyo, Japan) fitted with an 8 mm plunger. Soluble solid content (SSC) of the fruit juice was measured using a digital Atago PR-1 refractometer (Atago Co. Ltd, Tokyo, Japan) and expressed in Brix (%). Titratable acidity (TA) of the fruit juice was determined by titration using 0.1 N NaOH and phenolphthalein as a pH indicator and expressed as percentage citric acid equivalents. Evaluation of fruit quality indices was carried out using three fruit at each time point, and flesh samples were taken and stored at –80 °C for total RNA extraction and gene expression analysis.
Cloning of cDNA fragments encoding cell wall-modifying enzymes
RNA was extracted using a method for polysaccharide-rich fruit (Lopez-Gomez and Gomez-Lim, 1992; Ikoma et al., 1996) with slight modifications. First-strand cDNA was synthesized from total RNA using oligo(dT) according to the manufacturer’s instructions (Invitrogen Life Technologies, Carlsbad, CA, USA) and was used as template for amplifying cDNA fragments encoding the cell wall-modifying enzymes PG, PL, and EXP.
Expressed sequence tags (ESTs) of cell wall-modifying enzymes were obtained from A. chinensis ESTs (Crowhurst et al., 2008) registered in GenBank (National Center for Biotechnology Information, Washington, DC, USA) by TBLAST anaysis (http://www.ncbi.nlm.nih.gov/BLAST/), assembled, and grouped into contigs using Genetyx Macs, ver. 15 and ATGC ver. 4 software (GENETYX Corp., Tokyo, Japan). Selected contigs were used to design the gene-specific primers shown in Table 1. Using RT-PCR and TA cloning techniques (pGEM®-T Easy Vector Systems, Promega, Madison, WI, USA), cDNA fragments of the cell wall-modifying enzyme transcripts were isolated and designated as AC-PG, AC-PL, and AC-EXP, respectively. The fragments are highly homologous with the corresponding contigs, with >98% nucleotide sequence identity. AC-PG showed 96% homology at the amino acid sequence level with PG-C (Wang et al., 2000; accession no. AAF71158) from A. chinensis fruit tissues. AC-PL showed close similarities with the PL genes from other species in the database. Cloned AC-EXP showed 100% homology with Ad-EXP1 (accession no. AAR10411) isolated from A. deliciosa ‘Bruno’ kiwifruit tissues.
Table 1.
Oligonucleotide primers used for amplification of cDNA by PCR
| Primer DNA sequences | Gene |
| 5′-GGCAAAGCCTAGCAATGGATTTGTTAGG-3′ | AC-PG |
| 3′-AGTTTTATTGTAATATTCTACATTTGTA-5′ | |
| 5′-GGGAATCCGATCGATGACTGTTGG-3′ | AC-PL |
| 3′-TACATCTCCCAGTGGGTGTAGTC-5′ | |
| 5′-CTGAGCACGGCGCTTTTCAACAGC-3′ | AC-EXP |
| 3′-TCGTACTCGGCCTTCACCCCGGTC-5′ |
RNA gel blot analysis
An aliquot (5 μg) of total RNA from each sample was separated on a 1.2% agarose denaturing gel, subjected to electrophoresis, and transferred to a Hybond-N+ membrane (GE Healthcare, Bunkingham, UK). Digoxigenin (DIG)-labelled probes were prepared using cloned fragments and a PCR DIG probe synthesis kit (Roche Diagnostics, Mannheim, Germany). Membranes were hybridized with DIG-labelled probes in a hybridization buffer, 7% (w/v) SDS, 50% (v/v) de-ionized formamide, 5× SSC (sodium citrate/sodium chloride buffer), 50 mM sodium phosphate pH 7, 0.1% (w/v) N-lauroylsarcosine, and 2% (w/v) blocking reagent at 42 °C for 16 h. Membranes were washed twice for 5 min each in 2× SSC and 0.1% SDS at room temperature followed by high stringency washing, twice each for 30 min in 0.1× SSC and 0.1% SDS at 60 °C. Immunological detection was done according to the manufacturer’s instructions using CDP-star as a chemiluminescent substrate for alkaline phosphate (Roche Diagnostics) followed by exposure on Amersham high performance chemiluminescence film (GE Healthcare).
Results
Ethylene-dependent fruit ripening
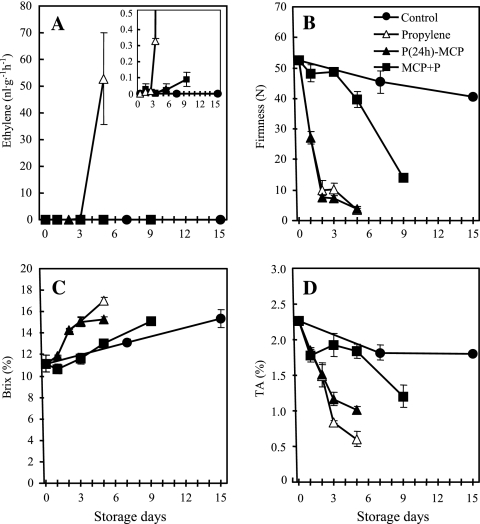
No measurable ethylene was detected in control fruit during the storage period (Fig. 1A). Ethylene production in propylene-treated fruit was detected at a level of 0.3 nl g−1 h−1 after 3 d of treatment and rapidly increased after 5 d of storage, while after propylene and subsequent 1-MCP application [P(24h)+MCP], the fruit produced no undetectable ethylene throughout the storage period. The induction of ethylene production in fruit treated with 1-MCP followed by propylene (MCP+P) was delayed until 5 d, and significant levels of ethylene were detected only after 9 d of storage.
Fig. 1.
Ethylene production and quality characteristics of ‘Sanuki Gold’ kiwifruit treated with 1-MCP and/or propylene. Control, non-treated; Propylene, continuously propylene treated until the induction of ethylene production; P(24h)+MCP, 24 h propylene treatment followed by a single exposure to 1-MCP for 12 h; MCP+P, a single exposure to 1-MCP for 12 h immediately after harvest, followed by continuous propylene treatment until ethylene production was detected. (A) Ethylene (inset: base ethylene production below 0.5 nl g−1 h−1), (B) flesh firmness, (C) SSC, (D) TA. The SE represents three replications at each time point, and error bars not shown are smaller than the symbol used.
Flesh firmness in control fruit remained high, >40 N, even after 15 d of storage (Fig. 1B). In propylene-treated fruit, flesh firmness decreased rapidly after 6 h of treatment, losing ∼50% of original flesh firmness after 24 h of exposure to propylene, and the fruit softened to <10 N after 2 d of treatment. Fruit in the P(24 h)+MCP treatment showed similar softening patterns in comparison with propylene-treated fruit. Fruit in the MCP+P treatment after 3 d had flesh that was as firm as that observed at harvest; these fruit softened slightly after 5 d and then rapidly softened to <20 N after 9 d of storage.
The SSC of fruit in the propylene and the P(24h)+MCP treatments increased rapidly, from 11.2% at harvest to 15% after 3 d of storage (Fig. 1C). After 5 d of storage, the SSC in propylene-treated fruit increased further to 17% while in the P(24h)+MCP treatment, the fruit SSC remained unchanged. The SSC slowly but steadily increased to 15% in MCP+P and control fruit after 9 d and 15 d of storage, respectively. The TA was rapidly reduced from 2.3% at harvest to 1.5% after 2 d of storage in the fruit treated with propylene or with propylene for 24 h followed by 1-MCP exposure [P(24h)+MCP] (Fig. 1D). The TA was reduced to 1.2% in P(24h)+MCP-treated fruit after 3 d and further declined to 1% after 5 d of storage, which was slightly higher than fruit treated with propylene which showed a rapid reduction to 0.8% and 0.6% after 3 d and 5 d of storage, respectively. Fruit exposed to the MCP+P treatment had a reduced TA of 1.8% after 5 d storage, which declined further to 1.2% after 9 d of storage. The TA of control fruit showed a slight reduction from 2.3% to 1.8% after 15 d of storage.
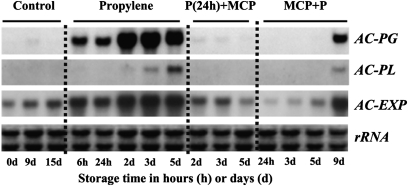
AC-PG and AC-PL transcripts were not observed in RNA from control fruit throughout the storage period (Fig. 2) although basal constitutive accumulation of AC-EXP mRNA was observed in control fruit. In propylene-treated fruit, AC-PG expression was induced within 6 h of treatment and AC-PG mRNA accumulation increased rapidly during the storage period and correlated with the reduction in firmness of the fruit flesh. Induction of AC-PL expression occurred after 3 d of propylene treatment and increased after 5 d. The level of AC-EXP transcript was elevated by propylene treatment. Expression of AC-PG and AC-PL in P(24h)+MCP-treated fruit was not observed between 2 d and 5 d of storage, while AC-EXP expression was reduced. Accumulation of AC-PG and AC-PL mRNA was not detected after 5 d of storage of MCP+P-treated fruit, but these genes showed increased expression after 9 d.
Fig. 2.
Expression of cell wall-degrading genes in ‘Sanuki Gold’ kiwifruit treated with 1-MCP and/or propylene. Control, non-treated; Propylene, continuously propylene treated until the induction of ethylene production; P(24h)+MCP, 24 h propylene treatment followed by a single exposure to 1-MCP for 12 h; MCP+P, a single exposure to 1-MCP for 12 h immediately after harvest, followed by continuous propylene treatment until ethylene production was detected. The vertical lanes represent treatments in either hours (h) or days (d) after commencement of the experiment. Each sample lane was loaded with 5 μg of total RNA.
Low-temperature-modulated fruit ripening
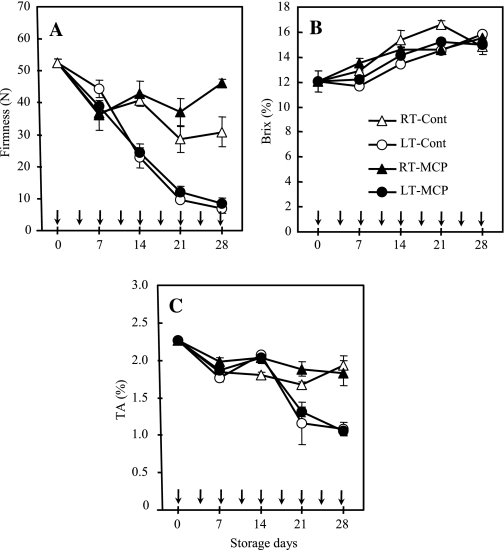
In all treatments, healthy fruit did not produce any detectable ethylene during storage. Flesh firmness in both LT-Cont and LT-MCP fruit reduced rapidly from 52 N to ∼25 N after 14 d of storage and to <10 N after 28 d of storage (Fig. 3A). However, flesh firmness in RT-Cont and RT-MCP fruit remained above 40 N after 14 d of storage, although RT-Cont fruit showed a further reduction to 28 N and 30 N after 21 d and 28 d of storage, respectively, compared with RT-MCP fruit that maintained flesh firmness above 37 N after 21 d and 28 d of storage. Previous reports demonstrated that kiwifruit softening and induction of ethylene production occurs within 1 or 2 weeks of storage at room temperature (Boquete et al., 2004; Koukounaras and Sfakiotakis, 2007; Birch et al., 2009). The longer storage life observed in the present experiments at room temperature may be due to the timely and careful elimination of diseased fruit that could be sources of exogenous ethylene for nearby fruit.
Fig. 3.
Quality characteristics of ‘Sanuki Gold’ kiwifruit stored under room temperature or low temperature with or without repeated 1-MCP treatments. (A) Flesh firmness, (B) SSC, (C) TA. RT-Cont, stored at 25 °C; RT-MCP, stored at 25 °C with repeated 1-MCP treatments; LT-Cont, stored at 4 °C; LT-MCP, stored at 4 °C with repeated 1-MCP treatments. The SE represents three replications at each time point, and error bars not shown are smaller than the symbol used. Arrows indicate 1-MCP treatment for 12 h.
The SSC remained unchanged in LT-Cont and LT-MCP fruit after 7 d of storage, but steadily increased from 12% to 14% after 14 d of storage and remained at <16% after 28 d of storage (Fig. 3B). The SSC in RT-Cont and RT-MCP fruit showed a slight increase from 12% to 13% after 7 d of storage, rose slightly to 14% after 14 d of storage, but remained below 16% after 28 d of storage. The reduction of the TA in RT-Cont and RT-MCP fruit was not significant after 14 d of storage and remained above 1.7% after 21 d and 28 d of storage. (Fig. 3C). However, TA in LT-Cont and LT-MCP fruit significantly decreased from 2% to ∼1.3% after 21 d of storage and further to 1.1% after 28 d of storage.
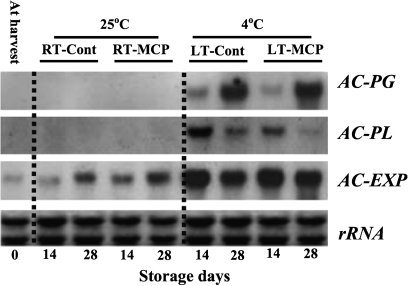
Expression of AC-PG and AC-PL was not observed in RT-Cont and RT-MCP fruit throughout the storage period (Fig. 4). However, expression of AC-PG in LT-Cont and LT-MCP fruit was detected after 14 d of storage and increased after 28 d of storage. In contrast, accumulation of AC-PL transcripts was higher after 14 d of exposure to LT-Cont or LT-MCP conditions but decreased after 28 d of storage. Although exposure to low temperature caused elevated expression of AC-EXP in LT-Cont and LT-MCP fruit after 14 d and 28 d of storage, only basal expression was observed in both RT-Cont and RT-MCP fruit throughout the storage period.
Fig. 4.
Expression of cell wall-degrading genes in ‘Sanuki Gold’ kiwifruit stored under room temperature or low temperature with or without repeated 1-MCP treatments. RT-Cont, stored at 25 °C; RT-MCP, stored at 25 °C with repeated 1-MCP treatments; LT-Cont, stored at 4 °C; LT-MCP, stored at 4 °C with repeated 1-MCP treatments. Each sample lane was loaded with 5 μg of total RNA.
Discussion
Ethylene-induced fruit ripening
In climacteric fruit, the ethylene signal is known to trigger many plant processes including ethylene production, fruit softening, colour change, and increases in flavour and aroma associated with fruit ripening (Hua and Meyerowitz, 1998). The rapid softening in response to exogenous ethylene (Kim et al., 1999) or propylene, an ‘ethylene analogue’ (Stavroulakis and Sfakiotakis, 1997), and its inhibition by 1-MCP, which is thought to bind the ethylene receptor irreversibly (Boquete et al., 2004), were previously reported using ‘Hayward’ kiwifruit. In this study, kiwifruit responded to propylene within 6 h by inducing AC-PG mRNA accumulation, elevating AC-EXP mRNA levels, and subsequently rapidly reducing flesh firmness (Figs 1, 2). Previous studies with ‘Hayward’ kiwifruit also showed that PG mRNA levels respond quickly to propylene treatment, and PG expression positively correlates with fruit softening (Wang et al., 2000). In contrast to the rapid softening response of fruit to propylene, 3 d exposure to propylene was required for the induction of endogenous production of ethylene and expression of AC-PL in kiwifruit. The response differences between fruit softening and ethylene production have been observed previously as kiwifruit ripen (Sfakiotakis et al., 1997). Based on the events that occur following exposure of fruit to ethylene, kiwifruit softening has been categorized into three main stages (MacRae and Redgwell, 1992). After exposure of fruit to ethylene, kiwifruit softening is characterized by pectin de-esterification and solubilization, loss of galactose, and degradation of solubilized pectins accompanied by starch degradation. These processes account for the ∼20% loss of firmness in harvested fruit. The production of endogenous ethylene accompanied by the generation of aroma and volatiles appears late during ripening when fruit have already significantly softened (Hallett et al., 1992; Redgwell et al., 1992, 1997).
Increases in the SSC and reduction in TA were accelerated in response to propylene (Fig. 1), while non-treated fruit showed slow but steady changes, similar to observations reported for ‘Hayward’ kiwifruit in response to ethylene (Beever and Hopkirk, 1990; Ikoma, 1996; Park et al., 2006). These observations suggest that the ethylene signal enhances the conversion of starch to sugars and the reduction in organic acids, but may not always be essential for the changes. The control of ripening-related changes by both ethylene and other developmental factors has been mentioned in bananas (Hubbard et al., 1990; Cordenunsi and Lajolo, 1995), mangoes (Castrillo et al., 1992), and melons (Guis et al., 1997; Silva et al., 2004). Application of 1-MCP after 24 h propylene treatment eliminated AC-PG mRNA accumulation within 1 d and restored AC-EXP mRNA levels nearby to the initial levels of non-treated fruit (Fig. 2). Further, 1-MCP treatment slowed the reduction in TA and the increase in SSC after 2 d and 3 d of storage, respectively, but failed to retard the changes in flesh firmness (Fig. 1), confirming that kiwifruit softening is highly sensitive to ethylene and that 1-MCP effectively disrupts ethylene signalling. Because ethylene signalling and inhibition by 1-MCP involve both transcription and translation regulation, these results suggest that transcription and translation of cell wall-modifying enzymes induced by the ethylene signal would be sufficient to initiate and sustain fruit softening even in the absence of detectable ethylene production.
In fruit treated with 1-MCP for 12 h immediately after harvest and stored in the presence of propylene, no response to propylene was detected until 5 d, when small changes in flesh firmness and ethylene production were observed (Fig. 1). In addition, the induction of both AC-PG and AC-PL expression and the slight elevation of AC-EXP expression accompanied by a rapid reduction in flesh firmness and in TA were observed after 9 d (Figs 1, 2). The duration of ethylene insensitivity after 1-MCP treatment varies between species (Sisler and Serek, 1997; Watkins, 2006). In higher plants, ethylene receptors and CTR1 function as negative regulators in the ethylene signalling pathway (Hua and Meyerowitz, 1998). In the presence of ethylene, receptor proteins undergo degradation through the ubiquitin–proteasome pathway, resulting in increased ethylene sensitivity and subsequent ethylene responses (Kevany et al., 2007). 1-MCP is assumed to bind the receptor proteins with greater affinity than ethylene (Jiang et al., 1999), becoming 1-MCP–receptor complexes which suppress ethylene signalling even in the presence of ethylene. Therefore, the duration of ethylene insensitivity after the end of 1-MCP treatment depends on the rate of turnover of the 1-MCP–receptor complex. It is logical to assume that the 1-MCP–ethylene receptor complex caused the ‘Sanuki Gold’ kiwifruit to be insensitive to propylene (and by analogy, ethylene) for at least 3 d after the end of 1-MCP treatment. Further, responses of the 1-MCP-pre-treated fruit to continuous propylene treatment (MCP+P in Figs 1 and 2) indicate that fruit regained partial sensitivity after 5 d and complete sensitivity after 9 d of storage, perhaps because 1-MCP–ethylene receptor complexes were degraded after 5–9 d.
Low-temperature-modulated fruit ripening is independent of ethylene
Low temperature storage is a major post-harvest technology that is used widely to extend the post-harvest life of fresh horticultural produce. Low temperature storage is thought to slow most cell metabolic activities and thereby to delay fruit ripening and plant senescence (McGlasson et al., 1979; Hardenburg et al., 1986). However, in chilling-sensitive plants, low temperature treatments induce physiological disorders such as tissue browning, woolly or dry texture, and abnormal cell metabolism such as membrane permeability disorders resulting in chilling injury. Previously in ‘Hayward’ kiwifruit, development of low temperature breakdown (LTB) symptoms was reported in 98% of fruit stored at –0.5 °C for 24 weeks, whereas only 9% showed such symptoms when stored at 2.5 °C (Lallu, 1997). The recommended commercial storage temperatures for kiwifruit range from 0 °C to 2 °C. In this study, no LTB symptoms were observed since fruit were stored at 4 °C.
Previous studies demonstrated that storage of ‘Hayward’ kiwifruit even at low temperature results in considerable loss of fruit firmness, compared with the firmness of the fruit at harvest (McDonald and Harman, 1982; Ritenour et al., 1999; Koukouranas and Sfakiotakis, 2007). Kim et al. (1999) observed that ‘Hayward’ kiwifruit soften extensively during storage at 0 °C without significant or detectable ethylene production. In previous studies, however, no comparisons were made with fruit stored at room temperature. In this study, ‘Sanuki Gold’ kiwifruit stored at 4 °C also softened significantly, losing >85% of original flesh firmness at harvest after 1 month of storage without any measurable ethylene production. Flesh softening was accompanied by the induction and accumulation of AC-PG and AC-PL mRNAs and an increase in AC-EXP mRNA abundance (Figs 3, 4). Thus far, the gradual softening of kiwifruit during storage at low temperature has been associated with ethylene signalling (Kim et al., 1999) even in the absence of any detectable ethylene production, since kiwifruit is known to be extremely sensitive to low concentrations of exogenous ethylene and/or basal levels of ethylene, referred to as system 1 ethylene, present in most fruit (Inaba, 2007; Yin et al., 2009). Notably, kiwifruit with fungal infection easily produces huge amounts of ethylene which may significantly influence the surrounding healthy fruit and often pose great post-harvest storage challenges. Exposure of kiwifruit to exogenous ethylene results in rapid fruit softening, although in order to induce endogenous ethylene, prolonged exposure to ethylene is required. Whether slow softening occurring during low temperature storage in the absence of any measurable ethylene production in kiwifruit is independent of ethylene is still not yet clear.
Since it had been established that kiwifruit treated with 1-MCP remain completely insensitive to ethylene with no detectable ethylene responses for 3 d (Figs 1, 2), fruit stored at both low and room temperature were repeatedly treated with 1-MCP twice a week throughout the storage period to determine whether the ethylene signal is involved in the gradual fruit softening induced by cold. Surprisingly, repeated 1-MCP treatments had no effect on fruit softening and accumulation of AC-PG, AC-PL, and AC-EXP mRNAs during storage at low temperature. On the other hand, non-treated fruit stored at room temperature maintained 60% of the initial flesh firmness, four times higher than fruit stored at low temperature, while accumulation of AC-PG and AC-PL mRNA was not detected after 4 weeks of storage. In fruit repeatedly treated with 1-MCP, flesh firmness scarcely changed during storage at room temperature, indicating that ethylene could be involved in the slight softening observed in control fruit stored at room temperature, even though no measurable ethylene was detected. Taken together, these observations indicate that the fruit softening and expression of cell wall-modifying enzymes elicited by low temperature treatment in kiwifruit occur in an ethylene-independent manner.
TA levels decreased considerably in fruit stored at low temperature regardless of 1-MCP treatment, whereas fruit stored at room temperature showed only a slight reduction after 4 weeks of storage (Fig. 3C). Koukouranas and Sfakiotakis (2007) showed that 1-MCP-treated and non-treated ‘Hayward’ kiwifruit stored at 0 °C had reduced TA after 8 weeks of storage. These observations suggest that cold temperature treatment induces the ethylene-independent reduction of TA in kiwifruit.
SSC showed a steady increase in both non-treated and 1-MCP-treated fruit stored at room and cold temperature (Fig. 3B). Similarly in both non-treated and 1-MCP-treated ‘Hayward’ kiwifruit stored at 20 °C, it was observed that the SSC gradually increased, although the increase was slower in 1-MCP-treated fruit (Boquete et al., 2004). Arpaia et al. (1987) also demonstrated that the SSC steadily increased in ‘Hayward’ kiwifruit stored at both room and cold temperature. Taken together, changes in flesh firmness, expression of cell wall-modifying enzymes, TA, and SSC in ‘Sanuki Gold’ kiwifruit suggest that low temperature storage modulates fruit ripening in an ethylene-independent manner.
In winter pears, a chilling treatment is necessary for uniform acceleration of fruit ripening (Lelievre et al., 1997). In ‘Passe-Crassane’ pears, chilling treatments induced fruit ripening after subsequent re-warming through the increased activities and accumulation of ACC oxidase and ACC synthase transcripts, which result in the production of ethylene (Looney, 1972; Lelievre et al., 1997). It was also demonstrated in ‘La France’ pears that storage of fruit at 1 °C for 3 weeks induced ethylene biosynthesis and PG mRNA accumulation after removal from cold storage, leading to fruit softening (Hiwasa et al., 2003). In both types of pear fruit, treatment with 1-MCP before and after chilling suppressed the acceleration of chilling-induced fruit ripening, indicating the involvement of ethylene signalling during ripening. In apples, it has been recently shown that cold binding factor (CBF) could be involved in the activation of PG transcription during chilling-induced softening in an ethylene-independent manner (Tacken et al., 2010). It has been suggested that cold and ethylene signalling pathways act independently and synergistically to induce fruit softening in apple. These examples are precedents for the hypothesis that low temperature modulates fruit ripening in kiwifruit independently of ethylene. Therefore, kiwifruit would be able to ripen in both an ethylene-dependent and independent manner. The combination of the two systems controlling ripening would be a contribution toward increasing the period available to provide edible fruit for seed dispersers before freezing temperatures start. The ripening of kiwifruit exposed to low temperature or ethylene provides a good model to reveal the differences and similarities of ripening mechanisms between climacteric and non-climacteric fruit.
Acknowledgments
We thank Dr Ann L. T. Powell, (University of California, Davis, USA) for careful reading of and useful comments on this paper. This work was supported in part by a Grant-in-Aid for Scientific Research (grant no. 20380022 to YK) from the Japan Society for the Promotion of Science, Japan.
Glossary
Abbreviations
- EXP
expansin
- 1-MCP
1-methylcyclopropene
- PG
polygalacturonase
- PL
pectate lyase
- SSC
soluble solid content
- TA
titratable acidity
References
- Arpaia ML, Labavitch JM, Greve C, Kader AA. Changes in cell wall components of kiwifruit during storage in air or controlled atmospheres. Journal of the American Society for Horticultural Science. 1987;112:474–481. [Google Scholar]
- Ayub R, Guis M, Ben-Amor M, Gillot L, Roustan JP, Latche A, Bouzayen M, Pech J. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nature Biotechnology. 1996;14:862–866. doi: 10.1038/nbt0796-862. [DOI] [PubMed] [Google Scholar]
- Beever DJ, Hopkirk G. Fruit development and fruit physiology. In: Warington IJ, Weston GC, editors. Kiwifruit science and management. Auckland: Ray Richards Publisher; 1990. pp. 97–126. [Google Scholar]
- Birch GG, Finglas PM, Roozen JP, Shahiji F. Postharvest structural changes of Hayward kiwifruit by means of magnetic resonance imaging spectroscopy. Food Chemistry. 2009;114:1583–1589. [Google Scholar]
- Boquete JE, Trinchero DG, Fraschina AA, Vilella F, Sozzi OG. Ripening of ‘Hayward’ kiwifruit treated with 1-methylcyclopropene after cold storage. Postharvest Biology and Technology. 2004;32:57–65. [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. The Plant Cell. 1999;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Molecular requirements for the biological activity of ethylene. Plant Physiology. 1967;42:144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo N, Kruger NJ, Whatley FR. Sucrose metabolism in mango fruit during ripening. Plant Science. 1992;84:45–51. [Google Scholar]
- Cordenunsi BR, Lajolo FM. Starch breakdown during banana ripening: sucrose synthase and sucrose phosphate synthase. Journal of Agriculture and Food Chemistry. 1995;43:347–351. [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Crowhurst RN, Gleave AP, MacRae EA, et al. Analysis of expressed sequence tags from Actinidia: applications of across species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genomics. 2008;9 doi: 10.1186/1471-2164-9-351. 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biology and Technology. 1998;14:87–98. [Google Scholar]
- Guis M, Botondi R, BenAmor M, Ayub R, Bouzayen M, Pech JC, Latche A. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. Journal of the American Society for Horticultural Science. 1997;122:748–751. [Google Scholar]
- Hallett IC, MacRae EA, Wegrzyn TF. Changes in kiwifruit cell wall ultrastructure and cell packing during postharvest ripening. International Journal of Plant Sciences. 1992;153:49–60. [Google Scholar]
- Hamilton A, Lycett G, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- Hardenburg RE, Watada AE, Wang CY. The commercial storage of fruits, vegetables, and florist and nursery stocks (USDA Handbook 66) Washington, DC: USDA; 1986. [Google Scholar]
- Hiwasa K, Kinugasu Y, Amano S, Hashimoto A, Nakano R, Inaba A, Kubo Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. Journal of Experimental Botany. 2003;54:771–779. doi: 10.1093/jxb/erg073. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hubbard NL, Pharr DM, Huber SC. Role of sucrose phosphate synthase in sucrose biosynthesis in ripening bananas and its relationship to the respiratory climacteric. Plant Physiology. 1990;94:201–208. doi: 10.1104/pp.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma M. Ripening of kiwifruit ‘Hayward’ by using ethylene treatment. Japanese Fruit Grower. 1996;51:66–76. [Google Scholar]
- Ikoma Y, Yano M, Ogawa K, Yoshioka T, Xu ZC, Hisada S, Omura M, Moriguchi T. Isolation and evaluation of RNA from polysaccharide-rich tissues in fruit for quality by cDNA library construction and RT-PCR. Journal of the Japanese Society for Horticultural Science. 1996;64:809–814. [Google Scholar]
- Inaba A. Studies on the internal feedback regulation of ethylene biosynthesis and signal transduction during fruit ripening, and the improvement of fruit quality. Journal of the Japanese Society for Horticultural Science. 2007;76:1–12. [Google Scholar]
- Jiang YM, Joyce DC, Macnish AJ. Extension of the shelf life of banana fruit by 1-methylcyclopropene in combination with polyethylene bags. Postharvest Biology and Technology. 1999;16:187–193. [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Dal Cin V, Klee HJ. Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant Journal. 2007;51:458–467. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]
- Kim HO, Hewett EW, Lallu N. The role of ethylene in kiwifruit softening. Acta Horticulturae. 1999;498:255–262. [Google Scholar]
- Koukouranas Sfakiotakis E. Effect of 1-MCP pre storage treatment on ethylene and CO2 production and quality of ‘Hayward’ kiwifruit during shelf-life after short medium and long term cold storage. Postharvest Biology and Technology. 2007;46:174–180. [Google Scholar]
- Lallu N. Low temperature breakdown in kiwifruit. Acta Horticulturae. 1997;444:579–585. [Google Scholar]
- Lelievre JM, Tichit L, Fillion L, Nam Y, Pech JC, Latche A. Effects of chilling on the expression of ethylene biosynthetic genes in Passe-Crassane pear (Pyrus communis L.) fruits. Plant Molecular Biology. 1997;33:847–855. doi: 10.1023/a:1005750324531. [DOI] [PubMed] [Google Scholar]
- Looney NE. Interaction of harvest maturity, cold storage and two growth regulators on ripening of Bartlett pears. Journal of the American Society for Horticultural Science. 1972;97:81–83. [Google Scholar]
- Lopez-Gomez R, Gomez-Lim MA. A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. HortScience. 1992;27:440–442. [Google Scholar]
- MacRae EA, Redgwell RJ. Softening in kiwifruit. Postharvest News Information. 1992;3:49N–52N. [Google Scholar]
- Manolopoulou H, Lambrinos G, Assimaki H. Modified atmosphere storage of ‘Hayward’ kiwifruit. Acta Horticulturae. 1997;444:619–624. [Google Scholar]
- McDonald B, Harman JE. Controlled-atmosphere storage of kiwifruit. I. Effect on fruit firmness and storage life. Scientia Horticulturae. 1982;17:113–123. [Google Scholar]
- McGlasson WB, Scott KJ, Mendoza JDB. The refrigerated storage of tropical and subtropical products. International Journal of Refrigeration. 1979;2:199–206. [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruits with propylene gives information about biogenesis of ethylene. Nature. 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Mitchell FG. Postharvest physiology and technology of kiwifruit. Acta Horticulturae. 1990;28:291–307. [Google Scholar]
- Murray AJ, Hobson GE, Schuch W, Bird CR. Reduced ethylene synthesis in EFE antisense tomatoes has differential effects on fruit ripening processes. Postharvest Biology and Technology. 1993;2:301–313. [Google Scholar]
- Mworia EG, Yoshikawa T, Yokotani N, Fukuda T, Suezawa K, Asiche W, Ushijima K, Nakano R, Kubo Y. The effect of MA storage and 1-MCP on storability and quality of ‘Sanuki Gold’ kiwifruit harvested at two different maturity stages. Journal of the Japanese Society forHorticultural Science. 2011;80:372–377. [Google Scholar]
- Mworia EG, Yoshikawa T, Yokotani N, Fukuda T, Suezawa K, Ushijima K, Nakano R, Kubo Y. Characterization of ethylene biosynthesis and its regulation during fruit ripening in kiwifruit, Actinidia chinensis ‘Sanuki Gold’. Postharvest Biology and Technology. 2010;55:108–113. [Google Scholar]
- Nishiyama K, Guis M, Rose JKC, Kubo Y, Bennett KA, Wangjin L, Kato K, Ushijima K, Nakano R, Inaba A. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. Journal of Experimental Botany. 2007;58:1281–1290. doi: 10.1093/jxb/erl283. [DOI] [PubMed] [Google Scholar]
- Park YS, Jung ST, Gorinstein S. Ethylene treatment of ‘Hayward’ kiwifruits (Actinidia deliciosa) during ripening and its influence on ethylene biosynthesis and antioxidant activity. Scientia Horticulturae. 2006;108:22–28. [Google Scholar]
- Pratt HK, Reid MS. Chinese gooseberry: seasonal patterns in fruit growth and maturation, ripening, respiration and the role of ethylene. Journal of the Science of Food and Agriculture. 1974;25:747–757. [Google Scholar]
- Redgwell RJ, MacRae E, Hallett I, Fischer M, Perry J, Harker R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta. 1997;203:162–173. [Google Scholar]
- Redgwell RJ, Melton LD, Brasch DJ. Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa) Plant Physiology. 1992;98:71–81. doi: 10.1104/pp.98.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour MA, Crisosto CH, Garner DT, Cheng GW, Zoffoli JP. Temperature, length of cold storage and maturity influence the ripening rate of ethylene-preconditioned kiwifruit. Postharvest Biology and Technology. 1999;15:107–115. [Google Scholar]
- Rose JKC, Lee HH, Bennett AB. Expression of a divergent expansin gene is fruit-specific and ripening regulated. Proceedings of the National Academy of Sciences, USA. 1997;94:5955–5960. doi: 10.1073/pnas.94.11.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit ME. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biology and Technology. 1999;15:279–292. [Google Scholar]
- Seymour G, Taylor J, Tucker G. Biochemistry of fruit ripening. London: Chapman and Hall; 1993. [Google Scholar]
- Sfakiotakis E, Antunes MD, Stavroulakis G, Niklis N, Ververidis P, Gerasopoulos D. Ethylene biosynthesis and its regulation in ripening ‘Hayward’ kiwifruit. In: Kanellis AK, Chang C, Kende H, Grierson D, editors. Biology and biotechnology of the plant hormone ethylene. Dordrecht: Kluwer Academic Publishers; 1997. pp. 47–56. [Google Scholar]
- Silva JA, Da Costa TS, Lucchetta L, Marini LJ, Zanuzo MR, Nora L, Nora FR, Twyman RM, Rombaldi CV. Characterization of ripening behavior in transgenic melons expressing an antisense 1-aminocyclopropane-1-carboxylate (ACC) oxidase gene from apple. Postharvest Biology and Technology. 2004;32:263–268. [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Stavroulakis G, Sfakiotakis E. Regulation of propylene-induced ripening and ethylene biosynthesis by oxygen in ‘Hayward’ kiwifruit. Postharvest Biology and Technology. 1997;10:189–194. [Google Scholar]
- Tacken E, Ireland H, Gunaseelan K, et al. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE 1 gene and fruit softening. Plant Physiology. 2010;153:294–305. doi: 10.1104/pp.109.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, MacRae EA, Wright MA, Bolitho KM, Ross GS, Atkinson RG. Polygalacturonase gene expression in kiwifruit: relationship to fruit softening and ethylene production. Plant Molecular Biology. 2000;42:317–328. doi: 10.1023/a:1006309529922. [DOI] [PubMed] [Google Scholar]
- Watkins CB. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnology Advances. 2006;24:389–409. doi: 10.1016/j.biotechadv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Whittaker JD, Smith SG, Gardner CR. Expression of ethylene biosynthetic genes in Actinidia chinensis fruit. Plant Molecular Biology. 1997;34:45–55. doi: 10.1023/a:1005789220668. [DOI] [PubMed] [Google Scholar]
- Wong DWS. Pectic enzymes. In: Wong DWS, editor. Food enzymes; structure and mechanisms. New York: Chapman and Hall; 1995. pp. 212–236. [Google Scholar]
- Xu CZ, Ikoma Y, Yano M, Ogawa K, Hyodo H. Varietal differences in the potential to produce ethylene and gene expression of ACC synthase and ACC oxidase between ‘Kui mi’ and ‘Hong xin’ of Chinese kiwifruit. Journal of the Japanese Society for Horticultural Science. 1998;67:204–209. [Google Scholar]
- Yang SF. Biosynthesis and action of ethylene. HortScience. 1985;20:41–45. [Google Scholar]
- Yano M, Hasegawa Y. Ethylene production in harvested kiwifruit with special reference to ripe rot. (In Japanese with English abstract) Journal of the Japanese Society for Horticultural Science. 1993;62:443–449. [Google Scholar]
- Yin XR, Allan AC, Zhang B, Wu RM, Burdon J, Wang P, Ferguson IB, Chen KS. Ethylene-related genes show a differential response to low temperature during ‘Hayward’ kiwifruit ripening. Postharvest Biology and Technology. 2009;52:9–15. [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y. Ripening-associated ethylene biosynthesis in tomato: ripening associated ethylene biosynthesis in tomato fruits is autocatalytically and developmentally regulated. Journal of Experimental Botany. 2009;60:3433–3442. doi: 10.1093/jxb/erp185. [DOI] [PMC free article] [PubMed] [Google Scholar]