Abstract
Cytokinin is an influential hormone in growth and developmental processes across many plant species. While several cytokinin-regulated genes have been well characterized in Arabidopsis, few have been identified in tomato, Solanum lycopersicum. Here a tomato family of 11 highly related cytokinin response factor genes designated as SlCRF1–SlCRF11 (Solanum lycopersicum cytokinin response factor) are identified and characterized. SlCRFs are AP2/ERF transcription factors and generally orthologous to Arabidopsis CRF clade members (AtCRFs). Some SlCRF genes lack a direct Arabidopsis orthologue and one SlCRF has a unique protein domain arrangement not seen in any other CRF protein. Expression analysis of SlCRF1–SlCRF11 revealed differential patterns and levels across plant tissues examined (leaf, stem, root and flower). Several SlCRFs show induction by cytokinin to various degrees, similar to AtCRFs. Additionally it is shown that some SlCRFs can be regulated by other factors, including NaCl, ethylene, methyl jasmonate, and salicylic acid. Examination of SlCRF proteins in transient Agrobacterium infiltration experiments indicates they can be nuclear localized in planta. Using a bimolecular fluorescence complementation (split-yellow fluorescent protein) system, it is also shown that SlCRF proteins can interact to form homo- and heterodimers. Overall this work indicates that some SlCRFs resemble previously identified CRFs in terms of structure, expression, and cytokinin regulation. However, SlCRFs have novel CRF protein forms and responses to abiotic factors, suggesting they may have a diverse set of roles in stress and hormone regulation in tomato.
Keywords: CRF, cytokinin, cytokinin response factor, SlCRF, tomato
Introduction
Cytokinin is an essential plant hormone known to be involved in numerous plant growth and developmental processes (Mok and Mok, 2001; Werner and Schmülling, 2009). Over the last decade, a model of cytokinin signalling in plants resembling bacterial two-component systems has become well established (To and Kieber, 2008; Werner and Schmülling, 2009). In this model, the binding of a sensor histidine kinase-like receptor to cytokinin initiates a multistep phosphorelay. Upon autophosphorylation, the receptor transfers the phosphoryl group to a histidine-containing phosphotransfer protein (HPt), which then transfers the phosphate to one of two types of response regulators (RRs) localized in the nucleus. Type-B RRs, transcription factors, then activate the expression of their target genes mediating cytokinin-regulated growth and developmental processes or other aspects of plant life, whereas type-A RRs act as part of a feedback control loop to regulate this process (To and Kieber, 2008).
Recently the cytokinin response factors (CRFs) were identified as several highly related AP2/ERF transcription factors induced by cytokinin from global expression analyses in Arabidopsis (Hoth et al., 2003; Rashotte et al., 2003; 2006; Brenner et al., 2005; Kiba et al., 2005; Hirose et al., 2007). CRFs appear to form a branch pathway of the cytokinin signalling pathway and may regulate downstream cytokinin targets independently or in conjunction with type-B response regulators (Rashotte et al., 2006; Werner and Schmülling, 2009). CRFs form a unique group of ERF proteins containing a clade-specific CRF domain that is always accompanied by an AP2/ERF DNA-binding domain. Furthermore, CRF domain-containing proteins are present in all land plants, but not in green algae, indicating that they may play important roles specific to land plants (Rashotte and Goertzen, 2010). Mutant analyses in Arabidopsis have implicated CRFs in the development of cotyledons, leaves, and embryos, as indicated by reduced size of cotyledons of the crf1,2,5 triple mutant and the embryo-lethal phenotype of the crf5,6 double mutant (Rashotte et al., 2006). In general, little is known of the function of CRFs outside of Arabidopsis, and very few CRF genes from other species have been examined in any detail. The genes that have been studied, PTI6/SlCRF1 and TSI1, are linked to processes other than cytokinin regulation, including disease resistance and stress responses (Zhou et al., 1997; Park et al., 2001; Gu et al., 2002). This study was conducted to completely identify and characterize all CRF genes in tomato Solanum lycopersicum, which here are designated as SlCRF genes. Eleven SlCRF genes were identified through a combination of existing sequence comparison and rapid amplification of cDNA ends (RACE)-PCR. Once SlCRF genes were identified, their expression was examined in different plant tissues, as was regulation by cytokinin, salt, and other hormones. In addition, the cellular localization of SlCRF genes in planta and the ability of SlCRF proteins to form homo- and heterodimers with each other was determined. Together this study generates a first complete picture of all CRF genes in any species, suggesting a broader function for CRF beyond cytokinin regulation and allowing functional parallels to be made between related clades of CRFs across species.
Materials and methods
Plant materials and growth conditions
The tomato dwarf cultivar Micro-Tom was used for all experiments. Plants were grown in Sunshine Mix #8 soil under a 16:8 h light:dark photoperiod at 150 μE, with a 26 °C day (light), 22 °C night (dark) temperature.
RNA isolation, cDNA synthesis, and expression analysis
Leaves, stems, flowers, and roots were harvested from 52-day-old Micro-Tom plants, and immediately flash-frozen in liquid nitrogen. RNA was extracted using a Qiagen RNeasy Kit according to the manufacturer’s instructions. A 500 ng aliquot of the total RNA was used for each tissue type in the subsequent reverse transcription with Qiagen qScript cDNA supermix. The first strand of cDNA was diluted 10 or 20 times before it was used in the reverse transcription-PCR (RT-PCR). PCR conditions were initiated for 2 min at 95 °C, followed by cycles of 30 s at 94 °C, a 30 s annealing step, a 35 s extension at 72 °C, and a 5 min final extension at 72 °C. RT-PCR was conducted for SlCRF1–SlCRF5, SlCRF11, and TIP41 over 29 cycles with a 56 °C annealing temperature step, and for SlCRF6–SlCRF10 over 35 cycles with a 54 °C annealing temperature step. The SlCRF-specific primers used in the RT-PCR are as follows: SlCRF1forward, 5′-GGAAAATTCAGTTCCGGTGA-3′; SlCRF1reverse, 5′-AAAATTGGTAACGGCGTCAG-3′; SlCRF2 forward, 5′-TGCCGGTCCTAGAGTTGTAA-3′; SlCRF2 reverse, 5′-CAGTGGCTGCTCTGCTCTAT-3′; SlCRF3 forward, 5′-AATGATGCAGTCGAGGAACC-3′; SlCRF3 reverse, 5′-CCTGGTCTTCCCATTCTCAA-3′; SlCRF4 forward, 5′-TGAATCCCTCTGTTCCAAGG-3′; SlCRF4 reverse, 5′-GTTTTGCCATTTCCACTGCT-3′; SlCRF5 forward, 5′-ACGATGACGACGAGAGGAAT-3′; SlCRF5 reverse, 5′-CTGACACCGCGAAACTTTTT-3′; SlCRF6 forward, 5′-GGTAATGGGAAGAAGCGAGTA-3′; SlCRF6 reverse, 5′-GAAGGAAACGTCTGTGGGTAAG-3′; SlCRF7 forward, 5′-GCTTCACGAAAATGAGGTTG-3′; SlCRF7 reverse, 5′-GGTTGATGGGGTCGATTTC-3′; SlCRF8 forward, 5′-CCACCAAGGATGAGCTAAAG-3′; SlCRF8 reverse, 5′-GTGGCACGGTGTTGATGG-3′; SlCRF9 forward, 5′-TGAGGAAATGGGGGAAATATG-3′; SlCRF9 reverse, 5′-TGTCATCAAAGCCTAGAAGTT-3′; SlCRF10 forward 5′-TGATGATGAAGGGGT TGATGTA-3′; SlCRF10 reverse, 5′-TGCTGGAGATGTGTGTGAAGTA-3′; SlCRF11 forward, 5′-AAGTGCCTGAGTTGGCTATG-3′; and SlCRF11 reverse, 5′-TCACCCTCGATCAGATAAAC-3′. All samples are compared with the control gene TIP41 (Expósito-Rodríguez et al., 2008).
SlCRF gene expression in response to hormone or salt treatment, as described below, was examined using RT-PCR initiated with 2 min at 95 °C, followed by 29–40 cycles of 30 s at 94 °C, 45 s at 57 °C, and 40 s at 72 °C, and a 5 min final extension at 72 °C. RT-PCR at different cycle lengths was performed for genes of varying intensities: SlCRF3 (29 cycles), SlCRF1, SlCRF2, SlCRF4, SlCRF6, SlCRF10, and SlCRF11 (30 cycles), SlCRF5 (30 cycles for salt, 35 for other treatments), SlCRF7 [35 cycles for methyljasmonate (MeJA), 40 for other treatments), and SlCRF8 and SlCRF9 (40 cycles). Primers used to examine SlCRF3–5 and TIP41 were as noted above. RT-PCR primers for SlCRF1, SlCRF2, and SlCRF6–11 are as follows: SlCRF1 forward, 5′-AACGATGTCGCTTTGTCACC-3′; SlCRF1 reverse, 5′-GGGCAAAATCGTCAAAGTCA-3′; SlCRF2 forward, 5′-ATGCTGCCGGTCCTAGAGTT-3′; SlCRF2 reverse, 5′-GAGCAGTTTCCGACGATGAC-3′; SlCRF6 forward, 5′-AGATGAGCTTTTTGGGCGTA-3′; SlCRF6 reverse, 5′-TCGCTTCTTCCCATTACCAC-3′; SlCRF7 forward, 5′-ACGTTGGTTGGGAAGTTTTG-3′; SlCRF7 reverse, 5′-TAATGGTTGATGGGGTCGAT-3′; SlCRF8 forward, 5′-ACGTTGGTTGGGAACTTTTG-3′; SlCRF8 reverse, 5′-GTGTTGATGGGGTTGATTCC-3′; SlCRF9 forward, 5′-GCGTTGCCTAAAGGAGTTAG-3′; SlCRF9 reverse, 5′-ACCAGGGCTCAAATTCTTAC-3′; SlCRF10 forward, 5′-CTCAGAGTTTGGTCTCACATAC-3′; SlCRF10 reverse, 5′-AACATGTCCATCTCCGTATC-3′; SlCRF11 forward, 5′-AAGTGCCTGAGTTGGCTATG-3′; and SlCRF11 reverse, 5′-TCACCCTCGATCAGATAAAC-3′. For characterizing SlCRF7 response to ethephon and SlCRF8 response to MeJA, primers used are the same as those utilized for examining the expression in different organs as noted above.
For quantitative real-time PCR (qRT-PCR) analysis, total RNA was extracted from cytokinin- or dimethylsulphoxide (DMSO) control-treated leaves using the same reagents and protocol as described for RT-PCR. A 500 ng aliquot of total RNA was converted into cDNA with Qiagen qScript cDNA supermix. A 2 μl aliquot of a 20-fold cDNA dilution was used for each reaction in the following qPCR. qPCR was performed with the SYBR-Green chemistry in a Eppendorf Mastercycler ep realplex with the same set of primers used for examining salt or hormone responses except SlCRF1 and SlCRF2. Primers for SlCRF1 and SlCRF2 are the same as used in the first RT-PCR experiment. Each reaction contains 9 μl of SYBR-Green supermix, 2 μl of cDNA template, 3 μl of 4 μM primers, and 3 μl of sterile water. The qPCR program consists of one cycle at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at 56 °C, and 35 s at 68 °C. The relative expression data used in the figure represent means±SE of two biological replicates. All samples are compared with the control gene TIP41 (Expósito-Rodríguez et al., 2008).
Hormone and salt treatments
For all hormone and salt (NaCl) treatments, plants were grown as described above and then leaves or other tissues were excised from 15-day-old Micro-Tom plants, placed in water, and gently shaken for 2 h prior to treatment. Then treatments or appropriate controls were added to shaking tissue for various times as indicated: 5 μM cytokinin (N6-benzyladenine; BA), 100 μM MeJA, and 2 mM SA (salicylic acid), each with the carrier solvent DMSO, and 200 mM NaCl and 1 mM Ethephon (of which ethylene is a breakdown product) with the appropriate level water controls. After designated treatment times (1 h or 3 h) leaves were removed from solution, patted dry, and immediately flash-frozen in liquid nitrogen, and stored at –80 °C until RNA extraction.
Phylogenetic analysis
Full-length sequences of SlCRF genes were originally identified by making use of existing sequence data from the four full-length SlCRF genes (SlCRF1, SlCRF3, SlCRF4, and SlCRF5) that were previously known either through 3′ RACE-PCR analysis of partial unigene constructs (SlCRF3, SlCRF4, and SlCRF5) or from an existing gene sequence for SlCRF1, also known as PTI6. BLAST analysis of the tomato unigene collection and now fully sequenced tomato genome was conducted using these four SlCRF genes and additional CRF sequences from other species, primarily Arabidopsis, at http://solgenomics.net using publicly available genome sequence data from the International Tomato Genome Sequencing Project and from the Kazusa Full-length Tomato cDNA Database at http://www.pgb.kazusa.or.jp/kaftom. Searches were done primarily using conserved AP2/ERF- or CRF-specific domain regions of the known SlCRF genes in a manner similar to that done in the identification of CRF genes in a wide range of plant species (Rashotte and Goertzen, 2010). Once all full-length SlCRF gene sequences were found, they were translated and aligned as proteins in CLC Sequence Viewer v6.5.1 using default parameters. A phylogenic cladogram was generated using the Neighbor–Joining method via bootstrap analysis of full-length aligned SlCRF proteins again in CLC Sequence Viewer v6.5.1 using default parameters. Arabidopsis genes examined herein are designated as follows: CRF9 (At1g49120), CRF10 (At1g68550), CRF11 (At3g25890), and CRF12 (At1g25470); and were previously noted as B-clade members of the CRF genes in Rashotte and Goertzen (2010), CRF9=CRF-B1, CRF10=CRF-B3, CRF11=CRF-B4, and CRF12=CRF-B2.
Protein examination
Vector construction:
All plasmids for BiFC (bimolecular fluorescence complementation) were generated using the Invitrogen GATEWAY™ cloning system according to the manufacturer’s instructions. Entry clones for SlCRF1, SlCRF2, SlCRF3, and SlCRF5 were prepared/generated via a BP reaction using the pDONR221 and the att-B PCR product containing att-B adaptor sites and full-length cDNA sequence except the stop codon. Through an LR reaction, coding sequence was transferred to destination vectors pSAT4-DEST-n (1–174) EYFP-C1 and pSAT5-DEST-c (175–end) EYFP-C1 which have N- and C-terminal parts of the yellow fluorescent protein (YFP) gene, respectively. These destination clones were later used to transform Micro-Tom protoplasts. To examine cellular localization in planta, SlCRF1, SlCRF2, and SlCRF5 were transferred, through an LR reaction, to the 35S:SlCRF:GFP (green fluorescent protein) constitutive expression destination vector pMDC84. These destination clones were later used to transform Agrobacterium tumefaciens that was injected into tobacco leaves. All destination vectors were obtained through the ABRC at Ohio State University.
Protoplast isolation and transformation for BiFC analysis
For isolating leaf protoplasts, leaves were taken from 15-day-old plants, cut into thin strips, and placed in enzyme solution [2% Cellulase R10, 1% Macerozyme R10, 0.6 M mannitol, 20 mM KCl, 25 mM MES solution, pH 5.7 which was heated at 55 °C for 10 min, then cooled down to room temperature before adding 10 mM CaCl2 and 1% bovine serum albumin (BSA)] under vacuum for 30 min. Next, leaf strips were gently shaken for 4 h or overnight at 40–60 rpm before increased shaking at 90–100 rpm for 10 min to release protoplasts. Enzyme solution containing the protoplasts was filtered with a 40 μm cell sifter into a 50 ml conical tube and spun at 100 g for 2 min to pellet the protoplasts. Pelleted protoplasts were resuspended in 2 ml of cold wash solution (0.6 M mannitol, 5 mM MES pH 5.7, 20 mM KCl, 10 mM CaCl2) and spun again. Then the pellet was resuspended in wash solution to obtain the final volume for electroporation and kept on ice until transformation. Electroporation of protoplasts was performed as in Rashotte et al. (2006) and then they left undisturbed in the dark at room temperature overnight prior to microscopic observation.
Agrobacterium infiltration and transformation for in planta examination of cellular location
Tobacco (Nicotiana tabacum) plants were grown under a long day 16 h light 26 °C, 8 h dark 22 °C cycle. Destination vectors used for transformation (SlCRF genes in pMDC84, as described above) were transformed into A. tumefaciens (C58-C1) by a method similar to that used in Rashotte et al. (2006), leading to a floral dip. However, once properly antibiotic-selected individual colonies were identified, further grown up in liquid culture, and spun down, they were then resuspended in infiltration media (10 mM MgCl2, 10 mM MES, 100 μM acetosyringone) and left at room temperature for 3 h similar to the method of Liu et al. (2002). Agrobacterium was then infiltrated into the abaxial side of 14- to 21-day-old plant leaves using a needle-less 2 ml syringe. Plants were then examined for transient transformation and GFP expression 48–72 h after injection using epifluorescence microscopy as in Cutcliffe et al. (2011).
Epifluorescence microscopy
BiFC and Agrobacterium-infiltrated tobacco leaves were examined using a Nikon Eclipse 80i epifluorescence microscope with a UV source in transformed protoplast. A standard UV filter was used in addition to 1 ng ml−1 of Hoechst 33342 dye initially to observe and identify nuclei in intact cells as a measure of the cell viability. A YFP filter that blocks both chlorophyll fluorescence and Hoechst 33342 fluorescence was used to examine the localization of any split-YFP fusions that occur due to BiFC between proteins. Cytokinin (2 μM BA) was routinely added to protoplasts prior to examination. A GFP filter that blocks both chlorophyll fluorescence and Hoechst 33342 fluorescence was used to examine cellular localization of any cells expressing GFP in Agrobacterium-infiltrated tobacco leaves. All photos were taken with a Qimaging Fast 1394 digital camera and are presented as composite images using Adobe Photoshop CS3 without altering the original integrity of the picture.
Results
Identification of novel tomato CRF genes (SlCRF genes)
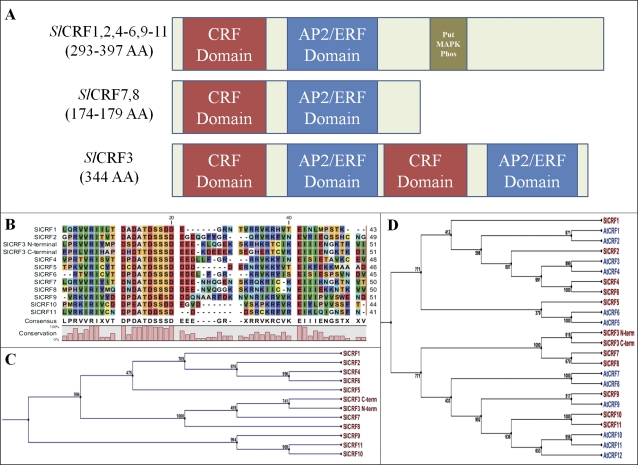
A family of 11 CRF genes from tomato, known as Solanum lycopersicum cytokinin response factor genes or SlCRF1–SlCRF11, have been identified and characterized (Fig. 1, Table 1; Supplementary Table S1 available at JXB online) These genes are members of the AP2/ERF transcription factor family, specifically related to clade VI and VI-L of the ERF subfamily of genes, known in Arabidopsis as AtCRF genes (Sakuma et al., 2002; Nakano et al., 2006; Rashotte and Goertzen, 2010). These genes were identified from a combination of BLAST searches of emerging tomato genome sequence resources using previously identified CRF genes in tomato, orthologous AtCRF sequences, and 3' RACE of incomplete expressed sequence tag (EST) unigene builds of SlCRF genes. Previous work identified transcription of four SlCRF sequences (SlCRF1, SlCRF3, SlCRF4, and SlCRF5), including the existing PTI6 gene, that has also been designated as SlCRF1 (Rashotte and Goertzen, 2010). From this base, 10 novel full-length expressed CRFs (SlCRF2–SlCRF11) have been identified, comprising all proteins in tomato containing a CRF domain, a defining characteristic of CRF proteins (Fig. 1, Table 1; Supplementary Table S1). In several cases 3′ RACE was used to generate full-length gene transcripts from assembled unigenes lacking a 3′ end region. Subsequent genome assemblage and sequenced bacterial artificial chromosome (BAC) contigs have verified the determined sequence identified from 3′ RACE experiments. Full-length transcripts for SlCRF1–SlCRF11 are presented (Supplementary Table S1). SlCRFs at a protein level fall into three classifications (Fig. 1A). One is a standard CRF protein (SlCRF1, SlCRF2, SlCRF4–SlCRF6, and SlCRF9– SlCRF11), which contains both a CRF and AP2 DNA-binding domain in addition to a putative mitogen-activated protein kinase (MAPK) phosphorylation motif, as seen in a wide range of plant species (Rashotte and Goertzen, 2010). The second is a shortened CRF protein (SlCRF7 and SlCRF8), which contains the CRF and AP2 DNA-binding domain, but lacks the 3' third of the protein and the phosphorylation motif, as is also seen in other species such as Arabidopsis (CRF7 and CRF8). The final classification is a unique CRF protein (SlCRF3), containing two CRF and AP2 DNA-binding domains in an alternating pattern. This is the only known CRF protein that contains more than a single CRF domain and is expressed, from >250 identified CRF proteins examined across all land plants. Interestingly its chromosomal position is very close to the highly related SlCRF8, only 9125 bp away, suggesting a possible gene duplication event (Table 1).
Fig. 1.
SlCRF protein form, alignment, and phylogenic relationships. (A) A model of SlCRF protein form including size, domains, and motifs for all 11 SlCRFs. (B) Protein sequence alignment of the CRF domain for SlCRF1– SlCRF11 is shown with a sequence consensus, including both SlCRF3 CRF domains. (C) Neighbor–Joining tree of SlCRF proteins based on alignment of the CRF domain with support values shown out of 1000 bootstrap replicates. (D) Neighbor–Joining tree of SlCRF and Arabidopsis CRF (AtCRF) proteins based on alignment of both the CRF and AP2 DNA-binding domains with support values shown out of 1000 bootstrap replicates.
Table 1.
SlCRF gene description
| Gene name | Chromosome/position (Build 2.40) | Gene model | Size (amino acids/bp) |
| SlCRF1/PTI6 | Ch 6 (44654446–44653700) | Solyc06g082590 | 248/747 |
| SlCRF2 | Ch 8 (62045738–62046757) | Solyc08g081960 | 340/1023 |
| SlCRF3 | Ch 1 (2911579–2910313) | Solyc01g008890 | 344/1035 |
| SlCRF4 | Ch 3 (2016125–2014935) | Solyc03g007460 | 396/1191 |
| SlCRF5 | Ch 1 (78502891–78503773) | Solyc01g095500 | 293/882 |
| SlCRF6 | Ch 6 (32043471–32044523) | Solyc06g051840 | 350/1053 |
| SlCRF7 | Ch 1 (14595809–14596333) | Solyc01g014720 | 174/525 |
| SlCRF8 | Ch 1 (2901188–2900649) | Solyc01g008880 | 175/540 |
| SlCRF9 | Ch 3 (62191449–62190256) | Solyc03g119580 | 397/1194 |
| SlCRF10 | Ch 5 (3622457–3621438) | Solyc05g009450 | 339/1020 |
| SlCRF11 | Ch 4 (874453–875505) | Solyc04g007180 | 350/1053 |
Alignment of these proteins revealed high similarity in domain regions, such as the core conserved region DPDATDSSSD of the CRF domain (Fig. 1B), similar to that seen in previous alignments of CRF proteins from a wide range of land plants (Rashotte and Goertzen, 2010). For ease of alignment and phylogenic analyses in this study, the full-length SlCRF3 was split into N- and C-terminal parts each containing a CRF and AP2 domain, although a full-length version yielded similar results (data not shown). Phylogenetic analysis based on similar domain sequences indicates that some SlCRFs have a paired relationship, suggesting an ancient duplication, as well as most SlCRFs having an Arabidopsis orthologue (Fig. 1C; D). Tomato and Arabidopsis do not have directly orthologous phylogenetic protein pairs since, in some cases, a single SlCRF protein is grouped with two Arabidopsis proteins (SlCRF2 with AtCRF1 and AtCRF2; SlCRF5 with AtCRF5 and AtCRF6). Additionally, SlCRF1 has no orthologous Arabidopsis gene partner (Fig. 1D), although it is part of a related subclade of CRF proteins found in a number of other species (Rashotte and Goertzen, 2010).
SlCRF genes are expressed in different plant tissues
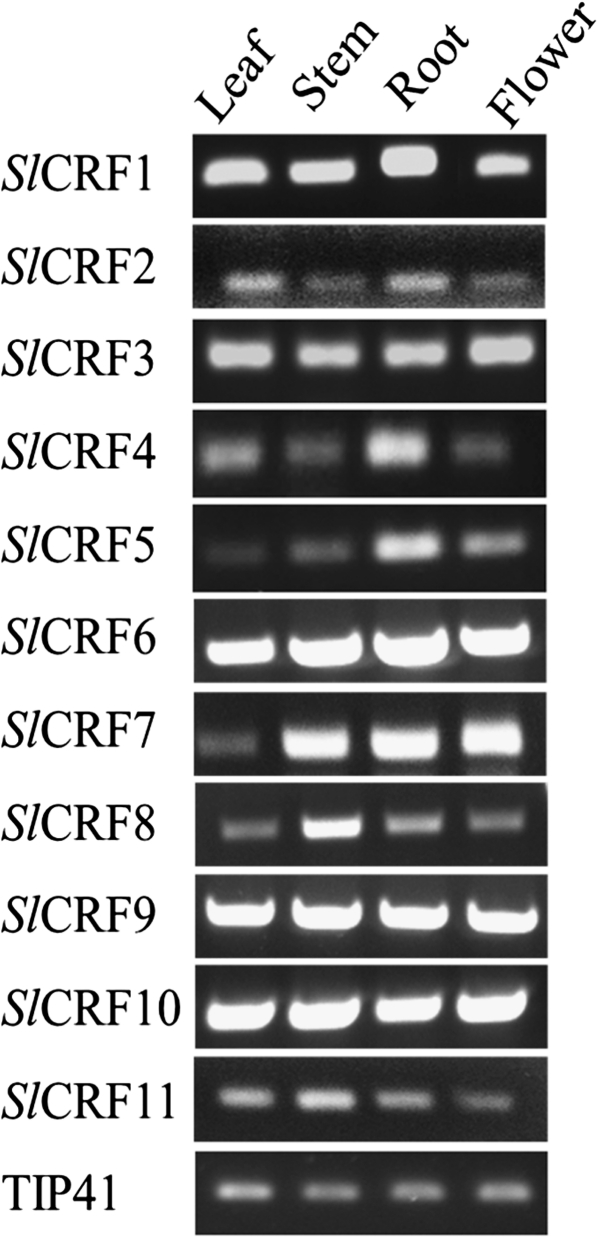
Previous work identified four SlCRF genes (SlCRF1, SlCRF3, SlCRF4, and SlCRF5) as expressed in leaf tissues (Rashotte and Goertzen, 2010). Here it is shown that SlCRF3– SlCRF11 are expressed in multiple different plant tissues throughout the plant (leaf, stem, root, and flowers) to varying degrees (Fig. 2). Generally, SlCRF expression levels were consistent across plant tissues examined. However, some genes showed preferential tissue expression, as seen for roots in SlCRF4 and SlCRF5 and for stems in SlCRF8 and SlCRF11 (Fig. 2).
Fig. 2.
SlCRF expression patterns in various tomato tissues. RT-PCR analysis of SlCRF1– SlCRF11 in leaf, stem, root, and flower tissues of 52-day-old plants is shown. The TIP41 gene serves as an internal control.
SlCRF transcript levels are regulated by cytokinin and salt
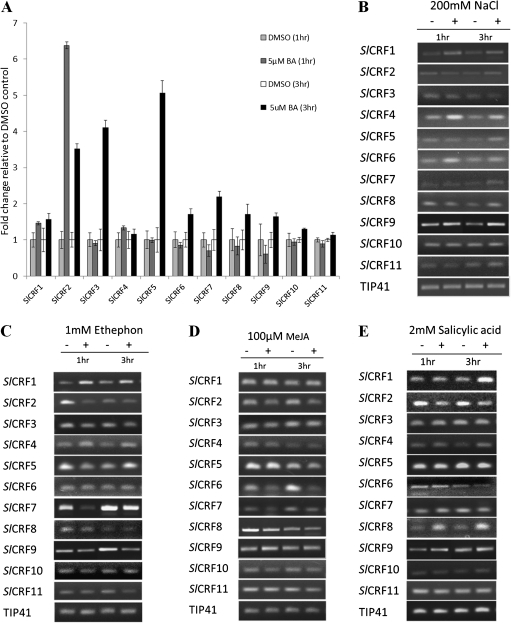
Knowing that several CRFs in Arabidopsis have previously been shown to be induced by cytokinin, the regulation of SlCRF genes by cytokinin was examined. Tomato leaves (15 d old) were treated with cytokinin (5 μM BA) or DMSO as a vehicle control for 1 h and 3 h and examined using real-time PCR. Three SlCRF genes (SlCRF2, SlCRF3, and SlCRF5) were found that are strongly (4- to 6-fold) induced by cytokinin (Fig. 3A). SlCRF2 showed rapid induction by cytokinin at 1 h after treatment to 6-fold over untreated levels and by 3 h was still induced, although at this point only ∼3.5-fold over control levels. Both SlCRF3 and SlCRF5 showed no induction at 1 h, but were highly induced (4- to 5-fold) after 3 h of cytokinin treatment. A few other SlCRF genes showed weaker levels (1.5- to 2-fold) of induction at 3 h of cytokinin treatment (SlCRF1, SlCRF6, SlCRF7, SlCRF8, and SlCRF9), whereas SlCRF4, SlCRF10, and SlCRF11 showed no change in expression (Fig. 3A). The results follow a pattern similar to that seen for AtCRF genes whereby some, but not all, members of this group are transcriptionally regulated by cytokinin (Rashotte et al., 2006).
Fig. 3.
Expression response of SlCRF genes to hormones and salt. Relative expression in 15-day-old leaves of SlCRF1–SlCRF11 in response to hormone or salt treatment at 1 h and 3 h after treatment versus non-treated controls. (A) qRT-PCR of cytokinin (5 μM BA) treatment. Data presented are a mean±SE (two biological replicates). Light grey bar, 1 h DMSO control; dark grey bar, 1 h BA treatment; white bar, 3 h DMSO control; black bar, 3 h BA treatment. (B) RT-PCR of salt (200 mM NaCl) treatment. (C) RT-PCR of ethylene (1 mM Ethephon) treatment. (D) RT-PCR of methyl jasmonate (100 μM MeJA) treatment. (E) RT-PCR of salicylic acid (2 mM SA) treatment. Data presented for RT-PCR are from a representative sample of experiments, with the TIP41 gene serving as an internal control.
SlCRF genes were also examined for changes in response to salt and other hormones in leaves treated at 1 h and 3 h versus controls using RT-PCR. The results revealed expression changes in several genes, although many showed little to no alterations (Fig. 3). Expression analysis of salt treatment (200 mM NaCl) revealed induction of SlCRF1, SlCRF4, and SlCRF6 at both 1 h and 3 h as well as a minor induction of SlCRF2, SlCRF5, and SlCRF7 at 3 h (Fig. 3B). This suggests a new potential role for SlCRF genes in stress regulation. Expression analysis of ethylene treatment (1 mM Ethephon) showed some induction of SlCRF1 and SlCRF4 at both 1 h and 3 h, while SlCRF2 was repressed at both 1 h and 3 h and SlCRF7 at 1 h (Fig. 3C). These are some of the first data linking any CRF to ethylene. Expression analysis of 100 μM MeJA treatment showed only a single transcript change, the repression of SlCRF6 at both 1 h and 3 h (Fig. 3D). Expression analysis of 2 mM SA treatment revealed induction of SlCRF1 at 3 h as well as induction of SlCRF4 and SlCRF8 at both 1 h and 3 h (Fig. 3E). Together these results suggest that SlCRF genes can be regulated by factors other than cytokinin.
SlCRF proteins show nuclear localization in planta
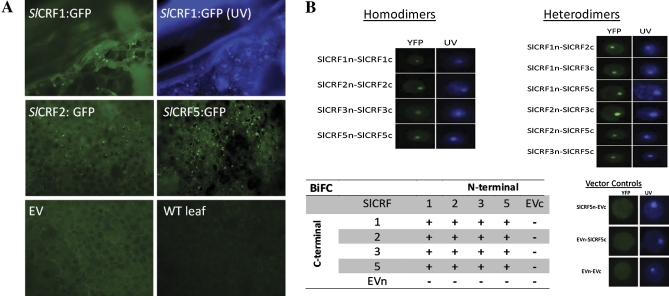
The cellular localization of specific SlCRF proteins (SlCRF1, SlCRF2, and SlCRF5) was examined by transiently expressing GFP-tagged SlCRF proteins in tobacco leaves via an Agrobacterium infiltration method (Fig. 4A). Leaves infiltrated with 35S:SlCRF:GFP vectors were examined for expression after 48 h. Each of the SlCRF proteins examined was found localized in the nucleus of leaf mesophyll cells and not other organelles in regions adjacent to infiltration sites as compared with empty transformed vectors or wild-type untransformed plants (Fig. 4A). Although localization of SlCRFs can be seen in the nucleus of cells, it is not obviously absent from the cytoplasm, which is consistent with previous models of AtCRFs that appear to move between the cytoplasm and nucleus. This is also in agreement with the cellular localization of SlCRFs as predicted by PSORT computer protein localization prediction models (data not shown), indicating preferences primarily for nuclear, cytoplasmic, or either nuclear or cytoplasmic protein localization.
Fig. 4.
SlCRF protein localization and protein–protein interactions. (A) Cellular localization of SlCRF1, SlCRF2, and SlCRF5 in tobacco leaves transiently transformed with 35S:SlCRF:GFP vectors via Agrobacterium infiltration. Representative examples of GFP expression from tagged SlCRF proteins indicate a strong nuclear localization in regions of transformed leaves visualized under UV light using a GFP wavelength filter (panels labelled SlCRF:GFP).The panel labelled SlCRF1:GFP (UV) is the same sample as SlCRF1:GFP shown without the GFP filter in the presence of Hoechst 33342 dye denoting the nucleus. EV denotes an empty vector control and WT leaf denotes an untransformed sample. (B) SlCRF proteins (SlCRF1, SlCRF2, SlCRF3, and SlCRF5) were analysed for potential homo- and heterodimerization using BiFC. Representative examples of positive SlCRF dimerizations are shown both under UV light in the presence of Hoechst 33342 dye denoting the nucleus and using a YFP wavelength filter to visualize BiFC interaction. Additionally, representative examples of empty vector (EV) controls for both N- and C-terminal BiFC vectors (EVn and EVc) are shown. A table of SlCRF interactions is shown, with (+) as positive and (–) for non-interactions.
SlCRF proteins interact among themselves
Protein–protein interactions can be important for functional regulation of proteins. In order to determine if this level of regulation occurs among SlCRFs, potential interactions were examined using the BiFC analysis split-YFP system. SlCRF proteins (SlCRF1, SlCRF2, SlCRF3, and SlCRF5) were placed into specific vectors which enabled their expression linked to either an N- or C-terminal half of a YFP protein, such that fluorescence would not be visible unless proteins containing each YFP half interact. Proteins were examined for interaction by electroporation of tomato leaf mesophyll protoplasts followed by epifluorescence microscopy (Fig. 4B). It was found that homodimers formed between all SlCRFs examined. In addition, heterodimers could also form with all SlCRF combinations examined (Fig. 4). In these experiments, while cytokinin is not required to observe nuclear localization, it is easier to visualize nuclear localization after its addition, so it is routinely added. Overall these findings are consistent with what has been found for AtCRFs and suggest that because there is a pattern for potential of all SlCRF proteins to interact, regulation of SlCRFs at the level of protein dimerization is unlikely to occur (Cutcliffe et al., 2011).
Discussion
Cytokinin is involved in various plant growth and developmental processes of great agronomic importance, yet few cytokinin-regulated genes have been studied in crop plants. This study presents the first examination of a complete set of CRF genes in a crop species, tomato (S. lycopersicum). Eleven SlCRF genes (SlCRF1– SlCRF11) were identified in this study as part of a larger group of CRF genes present in all land plants (Rashotte and Goertzen, 2010). SlCRF proteins contain the hallmark domains of this group; a CRF and AP2 DNA-binding domain, as well as a putative MAPK motif found in many other CRF proteins (Fig. 1; Rashotte and Goertzen, 2010). One SlCRF, SlCRF3, was found to have a unique protein structure containing two CRF and two AP2 domains (Fig. 1; Supplementary Table S1 at JXB online). While several AP2/ERF proteins contain two AP2 domains, including the founding member of this group, SlCRF3 is the only known protein to contain more than a single CRF domain. Despite this, it appears to be actively transcribed, induced by cytokinin, and able to interact with other SlCRFs proteins.
A phylogenetic analysis of SlCRFs shows relationships similar to that seen for AtCRFs and the overall group of CRFs in plants (Rashotte and Goertzen, 2010). Despite overall similarities between tomato and Arabidopsis CRFs, there are several differences that may suggest functional differences between species. An example is the existence of a single SlCRF gene where there are two paralogues in Arabidopsis, such as SlCRF5 compared with AtCRF5 and AtCRF6 (Fig. 1D). Another difference is that SlCRF1 has no direct Arabidopsis orthologue. In fact, most plant species appear to have a SlCRF1 orthologue, indicating that the condition in tomato is more common (Rashotte and Goertzen, 2010). It also suggests that the function of SlCRF1 is unlikely to be simply determined through studies of CRFs in Arabidopsis.
Expression of SlCRF1– SlCRF11 in tissues from roots to flowers suggests a broad role for these genes in the plant (Fig. 2). There also appears to be a range of transcript levels of SlCRFs potentially indicating different functional roles in different tissues. This is the most complete tissue analysis of a CRF group of genes from any species excluding Arabidopsis where microarray-generated data of AtCRFs reveal a pattern of expression across most tissue types and development, not unlike that seen for the SlCRFs in this study, suggesting that CRFs in most plants are likely to be expressed broadly across tissues (data not shown).
Several SlCRF genes were found to be induced by cytokinin, mirroring a pattern seen in Arabidopsis where only some CRF genes show strong induction by cytokinin (Rashotte et al., 2006). Interestingly these AtCRF genes parallel the SlCRF genes strongly induced in this study. SlCRF2, highly similarly to AtCRF2, shows the most rapid induction of tomato CRF genes comparable with very rapid induction of AtCRF2 (Fig. 3A; Rashotte et al., 2006). SlCRF5, similar to both AtCRF5 and AtCRF6, is also highly induced by cytokinin (Figs 1D, 3A; Rashotte et al., 2006). SlCRF5 is not as rapidly induced as SlCRF2, which parallels the slower cytokinin induction of AtCRF6 compared with other CRF genes (Rashotte et al., 2006). SlCRF3 is a unique gene, occurring only in tomato, and as such it is difficult to assess its role in cytokinin regulation, although it is clearly induced by cytokinin in a similar fashion to SlCRF5. The lack of cytokinin regulation of some highly related pairing of SlCRF genes also parallels expression studies of other AtCRF genes, such as SlCRF4 and SlCRF6 compared with AtCRF3 and AtCRF4. Overall, the pattern of transcriptional cytokinin regulation of SlCRF genes is similar to that of AtCRF genes and suggests that there may be similar regulation within specific clades of CRF genes.
Other factors that might transcriptionally affect SlCRFs as they had been shown to affect related ERF family members were examined: salt, ethylene, MeJA, and SA (Gu et al., 2000, 2002; Park et al., 2001; Sakuma et al., 2002; Nakano et al., 2006; Zarei et al., 2011). Treatment with salt (NaCl) induced about half of the SlCRFs to some degree (Fig. 3B), revealing that CRFs can be induced by abiotic factors. An investigation of related AtCRFs (AtCRF2, AtCRF5, and AtCRF6) also indicated induction by NaCl treatment from an examination of publically available microarray data. Previous examinations of the tobacco stress-induced 1 (TSI1) gene (a CRF member) has shown transcript induction during high salt stress in both overexpressing and RNAi (RNA interference) transgenic plants (Park et al., 2001; Han et al., 2006). The present finding that several SlCRFs are induced by salt treatment supports the previous finding for Tsi1 and suggests that CRFs play a role in salt stress response and may be involved in more general regulation of stress responses. Ethylene treatment resulted in a mixed set of responses from SlCRFs, from some induction to repression, with little effect on the majority of SlCRFs (Fig. 3C). Previous studies have shown that ethylene had little to no effect on AtCRFs and SlCRF1/Pti6, consistent with most SlCRFs in this study. The exception, SlCRF2 transcript repression, indicates that ethylene may play some role in SlCRF function, although a more detailed study is needed to determine further the extent. MeJA treatment showed almost no effect on any SlCRFs, suggesting that it plays little role in CRF function, although specific CRFs such as SlCRF6 may be exceptions (Fig. 3D). SA treatment resulted in minor induction of three SlCRFs similar to MeJA treatments, indicating that SA also appears to have little effect on the transcription of most SlCRFs. Together these results suggest that SlCRFs can be regulated by factors other than cytokinin and may fall into different groups of regulated genes: some (SlCRF3 and SlCRF5) regulated primarily by cytokinin, others (SlCRF1, SlCRF2, SlCRF4, SlCRF6, SlCRF7, and SlCRF8) regulated by several factors, and some (SlCRF9– SlCRF11) showing little response to factors examined in this study. A broader examination of SlCRF expression patterns, beyond this study, is needed to determine the functional role of each SlCRF.
Previous examinations of non-Arabidopsis CRF genes have shown links to pathogen response when overexpressed for Pti6 from tomato (SlCRF1) and Tsi1 from tobacco (Zhou et al., 1997; Park et al., 2001; Gu et al., 2002). While pathogen response was not examined in this study, the finding that SlCRF1 is induced by the factors ethylene and SA is linked to this process, and supports this previous reported role for SlCRF1 (Zhou et al., 1997; Gu et al., 2002). The finding that several other SlCRF genes are affected by these similar treatments may suggest that an effect on pathogen response could be a broader functional characteristic of some SlCRF genes.
Cellular localization is often an important factor for determining the function of proteins such as transcription factor localization to the nucleus required for their mode of action: binding to DNA. AtCRFs in protoplasts were previously shown to be throughout the cytoplasm and localized to the nucleus with the addition of exogenous cytokinin (Rashotte et al., 2006). Protoplasts are good single cell systems to examine cellular localization, but lack several aspects of a true in planta system that may reflect a more accurate result. To overcome this, GFP-tagged SlCRF proteins were transiently expressed in tobacco leaves where SlCRFs were found to be primarily nuclear localized in the absence of exogenous cytokinin, although some cytoplasmic localization as well cannot be ruled out (Fig. 4A). SlCRF localization to both the nucleus and cytoplasm would be consistent with previous results of AtCRFs and with protein localization prediction data for SlCRFs (Rashotte et al., 2006). It may be that CRFs act in a manner similar to the Arabidopsis histo-phospho transfer proteins (AHPs) known to move between the cytoplasm and the nucleus relaying a cytokinin signal in that pathway. Initial work examining AHP localization in protoplasts showed cytoplasmic expression followed by nuclear localization after the addition of exogenous cytokinin, similar to that of the AtCRFs (Hwang and Sheen, 2001). However, a recent in planta examination of AHPs revealed a strong nuclear expression of these proteins in root tissues, where there are high levels of endogenous cytokinin (Punwani et al., 2010). However, AHPs were also found to a lesser degree in the cytosol, consistent with a cycling between nucleus and cytosol needed for these proteins to function as phosphate carriers in cytokinin signalling (Punwani et al., 2010). The identification of SlCRFs primarily localized in the nucleus, without the addition of exogenous cytokinin, suggests a similar mechanism, in which intact leaf mesophyll cells contain levels of endogenous cytokinin high enough to focus SlCRF to the nucleus. It is contended that protoplasts contain very low levels of endogenous cytokinin, such that CRFs are not routinely found localized within their nucleus until exogenous cytokinin is added, consistent with the findings presented here.
Protein–protein interactions are very common and important in signal transduction, including the regulation of transcription factors by patterns of homo- or heterodimerization with other partners (Pawson and Scott, 1997; Pawson and Nash, 2000; Kasahara et al., 2001). It was found that each of the SlCRFs examined was able to form both homodimers and heterodimers with the other SlCRFs, suggesting that SlCRFs are unlikely to be regulated at this level. Although not all SlCRFs were examined in this study, the results of the representative SlCRFs examined here are consistent with a larger study of protein–protein interactions among AtCRFs, showing widespread homo- and heterodimerization and indicating that the CRF domain itself is likely to be involved in this interaction (Cutcliffe et al., 2011). Interestingly, the presence of an additional CRF and AP2 DNA-binding domain in SlCRF3 does not appear to affect these interactions.
In summary, this work identifies and characterizes 11 CRF genes in tomato (SlCRF1– SlCRF11). It is shown that SlCRF1– SlCRF11 are expressed at varying levels over a range of tissues. SlCRF proteins appear to show nuclear localization and can interact to form homo- and heterodimers amongst themselves. Several SlCRFs show strong induction by cytokinin similar to that previously noted for AtCRFs. Additionally, some SlCRFs were found to be regulated by factors other than cytokinin, potentially suggesting a diverse role for CRFs in stress and other hormone regulation in plants. This study indicates that SlCRFs appear to have multiple regulatory functions in tomato plants.
Supplementary data
Supplementary data are available at JXB online.
Table S1. SlCRF gene and protein sequences. Full-length DNA coding sequences as well as translated amino acid protein sequences for SlCRF1–SlCRF11 are shown.
Acknowledgments
We gratefully acknowledge all members of the Rashotte lab for critical reading of the manuscript. This work was funded by USDA-NRI grant 2008-35304-04457 and AAES-HATCH grant 370220-310007-2055.
References
- Brenner WG, Romanov GA, Kollmer I, Burkle L, Schmülling T. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. The Plant Journal. 2005;44:314–333. doi: 10.1111/j.1365-313X.2005.02530.x. [DOI] [PubMed] [Google Scholar]
- Cutcliffe JW, Hellmann E, Heyl A, Rashotte AM. CRFs form protein–protein interactions among each other and with members of the cytokinin-signaling pathway in Arabidopsis via the CRF domain. Journal of Experimental Botany. 2011;62:4995–5002. doi: 10.1093/jxb/err199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y-Q, Wildermuth M, Chakravarthy S, Loh Y-T, Yang C, He X, Han Y, Martin G. Tomato transcription factors Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. The Plant Cell. 2002;14:817–831. doi: 10.1105/tpc.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y-Q, Yang C, Thara VK, Zhou J, Martin GB. Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. The Plant Cell. 2000;12:771–785. doi: 10.1105/tpc.12.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B-K, Park J, Lee S-B, Kim M, Lee I-J, Kim K-J, Kwon C, Paek K- H. Tobacco Tsip1, a DnaJ-type Zn finger protein, is recruited to and potentiates Tsi mediated transcriptional activation. The Plant Cell. 2006;18:2005–2020. doi: 10.1105/tpc.106.043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Makita N, Kojima M, Kamada-Nobusad T, Sakakibara H. Overexpression of a type-A response regulator alters rice morphology. Plant and Cell Physiology. 2007;48:523–539. doi: 10.1093/pcp/pcm022. [DOI] [PubMed] [Google Scholar]
- Hoth S, Ikeda Y, Morgante M, Wang X, Zuo J, Hanafey MK, Gaasterland T, Tingey SV, Chua NH. Monitoring genome-wide changes in gene expression in response to endogenous cytokinin reveals targets in Arabidopsis thaliana. FEBS Letters. 2003;554:373–380. doi: 10.1016/s0014-5793(03)01194-3. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- Kasahara H, Usheva A, Ueyama T, Aoki H, Horikoshi N, Izumo S. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. Journal of Biological Chemistry. 2001;276:4570–4580. doi: 10.1074/jbc.M004995200. [DOI] [PubMed] [Google Scholar]
- Kiba T, Naitou T, Koizumi N, Yamashino T, Sakakibara H, Mizuno T. Combinatorial microarray analysis revealing Arabidopsis genes implicated in cytokinin responses through the His–Asp phosphorelay circuitry. Plant and Cell Physiology. 2005;46:339–355. doi: 10.1093/pcp/pci033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. The Plant Journal. 2002;31:777–786. doi: 10.1046/j.1365-313x.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- Mok DW, Mok MC. Cytokinin metabolism and action. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;89:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Park C-J, Lee S-B, Ham B-K, Shin R, Paek K- H. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. The Plant Cell. 2001;13:1035–1046. doi: 10.1105/tpc.13.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Nash P. Protein–protein interactions define specificity in signal transduction. Genes and Development. 2000;14:1027–1047. [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Punwani JA, Hutchison CE, Schaller GE, Kieber JJ. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. The Plant Journal. 2010;62:473–482. doi: 10.1111/j.1365-313X.2010.04165.x. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Carson SDB, To JPC, Kieber JJ. Expression profiling of cytokinin action in Arabidopsis. Plant Physiology. 2003;132:1998–2011. doi: 10.1104/pp.103.021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Goertzen LR. The CRF domain defines cytokinin response factor proteins in plants. BMC Plant Biology. 2010;10:74. doi: 10.1186/1471-2229-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Mason MM, Hutchison CE, Ferreria FJ, Schaller GE, Kieber JJ. A subset of Arabidopsis AP2 transcription factors mediate cytokinin responses in concert with a two-component pathway. Proceedings of the National Academy of Sciences, USA. 2006;103:11081–11085. doi: 10.1073/pnas.0602038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet J, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochemical and Biophysical Research Communications. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- To JP, Kieber JJ. Cytokinin signaling: two-components and more. Trends in Plant Sciences. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Werner T, Schmülling T. Cytokinin action in plant development. Current Opinion in Plant Biology. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zarei A, Körbes AP, Younessi P, Montiel G, Champion A, Memelink J. Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Molecular Biology. 2011;75:321–331. doi: 10.1007/s11103-010-9728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tang X, Martin G. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO. 1997;16:3207–3218. doi: 10.1093/emboj/16.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.