Abstract
Iron (Fe) is an essential mineral micronutrient for plants and animals. Plants respond to Fe deficiency by increasing root uptake capacity. Identification of gene networks for Fe uptake and homeostasis could result in improved crop growth and nutritional value. Previous studies have used microarrays to identify a large number of genes regulated by Fe deficiency in roots of three Arabidopsis ecotypes. However, a large proportion of these genes may be involved in secondary or genotype-influenced responses rather than in a universal role in Fe uptake or homeostasis. Here we show that a small percentage of the Fe deficiency transcriptome of two contrasting ecotypes, Kas-1 and Tsu-1, was shared with other ecotypes. Kas-1 and Tsu-1 had different timing and magnitude of ferric reductase activity upon Fe withdrawal, and different categories of overrepresented Fe-regulated genes. To gain insights into universal responses of Arabidopsis to Fe deficiency, the Kas-1 and Tsu-1 transcriptomes were compared with those of Col-0, Ler, and C24. In early Fe deficiency (24–48 h), no Fe-downregulated genes and only 10 upregulated genes were found in all ecotypes, and only 20 Fe-downregulated and 58 upregulated genes were found in at least three of the five ecotypes. Supernode gene networks were constructed to visualize conserved Fe homeostasis responses. Contrasting gene expression highlighted different responses to Fe deficiency between ecotypes. This study demonstrates the use of natural variation to identify central Fe-deficiency-regulated genes in plants, and identified genes with potential new roles in signalling during Fe deficiency.
Keywords: Arabidopsis, iron deficiency, microarray, natural variation, transcriptional profiling
Introduction
Iron (Fe) is an essential micronutrient for plants and most other forms of life. Iron is utilized in a wide variety of proteins that are involved in oxidation/reduction biochemical reactions, such as cytochrome- and iron-sulphur cluster-containing proteins of the electron transport chains of photosynthesis and respiration, and in enzymes involved in ameliorating oxidative stress, such as certain peroxidases, catalases, and superoxide dismutases (Hansch and Mendel, 2009). Excess Fe in cells or unbound Fe can stimulate the production of free radicals that are sources of oxidative stress damage (Halliwell and Gutteridge, 1992). Thus, the uptake, distribution, and storage of Fe, for example in ferritin proteins (Briat et al., 2010), must be tightly regulated (Walker and Connolly, 2008; Giehl et al., 2009).
Iron deficiency in dicotyledons results in increased capacity for roots to take up Fe by physiological responses including increased Fe(III) reduction, Fe(II) transport, and rhizosphere acidification. In Arabidopsis, these responses result from Fe-deficiency-regulated gene expression of the ferric reductase FRO2 (Robinson et al., 1999), the iron transporter IRT1 (Henriques et al., 2002; Varotto et al., 2002; Vert et al., 2002), and the H+-ATPase AHA2 (Santi and Schmidt, 2009). The transcription factor FIT is a key regulator of Fe-deficiency responses (Colangelo and Guerinot, 2004; Jakoby et al., 2004; Yuan et al., 2005), and is essential for FRO2 and IRT1 activity. To help understand additional genes that are involved in Fe-deficiency responses, several studies have used the Affymetrix ATH1 microarray (Buckhout et al., 2009; Colangelo and Guerinot, 2004; Dinneny et al., 2008; Garcia et al., 2010; Long et al., 2010; Yang et al., 2010) or previous versions of microarrays (Thimm et al., 2001; Wintz et al., 2003) to identify genes with altered transcript levels in Fe-deficient Arabidopsis roots. A proteomic study (Lan et al., 2010) has also identified Fe-regulated proteins in Arabidopsis roots. However, in these screens, typically hundreds to thousands of genes have increased or decreased expression, and many of these genes may not be involved directly in Fe uptake or homeostasis. Growth patterns or metabolic pathways may be altered by Fe limitation (Thimm et al., 2001; Lan et al., 2010), and it is possible that much of the Fe regulon may be involved in these processes. An improved method of filtering genes involved in early Fe-deficiency responses would be useful to help focus future research.
A large number of ecotypes of Arabidopsis thaliana have been collected from natural habitats in different regions of the world. These ecotypes represent homozygous inbred lines that are adapted to specific environments (Mitchell-Olds and Schmitt, 2006). Naturally occurring polymorphisms in DNA, such as single-nucleotide polymorphisms (SNPs), insertions, and deletions, are a source of genetic variability and can result in different alleles of genes or differences in promoter activity (Wray et al., 2003) that can give rise to differences in growth and responses to the environment between ecotypes (Alonso-Blanco et al., 2009). Research based on genotype × environment differences resulting from natural genetic variation among Arabidopsis ecotypes has led to identification of genes for uptake or accumulation of several minerals, such as sodium (Rus et al., 2006), molybdenum (Baxter et al., 2008a), and cobalt (Morrissey et al., 2009), and quantitative trait loci related to traits such as seed mineral concentration (Vreugdenhil et al., 2004; Waters and Grusak, 2008a; Ghandilyan et al., 2009), nitrogen use efficiency (Loudet et al., 2003), and water use efficiency (McKay et al., 2008). In bean (Phaseolus vulgaris), natural variation for root ferric reductase activity was the basis for mapping quantitative trait loci associated with this trait (Blair et al., 2010).
For analysis of the Fe regulon, one approach to filter the number of genes that are involved specifically in early Fe-deficiency responses may be to compare the Fe-regulated transcriptomes of different ecotypes (Schmidt and Buckhout, 2011). Most Arabidopsis resources have been developed for the ecotype Columbia-0 (Col-0), and the majority of microarray studies in Arabidopsis have utilized this ecotype (Colangelo and Guerinot, 2004; Dinneny et al., 2008; Garcia et al., 2010; Long et al., 2010; Yang et al., 2010). Two other ecotypes have been studied for Fe-deficiency-regulated gene expression thus far: Landberg erecta (Ler) (Buckhout et al., 2009), and C24 (Yang et al., 2010). Col-0 and Ler originated from regions with similar temperate climates in the central USA and Germany, whereas C24 originated from the somewhat warmer climate of Coimbra, Portugal. Although fold change of expression under Fe deficiency differed for many genes between Col-0 and C24, there were only six genes that were Fe-regulated in one ecotype but not the other (Yang et al., 2010).
In this work we compare root Fe-regulated transcriptomes of two divergent ecotypes, Kashmir-1 (Kas-1) and Tsushima-1 (Tsu-1), with each other and those of Col-0, Ler, and C24. Kas-1 and Tsu-1 are relatively large ecotypes that evolved in greatly contrasting climate zones (McKay et al., 2008). Kas-1 [Arabidopsis Biological Resource Center (ABRC), stock no. CS28376] was collected in the cold and dry high elevations of Kashmir, India (latitude 35 °N, longitude 77 °E; www.naturalvariation.org), whereas Tsu-1 (ABRC stock no. CS28781) was collected from a warm and humid region of Tsushima, Japan (34/35 °N, 136/137 °E; ABRC). These ecotypes have been genotyped with 149 genome-wide SNPs (www.naturalvariation.org/hapmap) and 48 microsatellites (McKay et al., 2008). Phenotypic differences between Kas-1 and Tsu-1 have been demonstrated for carbon isotope discrimination (McKay et al., 2003), drought adaptation (McKay et al., 2008), gene expression under drought (Juenger et al., 2010), flowering time (Werner et al., 2005), and mineral accumulation (Buescher et al., 2010), and Kas-1 was shown to be resistant to powdery mildew, in contrast to Col-0 (Wilson et al., 2001). We show that Kas-1 and Tsu-1 respond to Fe deficiency on different timescales than each other or Col-0. We hypothesized that these differences in timing could be exploited to refine genes that are likely involved specifically in early Fe uptake and distribution responses while excluding genes that are differentially regulated under Fe deficiency but are not specifically related to Fe uptake. Here we describe a relatively small number of genes that respond to Fe deficiency in all or most ecotypes studied and discuss categories of genes that differ between ecotypes.
Materials and methods
Plant material and growth
Seeds of the three A. thaliana ecotypes used in this study, Col-0, Kas-1, and Tsu-1, were imbibed in 0.1% agar at 4 °C for 72 h. Seeds were planted onto rockwool loosely packed into 1.5 ml centrifuge tubes with the bottoms removed. The tubes were floated in foam rafts in containers of nutrient solution, composed of 0.8 mM KNO3, 0.4 mM Ca(NO3)2, 0.3 mM NH4H2PO4, 0.2 mM MgSO4, 50 μM Fe(III)-ethylenediamine-N,N'-bis(2-hydroxyphenylacetic acid) (EDDHA), 25 μM CaCl2, 25 μM H3BO3, 2 μM MnCl2, 2 μM ZnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4, and 1 mM 2-(N-morpholino)ethanesulphonic acid (MES) buffer (pH 5.5). Lighting was provided at a photoperiod of 16 h of 150 μmol m−2 s−1 4100K fluorescent light (on at 06:00 and off at 22:00). After 10 days, seedlings and the tubes were transferred to holes in lids of containers containing 0.75 l of the same nutrient solution with constant aeration for an additional 14 days before plants were transferred to treatments. The +Fe solution contained 50 μM Fe(III)-EDDHA (Sprint 138; Becker-Underwood, Ames, IA, USA); Fe was omitted from the −Fe solution. All the experiments were initiated at 14:00, 8 h before the end of the photoperiod.
Root ferric reductase assays were performed for 30 min on individual roots, using 10 ml of an assay solution of 0.1 mM ferrozine (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mM Fe(III)-ethylenediaminetetra-acetic acid, and 5 mM MES buffer (pH 5.5) (Fisher Scientific, Fair Lawn, NJ, USA). Reduced Fe was calculated using absorbance at 562 nm with the extinction coefficient of 28.6 mM−1 cm−1.
Real-time reverse transcriptase-PCR
Total RNA was extracted from roots using the Plant Total RNA kit (IBI Scientific, Peosta, IA, USA). RNA quality and concentration was determined by UV spectrophotometry. DNase-treated RNA (1 μg; RNase-free DNase I, New England Biolabs, Ipswich, MA, USA) was used for cDNA synthesis, using the High Capacity cDNA Reverse Transcription kit (ABI, Foster City, CA, USA) with random hexamers at 2.5 μM final concentration. cDNA corresponding to 50 ng of total RNA was used in a 15 μl real-time PCR reaction performed in a MyIQ (Bio-Rad, Hercules, CA, USA) thermal cycler using SYBR GreenER qPCR SuperMix (Invitrogen Technology, Carlsbad, CA, USA) and 0.2 μM gene-specific primers (see below). The following standard thermal profile was used for all PCRs: 50 °C for 2 min, 95 °C for 8 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. At the end of each reaction, a dissociation curve was performed to ensure primer specificity. The Ct values for all genes were calculated using BioRad IQ5 System Software version 2.0 (BioRAD, Hercules, CA, USA) and normalized to the Ct value of ubiquitin (UBQ10) using the equation Y = 2−ΔCt , where ΔCt = CtUBQ10 – Cttarget gene. The PCR efficiency from the exponential phase (E) was calculated for each individual amplification plot using the equation (1+E) = 10slope calculated by the LinRegPCR program (Ramakers et al., 2003). PCR efficiency ranged from 97 to 99.5%, with correlation coefficient (r2) ranging from 0.98 to 1.0. The calculated PCR efficiency was then used to normalize each obtained Ct value.
The primers used were as follows: AHA2 (At4g30190; 5′- TGACTGATCTTCGATCCTCTCA-3′, 5′-GAGAATGTGCATGTGCCAAA-3′), FIT (At2g28160; 5′-GGAGAAGGTGTTGCTCCATC-3′, 5′-TCCGGAGAAGGAGAGCTTAG-3′), FRO2 (At1g01580; 5′-CCACATCTGCGTATCAAGTT-3′, 5′-TCCCAAACAAGCTACGACCA-3′), NRAMP4 (At5g67330; 5′-AGCGGACACCATCGGTCTTGC-3′, 5′-CGGGAAAAACCCACCCCCGT-3′), OPT3 (At4g16370; 5′-CTCGATGCAGGGACCGCGTT-3′, 5′-TTCCAGGAGCCGTGGGACAGG-3′), and UBQ10 (At4g05320; 5′-TGGTGGTATGCAGATTTTCG-3′, 5′-GGCTTTCAGGTTATCAATGG-3′). To correct for SNPs and amplify the same amplicons, different reverse primers were used for Kas-1 and Tsu-1 for the following: FRO3 (At1g23020; forward 5′-GATTCTACTGGCTTCTCTTGG-3′, reverse 5′-CTAATCCGGCCTTCACTAAC-3′ for Kas-1, reverse 5′-TAACCCTAATCCCTTCACTAAC-3′ for Tsu-1), IRT1 (At4g19690; forward 5′-CGGTTGGACTTCTAAATGC-3′, reverse 5′-CGATAATCGACATTCCACCG-3′ for Kas-1, reverse 5′-CGATAATGGACATTCCACCG-3′ for Tsu-1). All primer pairs generated amplicons ranging from 100 to 300 bp.
Microarrays and analysis
Total RNA was isolated from roots using the RNeasy Plant Kit (Qiagen, Chatsworth, CA, USA), and quality was assessed using the Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). The Affymetrix GeneChip Arabidopsis ATH1 Genome Array was used for microarray analysis, with three biological replicates for each treatment and time point (+Fe, −Fe 24 h, −Fe 48 h), and each ecotype (Kas-1 and Tsu-1), for a total of 18 arrays. DNase I (5 μg; Qiagen)-treated RNA was used and all further procedures (hybridization, washing, staining, and scanning) were carried out at the Genomics Core Research Facility of the University of Nebraska according to manufacturer's instructions. For data analysis, the probe intensity files (.cel files) were imported into GeneSpringX software (Agilent), and the set of nine arrays from each ecotype was normalized using the MAS5 algorithm, with only the entities present or marginal in at least three replicates. To identify differentially expressed genes, an analysis of variance (using P ≤ 0.05) was performed on the filtered and normalized dataset, and genes that showed significant differences as up- or downregulated by at least 1.5-fold were considered to be differentially expressed. Averages from three biological replicates for each sample were used for analysis. The complete dataset is available as GEO Accession GSE29086 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29086).
Bioinformatics
Overrepresented functional categories of Fe-regulated genes were identified for Gene Ontology (GO) and Munich Information Center for Protein Sequences (MIPS) classification using BioMaps software on the Virtual Plant (Katari et al., 2010) website. Sets of Fe-upregulated or -downregulated genes were used for multifactor comparison to generate five-way Venn diagrams using Sungear software on the Virtual Plant website. Supernode networks were constructed with Gene Networks software on the Virtual Plant website based on MIPS functional classification with settings specifying direct associations and primary enzymatic reactions. MapMan software (Thimm et al., 2004) was used with fold-change values to categorize expression of Fe-regulated genes from this study and previous studies (Colangelo and Guerinot, 2004; Garcia et al., 2010; Long et al., 2010; Yang et al., 2010) into metabolic functional bins. Output tables of these bins were combined and selected genes were graphically represented as heatmaps using the Matrix2png interface (www.bioinformatics.ubc.ca/matrix2png/index.html),
Results
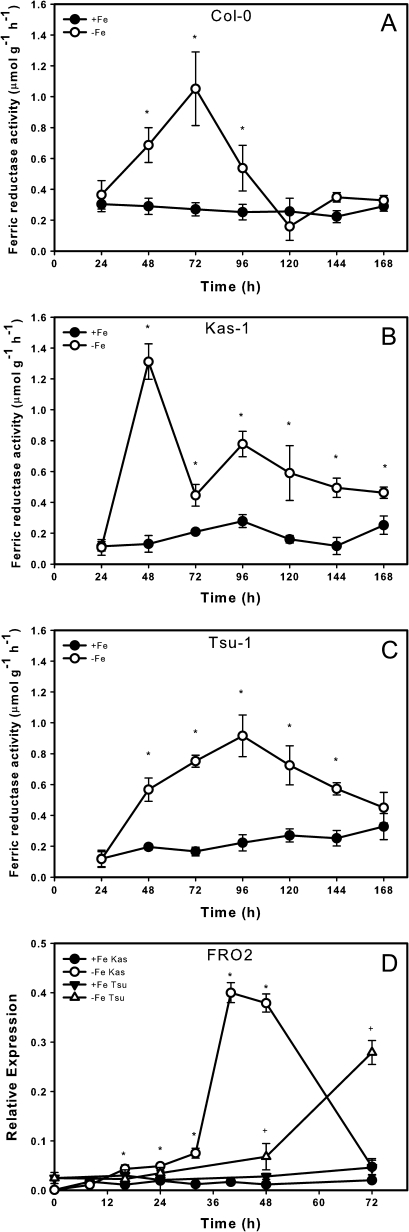
Several A. thaliana ecotypes that are parents of recombinant inbred line populations (Col-0, Ler, Bur-0, Bay-0, Est-1, Sakhdara, Cvi, Kondara, Kas-1, and Tsu-1) were tested for suitability of growth in our hydroponic system. Kas-1 and Tsu-1 were chosen for further study. Over a time course of Fe-deficiency-induced root ferric reductase activity, ecotypes Col-0, Kas-1, and Tsu-1 exhibited substantially different timing and magnitude of response (Fig. 1A–C). None of the ecotypes had increased root ferric reductase activity at 24 h. At 48 h of Fe deficiency, Kas-1 ferric reductase activity was increased by 10-fold, whereas Col-0 and Tsu-1 had increased activities of 2.4-fold and 2.9-fold, respectively. By 72 h, Kas-1 activity declined markedly, then recovered slightly at 96 h, and remained elevated throughout the remainder of the experiment. This decline and recovery pattern was observed in multiple replications of this experiment. In contrast, Col-0 reached peak activity at 72 h, with total ferric reductase activity 20% less than that of Kas-1 at 24 h. Fe-deficient Col-0 ferric reductase activity declined to levels equivalent to those of Fe-replete plants at 120–168 h (5–7 days). Tsu-1 reached its peak activity at 96 h, at a magnitude 27% less than that of Kas-1 on 24 h, and root ferric reductase activity remained elevated in Fe-deficient plants until 144 h. The pattern of expression of the FRO2 gene was very similar to total root reductase activity in Kas-1 and Tsu-1 over the time course (Fig. 1D). FRO2 gene expression was slightly but significantly upregulated in Kas-1 after 16 h Fe deficiency; however, the most substantial increases were observed at 40 and 48 h, with a 20-fold induction, which coincided with the highest root ferric chelate reductase activity observed in this ecotype. The earliest significant upregulation of FRO2 in Tsu-1 was at 48 h, and expression continued to increase afterwards, reflecting the pattern of whole-root ferric reductase activity.
Fig. 1.
Time course of root ferric chelate reductase activity and FRO2 gene expression. Ferric reductase activity of (A) Col-0, (B) Kas-1, and (C) Tsu-1. Expression of FRO2 (D) in Kas-1 and Tsu-1 roots over time. Values of a representative experiment, n = 3 ± SD. * Denotes statistical significance for Kas-1, + denotes statistical significance for Tsu-1 (P ≤ 0.05) between treatments at each time point. +Fe, 50 μM Fe, −Fe, no added Fe.
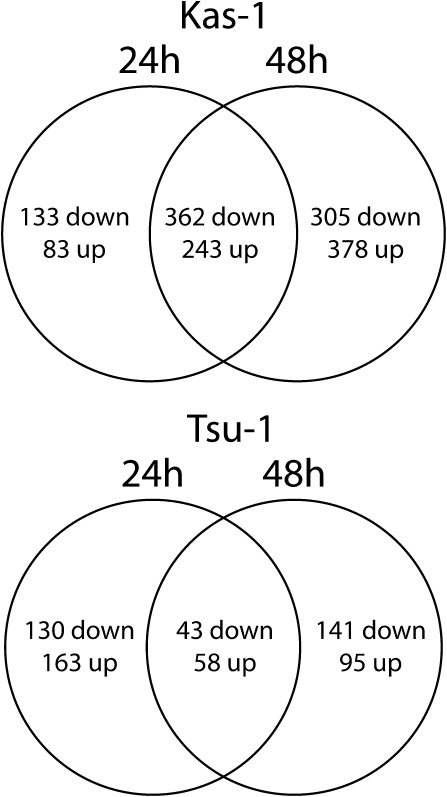
Based on the differences in timing of ferric reductase activity and FRO2 expression after transfer of plants to Fe-deficiency solution, we conducted microarray transcriptional profiling of Kas-1 and Tsu-1 at 24 and 48 h of −Fe treatment. A total of 1504 entities were Fe-regulated ≥1.5-fold in Kas-1, and 630 were Fe-regulated ≥1.5-fold in Tsu-1. A notable pattern of expression was that the majority of Tsu-1 genes were expressed at one but not both time points, exclusively at the earlier (41% of all downregulated, 51% of all upregulated) or later (45% of all downregulated, 30% of all upregulated) time points (Fig. 2), whereas in Kas-1 a greater portion of genes was differentially expressed at both time points (45% of all downregulated, 35% of all upregulated) or only at the later time point.
Fig. 2.
Venn diagram of microarray expression of Arabidopsis root transcripts in response to Fe deficiency. Numbers of differentially regulated genes in Kas-1 (A) and Tsu-1 (B) ecotypes 24h, 48 h, or at both 24 and 48 h after transfer to control (+Fe, 50 μM Fe) or Fe-deficiency (no added Fe) conditions.
All genes that were upregulated or downregulated in Kas-1 or Tsu-1 (at either time point) were analysed for overrepresented GO and MIPS categories. Kas-1 Fe-deficiency-downregulated genes had 20 overrepresented GO categories and 18 overrepresented MIPS categories (Supplementary Table 1). Many of these GO and MIPS categories were for genes that respond to various chemical, biotic or abiotic stress, wounding, or certain plant hormones. There were also genes categorized as involved in amino acid, aromatic compound, and secondary metabolite metabolism. For Fe-deficiency-upregulated genes, three GO- and five MIPS-overrepresented categories were primarily in cell-wall metabolism (Supplementary Table S1). The results for Tsu-1 Fe-regulated genes were very different, in that there were no enriched GO categories and only one category of ‘unannotated’ for MIPS classification for Fe-downregulated Tsu-1 genes (Supplementary Table 1). Similarly, Fe-deficiency upregulated genes were enriched in only one GO category, for suberin biosynthesis, and only one MIPS category of ‘mitochondrion’.
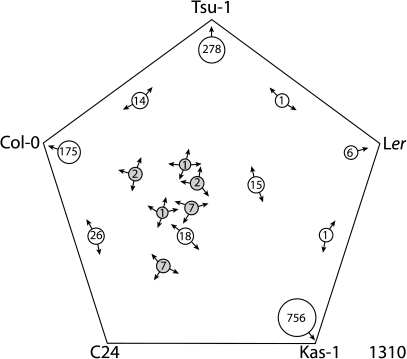
To compare the Fe regulon in the rapid responding Kas-1 and the more slowly responding Tsu-1 with that of other ecotypes, we utilized the Sungear software on the Virtual Plant website to create a five-way Venn diagram between our datasets and those of Col-0, C24, and Ler. There have been several microarray studies for Col-0 previously, so to account for possible variation between experiments and growth conditions differences, we created a union set of all the up- or downregulated Fe-regulated transcripts from Colangelo and Guerinot (2004), Garcia et al. (2010), Dinneny et al. (2008) (Fe-deficiency time course dataset only, not including any salt stress, root zone, or cell-specific datasets), Long et al. (2010), and Yang et al. (2010). When comparing all five ecotypes (Fig. 3), surprisingly, no genes were significantly downregulated by Fe deficiency in all ecotypes. Only two genes (At5g49770 and At2g36885) were significantly downregulated by Fe deficiency in four of the five ecotypes, and only 18 genes were downregulated in three of the five ecotypes (Table 1), a very small percentage of the total number (1310) of Fe-deficiency-downregulated genes. We considered genes found in at least three of the five ecotypes to be a set of ‘common’ Fe-regulated genes (Table 1). The genes not included in this table can be found in Supplemental Dataset 1. The Venn set analysis also revealed that the greatest percentage of the total set of Fe-downregulated genes was observed in only one ecotype.
Fig. 3.
Five-way generalized Venn diagram of Fe-deficiency-downregulated genes from A. thaliana ecotypes Tsu-1, Kas-1, Ler, Col-0, and C24. Arrows on the circles indicate ecotypes in which genes of that set were significantly downregulated. Common gene sets are shaded. Total number of genes for all ecotypes is indicated in the lower right corner.
Table 1.
Common set of root Fe-deficiency-downregulated genes found in at least three of five A. thaliana ecotypes (Tsu-1, Kas-1, Ler, Col-0, and C24). Fold change for −Fe as compared to +Fe is provided when available in original reference, otherwise ‘up’ indicates upregulation in −Fe as compared to +Fe, and ‘down’ indicates downregulated in −Fe as compared to +Fe.
| Fold change (−Fe versus +Fe) |
||||||||||||||
| Gene locus | Description | Tsu-1 24 h | Tsu-1 48 h | Kas-1 24 h | Kas-1 48 h | Ler 24 ha | Col-0 24 hb | Col-0 24 hc | Col-0 24 h d | Col-0 48 h d | Col-0 72 h d | Col-0 72 h e | Col-0 72 h f | C24 72 h f |
| At5g59520 | ZIP2, metal transporter | −7.9 | −12.7 | −5.0 | −5.0 | |||||||||
| At2g32270 | ZIP3, metal transporter | 1.5 | 2.4 | Down | Down | Down | Down | −2.9 | −2.4 | |||||
| At3g56240 | CCH, copper chaperone | −2.3 | −2.7 | −1.7 | −2.0 | |||||||||
| At5g01600 | FER1, ferritin | Down | −2.5 | Down | Down | Down | −6.7 | −4.8 | ||||||
| At3g56090 | FER3, ferritin | Down | Down | −2.1 | −1.6 | |||||||||
| At2g40300 | FER4, ferritin | Down | −1.5 | −3.4 | −2.6 | |||||||||
| At3g09220 | LAC7, putative laccase | −2.0 | Down | Down | −3 | −3.8 | ||||||||
| At3g25190 | Nodulin-like 21 | 2.3 | Down | 7.4 | Down | Down | Down | −3.8 | −5.3 | |||||
| At1g21140 | Nodulin-like 1 | Down | 6.2 | −5.9 | −5.0 | |||||||||
| At5g49360 | BXL1, bifunctional β-D-xylosidase/α-L-arabinofuranosidase | −1.7 | 1.8 | −2.4 | −3.6 | Down | ||||||||
| At5g18670 | BMY3, putative β-amylase | −1.8 | −2.5 | −3.5 | Down | |||||||||
| At4g36220 | F5H, ferulate 5-hydroxylase | 1.6 | −2.0 | −2.3 | −1.8 | |||||||||
| At5g49780 | Leucine-rich repeat transmembrane protein kinase | −1.8 | −2.1 | −2.5 | ||||||||||
| At5g49770 | Leucine-rich repeat transmembrane protein kinase | −2.2 | −1.7 | −2.7 | −1.8 | |||||||||
| At5g49760 | Leucine-rich repeat transmembrane protein kinase | −1.5 | −2.0 | −2.4 | ||||||||||
| At1g79320 | Caspase/cysteine-type endopeptidase | −4.1 | 2.4 | 2.5 | −1.9 | Down | −3 | −3.7 | ||||||
| At4g13250 | Similar to short-chain dehydrogenase/reductase (SDR) family protein | −1.9 | −2.4 | −2 | −2 | |||||||||
| At4g18550 | Lipase class 3 family protein | −1.6 | −1.5 | −2.4 | −2.8 | |||||||||
| At2g36885 | Unknown protein | −1.7 | Down | Down | Down | −2.8 | −2.5 | |||||||
| At1g68650 | Unknown protein | Down | Down | −2.4 | −2.3 | |||||||||
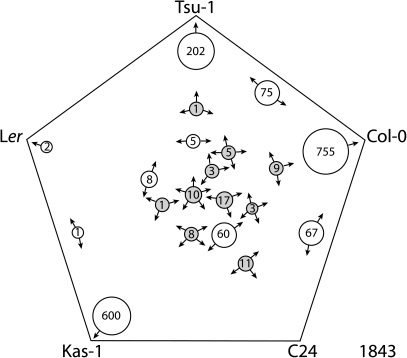
In a 5-way Venn diagram for genes that were upregulated under Fe deficiency, similar to Fe-deficiency downregulated genes, by far the largest sets were those that were upregulated in only one of the ecotypes (Fig. 4). However, there was a small set of 10 genes that was upregulated by Fe deficiency in all five ecotypes, which we refer to as the ‘core’ Fe-deficiency genes (Table 2). There were also relatively small sets of ‘common’ Fe-upregulated genes in four of the five ecotypes (16 genes, Table 2) and three of the five ecotypes (42 genes, Table 2). The genes not included in this table can be found in Supplemental Dataset 1.
Fig. 4.
Five-way generalized Venn diagram of Fe-deficiency-upregulated genes from A. thaliana ecotypes Tsu-1, Kas-1, Ler, Col-0, and C24. Arrows on the circles indicate ecotypes in which genes of that set were significantly upregulated. Core and common gene sets are shaded. Total number of genes for all ecotypes is indicated in the lower right corner.
Table 2.
Core and common set of root Fe deficiency upregulated genes from Arabidopsis thaliana ecotypes (Tsu-1, Kas-1, Ler, Col-0 and C24). Fold change for −Fe as compared to +Fe is provided when available in original reference, otherwise ‘up’ indicates upregulation in −Fe as compared to +Fe, and ‘down’ indicates downregulated in −Fe as compared to +Fe.
| Fold change (−F versus +Fe) |
|||||||||||||||
| Gene locus | Description | Tsu-1 24 h | Tsu-1 48 h | Kas-1 24 h | Kas-1 48 h | Ler 24 ha | Col-0 24 hb | Col-0 24 hc | Col-0 24 h d | Col-0 48 h d | Col-0 72 h d | Col-0 72 h e | Col-0 72 h f | C24 72 h f | Col-0 g |
| Core set of root upregulated genes (observed in five of five ecotypes) | |||||||||||||||
| At4g19690 | IRT1, metal transporter | 3.8 | 6.3 | 29.3 | 61.7 | Up | 27.4 | 29.3 | Up | Up | Up | 16.3 | 13.7 | 6.6 | |
| At4g16370 | OPT3, metal transporter | 1.7 | 5.2 | −1.6 | 1.7 | Up | 5.2 | Up | Up | Up | 7.3 | 5.7 | |||
| At1g23020 | FRO3, metal transporter | 2.8 | 5.4 | 2.0 | 2.8 | Up | 4.8 | Up | Up | Up | 7.8 | 6.7 | |||
| At3g58810 | MTP3/MTPa2, metal transporter | 3.4 | 7.1 | 3.4 | 6.5 | Up | 4.8 | 5.4 | Up | Up | Up | 11.9 | 9.4 | 6.7 | Up |
| At3g56980 | bHLH039, transcription factor | 5.4 | 10.2 | 2.6 | Up | 9.5 | Up | Up | Up | 24.5 | 20.3 | Up | |||
| At3g12900 | 2OG-Fe(II) oxygenase family protein | 2.4 | 9.7 | 6.0 | 19.2 | Up | 24.9 | 11.2 | Up | Up | Up | 18.5 | 95.5 | 35.3 | |
| At3g07720 | Galactose oxidase/kelch repeat-containing protein | 1.6 | 10.5 | 4.2 | 4.0 | Up | 8.3 | 11.2 | Up | Up | Up | 12.9 | 11.3 | 12.1 | Up |
| At5g53450 | ORG1, OBP3 responsive gene (protein kinase) | 1.7 | 3.4 | 2.4 | Up | 4.4 | Up | Up | Up | 5.3 | 3.5 | ||||
| At1g47400 | Unknown protein | 3.0 | 5.2 | 2.7 | 5.9 | Up | 5.2 | Up | Up | Up | 13.6 | 6.6 | |||
| At1g74770 | Unknown protein | 2.0 | 3.7 | 2.4 | Up | Up | Up | 5.4 | 4.9 | ||||||
| Common set of root upregulated genes (observed in four of five ecotypes) | |||||||||||||||
| At4g19680 | IRT2, metal transporter | 4.3 | 5.6 | Up | 3.0 | 3.8 | 21.0 | 14.3 | 7.1 | Up | |||||
| At5g67330 | NRAMP4, metal transporter | 3.0 | Up | 2.0 | Up | Up | Up | 2.2 | 2.0 | ||||||
| At3g58060 | MTPc3, metal transporter | 3.5 | 3.9 | Up | 3.0 | Up | Up | Up | 8.9 | 21.2 | 22.4 | ||||
| At5g02780 | Glutathione S-transferase (flavonoid synthesis) | 3.7 | 7.7 | 3.8 | 7.4 | 7.0 | 11.2 | Up | Up | Up | 19.1 | 27.4 | 21.6 | ||
| At3g50740 | UGT72E1, UDPG:coniferyl alcohol glucosyltransferase | 2.4 | 3.6 | 4.0 | 4.7 | 2.6 | Up | Up | Up | 8.6 | 4.2 | 3.9 | Up | ||
| At1g09560 | GLP5 germin-like protein | 2.3 | 3.7 | Up | 3.8 | 3.0 | Up | Up | Up | 3.0 | 2.4 | ||||
| At1g34760 | GRF11/GR14, 14-3-3 protein, binds H+-ATPase (root hairs) | 2.0 | 2.9 | Up | 2.3 | 3.7 | Up | Up | Up | 18.4 | 9.4 | 10.9 | |||
| At3g06890 | Similar to oxidoreductase/transition metal ion binding | 1.7 | Up | 2.7 | Up | Up | 2.8 | 3.7 | 4.6 | ||||||
| At3g47640 | PYE, bHLH transcription factor | 1.9 | Up | 1.5 | Up | Up | Up | 2.6 | 2.2 | ||||||
| At3g18290 | BTS, E3 ligase | 1.6 | 2.7 | Up | 2.2 | Up | Up | Up | 2.7 | 2.5 | |||||
| At2g20030 | RING-H2 finger protein ATL2D precursor | 1.6 | Up | 2.0 | 3.0 | 3.0 | 2.5 | ||||||||
| At5g05250 | Unknown protein | 2.5 | 7.2 | Up | 3.0 | Up | Up | Up | 6.0 | 4.4 | |||||
| At3g56360 | Unknown protein | 2.2 | 1.6 | Up | 2.7 | Up | Up | Up | 2.3 | 1.6 | |||||
| At1g49000 | Unknown protein | 3.3 | −1.5 | 1.5 | 1.9 | 2.7 | Up | Up | 5.8 | 3.4 | |||||
| At2g29995 | Unknown protein | 1.6 | 1.8 | Up | 2.3 | 3.3 | |||||||||
| At3g61930 | Unknown protein | 3.3 | 8.7 | Up | 11.8 | 6.9 | Up | Up | Up | 32.5 | 23.9 | 11.7 | |||
| Common Set of root upregulated genes (observed in three of five ecotypes) | |||||||||||||||
| At4g30120 | HMA3, P1B-type Zn-ATPase | 1.7 | 1.6 | Up | 5.5 | 5.1 | 4.3 | ||||||||
| At1g80830 | NRAMP1, metal transporter | 1.5 | 4.2 | 2.8 | |||||||||||
| At5g13740 | ZIF1, zinc transporter | Up | 2.5 | Up | Up | Up | 2.6 | 2.3 | |||||||
| At3g46900 | COPT2, Cu transporter | Up | 3.7 | 4.0 | Up | Up | Up | 5.8 | 15.4 | 7.5 | |||||
| At5g03570 | FPN2/IREG2, metal transporter | Up | 3.0 | Up | Up | Up | 6.6 | 6.4 | 4.2 | ||||||
| At5g26820 | IREG3, transporter | Up | Up | 2.3 | 1.9 | ||||||||||
| At5g38820 | Amino acid transporter family protein | 1.7 | 3.3 | Up | 3.7 | 4.5 | Up | Up | Up | 6.8 | 16.3 | ||||
| At3g60330 | AHA7, H+-ATPase | 1.7 | 2.0 | 2.1 | 3.0 | 2.6 | 2.8 | ||||||||
| At5g07390 | RBOHA | 2.6 | Up | Up | 2.3 | 1.6 | |||||||||
| At5g47910 | RBOHD | 1.5 | 2.7 | 2.9 | Up | Up | Up | 3.8 | 4.2 | 4.4 | |||||
| At3g12820 | MYB10, transcription factor | Up | 3.5 | 2.6 | Up | Up | Up | 11.7 | 24.0 | 24.2 | |||||
| At1g56160 | MYB72, transcription factor | Up | 2.2 | 3.4 | Up | Up | Up | 23.4 | 84.5 | 72.2 | |||||
| At2g28160 | FIT, transcription factor | Up | 2.8 | 3.5 | 2.4 | 2.8 | |||||||||
| At5g04150 | bHLH101, transcription factor | Up | 4.1 | Up | Up | Up | 13.9 | 6.1 | |||||||
| At4g01250 | WRKY22, transcription factor | 1.6 | 2.2 | 2.0 | 1.6 | 2.0 | |||||||||
| At2g14210 | MADS box gene, transcription factor | 2.0 | 2.0 | 2.7 | 2.8 | ||||||||||
| At1g02500 | SAM1, S-adenosylmethionine synthetase | 1.8 | 2.1 | Up | Up | Up | 2.6 | 2.2 | |||||||
| At5g04950 | NAS1, nicotianamine synthase | 1.9 | 2.2 | 7.5 | 5.9 | Up | Up | Up | 3.5 | 3.1 | 3.1 | ||||
| At5g13910 | LFY, ethylene response factor subfamily B-1 | 1.8 | Up | Up | 3.1 | 3.7 | |||||||||
| At2g45400 | BEN1, dihydroflavinol 4-reductase-like protein | Up | 2.3 | Up | 2.7 | 3.3 | |||||||||
| At1g14185 | Glucose-methanol-choline (GMC) oxidoreductase family protein | 2.6 | 2.1 | 9.9 | 7.1 | ||||||||||
| At2g16060 | HB1/GLB1, nonsymbiotic haemoglobin | 15.9 | 6.2 | 2.4 | Up | Up | |||||||||
| At5g61250 | Heparanase-like protein 2 | 1.7 | 1.8 | Up | 2.6 | 2.2 | |||||||||
| At4g36010 | Pathogenesis-related thaumatin family protein | 2.6 | 2.0 | 1.8 | Up | Up | |||||||||
| At5g16570 | GLN1, glutamine synthetase | 1.7 | Up | 2.4 | 2.0 | ||||||||||
| At5g36890 | Glycosyl hydrolase family 1 protein | 1.6 | 3.4 | 4.1 | Up | Up | Up | 6.2 | 8.7 | 5.7 | |||||
| At4g17260 | Lactate/malate dehydrogenase family protein | 6.3 | 1.6 | 1.5 | Up | Up | |||||||||
| At2g46710 | rac GTPase activating protein family | 2.5 | 2.2 | 2.1 | |||||||||||
| At3g21240 | Isoform of 4-coumarate:CoA ligase (4CL) | 1.8 | 2.2 | Up | Up | Up | 3.0 | 3.9 | 3.7 | ||||||
| At2g42750 | 4Fe-4S ferredoxin, iron-sulphur-binding family | 1.7 | Up | 1.8 | Up | Up | Up | ||||||||
| At4g30490 | AFG1-like ATPase family protein | 1.5 | 1.7 | 3.3 | 2.5 | ||||||||||
| At4g10510 | Subtilase family protein | 1.7 | Up | Up | 2.1 | 6.2 | 4.8 | ||||||||
| At4g12910 | Serine carboxypeptidase family | −1.5 | 1.6 | Up | Up | 2.3 | 2.4 | 2.1 | |||||||
| At1g18910 | Zinc finger (C3HC4-type RING finger) family protein | Up | 1.7 | Up | Up | 2.8 | 3.2 | 3.0 | |||||||
| At3g53280 | CYP71B5, cytochrome P450 monooxygenase | Up | 3.5 | Up | Up | 19.4 | 40.1 | 33.8 | |||||||
| At3g61410 | U-box domain-containing protein kinase | Up | 2.1 | Up | Up | 3.6 | 4.2 | 4.8 | |||||||
| At4g29220 | PFK1, phosphofructokinase family protein | Up | 1.9 | Up | Up | 2.7 | 2.5 | 2.8 | |||||||
| At4g38950 | Similar to microtubule motor; similar to kinesin heavy chain | −1.5 | Up | 2.1 | 2.0 | 2.1 | |||||||||
| At4g19160 | Unknown protein | 1.5 | Up | Up | 3.3 | 2.6 | |||||||||
| At5g55620 | Unknown protein | Up | 2.1 | Up | Up | 11.6 | 12.3 | ||||||||
| At5g67370 | Unknown protein | Up | 2.6 | Up | Up | Up | 7.3 | 13.3 | |||||||
| At1g01570 | Similar to fringe-related protein | Up | 2.9 | 3.3 | |||||||||||
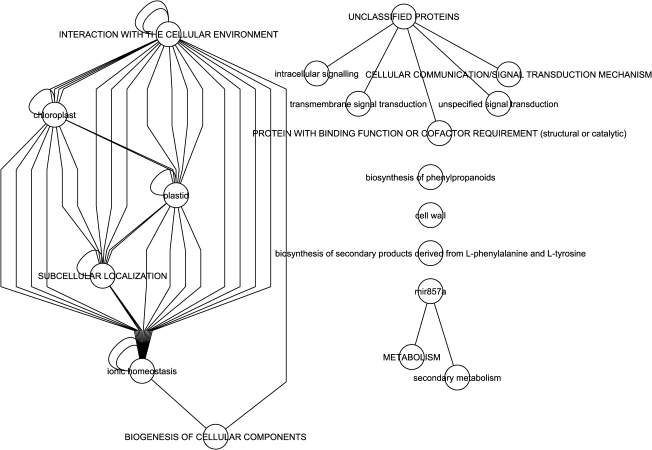
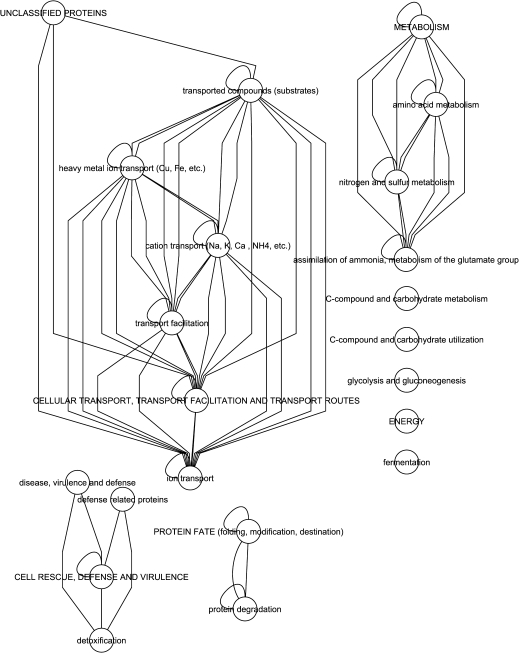
To gain insight into possible functional networks of these core and common genes, hierarchical supernode networks based on MIPS functional categories were constructed. For downregulated common genes (Fig. 5), ion homeostasis within subcellular compartments comprised a major network. A smaller network of unclassified proteins included signalling genes, while standalone nodes included cell-wall, phenylpropanoid, and secondary product metabolism. For the core and common upregulated genes supernode networks (Fig. 6), in addition to cellular transport networks, metabolic networks for amino acids and nitrogen and sulphur compounds, cell rescue, defense, and virulence, protein fate, and energy were found to be associated with these highly conserved Fe-regulated genes.
Fig. 5.
Hierarchical super node gene networks of Fe-deficiency-downregulated genes common among A. thaliana accessions Tsu-1, Kas-1, Ler, Col-0, and C24. Each super node represents a group of genes with common protein function based on MIPS categories. Each edge represents interactions between a gene in each super node.
Fig. 6.
Hierarchical supernode gene networks of core and common Fe-deficiency-upregulated genes from A. thaliana accessions Tsu-1, Kas-1, Ler, Col-0, and C24. Each super node represents a group of genes with common protein function based on MIPS categories. Each edge represents interactions between a gene in each super node.
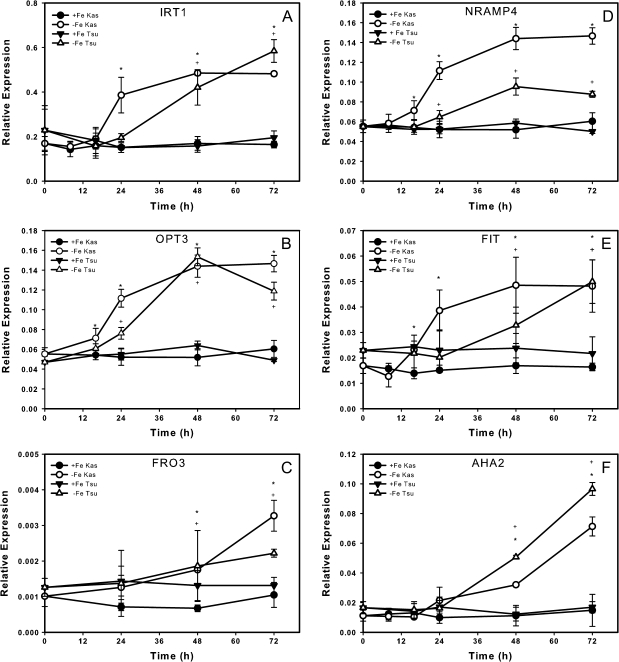
To validate the microarray results and further characterize the molecular responses of Kas-1 and Tsu-1 to Fe deficiency, we carried out a time course of gene expression by real-time reverse transcriptase (RT)-PCR for some of the genes detected on the microarrays and some other genes known to respond to Fe deficiency. Our microarray analysis placed IRT1 in the core set of Fe-deficiency-upregulated genes (Table 2), and indicated that IRT1 was more highly expressed in Kas-1 than Tsu-1 at each time point. Our time course results were similar, indicating that IRT1 was significantly upregulated in Kas-1 but not in Tsu-1 at 24 h, and was upregulated in both ecotypes at 48 h and afterwards (Fig. 7A). OPT3 is another member of the core Fe responsive genes, and was upregulated earlier in Kas-1 than Tsu-1 (Fig. 7B), although in Tsu-1 this gene was upregulated at both time points by microarray analysis. Another core gene is FRO3, expression of which was significantly upregulated in Fe-deficient Kas-1 and Tsu-1 roots at 48 and 72 h as shown by real-time RT-PCR (Fig. 7C), and at both time points in both ecotypes by microarray (Table 2). By microarray, NRAMP4 was upregulated by Fe deficiency in four of five ecotypes. By RT-PCR, NRAMP4 was significantly upregulated after 16 h of Fe deprivation and afterwards in Kas-1 by RT-PCR, and in Tsu-1 at 24 h and later (Fig. 7D). The common Fe-responsive transcription factor FIT was detected in single microarray experiments for C24 and Ler, and in some but not all experiments using Col-0 (Table 2). FIT transcripts were not significantly upregulated in our microarray experiments, but by real-time RT-PCR we observed upregulation to nearly 3-fold (Fig. 7E). Kas-1 upregulated this gene before Tsu-1 did, by 24 h. The H+-ATPase AHA2 gene was 1.5-fold-upregulated in the 48 h Kas-1 arrays. Expression of AHA2 by real-time RT-PCR resembled that of FRO3, in that expression was similar between Kas-1 and Tsu-1, and was upregulated in both ecotypes at 48 h and later (Fig. 7F).
Fig. 7.
Time course expression of Fe-uptake-related genes in Kas-1 and Tsu-1 roots. (A) IRT1, (B) OPT3, (C) FRO3, (D) NRAMP4, (E) FIT, (F) AHA2; n = 3 ± SD. * Denotes statistical significance for Kas-1, + denotes statistical significance for Tsu-1 (P ≤ 0.05) between treatments at each time point. +Fe, 50 μM Fe; −Fe, no added Fe.
Discussion
We have established a genotype × environment interaction for timing of maximal induction of ferric reductase activity using a time-course approach with three diverse Arabidopsis ecotypes, Kas-1, Tsu-1, and Col-0, where Kas-1 responded the most rapidly, Tsu-1 the most slowly, and Col-0 was intermediate. Our overall hypothesis for this study was that the most important early Fe-responsive genes could be determined by comparing the Fe-regulated transcriptomes of these diverse Arabidopsis ecotypes at fixed time points early in the progression of responses to the onset of Fe deficiency. We chose 24 and 48 h to sample our plants, because in Kas-1 24 h is prior to ferric reductase activity upregulation, and 48 h is at the peak, while both of these time points are prior to the peak for Tsu-1. This analysis revealed a small overlapping set of genes from among the many with altered expression under Fe deficiency. This filtering method could narrow the focus of follow-up studies to determine the roles of universally Fe-regulated genes by avoiding genes that respond to Fe deficiency in a genotype-influenced manner; that is, in some but not in all ecotypes. We also propose that this analysis could offer insight into new transcription factor and/or signalling proteins that might be upstream regulators of conserved Fe-deficiency responses.
Diversity in root ferric reductase activity at a single time point was described for a number of ecotypes (Saleeba and Guerinot, 1995). Under our time-course conditions, Kas-1 and Tsu-1 were one day sooner or later to reach maximum root ferric reductase activity than Col-0, offering the potential for detecting diverse molecular responses between these ecotypes that are involved in perception and signalling of Fe-deficiency responses. Root ferric reductase activity is carried out by FRO2 (Robinson et al., 1999), which is regulated by FIT (Colangelo and Guerinot, 2004). Consistent with ferric reductase activity, FIT and FRO2 were upregulated in Kas-1 one day sooner than in Tsu-1, suggesting that Kas-1 has genetically determined differences in Fe status perception or signalling. Similarly, IRT1 and NRAMP4 were upregulated in Kas-1 earlier than in Tsu-1. Other, later-responding genes, such as FRO3 and AHA2, did not show significant differences in timing of induction, suggesting that multiple regulatory pathways are required for the complete Fe regulon.
We hypothesize that genes that do not respond to Fe deficiency in multiple ecotypes are less likely to be primary Fe-responsive genes and may represent genotype × environment responses that differ between ecotypes. To produce our dataset, Kas-1 and Tsu-1 were grown side by side in the same batch of nutrient solution and collected simultaneously; however, only 17 downregulated (Fig. 3) and 24 upregulated (Fig. 4) genes were in common between these ecotypes, demonstrating that genotype effects were important in the same environment. This is in contrast to the study of Yang et al., (2010), which observed a small number of differentially expressed genes between Col-0 and C24 ecotypes. A surprisingly small number of genes, 10 in total, were upregulated at one or more early time points in all ecotypes studied, which we termed the ‘core’ Fe-deficiency-response genes. Although not present on the Affymetrix ATH1 Genome Array, FRO2 probably also belongs to this set of core genes, since this gene has consistently been upregulated in this and previous Arabidopsis Fe-deficiency studies (Colangelo and Guerinot, 2004; Wu et al., 2005; Mukherjee et al., 2006; Buckhout et al., 2009; Garcia et al., 2010). The core genes include IRT1, known to be important for Fe uptake (Vert et al., 2002). These genes also include MTP3, which is thought to sequester Zn into the vacuole under Fe deficiency (Arrivault et al., 2006), OPT3, potentially involved in translocation of Fe into or from vascular tissues (Stacey et al., 2008), and FRO3, a ferric reductase with undefined function. Only four out of the 10 core genes are FIT-dependent, suggesting that transcription factors in addition to FIT take part in the Fe-deficiency regulation. Some of the core genes were not significant in all of the previous Col-0 microarray experiments. Although our Tsu-1 and Kas-1 plants were grown in identical conditions, to allow for some degree of experimental variability between labs, we also considered ‘common’ genes that were Fe-regulated in three or four of the five ecotypes, which is still a relatively small number of only 58 out of 1842 genes. One striking feature of the core and common Fe-deficiency genes is that there are several genes annotated as ‘unknown’, or their specific function in the Fe-deficiency response has not been determined. This result illustrates that there are crucial processes yet to be discovered in the early responses and adaptation to Fe deprivation in plants. By far, the largest number of Fe responsive genes, both upregulated and downregulated, for Kas-1, Tsu-1, and the Col-0 combined set, were not observed in any of the other ecotypes. Thus, it seems likely that there is a small set of universal Fe responsive genes and a large number of genes that represent genotype-influenced responses in different ecotypes, based on their evolutionary history. A similar result was observed for the transcriptome response to salicylic acid, where most responsive genes were observed in only one or a few of seven different Arabidopsis ecotypes, and only a small set of genes (38 in total) were commonly regulated in the majority of ecotypes (van Leeuwen et al., 2007).
It is not clear at this point what roles the ‘ecotype-specific’ Fe-regulated genes play in the root adaptation to Fe deprivation. We note that since ecotypes can respond to Fe deficiency in different timescales, if each ecotype were sampled at the same relative stage of response, the number of genes in the core set may increase. A caveat is that the number of secondary stress-responsive genes would also increase. Thus, we believe our approach to compare diverse ecotypes at temporally chosen time points allows identification of the earliest and most robust primary Fe-response genes. Potentially, the genes observed in only one ecotype are not involved with Fe uptake per se, but with ecotype-specific growth patterns that may be perturbed by Fe deficiency. For example, we have observed that Kas-1 seedlings produce more lateral roots than Tsu-1 seedlings on both Fe-replete and Fe-deficient agar medium (Supplementary Fig. S1). The notable number of genes involved in cell-wall processes by GO or MIPS categorization in Fe-deficient Kas-1 may reflect initiation or elongation of additional lateral roots. Another contrasting trait between Kas-1 and Tsu-1 is water-use efficiency, with Kas-1 showing superior drought avoidance and constitutive lower stomatal conductance (McKay et al., 2008; Juenger et al., 2010). Whether constitutive differences in water-use efficiency among ecotypes could impact the sensitivity and tolerance to mineral deficiencies remains to be tested, but it is known that water deficit has a significant effect on mineral accumulation in Arabidopsis (Ghandilyan et al., 2009).
Another interesting observation from the common Fe-deficiency genes is the dissimilarity in genes expressed in Kas-1 and Ler. In genes downregulated in four ecotypes, seven genes that were downregulated in Ler were not significant in Kas-1, and there were 10 downregulated genes in Kas-1 that were not significant in Ler (Table 1). Only one gene out of this set, ZIP3, was significant in both Kas-1 and Ler, but this gene was upregulated in Kas-1, and in contrast was downregulated in Ler and Col-0. In the common upregulated Fe-responsive genes upregulated in three of the five ecotypes (Table 2), a similar lack of overlap between Kas-1 and Ler was observed. There was much higher agreement between Ler and Col-0, which may reflect their adaptations to similar climate zones that are quite different than the native Kas-1 climate.
To gain additional insight into ecotype-specific metabolic responses, we compared gene expression as fold change among Kas-1, Tsu-1, Col-0, and C24 ecotypes for MapMan (Thimm et al., 2004) functional categories, an approach that has also been taken to compare Col-0 and an Fe homeostasis mutant (Schuler et al., 2011), with a number of similar categories observed. Within the transport bin (Supplementary Fig. S2), differences in expression of transporters for minerals other than Fe may indicate that the different ecotypes have differing homeostasis mechanisms for handling excess non-Fe metals that may be taken up by IRT1 (Korshunova et al., 1999; Rogers et al., 2000) or other upregulated transporters. Additionally there were several transporters for oligopeptides, phosphate, potassium, sulphate, nitrate, amino acids, and calcium that were primarily Fe-regulated in Kas-1 and/or Tsu-1 but not Col-0 or C24. These differences may explain variability in mineral concentrations between Arabidopsis ecotypes that have been reported (Baxter et al., 2008b; Waters and Grusak, 2008b; Buescher et al., 2010). In the metal-handling bin, all four ferritin (FER) genes were downregulated, but only in Col-0 and C24 (Supplementary Fig. S3). Ferritin protein is important for Fe storage in plants, and differences in expression may be representative of differences in internal metal accumulation. Under non-stress growth conditions, Tsu-1 had higher accumulation of Mn (+23% in relation to Col-0), Fe (+21%), Na (+127%), and K (+20%), whereas Kas-1 showed a decreased accumulation of Zn (−33% in relation to Col-0), Mo (−59%), and Cd (−26%) (Buescher et al., 2010). Similarly, in the redox bin (Supplementary Fig. S4), the Fe superoxide dismutase FSD1 was downregulated in Col-0 and C24, but not in Kas-1 or Tsu-1. Kas-1 had Fe regulation of several genes involved in sulphur-containing redox processes that were not observed in the other ecotypes, suggesting differences in metabolism that may interact with responses to Fe deficiency. Further suggestions of fundamental metabolic differences are seen when the cytochrome P450 and peroxidase genes from the ‘miscellaneous’ MapMan bin are displayed (Supplementary Fig. S5). There are several downregulated and upregulated genes in both of these categories that were Fe-regulated in only one ecotype.
Transcription factors of selected families are shown in Supplementary Fig. S6. Some MYB family genes are important for regulating flavonoid synthesis under mineral nutrient deficiency (Lillo et al., 2008). Fifteen MYB family transcription factors were Fe-regulated only in Kas-1. Similarly, there were several NAC and WRKY family transcription factor genes that were Fe-regulated only in Kas-1, while three NAC genes had opposite directions of regulation in Kas-1 and Col-0 or Tsu-1, suggesting a genotype-influenced response. The NAC family proteins regulates a wide array of plant development and responses to stress (Olsen et al., 2005), while WRKY proteins regulate diverse responses to pathogens, development, and abiotic stress (Rushton et al., 2010). Regulation of a number of WRKY genes was enriched in genes differentially expressed between WT and nas4x-1 mutants (Schuler et al., 2011). It is possible that differences in Fe regulation of these transcription factors result in genotype-influenced Fe-deficiency responses.
As with other transcription factor families, several basic helix-loop-helix (bHLH) family genes were Fe-upregulated in Kas-1 only. Several bHLH genes were previously shown to be Fe-regulated and/or have been implicated in regulation of Fe-deficiency responses, including the core Fe-response gene bHLH039 and common Fe-response genes bHLH101, PYE, and FIT. Based on phenotypes of mutants, FIT has been the most important Fe-responsive transcription factor gene described; however, it is not the most robustly Fe-regulated, suggesting that fold-change regulation does not necessarily correlate to functional importance. Overexpression of Arabidopsis bHLH039 in tobacco roots resulted in production of riboflavin (Vorwieger et al., 2007), a common root Fe-deficiency response. bHLH039 has been reported to act as a heterodimer with FIT in binding to FRO2 and IRT1 promoters (Yuan et al., 2008). Constitutive overexpression of FIT, bHLH038, or bHLH039 alone had no effect on Fe reduction under Fe-replete conditions, but co-overexpression of FIT and bHLH038 or FIT and bHLH039 resulted in a constitutive reduction of ferric iron at the roots (Yuan et al., 2008). Like many transcription factors, PYE protein can also interact with other bHLH proteins (Long et al., 2010), and WRKY and NAC proteins are known to form heterodimers (Olsen et al., 2005; Rushton et al., 2010). An extensive network of genes is associated with PYE (Ivanov et al., 2011), incluing metal homeostasis genes. These results suggest that differences in expression of partner bHLH genes between ecotypes for genes that interact with FIT (e.g. bHLH039, bHLH038, other unknown proteins) or other key transcription factors may contribute to different Fe regulons in different ecotypes.
As with other MapMan functional categories, there were a number of signalling genes that were Fe-regulated in some, but not all ecotypes (Supplementary Fig. S7). However, several interesting gene-expression patterns were conserved among several ecotypes. Two leucine-rich repeat protein kinases and one serine/threonine kinase were downregulated in multiple ecotypes, and one (At5g49760) was also downregulated at the protein level (Lan et al., 2010), suggesting that the proteins encoded by these genes may be involved in early Fe-deficiency responses. Decreased expression of these kinases could modulate the activity of a number of undetermined proteins that carry out physiological responses to Fe deficiency.
An additional gene of interest that was conserved across ecotypes is GRF11/GR14, a 14-3-3 protein, expression of which depended on presence of a functional FIT gene (Colangelo and Guerinot, 2004). The 14-3-3 proteins activate or deactivate other proteins by binding phosphorylated motifs in the target protein and changing its conformation (Oecking and Jaspert, 2009). GF14 was demonstrated to activate the AHA2 H+-ATPase protein (Jahn et al., 1997), which has been identified as the primary source of rhizosphere acidification under Fe deficiency (Santi and Schmidt, 2009). AHA2 gene expression increases under Fe deficiency, but at time points later than FIT, IRT1, or FRO2. Activation of the protein could be a more rapid and additional level of regulation for rhizosphere acidification activity, and modification by 14-3-3 proteins may be a mechanism for activation of other Fe-deficiency responses. We searched the Arabidopsis proteome for potential 14-3-3-binding sites (RSX(S/T)XP) using Patmatch, then compared these putative target proteins to the core and common Fe response genes. This identified additional potential targets in metabolic (At3g21240, 4-coumorate:CoA ligase in the phenylpropanoid pathway), signalling (rac GTPase activating protein), and regulatory (bHLH101, WRKY22) targets, suggesting that GRF11 may be a key control point in the cascade of Fe-deficiency responses in Arabidopsis by modulating protein activity prior to transcriptional upregulation, which occurs for these genes in most or all of the ecotypes studied. Proteomic profiling has demonstrated that 14-3-3 proteins can interact with WRKY family transcription factors (Chang et al., 2009). A model of the potential GRF11 network is presented in Supplementary Fig. S8.
Our study suggests that comparison of transcriptomes of diverse germplasm using similar experiments can reveal robustly differentially Fe-regulated genes and exclude a substantial number of genes that are more slowly or non-Fe-responsive in some ecotypes. We have also identified conserved signalling networks that may play key roles in universal Fe responses at the level of protein activity. Future studies can further exploit natural variation between ecotypes to further understand the biology of different ecotypes on a systems level and uncover the factors that control sensing of and responses to plant Fe status.
Supplementary material
Supplementary material is available at JXB online.
Supplementary Table S1. Overrepresented GO and MIPS categories in Kas-1 and Tsu-1.
Supplementary Fig. S1. Number of lateral roots of Kas-1 and Tsu-1 seedlings.
Supplementary Fig. S2. Heat map of the MapMan ‘transport’ functional bin.
Supplementary Fig. S3. Heat map of the MapMan ‘metal-handling’ functional bin.
Supplementary Fig. S4. Heat map of the MapMan ‘redox’ functional bin.
Supplementary Fig. S5. Heat map of the MapMan ‘miscellaneous’ functional bin.
Supplementary Fig. S6. Heat map of the MapMan ‘regulation’ functional bin.
Supplementary Fig. S7. Heat map of the MapMan ‘signalling’ functional bin.
Supplementary Fig. S8. Model of the involvement of GF14/GRF11, a 14-3-3 protein, in a potential network of root Fe-deficiency-regulated responses.
Supplementary Dataset. Iron-regulated genes from sets represented in Fig. 3 and Fig. 4.
Acknowledgments
This work was supported in part by a grant from the University of Nebraska Layman Fund to BMW. We thank Laura Hock for excellent technical assistance.
Glossary
Abbreviations:
- ABRC
Arabidopsis Biological Resource Center
- SNP
single-nucleotide polymorphism
- EDDHA
ethylenediamine-N,N'-bis(2-hydroxyphenylacetic acid)
- MES
2-(N-morpholino)ethanesulphonic acid
- RT
reverse transcriptase
- Ct
cycle threshold
- GO
Gene Ontology
- MIPS
Munich Information Center for Protein Sequences
- bHLH
basic helix-loop-helix
References
- Alonso-Blanco C, Aarts MGM, Bentsink L, Keurentjes JJB, Reymond M, Vreugdenhil D, Koornneef M. What has natural variation taught us about plant development, physiology, and adaptation? The Plant Cell. 2009;21:1877–1896. doi: 10.1105/tpc.109.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Senger T, Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. The Plant Journal. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- Baxter I, Muthukumar B, Park HC, Buchner P, Lahner B, Danku J, Zhao K, Lee J, Hawkesford MJ, Guerinot ML, Salt DE. Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1) PLOS Genetics. 2008a;4:12. doi: 10.1371/journal.pgen.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Guerinot ML, Salt DE. The leaf ionome as a multivariable system to detect a plant's physiological status. Proceedings of the National Academy of Sciences, USA. 2008b;105:12081–12086. doi: 10.1073/pnas.0804175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair MW, Knewtson SJB, Astudillo C, Li CM, Fernandez AC, Grusak MA. Variation and inheritance of iron reductase activity in the roots of common bean (Phaseolus vulgaris L.) and association with seed iron accumulation QTL. BMC Plant Biology. 2010;10:12. doi: 10.1186/1471-2229-10-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Duc C, Ravet K, Gaymard F. Ferritins and iron storage in plants. Biochimica et Biophysica Acta General Subjects. 2010;1800:806–814. doi: 10.1016/j.bbagen.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Buckhout TJ, Yang TJW, Schmidt W. Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genomics. 2009;10:147. doi: 10.1186/1471-2164-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher E, Achberger T, Amusan I, et al. Natural genetic variation in selected populations of Arabidopsis thaliana is associated with ionomic differences. PLOS One. 2010;5:10. doi: 10.1371/journal.pone.0011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I-F, Curran A, Woolsey R, Quilici D, Cushman JC, Mittler R, Harmon A, Harper JF. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics. 2009;9:2967–2985. doi: 10.1002/pmic.200800445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo EP, Guerinot ML. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. The Plant Cell. 2004;16:3400–3412. doi: 10.1105/tpc.104.024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Lucena C, Romera FJ, Alcantara E, Perez-Vicente R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. Journal of Experimental Botany. 2010;61:3885–3899. doi: 10.1093/jxb/erq203. [DOI] [PubMed] [Google Scholar]
- Ghandilyan A, Ilk N, Hanhart C, et al. A strong effect of growth medium and organ type on the identification of QTLs for phytate and mineral concentrations in three Arabidopsis thaliana RIL populations. Journal of Experimental Botany. 2009;60:1409–1425. doi: 10.1093/jxb/erp084. [DOI] [PubMed] [Google Scholar]
- Giehl RFH, Meda AR, von Wiren N. Moving up, down, and everywhere: signaling of micronutrients in plants. Current Opinion in Plant Biology. 2009;12:320–327. doi: 10.1016/j.pbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Letters. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- Hansch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Current Opinion in Plant Biology. 2009;12:259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Henriques R, Jasik J, Klein M, Martinoia E, Feller U, Schell J, Pais MS, Koncz C. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Molecular Biology. 2002;50:587–597. doi: 10.1023/a:1019942200164. [DOI] [PubMed] [Google Scholar]
- Ivanov R, Brumbarova T, Bauer P. Fitting into the Harsh Reality: Regulation of Iron-deficiency Responses in Dicotyledonous Plants. Molecular Plant. 2011 doi: 10.1093/mp/ssr065. in press. [DOI] [PubMed] [Google Scholar]
- Jahn T, Fuglsang AT, Olsson A, Brüntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. The Plant Cell. 1997;9:1805–1814. doi: 10.1105/tpc.9.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P. FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Letters. 2004;577:528–534. doi: 10.1016/j.febslet.2004.10.062. [DOI] [PubMed] [Google Scholar]
- Juenger TE, Sen S, Bray E, Stahl E, Wayne T, Mckay J, Richards JH. Exploring genetic and expression differences between physiologically extreme ecotypes: comparative genomic hybridization and gene expression studies of Kas-1 and Tsu-1 accessions of Arabidopsis thaliana. Plant, Cell & Environment. 2010;33:1268–1284. doi: 10.1111/j.1365-3040.2010.02146.x. [DOI] [PubMed] [Google Scholar]
- Katari MS, Nowicki SD, Aceituno FF, et al. VirtualPlant: a software platform to support systems biology research. Plant Physiology. 2010;152:500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Molecular Biology. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Lan P, Li WF, Wen TN, Shiau JY, Wu YC, Lin WD, Schmidt W. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiology. 2010;155:821–834. doi: 10.1104/pp.110.169508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell and Environment. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. The Plant Cell. 2010;22:2219–2236. doi: 10.1105/tpc.110.074096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O, Chaillou S, Merigout P, Talbotec J, Daniel-Vedele F. Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiology. 2003;131:345–358. doi: 10.1104/pp.102.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology. 2003;12:1137–1151. doi: 10.1046/j.1365-294x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Nemali KS, Sen S, Mitchell-Olds T, Boles S, Stahl EA, Wayne T, Juenger TE. Genetics of drought adaptation in Arabidopsis thaliana II. QTL analysis of a new mapping population, Kas-1 x Tsu-1. Evolution. 2008;62:3014–3026. doi: 10.1111/j.1558-5646.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Schmitt J. Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature. 2006;441:947–952. doi: 10.1038/nature04878. [DOI] [PubMed] [Google Scholar]
- Morrissey J, Baxter IR, Lee J, Li LT, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. The Plant Cell. 2009;21:3326–3338. doi: 10.1105/tpc.109.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee I, Campbell NH, Ash JS, Connolly EL. Expression profiling of the Arabidopsis ferric chelate reductase (FRO) gene family reveals differential regulation by iron and copper. Planta. 2006;223:1178–1190. doi: 10.1007/s00425-005-0165-0. [DOI] [PubMed] [Google Scholar]
- Oecking C, Jaspert N. Plant 14-3-3 proteins catch up with their mammalian orthologs. Current Opinion in Plant Biology. 2009;12:760–765. doi: 10.1016/j.pbi.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Olsen AN, Ernst HA, Lo Leggio L, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends in Plant Science. 2005;10:79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proceedings of the National Academy of Sciences, USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, Salt DE. Natural variants of AtHKT1 enhance Na+ accumulation in two wild Populations of Arabidopsis. PLOS Genetics. 2006;2:1964–1973. doi: 10.1371/journal.pgen.0020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QXJ. WRKY transcription factors. Trends in Plant Science. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Saleeba JA, Guerinot ML. Induction of ferric reductase activity in response to iron deficiency in Arabidopsis. Biometals. 1995;8:297–300. [Google Scholar]
- Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytologist. 2009;183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Buckhout TJ. A hitchhiker's guide to the Arabidopsis ferrome. Plant Physiology and Biochemistry. 2011;49:462–470. doi: 10.1016/j.plaphy.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Schuler M, Keller A, Backes C, Philippar K, Lenhof HP, Bauer P. Transcriptome analysis by GeneTrail revealed regulation of functional categories in response to alterations of iron homeostasis in Arabidopsis thaliana. BMC Plant Biology. 2011;11:10. doi: 10.1186/1471-2229-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MG, Patel A, McClain WE, Mathieu M, Remley M, Rogers EE, Gassmann W, Blevins DG, Stacey G. The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiology. 2008;146:589–601. doi: 10.1104/pp.107.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Essigmann B, Kloska S, Altmann T, Buckhout TJ. Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiology. 2001;127:1030–1043. [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, et al. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal. 2004;37:914–939. doi: 10.1111/j.1365-313x.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen H, Kliebenstein DJ, West MAL, Kim K, van Poecke R, Katagiri F, Michelmore RW, Doerge RW, Clair DA. Natural variation among Arabidopsis thaliana accessions for transcriptome response to exogenous salicylic acid. The Plant Cell. 2007;19:2099–2110. doi: 10.1105/tpc.107.050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varotto C, Maiwald D, Pesaresi P, Jahns P, Salamini F, Leister D. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. The Plant Journal. 2002;31:589–599. doi: 10.1046/j.1365-313x.2002.01381.x. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorwieger A, Gryczka C, Czihal A, et al. Iron assimilation and transcription factor controlled synthesis of riboflavin in plants. Planta. 2007;226:147–158. doi: 10.1007/s00425-006-0476-9. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil D, Aarts MGM, Koornneef M, Nelissen H, Ernst WHO. Natural variation and QTL analysis for cationic mineral content in seeds of Arabidopsis thaliana. Plant Cell and Environment. 2004;27:828–839. [Google Scholar]
- Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology. 2008;11:530–535. doi: 10.1016/j.pbi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Waters BM, Grusak MA. Quantitative trait locus mapping for seed mineral concentrations in two Arabidopsis thaliana recombinant inbred populations. New Phytologist. 2008a;179:1033–1047. doi: 10.1111/j.1469-8137.2008.02544.x. [DOI] [PubMed] [Google Scholar]
- Waters BM, Grusak MA. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytologist. 2008b;177:389–405. doi: 10.1111/j.1469-8137.2007.02288.x. [DOI] [PubMed] [Google Scholar]
- Werner JD, Borevitz JO, Uhlenhaut NH, Ecker JR, Chory J, Weigel D. FRIGIDA-independent variation in flowering time of natural Arabidopsis thaliana accessions. Genetics. 2005;170:1197–1207. doi: 10.1534/genetics.104.036533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IW, ClL Schiff, Hughes DE, Somerville SC. Quantitative trait loci analysis of powdery mildew disease resistance in the Arabidopsis thaliana accession Kashmir-1. Genetics. 2001;158:1301–1309. doi: 10.1093/genetics/158.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H, Fox T, Wu YY, Feng V, Chen WQ, Chang HS, Zhu T, Vulpe C. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. Journal of Biological Chemistry. 2003;278:47644–47653. doi: 10.1074/jbc.M309338200. [DOI] [PubMed] [Google Scholar]
- Wray GA, Hahn MW, Abouheif E, Balhoff JP, Pizer M, Rockman MV, Romano LA. The evolution of transcriptional regulation in eukaryotes. Molecular Biology and Evolution. 2003;20:1377–1419. doi: 10.1093/molbev/msg140. [DOI] [PubMed] [Google Scholar]
- Wu HL, Li LH, Du J, Yuan YX, Cheng XD, Ling HQ. Molecular and biochemical characterization of the Fe(III) chelate reductase gene family in Arabidopsis thaliana. Plant and Cell Physiology. 2005;46:1505–1514. doi: 10.1093/pcp/pci163. [DOI] [PubMed] [Google Scholar]
- Yang TJW, Lin W-D, Schmidt W. Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiology. 2010;152:2130–2141. doi: 10.1104/pp.109.152728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YX, Zhang J, Wang DW, Ling HQ. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Research. 2005;15:613–621. doi: 10.1038/sj.cr.7290331. [DOI] [PubMed] [Google Scholar]
- Yuan YX, Wu HL, Wang N, Li J, Zhao WN, Du J, Wang DW, Ling HQ. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Research. 2008;18:385–397. doi: 10.1038/cr.2008.26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.