Abstract
The Notch signaling pathway regulates embryonic development of the pancreas, inhibiting progenitor differentiation into exocrine acinar and endocrine islet cells. The adult pancreas appears to lack progenitor cells, and its mature cell types are maintained by the proliferation of pre-existing differentiated cells. Nonetheless, Notch remains active in adult duct and terminal duct/centroacinar cells (CACs), in which its function is unknown. We previously developed mice in which cells expressing the Notch target gene Hes1 can be labeled and manipulated, by expression of Cre recombinase, and demonstrated that Hes1+ CACs do not behave as acinar or islet progenitors in the uninjured pancreas, or as islet progenitors after pancreatic duct ligation. In the current study, we assessed the function of Notch signaling in the adult pancreas by deleting the transcription factor partner of Notch, Rbpj, specifically in Hes1+ cells. We find that loss of Rbpj depletes the pancreas of Hes1-expressing CACs, abrogating their ongoing contribution to growth and homeostasis of more proximal duct structures. Upon Rbpj deletion, CACs undergo a rapid transformation into acinar cells, suggesting that constitutive Notch activity suppresses the acinar differentiation potential of CACs. Together, our data provide direct evidence of an endogenous genetic program to control interconversion of cell fates in the adult pancreas.
Keywords: pancreas, Notch, Rbpj, transdifferentiation, acinar, centroacinar
INTRODUCTION
The adult pancreas experiences relatively little cell turnover during normal homeostasis, and most evidence to date indicates that its cell types are maintained by faithful replication of pre-existing cells. In endocrine islets, replication is the main mode of generating new insulin-producing β-cells (Brennand et al., 2007; Dor et al., 2004; Georgia and Bhushan, 2004; Teta et al., 2007). Replication also appears to be the mechanism by which acinar cells, belonging to the exocrine pancreas, are maintained during homeostasis and regeneration (Desai et al., 2007; Strobel et al., 2007). Although it remains controversial whether adult stem or progenitor cells contribute to maintenance and repair of the pancreas, embryonic pancreatic organogenesis relies on multipotent and lineage-restricted progenitor cells, the differentiation of which is controlled by intrinsic and extrinsic factors (reviewed in Pan and Wright, 2011). Notch signaling is a major regulator of progenitor cell differentiation, in the embryonic pancreas as well as numerous other developing and adult tissues (Chiba, 2006). Although Notch appears to be active in the adult pancreas, its potential contribution to tissue homeostasis is unknown and is the focus of this study.
The Notch pathway is activated by juxtacrine interactions between Delta/Serrate family ligands and Notch family receptors, which trigger the protease-induced release and nuclear translocation of the Notch intracellular domain (NIC). Nuclear NIC binds the transcription factor Su(H)/CSL/Rbpjκ (henceforth referred to as Rbpj), and co-activates target genes including the Hes/Hey family of transcriptional repressors (Kageyama et al., 2007; Kopan and Ilagan, 2009). A central output of Notch signaling, across tissues and phyla, is control of cell fate (Chiba, 2006), and Notch activation in the embryonic pancreas inhibits acinar and islet cell differentiation while promoting development of duct cells (Esni et al., 2004; Hald et al., 2003; Kopinke et al., 2011; Murtaugh et al., 2003; Yee et al., 2005). In the mature pancreas, gain-offunction studies suggest that Notch signaling promotes acinar cell transdifferentiation to duct or progenitor-like cells (De La O et al., 2008; Miyamoto et al., 2003; Mukhi and Brown, 2011). Whether endogenous Notch plays such a role remains unclear, as the only phenotype observed after pan-pancreatic deletion of the Notch1 receptor is impaired regeneration of adult acinar cells (Siveke et al., 2008). Nonetheless, Notch signaling appears to be active in the adult pancreas, as evidenced by expression of its target gene Hes1 in centroacinar cells (CACs) and ducts (Kopinke et al., 2011; Miyamoto et al., 2003; Parsons et al., 2009; Stanger et al., 2005). CACs constitute the terminal element of the ductal tree and are characterized by their central position within individual acinar rosettes (Ekholm et al., 1962). These cells have been proposed to represent an adult progenitor-like cell in the pancreas and to produce new β-cells following injury (Hayashi et al., 2003; Nagasao et al., 2003) and in vitro (Rovira et al., 2010). Whether CACs actually behave as adult progenitor cells in vivo has remained controversial, as tools for lineage tracing these cells have been lacking until now.
We recently generated a tamoxifen-inducible Cre line under the control of the Hes1 promoter (Hes1CreERT2, abbreviated Hes1C2), which faithfully marks Hes1+ CACs (Kopinke et al., 2011). Lineage tracing experiments in adult mice indicate that adult Hes1+ CACs do not normally contribute to new β-cells or acini. In utero, however, Hes1+ cells represent bipotent exocrine progenitors in which ectopic Notch promotes duct specification at the expense of acinar fate (Kopinke et al., 2011). Thus, sustained Notch signaling in Hes1+ CACs might enforce their ductal fate and restrain their full differentiation potential. In the current study, we challenge the system by disrupting Notch signaling specifically in Hes1-expressing cells, and demonstrate that Notch controls interconversion of cell fates in the adult pancreas.
MATERIAL AND METHODS
Animal experiments
Hes1C2 (Kopinke et al., 2011), R26REYFP (Srinivas et al., 2001) and Rbpj lox (Han et al., 2002) mice have been described previously. Ptf1aCre-ERTM mice were generated by recombinase-mediated cassette exchange (Burlison et al., 2008), inserting the Cre-ERTM coding region (Danielian et al., 1998) into the first exon of Ptf1a (full details of this allele will be published elsewhere). Rbpj lox mice, kindly provided by Tasuku Honjo (Kyoto University, Kyoto, Japan) and Sean Morrison (University of Michigan, Ann Arbor, MI), were crossed to Hprt-Cre deletor mice (Tang et al., 2002) to generate a null (RbpjΔ) allele. PCR genotyping for the floxed allele of Rbpj was performed as described (Han et al., 2002); for the null allele, the following oligos were used: forward 5'-TAACTATCTTGGAAGGCTAAAAT-3' and reverse 5'-GCTTGAGGCTTGATGTTCTGTATTGC-3' (598 bp product).
Tamoxifen (Sigma T-5648) was dissolved in corn oil, and administered by oral gavage at doses of 5 mg (Ptf1aCre-ERTM) or 10 mg (Hes1C2) per mouse between 6–8 weeks of age. BrdU (Sigma) was dissolved in drinking water (1 mg/ml) and provided to mice ad libitum, beginning 3 days prior to tamoxifen administration and continuing for 7 days thereafter. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Staining and analysis
Immunostaining and analysis were performed as previously described (Kopinke et al., 2011; Kopinke and Murtaugh, 2010). The following primary antibodies were used: sheep anti-amylase 1:2500 (BioGenesis), rat anti-BrdU 1:2000 (Abcam), rabbit anti-cytokeratin-19 1:1500 (gift from Ben Stanger, University of Pennsylvania, Philadelphia, PA), rabbit monoclonal anticytokeratin-19 1:500 (Epitomics), rat anti-cytokeratin-19 1:50 (Developmental Studies Hybridoma Bank), rabbit anti-cleaved Caspase3 1:1000 (Cell Signaling), rat anti-E-cadherin 1:2000 (Zymed), rabbit anti-GFP 1:4000 (Abcam), goat anti-GFP 1:2500 (Rockland), guinea pig anti-glucagon 1:2500 (Linco), rabbit anti-glucagon 1:2500 (Zymed), guinea pig anti-Insulin 1:2000 (Dako), rabbit anti-Ki67 1:150 (Vector labs) and rabbit anti-Ptf1a 1:800 (gift from Helena Edlund, Umea University, Umea, Sweden). All secondary antibodies (raised in donkey) were obtained from Jackson Immunoresearch. For Ki67 and BrdU immunofluorescence, a 15 min DNase I digestion (700 U/µl, in 40 mM Tris-HCl pH 7.4, 10 mM NaCl, 6 mM MgCl2, 10 mM CaCl2) was necessary (Ye et al., 2007). Periodic Acid Schiff (PAS) staining was carried out according to the manufacturer's instructions (Sigma). For quantifications, co-immunofluorescence was determined using the Analyze Particles function of ImageJ (NIH) and confirmed by eye in Adobe Photoshop. Calculations and graphs were generated with Microsoft Excel and R (www.r-project.org). P-values were determined by Tukey’s HSD test in R, and data are represented as mean +/− SEM. The numbers of mice used for each experiment are indicated in each graph. Acinar dissociation was performed as previously described (Kopinke and Murtaugh, 2010). The total number of cells counted for each graph is listed in Table S1.
RESULTS
Hes1-specific deletion of Rbpj in adult intestine and pancreas
Rbpj encodes the transcription factor through which Notch activates target genes (Kopan and Ilagan, 2009). To determine a potential role for Notch signaling in Hes1+ cells of the adult pancreas, we used our inducible Hes1CreERT2 line (Hes1C2) to delete a floxed Rbpj allele (Han et al., 2002; Kopinke et al., 2011). Our breeding scheme (Fig. 1A) yielded both Hes1C2/+; Rbpjlox/+ mice, which are heterozygous for the floxed allele (henceforth referred to as RbpjHes1-lox), and Hes1C2/+; Rbpj lox/Δ animals, which carry a null (Δ) and a floxed allele of Rbpj (RbpjHes1-cKO). All genotypes also included an R26REYFP reporter (Srinivas et al., 2001), to follow the fate of recombined cells (see below). RbpjHes1-cKO mice reached adulthood at a Mendelian ratio, and were indistinguishable from wild-type or RbpjHes1-lox animals before tamoxifen (TM) administration. It should be noted, however, that RbpjHes1-cKO animals are compound heterozygotes for two major Notch components, Hes1 and Rbpj. As an additional control, therefore, we generated mice that were heterozygous for the null rather than the floxed allele of Rbpj (Hes1C2/+; R26REYFP/+; RbpjΔ/+; referred to as RbpjHes1-het). Comparisons between these mice and RbpjHes1-cKO allowed us to distinguish potential phenotypes caused by compound Hes1/Rbpj heterozygosity from those attributable to complete loss of Rbpj.
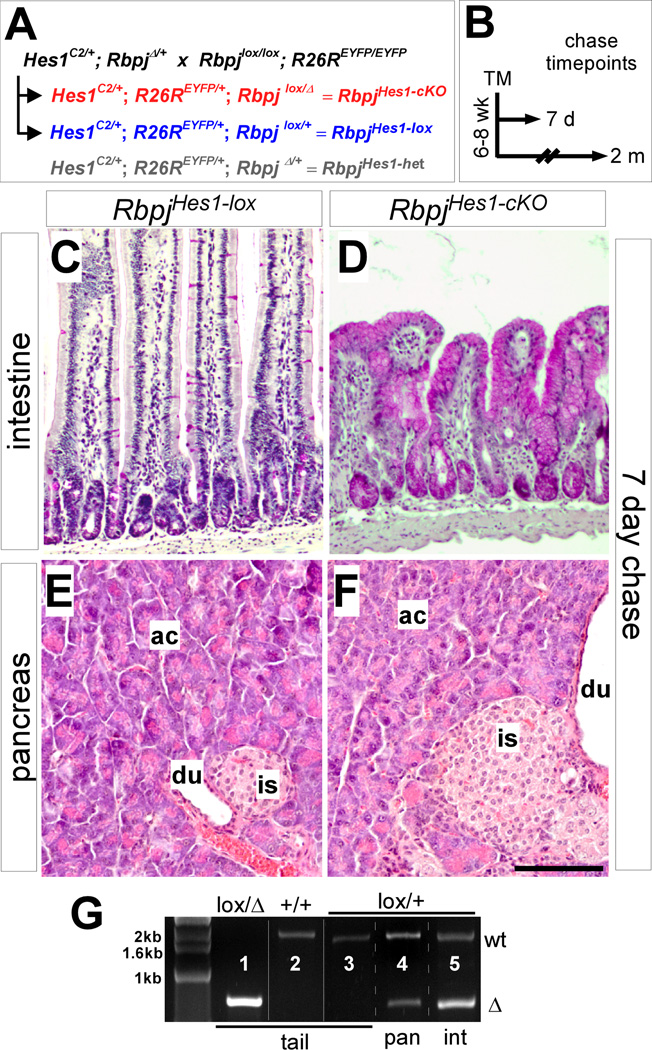
Figure 1. Hes1-specific deletion of Rbpj in the pancreas and intestine.
(A) Breeding strategy for conditional knockout of Rbpj. Animals heterozygous for a null (Δ) and a floxed (lox) allele of Rbpj (Rbpj lox/Δ) are abbreviated RbpjHes1-cKO, while Rbpjlox/+ mice serve as controls (RbpjHes1-lox). In some experiments, RbpjΔ/+ mice were included as an additional control (RbpjHes1-het). All mice also carry a R26REYFP reporter allele, allowing for lineage tracing of recombined cells. (B) Pulse-chase strategy. Recombination was induced by TM administration in 6–8 week old adults and animals were chased for 7 days (short term) or 2 months (long term) before analyzing. (C–F) Comparison of PAS-stained intestine (C–D) and H&E-stained pancreata (E–F) between RbpjHes1-lox and RbpjHes1-cKO mice after a 7 day chase. Intestinal KO of Rbpj leads to widespread transformation of the gut epithelium into PAS+ goblet cells. In contrast, no morphological differences were detected between RbpjHes1-lox and RbpjHes1-cKO pancreata. (G) PCR to detect recombination of floxed Rbpj. While the deletion-specific Rbpj amplification product (Δ) can be detected in tail DNA from Rbpjlox/Δ (lane l) but not Rbpj+/+ or Rbpjlox/+ (lanes 2–3) mice, the Δ band can be amplified from the pancreas or duodenum of an Rbpjlox/+ mouse after TM treatment (lanes 4–5). Abbreviations: ac, acinar; is, islet; du, duct. Scale bar: 100 µm.
In all experiments, unless otherwise indicated, 10 mg TM was administered to 6–8 week old adult mice, which were chased for 7 days (short term) or 2 months (long term) (Fig. 1B). To monitor proliferation of labeled cells, mice used for 7 day chase experiments were also continuously supplied with the thymidine analogue BrdU in the drinking water, from 3 days prior to TM treatment through sacrifice. This approach has previously been shown to capture all cells entering S-phase during the chase period (Teta et al., 2007).
Inhibiting Notch in the small intestine causes overproduction of goblet cells (Riccio et al., 2008; van Es et al., 2005), and we assayed this phenotype as an indicator of successful Rbpj deletion. Hes1C2 is active in intestinal stem cells (Kopinke et al., 2011), and deletion of Rbpj with Hes1C2 caused robust transformation of the gut epithelium into PAS-positive goblet cells (Fig. 1C–D). Importantly, the pancreata of these mice exhibited no obvious morphological differences from controls (Fig. 1E–F). To confirm successful recombination in the pancreas, we performed PCR to detect the deletion (Δ) allele of Rbpj (Fig. 1G). As expected, the deletion-specific product can be detected in the pancreas and intestine of TM-treated RbpjHes1-lox mice, indicating recombination of the floxed allele.
Deletion of Rbpj in Hes1-expressing duct cells blocks their expansion
We previously showed that Hes1C2 marks not only CACs but also a preferentially-expanding subset of cells within adult ducts, consistent with a recent study suggesting that Jagged1-Notch signaling is mitogenic for ducts (Golson et al., 2009; Kopinke et al., 2011). We therefore analyzed Rbpj knockouts for any defects of the ductal tree, using the R26REYFP reporter allele to monitor the fate of cells deleting Rbpj. This approach should allow us to quantitatively compare RbpjHes1-lox to RbpjHes1-cKO cells by virtue of EYFP expression.
As previously (Kopinke et al., 2011), we could detect an increase in the fraction of labeled CK19+ duct cells between 7 days and 2 months in RbpjHes1-lox mice. By comparison, the labeling index of RbpjHes1-cKO ducts remained the same regardless of the chase period (Fig. 2A–E; see also Table S1 for details on this and other quantitative analyses), suggesting that the expansion of Hes1 lineage-derived ducts requires Notch activity. The ductal tree can be divided into proximal (intra- and interlobular) and distal ducts (intercalated ducts, terminal ducts and CACs) (Kopinke and Murtaugh, 2010). To determine if loss of Rbpj results in different outcomes at different positions within the ductal network, we analyzed RbpjHes1-lox and RbpjHes1-cKO pancreata for the contribution of EYFP+ cells specifically to proximal and distal ducts at 7 days post-TM. Since terminal ducts and CACs are phenotypically similar and difficult to distinguish under the microscope (Ekholm et al., 1962), we analyzed them together and henceforth refer to them collectively as CACs. We found that although the EYFP+ fraction of intra- and interlobular ducts did not change within 7 days of Rbpj deletion (Fig. 2F), Rbpj-deleted intercalated ducts and CACs experienced an approximately 2-fold reduction in their EYFP labeling index (Fig. 2G). In addition, the fraction of EYFP-labeled cells that had progressed through the cell cycle, as indicated by BrdU incorporation, did not change among proximal ducts but decreased ~2-fold in distal ducts (Fig. 2F–I).
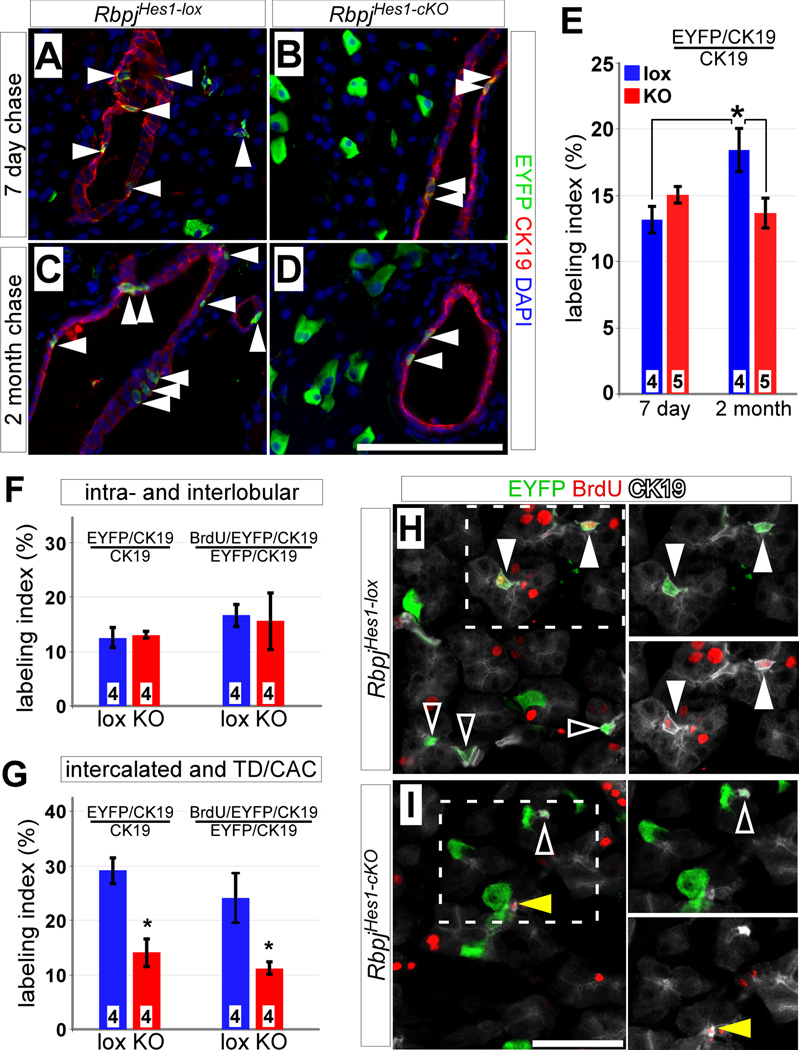
Figure 2. Deletion of Rbpj inhibits expansion of Hes1C2-labeled ducts.
(A–E) Adult RbpjHes1-lox and RbpjHes1-cKO mice were treated with tamoxifen and stained for EYFP (green) and the duct marker CK19 (red) after 7 days (A–B) and 2 months (C–D). While there is no difference in the fraction of EYFP-labeled duct cells (arrowheads) between 7 day chased RbpjHes1-lox and RbpjHes1-cKO animals, an increase is detected between 7 days and 2 month in RbpjHes1-lox mice, which is inhibited in RbpjHes1-cKO animals (E). * P < 0.05. (F) Quantifications of EYFP and BrdU labeling indices of intra- and interlobular ducts indicate no differences between RbpjHes1-lox and RbpjHes1-cKO mice (P=0.75). (G) Quantifications of EYFP and BrdU labeling indices of intercalated ducts, terminal ducts (TD) and centroacinar cells (CACs) reveal a 2-fold decrease in EYFP labeling in RbpjHes1-cKO mice, as well as a 2-fold reduction in the fraction of cycling (BrdU+) EYFP-labeled intercalated ducts, TDs and CACs. * P < 0.05. (H–I) EYFP (green) and BrdU (red) labeling of CK19+ CACs (white), 7 days post-TM and following a 10 day BrdU pulse. CACs expressing EYFP only (open arrowheads) or positive for both EYFP and BrdU (closed arrowheads) can be found in RbpjHes1-lox mice. In contrast, RbpjHes1-cKO animals have fewer EYFP+ CACs, and most of the BrdU+ CACs are EYFP-negative (yellow arrowhead). Numbers in bars (E–G) indicate mice analyzed per group. Scale bars: A–D and J, 100 µm; F–G, 50 µm.
In wild-type pancreata, both the EYFP and BrdU labeling indices of distal duct cells are approximately double those of proximal cells (Fig. 2F–G). The fact that proliferation of distal cells declined to a more proximal-like level, following Rbpj deletion, could reflect a mitogenic role for Notch in this population, but a change in proliferation alone seemed insufficient to explain the observed rapid decrease in EYFP labeling of distal duct cells. Staining for cleaved Caspase-3 revealed minimal apoptosis in either genotype, after a 2 or 7 day post-TM chase, with no increase upon Rbpj deletion (Fig. S1 and data not shown), suggesting that apoptosis was not responsible for the disappearance of labeled cells from the distal ducts. To determine if loss of Rbpj might cause necrosis or other injury of duct cells, we stained for the exocrine lumenal marker Muc1, which is expressed preferentially in distal ducts and acini (Kopinke and Murtaugh, 2010). Consistent with the normal histological appearance of RbpjHes1-cKO pancreata (Fig. 1E–F), we found no gross or subtle morphological abnormalities in the Muc1+ ductal network following Rbpj deletion (Fig. S2). We therefore considered the possibility that a subset of Rbpj-depleted distal duct cells had adopted a non-ductal fate.
Dramatic increase of Hes1-labeled acinar cells after loss of Rbpj
Notch signaling inhibits embryonic islet cell development (Apelqvist et al., 1999; Jensen et al., 2000), suggesting that Rbpj-deleted CACs might adopt an endocrine fate. Nonetheless, we did not detect a single insulin+ β-cell labeled by EYFP after a 7-day or 2-month chase in RbpjHes1-cKO mice, nor was there any increase in the small fraction of glucagon+ α-cells normally labeled by Hes1C2 (Kopinke et al., 2011) (Fig. S3). We conclude that inhibiting Notch activity does not permit CAC-to-islet differentiation. By contrast, casual inspection revealed a considerable increase in EYFP labeling of RbpjHes1-cKO acinar cells compared to RbpjHes1-lox (Fig. 3A–D). This effect was quite rapid: within 7 days of tamoxifen administration, RbpjHes1-cKO pancreata exhibited an approximately 3.5-fold increase in labeled acinar cells, which did not increase further after 2 months (Fig. 3E). This increase was not due to compound haploinsufficiency for Rbpj and Hes1, as the acinar labeling index of RbpjHes1-het mice was indistinguishable from RbpjHes1-lox. The increase in EYFP+ acinar cells was not accompanied by a detectable change in total pancreas mass despite what should correspond to a 10% increase in total acinar numbers (Fig. S4), although such a small change might be difficult to detect in the face of even modest experimental noise.
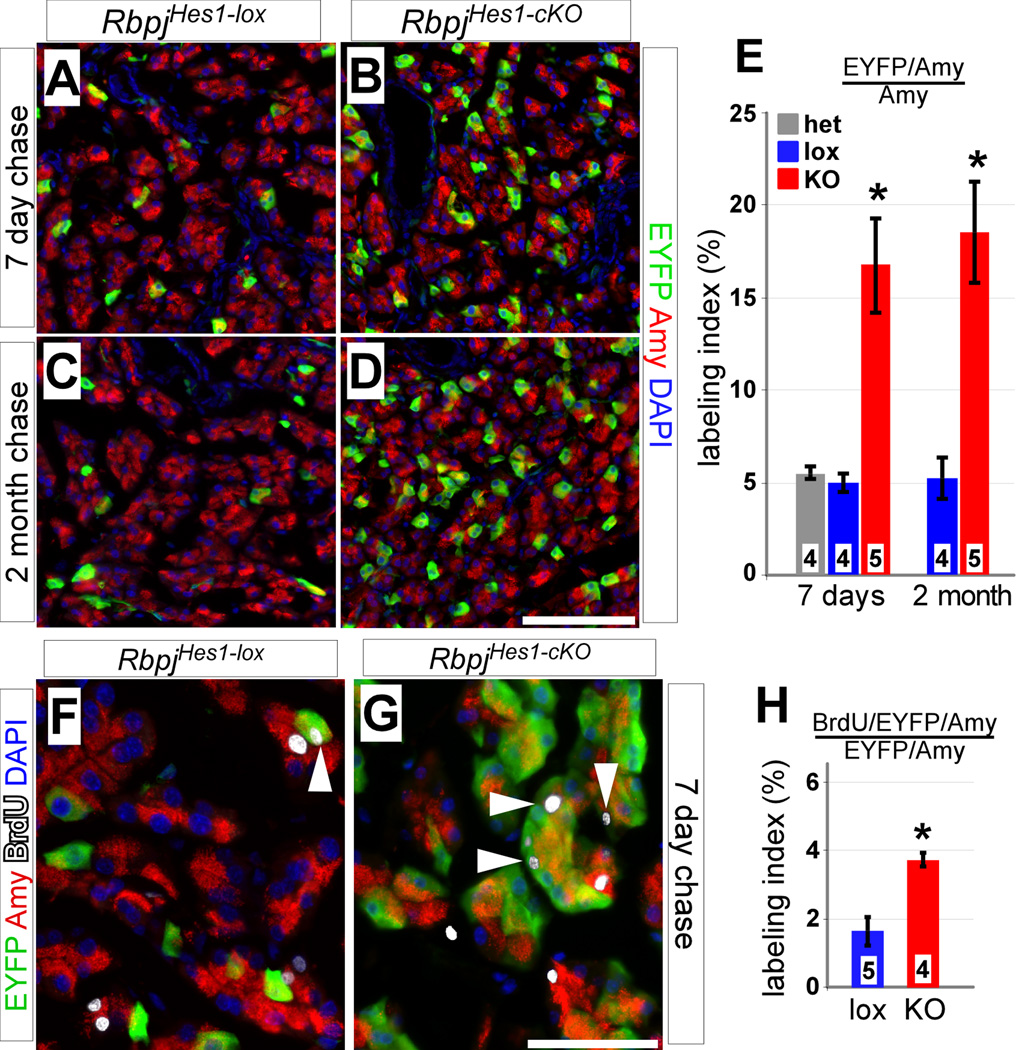
Figure 3. Loss of Rbpj in Hes1+ cells results in dramatic increase of labeled acinar cells.
(A–E) Adult RbpjHes1-lox and RbpjHes1-cKO pancreata were analyzed for co-expression of EYFP (green) with the acinar marker amylase (red), 7 days (A–B) and 2 months (C–D) post-TM. The fraction of EYFP+ acini in RbpjHes1-lox animals remains constant between 7 days and 2 months. In contrast, a drastic increase (~3.5-fold) in labeled acinar cells is seen both 7 days and 2 months after loss of Rbpj (E). * P < 0.005. (F–H) BrdU/EYFP labeling at 7 days post-TM. RbpjHes1-cKO animals display a 2-fold increase over RbpjHes1-lox in the fraction of EYFP+ acinar cells that have incorporated BrdU (white arrowheads). * P < 0.005. Numbers in bars (E, H) indicate mice analyzed per group. Scale bars: A–D, 100 µm; F–G, 50 µm.
Notch signaling has previously been suggested to inhibit acinar cell proliferation (Siveke et al., 2008). To determine whether the increased acinar labeling in RbpjHes1-cKO could be attributed entirely to division of rare Hes1C2-labeled acinar cells (Kopinke et al., 2011), we analyzed BrdU incorporation rates in RbpjHes1-lox and RbpjHes1-cKO mice (see above). After a 7 day chase, ~2% of EYFP+ acinar cells were positive for BrdU in RbpjHes1-lox mice, compared to ~4% in RbpjHes1-cKO mice (Fig. 3G–I). Because the initial fraction of BrdU+ acinar cells even after continuous administration of BrdU for 10 days was very low, and increased only to 4% in RbpjHes1-cKO mice, accelerated proliferation of Hes1+ acinar cells cannot explain fully the dramatic increase in EYFP+ acinar cells following Rbpj deletion.
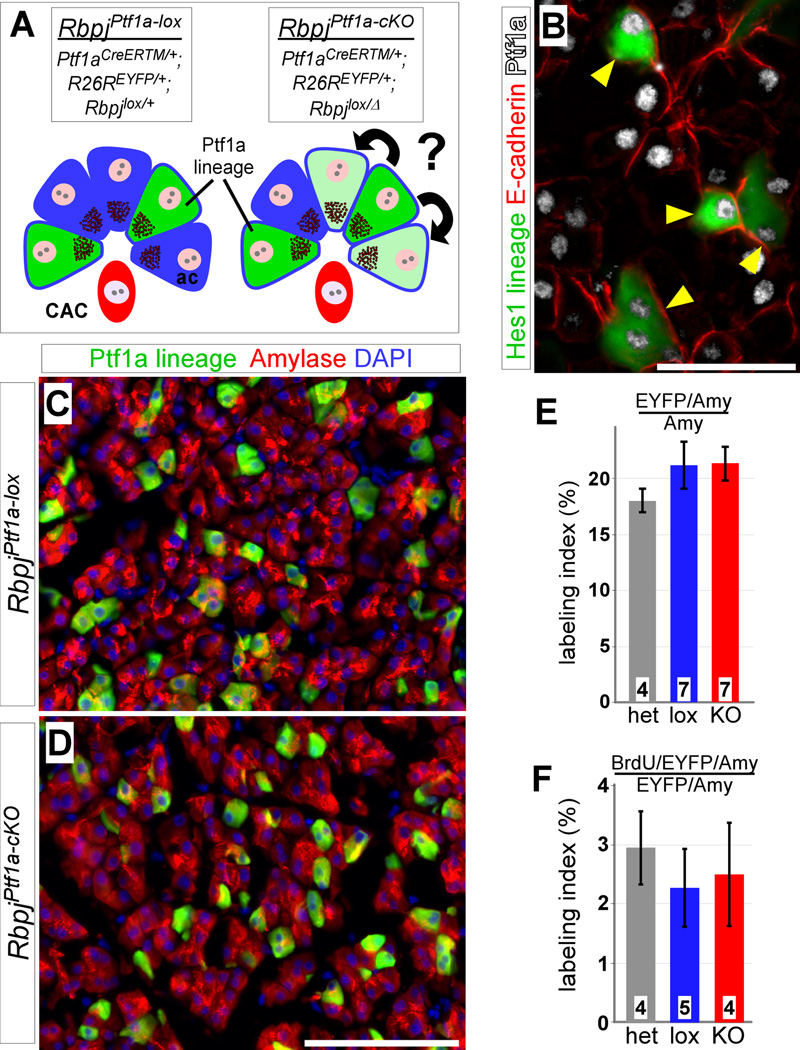
To analyze acinar cells more directly, we deleted Rbpj using a TM-inducible Cre line under control of the acinar-specific transcription factor Ptf1a (Ptf1aCre-ERTM) (Fig. 4A). Immunostaining confirmed that all acinar cells expressed Ptf1a, including the subpopulation labeled by Hes1C2 (Fig 4B). Similar to our Hes1C2 breeding scheme (Fig. 1A), we generated mice containing floxed and wild-type Rbpj alleles (RbpjPtf1a-lox), or floxed and null alleles (RbpjPtf1a-cKO), all on a R26REYFP/+ background (Fig. 4A). We found no difference between RbpjPtf1a-lox and RbpjPtf1a-cKO in the EYFP labeling of acinar cells at 7 days post-TM (Fig. 4C–E). Using the same BrdU labeling scheme as above, we also detected no change in the fraction of EYFP-expressing, BrdU+ acinar cells between genotypes (Fig. 4F). These results argue against a role for Notch in regulating proliferation of mature acinar cells, and raise the possibility of a Notch-regulated influx to the acinar compartment from another cell type.
Figure 4. No increased proliferation after acinar-specific loss of Rbpj.
(A) To determine whether Notch generally represses expansion of acinar cells, Rbpj was deleted using an inducible Ptf1aCre-ERTM Cre driver, which induces mosaic recombination in acinar cells but not ducts or CACs. Blue, acinar cells; red, CAC; green, Ptf1a lineage-labeling. (B) Short term lineage tracing of Hes1+ cells (green) demonstrates that all EYFP+ acinar cells (arrowheads) also express Ptf1a (white). (C–D) At 7 days post-TM, no difference is detected, between RbpjPtf1a-lox and RbpjPtf1a-cKO, in EYFP labeling (green) of amylase+ acinar cells (red). (E) There is no change in the fraction of EYFP+ acinar cells after a 7 day chase (P=0.52). (F) Quantification of BrdU labeling analysis 7 days post-TM. The EYFP/BrdU labeling index of acinar cells remains the same between control (lox and het) and Ptf1a1-KO animals (P=0.81). Numbers in bars (E–F) indicate mice analyzed per group. Scale bars: B, 50 µm; C–D, 100 µm.
Loss of Rbpj causes rapid transformation of CAC into acinar cells
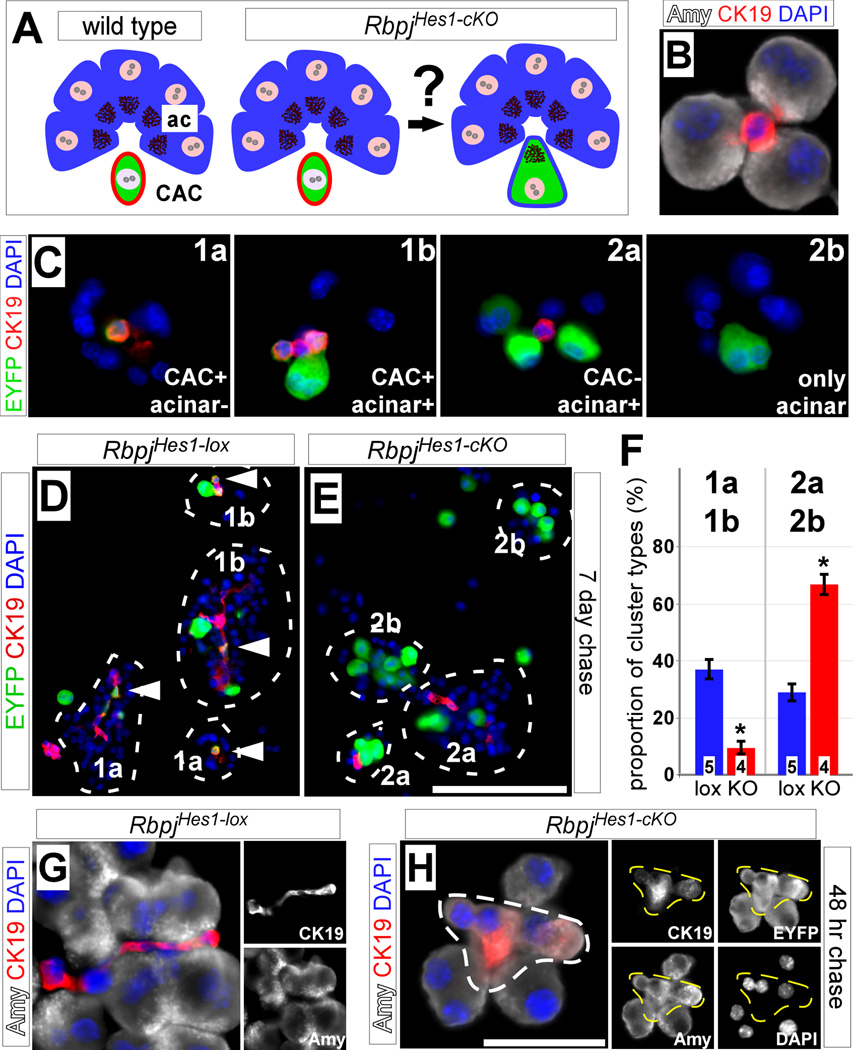
The disappearance of EYFP+ intercalated ducts and CACs (Fig. 2), and our finding that Notch does not inhibit acinar proliferation (Fig. 4), prompted us to investigate whether the observed increase of EYFP+ acinar cells in RbpjHes1-cKO mice was due to a cell fate switch of CACs (Fig. 5A). To analyze individual acinar units, and avoid missing small CACs due to sectioning artifacts, we performed enzymatic digestion to dissociate the pancreas into clusters containing only acinar cells and CACs (referred to as acinar preps; Fig. 5B) (Kopinke and Murtaugh, 2010; Kurup and Bhonde, 2002). Immediately after digestion, acinar preps were spun onto slides, fixed and processed for immunostaining. Using this method, we scored two major categories of clusters at 7 days post-TM treatment, based on the presence (class 1) or absence (class 2) of EYFP-labeled CACs. Class 1 comprised clusters in which only CACs were labeled (1a) or in which both CACs and acini were labeled (1b). Class 2 comprised clusters containing labeled acini with unlabeled CACs (2a) or labeled acini with no CACs at all (2b). If CACs were converting to acinar cells after Rbpj deletion, we would expect a decrease in class 1 clusters and an increase in class 2. Indeed, we found that the majority of RbpjHes1-lox clusters were of class 1, while class 2 predominated in RbpjHes1-cKO (Fig. 5G–H). Quantification revealed a 3.5-fold reduction, in RbpjHes1-cKO mice, of the class 1 cluster frequency, and a concomitant increase (2.5-fold) of class 2 clusters (Fig. 5I), suggesting that CACs convert to acinar cells after loss of Rbpj.
Figure 5. Hes1+ CACs contribute to acinar cells upon Rbpj deletion.
(A) We hypothesize that the increase in acinar labeling after loss of Rbpj is due to CACs adopting an acinar fate. Blue, acinar cells; red, CAC; green, Hes1 lineage-labeling. (B) Dissociation of whole pancreata generates small clusters containing amylase+ acinar cells (white) and CK19+ CACs (red). (C) Clusters from Hes1 lineage traced (green) pancreata were divided into two major categories, based on the presence (class 1) or absence (class 2) of labeled CACs as indicated. Class 1 clusters contain EYFP+ CACs with acinar cells unlabeled (1a) or EYFP+ CACs and acinar cells together (1b), while class 2 clusters contain EYFP+ acinar cells with unlabeled CACs (2a) or EYFP+ acinar cells with no CACs present (2b). Inserts represent schematic representations of cluster types. (D–E) Immunofluorescence for EYFP (green) and CK19 (red) of clusters from dissociated pancreata of RbpjHes1-lox and RbpjHes1-cKO animals at 7 days post-TM. Only clusters containing 3 or more closely-attached acinar cells were scored (circle). Most of the RbpjHes1-lox clusters contain labeled CAC (arrowhead) and belong to class 1, while CACs are either unlabeled or absent altogether in RbpjHes1-cKO clusters (classes 2a and 2b). (F) Scoring of cluster types from two independent experiments demonstrates a shift in the proportion of clusters from type 1a and 1b to 2a and 2b between RbpjHes1-lox and RbpjHes1-cKO mice (* P<0.0005). Note that the denominator represents the number of total clusters present per field, including entirely EYFP-negative ones. (G–H) Immunofluorescence for amylase (white) and CK19 (red) of cell clusters from RbpjHes1-lox (J) and RbpjHes1-cKO (K) pancreata 48 hrs after TM administration. In RbpjHes1-lox mice (J), CK19 and amylase expression are restricted to CAC and acinar cells, respectively. After TM induction (K), some amylase+ acinar cells are also positive for CK19 (dotted outline). Co-expression of duct and acinar markers is seen only in EYFP+ cells (right). Right, single-channel amylase, CK19, EYFP and DAPI staining as indicated. Numbers in bars (I) indicate mice analyzed per group. Scale bar: D–E, 50 µm; G–H, 25 µm.
If CACs were indeed capable of adopting an acinar fate, we should observe transitional cells expressing both duct and acinar markers. By analyzing acinar preps from a 48 hr chase, we were able to detect EYFP+ cells co-expressing the duct marker CK19 and the mature acinar marker amylase in RbpjHes1-cKO mice specifically (Fig. 5J–K). At later chase time points, we no longer observed EYFP-labeled cells co-expressing CK19 and amylase, nor were such cells observed in RbpjHes1-lox acinar preps at any time point. Thus, loss of Rbpj in CACs causes a rapid transition to an acinar fate.
DISCUSSION
The mammalian pancreas is a generally static organ, and numerous studies support replication as the major mode of postnatal expansion, adult homeostasis and regeneration (Brennand et al., 2007; Desai et al., 2007; Dor et al., 2004; Georgia and Bhushan, 2004; Kopinke and Murtaugh, 2010; Solar et al., 2009; Strobel et al., 2007; Teta et al., 2007). Nonetheless, adult cell fates can be overridden by the ectopic activation of developmental regulatory factors (Collombat et al., 2009; De La O et al., 2008; Zhou et al., 2008). While such gain-of-function experiments reveal the potential of adult cells to change fates, they do not address the mechanisms by which lineages normally maintain phenotypic fidelity. Here, we provide evidence that an endogenous signaling pathway acts to prevent cell type interconversion in the adult pancreas.
Notch signaling regulates cell fate decisions in numerous contexts, in some cases promoting one fate at the expense of another while in others suppressing differentiation altogether (Chiba, 2006). In the intestine, for example, Notch is not only required for absorptive cell specification but also to maintain a self-renewing stem cell compartment (Riccio et al., 2008; van Es et al., 2005). Similarly, Notch acts during early pancreas development to suppress progenitor cell differentiation, while in later organogenesis it promotes duct development at the expense of acinar (Esni et al., 2004; Hald et al., 2003; Kopinke et al., 2011; Murtaugh et al., 2003; Yee et al., 2005). We have previously shown that Hes1 lineage-derived cells represent a preferentially expanding population within the adult duct epithelium (Kopinke et al., 2011), suggesting that Notch might act in the adult primarily as a ductal mitogen. Surprisingly, we find that although Hes1IC2 is expressed in both proximal and distal duct cells, indicating Notch activity, deletion of Rbpj impairs proliferation of distal duct cells specifically (Fig. 2). These findings raise the possibility that Rbpj-mediated Notch signaling does not directly regulate proliferation of main duct cells, but instead drives the maintenance of a distal centroacinar cell pool that contributes to more proximal ducts during postnatal organ growth.
While this model emphasizes the cell fate determination role of Notch signaling in the adult pancreas, it leaves open the question of why CACs should retain the ability to generate acinar cells. Histological studies previously suggested that CACs could give rise to β-cells following injury, implicating this cell type as a facultative progenitor (Hayashi et al., 2003; Nagasao et al., 2003), and CACs have more recently been shown to have a unique capacity for multi-lineage differentiation in vitro (Rovira et al., 2010). Intriguingly, the cells isolated in that study were actually Hes1-negative (Rovira et al., 2010), consistent with the possibility that Hes1 is normally expressed by CACs with restricted differentiation potential. It will be interesting to decipher the lineage relationship, if any, between Hes1-positive and -negative CACs, and to determine whether the latter population contributes to new acinar cells in vivo.
Taken together, our lineage tracing and Rbpj knockout results suggest that Hes1 lineage-derived CACs behave as bipotent, exocrine-restricted progenitor cells, similar to Hes1-expressing cells in the late embryonic pancreas (Kopinke et al., 2011), with their acinar differentiation potential suppressed by constitutive Notch signaling. Are there circumstances in which wild-type CACs might reacquire acinar potential? One possibility is that CACs represent an "emergency reserve” for replacement of acinar cells lost to injury, and that sustained Notch signaling ensures that these cells remain available. Intriguingly, Notch activity is required for regeneration from caerulein-induced pancreatitis (Siveke et al., 2008), which might reflect the role of Notch in maintaining Hes1+ CACs. Although lineage tracing analyses indicate that regeneration from pancreatitis is driven primarily by proliferation of surviving acinar cells (Desai et al., 2007; Strobel et al., 2007), a recent study suggests that more extreme acinar loss can be repaired by differentiation of non-acinar cells, most likely ducts (Criscimanna et al., 2011). Our findings provide a basis to address the role of Hes1-expressing CACs and Notch signaling in regeneration, and raise the question of whether other pathways controlling pancreatic organogenesis continue to play analogous roles in postnatal life.
HIGHLIGHTS.
Notch is required for expansion of adult pancreatic ducts
Notch-deficient centroacinar cells do not give rise to endocrine cells
Endogenous Notch signaling prevents centroacinar-to-acinar cell differentiation in adult pancreas
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tasuku Honjo, Sean Morrison, Ben Stanger and Helena Edlund for generous gifts of reagents, and Nadja Makki, Jean-Paul De La O and Kristen Kwan for helpful comments on the manuscript. This work was supported by grants from the NIH (L.C.M., R01-DK075072; C.V.E.W., P01-DK42502) and Beta Cell Biology Consortium (L.C.M., U01-DK072473, subaward VUMC35146), and by graduate fellowships from the Boehringer Ingelheim Fonds and University of Utah to D.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Brennand K, Huangfu D, Melton D. All beta Cells Contribute Equally to Islet Growth and Maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimanna A, Speicher JA, Houshmand G, Shiota C, Prasadan K, Ji B, Logsdon CD, Gittes GK, Esni F. Duct Cells Contribute to Regeneration of Endocrine and Acinar Cells Following Pancreatic Damage in Adult Mice. Gastroenterology Jul. 2011 Jul 13; doi: 10.1053/j.gastro.2011.07.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- De La O JP, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Ekholm R, Zelander T, Edlund Y. The ultrastructural organization of the rat exocrine pancreas. II. Centroacinar cells, intercalary and intralobular ducts. J. Ultrastruct. Res. 1962;7:73–83. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golson ML, Loomes KM, Oakey R, Kaestner KH. Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology. 2009;136:1761–1771. e1761. doi: 10.1053/j.gastro.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev. Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch. Histol. Cytol. 2003;66:163–174. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup S, Bhonde RR. Analysis and optimization of nutritional set-up for murine pancreatic acinar cells. JOP. 2002;3:8–15. [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Brown DD. Transdifferentiation of tadpole pancreatic acinar cells to duct cells mediated by Notch and stromelysin-3. Dev. Biol. 2011;351:311–317. doi: 10.1016/j.ydbio.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasao J, Yoshioka K, Amasaki H, Mutoh K. Centroacinar and intercalated duct cells as potential precursors of pancreatic endocrine cells in rats treated with streptozotocin. Ann Anat. 2003;185:211–216. doi: 10.1016/s0940-9602(03)80025-0. [DOI] [PubMed] [Google Scholar]
- Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev. Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. U. S. A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, Depinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SH, Silva FJ, Tsark WM, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvIm. J. Genesis. 2002;32:199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Ye W, Mairet-Coello G, DiCicco-Bloom E. DNAse I pre-treatment markedly enhances detection of nuclear cyclin-dependent kinase inhibitor p57Kip2 and BrdU double immunostaining in embryonic rat brain. Histochem. Cell Biol. 2007;127:195–203. doi: 10.1007/s00418-006-0238-6. [DOI] [PubMed] [Google Scholar]
- Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev. Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.