Abstract
Background
Factors affecting risk for impingement and dislocation can be related to the patient, implant design, or surgeon. While these have been studied independently, the impact of each factor relative to the others is not known.
Questions/purposes
We determined the effect of three implant design factors, prosthetic placement, and patient anatomy on subject-specific ROM.
Methods
We virtually implanted hip geometry obtained from 16 CT scans using computer models of hip components with differences in head size, neck diameter, and neck-shaft angle. A contact detection model computed ROM before prosthetic or bony impingement. We correlated anatomic measurements from pelvic radiographs with ROM.
Results
When we implanted the components for best fit to the subject’s anatomy or in the recommended orientation of 45° abduction and 20° anteversion, ROM was greater than 110° of flexion, 30° of extension, 45° of adduction-abduction, and 40° of external rotation. Changes in head size, neck diameter, and neck-shaft angle generated small gains (3.6°–6°) in ROM when analyzed individually, but collectively, we noted a more substantial increase (10°–17°). Radiographic measurements correlated only moderately with hip flexion and abduction.
Conclusions
It is feasible to tailor implant placement to each patient to maximize bony coverage without compromising ROM. Once bony impingement becomes the restricting factor, further changes in implant design may not improve ROM. Radiographic measurements do not appear to have value in predicting ROM.
Introduction
Dislocation is often the most common major early complication after THA [12, 14, 29, 42, 48]. Clinical series of primary hip arthroplasty report incidences from 0.6% to 3% [9, 18, 26, 44, 46], with even higher dislocation rates in certain subsets of primary patients (such as obesity and hip dysplasia) and after revision surgery [4, 7, 13, 16, 17, 20, 25, 27, 32]. ROM before impingement is an important indicator of joint stability, and surgeons often use it as an intraoperative test to assess hip stability [3, 22, 33, 36, 37]. ROM predicted by computer models also correlates with clinical hip dislocation rates [2, 34].
Factors contributing to dislocation are related to the patient, prosthetic design, and surgeon. Patient-related factors include bony anatomy, soft-tissue stability, muscle tone, and postoperative activity, as well as behavioral factors, such as alcoholism, and disorders, such as Parkinson’s disease [19, 35]. The morphology of the bony anatomy around the hip determines the ROM before bony impingement [20, 22, 23, 27]. For example, acetabular dysplasia alone and in combination with increased anteversion of the femur is associated with a greater incidence of dislocation [40]. Unstable hips are often a result of poor soft-tissue tension, due to either inadequate neck length or poor quality of soft tissues in revision surgery.
Factors specific to prosthetic design that can affect ROM include head size, head-neck ratio, and neck-shaft angle [11]. The ratio between the diameters of the head and neck affects the net ROM before impingement of the neck on the liner [6]. A larger head size increases the ROM and the distance the head has to translate before dislocating (sometimes called the “jump distance”) [1, 5, 8]. The neck-shaft angle alters the position of the femoral shaft relative to the pelvis and can affect ROM.
While surgeons have the option of choosing among available designs, several additional factors are directly under their control. The type of surgical approach can have a major impact on the incidence of dislocation, with the anterolateral approach being protective against posterior dislocation [42, 46]. When using the posterior approach, preserving hip rotator muscles and repairing the capsule can reduce the risk for dislocation from 4.8% to less than 1% [43, 44]. Component orientation such as acetabular abduction and anteversion and femoral anteversion directly affect the joint angle where prosthetic impingement occurs [11]. Surgeons can adjust the neck length to modulate the passive tension in the soft tissues around the hip.
All of the above factors are implicated as affecting the incidence of hip dislocation. However, the relative contribution of these factors is unclear. Additionally, the importance of these factors in the context of variable bony anatomy is not known. We previously described a model computing ROM before prosthetic and bony impingement [22]. We found bony impingement substantially changed the ROM compared to when only prosthetic impingement was taken into account. In a probabilistic study of the same model, we reported means and SDs and predicted upper and lower bounds for the ROM given reported variability in acetabular component orientation [36]. We found the variation in implant position reported using surgical navigation did not result in poor ROM, while using the variation in implant position reported using manual instrumentation resulted in poor ROM in 3% to 5% of the 1000 trials analyzed. However, in those studies, since we only modeled the anatomy of one subject, we could not assess the effect of between-subject anatomic variation on hip ROM.
Restriction of passive hip ROM due to impingement is multifactorial and depends on anatomic morphology of the bones, implant design parameters, and implant orientation [11, 22, 36]. Our objectives were to determine (1) the variability in bony anatomy and the effect of bony impingement on restricting hip ROM; (2) the effect of common implant design factors, such as head size, neck diameter, and neck-shaft angle, relative to the patient’s bony anatomy; and (3) whether anatomic landmarks obtained from routine radiographs of the hip predict ROM.
Materials and Methods
We screened all patients who underwent a CT scan of the hip during 2008 and excluded those with fractures, visible dysplasia, severe arthritis, or any visible deformity but included CT scans with 0.5-mm axial slice distance and that included the pelvis and the proximal 1/3 of the femur. This resulted in 16 CT scans for five men and 11 women, with a mean (± SD) age of 68 (± 15) years; six had evidence of early osteoarthritis, four were to rule out stress fractures, three had early avascular necrosis of the femoral head, two had a clinical diagnosis of femoroacetabular impingement, and one was for suspected sepsis. IRB approval and patient consents were obtained. CT scans were imported into a volume segmentation program (MIMICS®; Materialise, Leuven, Belgium). The program reconstructed the surface geometry of the femur and pelvis as a triangle mesh. We chose an orthogonal coordinate system based on the recommendation of the International Society of Biomechanics [47] and oriented the pelvis so that the AP iliac spines were level and in the same frontal plane as the pubic symphysis (no lordosis or pelvic obliquity). We located the center of the natural femoral head by fitting a sphere to the articular surface of femoral head, using the same method to locate the center of the natural acetabulum. Then, we lined up the center of the femoral head with center of the acetabulum, orienting the long axis of the femur (the line joining the femoral head center to the midpoint of the intercondylar notch) perpendicular to the transverse axis of the pelvis (defining neutral hip abduction) and parallel to the frontal plane of the pelvis (defining neutral hip flexion). We used a line passing through the medial and lateral epicondyles to define neutral rotation of the femur. The center of the hip axis was located at the center of the head. In neutral position, the x-axis was pointed in the anterior direction, the y-axis superiorly, and the z-axis toward the medial aspect of the patient’s right side (Fig. 1). Flexion-extension around the pelvic z-axes (fixed to the pelvis) and axial rotation of the hip around the femoral y-axis (fixed to the femur) were described. The axis for abduction-adduction was the floating axis (perpendicular to both the y- and z-axes) [47]. We defined abduction of the acetabular cup from the horizontal line around the x-axis of the pelvis, whereas the true anteversion was the rotation of the cup around the y-axis of the pelvis (as opposed to apparent radiographic anteversion, which was rotation of the cup about its abducted axis).
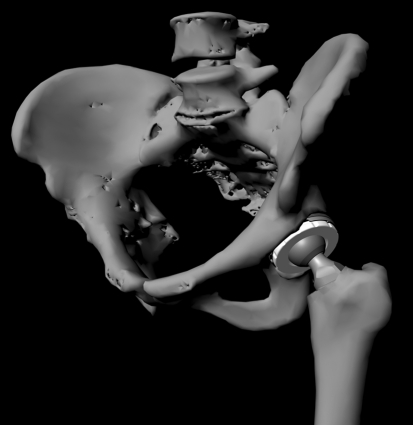
Fig. 1.
A computer-generated image shows a representative pelvis and femur implanted with hip arthroplasty components.
We analyzed the Secur-Fit Max® and Super Secur-Fit® THA component designs (Stryker Orthopaedics, Mahwah, NJ). We chose these designs because of their overall similarities and availability in the different neck diameter and neck-stem angle options and because these design features are generic and broadly applicable to other designs. The Secur-Fit Max® had a neck diameter of 12.5 mm and the Super Secur-Fit® had a neck diameter of 11 mm. Both stem designs were available in 127° and 132° neck-stem angles. We paired all femoral component designs with the same acetabular component design (Trident®; Stryker Orthopaedics). Each stem design was tested with 28-, 32-, and 36-mm-diameter heads. We obtained computer-aided design models from the manufacturer and converted them into triangle mesh surfaces (Fig. 2). The major design differences analyzed were head diameter (28, 32, and 36 mm), neck diameter (12.5 and 11 mm), and neck-stem angle (127° and 132°).
Fig. 2.
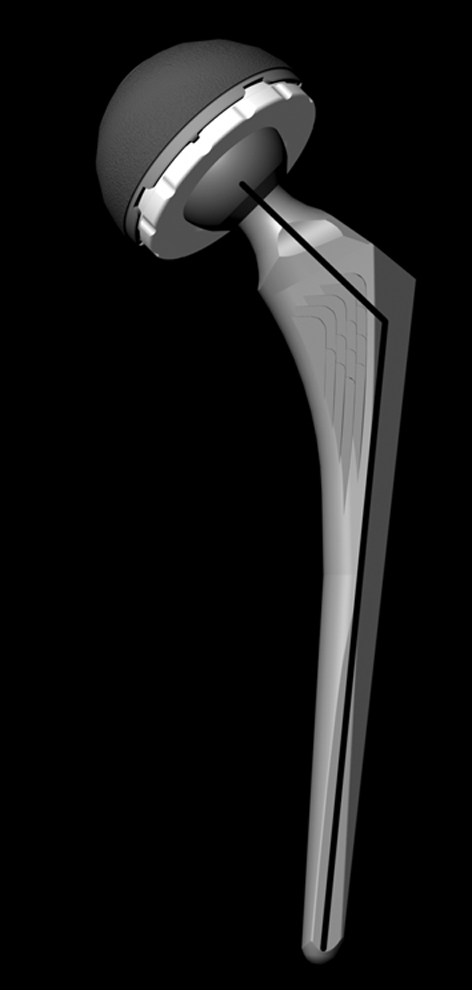
A computer-generated image shows the hip arthroplasty design. Implant design variables included head size, neck diameter, and neck-shaft angle (thick black line).
We virtually implanted these models into each of the reconstructed anatomic models under the direction of a joint arthroplasty surgeon (CWC). In the computer model, we resected the bony anatomy of the femoral head and neck 13.5 mm above the lesser trochanter at a 45° to the vertical axis. Then, we reamed the natural acetabulum by creating a sphere (sphere diameter = outer diameter of cup). This sphere was subtracted (using Boolean subtraction) from the natural acetabulum to generate a surface resembling a reamed acetabulum. Next, we virtually implanted the hip components in the pelvis and the femur (Fig. 1). For initial placement, we chose the implant sizes and the locations of the cup relative to the pelvis and the stem relative to the femur. We used the equivalent of preoperative radiographic templating to select prosthetic size for best fit of the femoral stem in the femoral canal and the acetabular component in the acetabulum and for initial implantation position. We maintained the original hip center of rotation (center of the sphere defining the native acetabulum) and limb length (vertical level of tip of the lesser trochanter relative to the hip center) while aligning the long axis of the stem with the long axis of the intramedullary canal of the femur. The acetabular center was unchanged since the center of the acetabular component was lined up with the center of the natural acetabulum during the simulated implantation. We chose two implantation orientations for the acetabular component: anatomic and recommended. In the anatomic orientation, we aligned the component to the native acetabular abduction and anteversion, the cup was abducted and anteverted for maximum bony coverage in the socket, and the femoral stem was anteverted to match the natural anteversion of the femoral neck. In the recommended orientation, the cup was abducted 45° and anteverted 20° using the previously defined pelvic coordinate system.
A previously reported automated contact detection model [22] determined the ROM in each degree of freedom (flexion/extension, abduction/adduction, internal/external rotation) and the type of contact that occurred (implant-implant, implant-bone, bone-bone). We recorded impingement as prosthetic if the neck of the femoral component impinged on the liner (implant-implant) and as bony if either the pelvis or the femur was involved (implant-bone or bone-bone). Since we did not model the soft tissues, this represented the maximum possible ROM.
To determine potential for anterior dislocation, we recorded maximum extension with the hip at 30° abduction and 30° external rotation. To determine potential for posterior dislocation, we recorded maximum adduction with the hip at 90° flexion and 20° internal rotation. These compound motions simulated the typical intraoperative maneuvers we perform to assess hip stability.
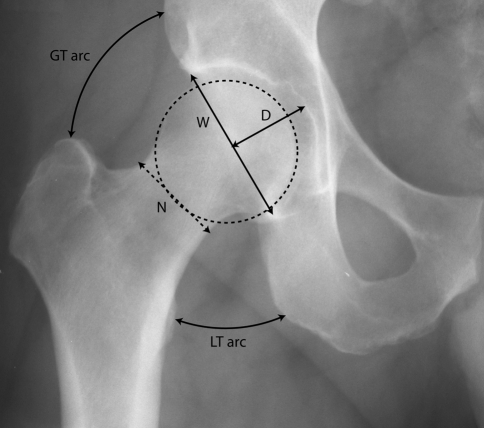
We measured the following based on their potential effect on ROM using a plain AP radiograph of the pelvis (Fig. 3): head diameter, acetabular inclination, neck diameter, acetabular depth ratio, the arc length between the tip of greater trochanter and ilium, the arc length between lesser trochanter and ischium, and the angle of the flare of the wing of the ilium.
Fig. 3.
A plain AP radiograph of the pelvis shows the measurement of head diameter (dotted circle), neck diameter (N), acetabular depth ratio (= D × 1000/W), the normalized arc length between the tip of greater trochanter and ilium (GT arc), and the arc length between lesser trochanter and ischium (LT arc).
We used repeated-measures ANOVA (Systat®; Systat Software Inc, Chicago, IL) to determine differences between mean hip ROM among the design variable groups: head size, neck diameter, and neck-stem angle. We used linear regression (Systat®) to determine the correlations between each of the anatomic measurements (head diameter, acetabular inclination, neck diameter, acetabular depth ratio, the arc length between the tip of greater trochanter and ilium, the arc length between lesser trochanter and ischium, and the angle of the flare of the wing of the ilium) on the pelvic radiograph and hip ROM.
Results
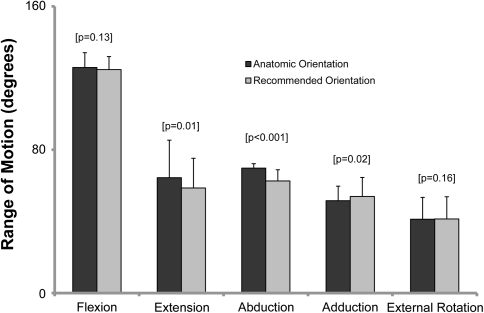
Measurements of hip morphometry on pelvic radiographs revealed substantial between-subject variability, with SDs ranging from 7% to more than 300% of the average value (Table 1). This variability in radiographic measurements was also reflected in the large SD bars in hip ROM (Figs. 4–6). These bars show the range of results in flexion, extension, abduction, adduction, and external rotation being 29°, 69°, 21°, 30°, and 37°, respectively. As a third measure, we compared average ROM between two implant positions: (1) cup and stem implanted for best fit to the subject’s anatomy (ie, aligned to native acetabular abduction and anteversion and femoral anteversion) and (2) the commonly recommended orientation of 45° abduction and 20° anteversion. When we implanted the cup and stem for best fit to the subject’s anatomy, excellent ROM (average flexion, 126° ± 8°; average extension, 64° ± 21°; average abduction, 70° ± 3°; average adduction, 52° ± 8°; average external rotation, 41° ± 12°) was achieved in all directions (Fig. 4). For comparison between the anatomic and recommended cup orientations, we averaged the ROM across all eight implant designs (Fig. 4). Realigning the cup to the recommended orientation of 45° abduction and 20° anteversion did not change mean hip ROM in flexion (p = 0.13) or external rotation (p = 0.16) but reduced ROM in abduction (p < 0.001), adduction (p = 0.02), and extension (p = 0.01). Internal rotation was always above 80°. These data are not included as being unlikely to be affected by prosthetic or bony impingement.
Table 1.
Radiographic measurements
| Measurements of bony anatomy | Average | SD | Range |
|---|---|---|---|
| Head size (mm) | 51.3 | 3.7 | 44.5–56.4 |
| Neck diameter (mm) | 41.3 | 3.7 | 34.2–47.2 |
| Acetabular abduction angle (degrees) | 60.2 | 3.9 | 54.9–65.9 |
| Acetabular depth index | 459.7 | 60.0 | 350.4–556.4 |
| Mediolateral distance of tip of greater trochanter to head center (mm) | 52.6 | 6.4 | 40.1–60.5 |
| Superoinferior distance of tip of greater trochanter to head center (mm) | 2.8 | 10.4 | −14.2–20.7 |
| Mediolateral distance of tip of lesser trochanter to head center (mm) | 7.6 | 7.0 | −1.2–23.9 |
| Superoinferior distance of tip of lesser trochanter to head center (mm) | 61.6 | 6.0 | 53–75.6 |
| Angle of iliac flare (degrees) | 68.0 | 5.7 | 58.6–75.1 |
| Angle subtended by the arc from greater trochanter to ilium (degrees) | 66.3 | 12.0 | 43.7–84.6 |
| Angle subtended by arc from lesser trochanter to ischial ramus (degrees) | 25.6 | 8.7 | 14.3–40.6 |
Fig. 4.
A graph shows the mean maximum ROM in each direction for anatomic and recommended orientations. Anatomic orientation is when surgeons implant the acetabular component to match each patient’s native acetabular abduction and anteversion; recommended is implanted in 45° acetabular abduction and 20° anteversion.
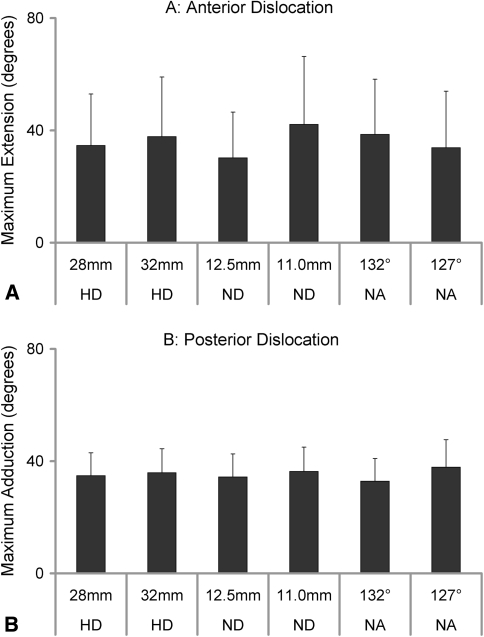
Fig. 6A–B.
Graphs show the ROM in the directions considered likely to be at risk for dislocation. (A) Neck diameter and neck angle, but not head size, have an effect on ROM in the direction of maximum risk for anterior dislocation. (B) Neck diameter and neck angle also affect the ROM in the direction of maximum risk for posterior dislocation. However, reducing the neck angle has the opposite effect on posterior dislocation. HD = head diameter; ND = neck diameter, NA = neck angle.
Increasing head size from 28 to 36 mm increased ROM between 0° and 6.5° (most commonly in extension and abduction) (Fig. 5). Reducing neck diameter only increased extension and abduction (maximum mean increase, 6°) (Fig. 5). A 127° neck-shaft angle had mixed results: modestly increasing flexion at the expense of abduction (Fig. 5). Neck diameter and neck-shaft angle, but not head size, influenced ROM in the direction of maximum risk for anterior dislocation. The thinner diameter increased ROM (p = 0.003) before impingement by a mean of nearly 10° and the 127° neck-shaft angle increased ROM (p < 0.01) before impingement by nearly 5° (Fig. 6). To determine potential for posterior dislocation, we recorded maximum adduction with the hip at 90° flexion and 20° internal rotation. Neck diameter and neck angle also influenced the ROM in the direction of maximum risk for posterior dislocation (Fig. 5B). Although significant (p = 0.005), the increase in ROM due to the smaller neck diameter was small (2°) for posterior dislocation. Additionally, reducing the neck angle increased the risk for posterior dislocation while reducing the risk for anterior dislocation. The combined effect of head size, neck diameter, and neck angle increased ROM in the direction at risk for anterior dislocation by 17° (± 19°) and for posterior dislocation by 10° (± 5°).
Fig. 5A–E.
Graphs show the results with the acetabular component in the recommended orientation of 45° abduction and 20° anteversion: (A) flexion; (B) extension; (C) abduction; (D) adduction; and (E) external rotation. HD = head diameter; ND = neck diameter, NA = neck angle.
On analysis of plain AP radiographs, mean head size was 51 mm (± 4 mm), mean neck diameter was 41 mm (± 4 mm), mean anatomic acetabular inclination was 41° (± 2°), and mean acetabular depth ratio was 460 (± 60). Hip flexion correlated (R2 = 0.59, p = 0.03) with acetabular abduction angle and the angle of the flare of the iliac wing. Hip abduction correlated (R2 = 0.50, p = 0.05) with the angle of the flare of the iliac wing and the length of the arc from the tip of the greater trochanter to the ilium. We observed no correlations between hip ROM and head size, neck diameter, or mean acetabular depth ratio.
Discussion
Factors implicated in hip dislocation include the patient’s anatomy, implant design features, and surgical placement [1, 5, 6, 8, 11]. However, the magnitudes of the contributions of these factors, singly and in combination, are not fully known. Newer designs are being developed and marketed to reduce impingement, and computer-assisted navigation systems are being utilized to improve surgical alignment. However, the importance of these factors in the context of variable bony anatomy is unknown. In this study, we took a subject-specific approach to determine the variability in bony anatomy and the effect of bony impingement on restricting hip ROM. We compared the effect of common and broadly applicable implant design factors, such as head size, neck diameter, and neck-shaft angle, and measured anatomic landmarks on pelvic radiographs in an attempt to predict hip ROM.
Our study included some limitations. First, we ignored any influence of soft-tissue tightness or impingement on hip ROM. Therefore, our model predicted the maximum possible ROM, which supported the analysis of the maximum effect that each variable we studied had on hip ROM. Second, we only analyzed the ROM before impingement. Hip dislocation involves levering of the head out of the socket after impingement, and a larger head size may improve resistance to dislocation, even if the ROM to impingement remains the same. However, surgeons commonly use hip ROM as a marker for hip dislocation, which correlates with hip dislocation [2, 34]. Third, femoral stem anteversion and combined anteversion of the cup and stem were also important factors that could influence ROM. In addition, we restricted our study to the effect of anatomic variables relative to implant design variables. Fourth, we studied only design variations from one manufacturer. Additional features that are more specific to individual designs, such as the depth of the cup design (whether greater than or less than a hemisphere), and the cross-sectional geometry of the neck (trapezoidal versus circular), can also influence ROM. Nevertheless, we selected generic design variables that can be extrapolated to a wide set of designs.
The variation in ROM among subjects was high: with the range of differences in flexion, extension, abduction, adduction, and external rotation being 29°, 69°, 21°, 30°, and 37°, respectively. This range of differences was much higher than the change in ROM generated by individual design parameters (such as differences in head-neck ratio, 6.5°) or component orientation (between ideal and anatomic, 7°). When implants were positioned to minimize impingement, bony impingement became much more common than prosthetic impingement [22]. Under these circumstances, modest gains (> 6.5°) in ROM were achieved by increasing head size from 28 to 36 mm. These gains were most commonly noted in extension and abduction, the directions in which prosthetic impingement was more often the restricting factor than in flexion and adduction. Since the average ROM in abduction and extension with the smallest head size (28 mm) and the largest neck diameter (12.5 mm) was high (approaching 60°), the clinical value of further increases in ROM was questionable. These values were similar to the ROM reported before prosthetic impingement when implants were positioned at or near optimum orientation [11, 38, 45].
We previously identified “safe zones” for orienting acetabular and femoral components to minimize prosthetic impingement [11]. Computer models from other investigators also predicted similar combinations of orientations as being optimal [45]. However, these computer models did not account for bony impingement. We found bony impingement substantially altered the ROM and moderated the magnitude of benefit of several implant design features, including head size [22]. Our results indicated a single cup position generating an optimal ROM in all patients was elusive. Aligning the components directly to the variable patient anatomy resulted in overall excellent ROM. Aligning the components in the recommended orientation of 45° abduction and 20° anteversion did little to improve overall hip ROM from the perspective of implant stability. Aligning the acetabular component to the patient’s acetabulum had the added advantage of maximizing bony coverage, which could be important in cementless cups. Since patient-related factors overshadowed individual parameters of implant design, cup position should be tailored to the individual patient and corrected only to address the direction of restricted ROM (without jeopardizing implant wear performance and interface stability).
Implant design parameters affected ROM and the potential for dislocation. We previously reported increasing head size from 22 to 32 mm increased ROM before prosthetic impingement by approximately 15° to 20° [11]. However, when bony impingement was also taken into account, ROM reduced in up to 44% of the conditions tested [22]. One study in a cadaver bone model reported increases of ROM between 10° and 20° when head size was increased from 22 to 32 mm, which was relatively higher than in our study [6]. One explanation for this difference was that the neck diameter appeared larger than that used in our study, resulting in higher incidence of prosthetic impingement. In our study, gains in ROM due to changes in individual design parameters were not impressive, especially when compared to the variability within subjects. While individual design features had a modest effect on risk for dislocation, the combination of head size, neck diameter, and neck-shaft angle resulted in a more sizeable improvement in reducing impingement in the direction of anterior dislocation (17° ± 19°) and posterior dislocation (10° ± 5°).
We compared the ROM predicted by our model to previously published reports (Table 2). The wide variation in clinically reported ranges can be attributed to differences in implant design, surgical technique, patient population, and method of measuring ROM. In general, clinical ROMs after THA were lower than our results. Davis et al. [10] reported 9% of 1517 hips with high motion (flexion > 115°, abduction > 25°, and external rotation > 20°). Since we placed our components in “optimal” alignment and did not simulate the soft tissues, our results reflect the scenario of maximum possible ROM achievable before prosthetic or bony impingement. Relative differences reported in ROM due to differences in head size were similar to our findings: ranging from a average 4° improvement in maximum flexion (between 28-mm and 32- to 60-mm head sizes) [49] to a 9° improvement (between 26-mm and 32-mm head sizes) [28]. A cadaver study, which permits paired comparison in the same anatomy, reported an improvement of only 1.7° in maximum flexion (between 28-mm and 32-mm head sizes) [3].
Table 2.
Hip ROM reported after THA
| Study | Flexion (°) | Extension (°) | Abduction (°) | Adduction (°) | External rotation (°) | Head size (mm) |
|---|---|---|---|---|---|---|
| Fowble et al. [15] | 119 (90–170) | 1 (−10–25) | 45 (25–70) | 19 (0–45) | 41 (20–70) | 28–36 |
| Miki et al. [31] | 95 ± 12 | 10 ± 10 | 30 ± 9 | 21 ± 9 | ||
| McGrory et al. [30] | 96 ± 24 | 21 ± 11 | 12 ± 8 | 19 ± 13 | ||
| Imai et al. [21] | 93 ± 11 | 30 ± 6 | 21 ± 4 | 34 ± 14 | 22 | |
| Le Duff et al. [24] | 121 (70–140) | 43 (30–55) | 28 (20–40) | 36 (5–55) | ||
| Trudelle-Jackson et al. [41] | 94 ± 19 | 5 ± 10 | 24 ± 6 | 18 ± 6 | 21 ± 5 | |
| Sun et al. [39] | 25 | 26–28 | ||||
| Matsushita et al. [28] | 89 ± 6 | 29 ± 7 | 37 ± 12 | 26 | ||
| Matsushita et al. [28] | 98 ± 7 | 36 ± 8 | 39 ± 16 | 32 | ||
| Zijlstra et al. [49] | 106 ± 13 | 2 ± 4 | 41 ± 8 | 27 ± 10 | 29 ± 11 | 28 |
| Zijlstra et al. [49] | 110 ± 10 | 2 ± 5 | 40 ± 8 | 26 ± 8 | 28 ± 10 | 38–60 |
| Bartz et al. [3]* | 113 ± 2 | 32 | ||||
| Bartz et al. [3]* | 111 | 28 | ||||
| Davis et al. [10]† | > 115 (9%) | > 25 (9%) | > 20 (9%) | |||
| Bunn et al. | 126 ± 8 | 64 ± 21 | 70 ± 3 | 52 ± 8 | 41 ± 12 | 28–36 |
Values are expressed as mean or mean ± SD, with range in parentheses; empty cells represent data that were not reported; * this was a cadaver study and maximum hip flexion in 10° abduction before impingement was reported; †reported 9% of their patients with high motion: flexion > 115°, abduction > 25°, and external rotation > 20°; and 81% of their patients with flexion between 90° and 114°, abduction between 16° and 24°, and external rotation between 11° and 19°.
Surgeons routinely obtain a preoperative, plain AP radiograph of the pelvis, which provides useful information about anatomic landmarks. We presumed an arc described between tip of the greater trochanter and the pelvis would correlate with hip abduction, and a similar arc described between the tip of the lesser trochanter and the pelvis would correlate with hip adduction. We also anticipated the head-neck ratio of the native femur would correlate with overall hip ROM before bony impingement (similar to the correlation of prosthetic head-neck ratio with ROM). Hip abduction correlated with the length of the arc from the tip of the greater trochanter to the ilium. Hip flexion also correlated with the measured anatomic acetabular abduction angle. Although statistically significant, the strength of these correlations (r2 < 0.6) was not sufficient to accurately predict ROM on a subject-specific basis; therefore, we did not succeed in our third goal of predicting anatomic landmarks from routine pelvic radiographs.
In summary, variability in ROM among subjects is higher than the increased ROM generated by individual prosthetic design features implant design. The small differences in ROM between anatomic placement or placement of the components in the recommended 45° abduction and 20° anteversion indicates surgeons can tailor implant placement to each patient without compromising ROM if maximizing bony coverage is necessary. Individual prosthetic design features have small effects on ROM and even an increase in head size from 28 to 36 mm generated only modest increases in ROM. Combinations of multiple design features can increase ROM by more than 10°; however, once bony impingement becomes the restricting factor, further changes in implant design may not improve ROM. Measurements from plain radiographs appear unlikely to provide information that predicts individual ROM.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or the device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bader R, Scholz R, Steinhauser E, Zimmermann S, Busch R, Mittelmeier W. The influence of head and neck geometry on stability of total hip replacement: a mechanical test study. Acta Orthop Scand. 2004;75:415–421. doi: 10.1080/00016470410001178-1. [DOI] [PubMed] [Google Scholar]
- 2.Barrack RL, Butler RA, Laster DR, Andrews P. Stem design and dislocation after revision total hip arthroplasty: clinical results and computer modeling. J Arthroplasty. 2001;16:8–12. doi: 10.1054/arth.2001.28359. [DOI] [PubMed] [Google Scholar]
- 3.Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82:1300–1307. doi: 10.2106/00004623-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Berry DJ, Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2456–2463. doi: 10.2106/JBJS.D.02860. [DOI] [PubMed] [Google Scholar]
- 5.Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes. J Arthroplasty. 2005;20:11–19. doi: 10.1016/j.arth.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Chandler DR, Glousman R, Hull D, McGuire PJ, Kim IS, Clarke IC, Sarmiento A. Prosthetic hip range of motion and impingement: the effects of head and neck geometry. Clin Orthop Relat Res. 1982;166:284–291. [PubMed] [Google Scholar]
- 7.Chee YH, Teoh KH, Sabnis BM, Ballantyne JA, Brenkel IJ. Total hip replacement in morbidly obese patients with osteoarthritis: results of a prospectively matched study. J Bone Joint Surg Br. 2010;92:1066–1071. doi: 10.1302/0301-620X.92B8.22764. [DOI] [PubMed] [Google Scholar]
- 8.Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don’t do. Clin Orthop Relat Res. 2004;429:102–107. doi: 10.1097/01.blo.0000150117.42360.f9. [DOI] [PubMed] [Google Scholar]
- 9.Daly PJ, Morrey BF. Operative correction of an unstable total hip arthroplasty. J Bone Joint Surg Am. 1992;74:1334–1343. [PubMed] [Google Scholar]
- 10.Davis KE, Ritter MA, Berend ME, Meding JB. The importance of range of motion after total hip arthroplasty. Clin Orthop Relat Res. 2007;465:180–184. doi: 10.1097/BLO.0b013e31815c5a64. [DOI] [PubMed] [Google Scholar]
- 11.D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW., Jr The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315–321. doi: 10.2106/00004623-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop Relat Res. 1998;355:144–151. doi: 10.1097/00003086-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Eskelinen A, Helenius I, Remes V, Ylinen P, Tallroth K, Paavilainen T. Cementless total hip arthroplasty in patients with high congenital hip dislocation. J Bone Joint Surg Am. 2006;88:80–91. doi: 10.2106/JBJS.E.00037. [DOI] [PubMed] [Google Scholar]
- 14.Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop Relat Res. 1980;151:169–178. [PubMed] [Google Scholar]
- 15.Fowble VA, dela Rosa MA, Schmalzried TP. A comparison of total hip resurfacing and total hip arthroplasty—patients and outcomes. Bull NYU Hosp Jt Dis. 2009;67:108–112. [PubMed] [Google Scholar]
- 16.Grant JA, Viens N, Bolognesi MP, Olson SA, Cook CE. Two-year outcomes in primary THA in obese male veterans administration medical center patients. Rheumatol Int. 2008;28:1105–1109. doi: 10.1007/s00296-008-0575-y. [DOI] [PubMed] [Google Scholar]
- 17.Grigoris P, Grecula MJ, Amstutz HC. Tripolar hip replacement for recurrent prosthetic dislocation. Clin Orthop Relat Res. 1994;304:148–155. [PubMed] [Google Scholar]
- 18.Hedlundh U, Ahnfelt L, Hybbinette CH. Dislocations and the femoral head size in primary total hip arthroplasty. Clin Orthop Relat Res. 1996;333:226–233. doi: 10.1097/00003086-199612000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P, Filippini P, Flouzat-Lachaniette CH, Batista SU, Poignard A. Constrained liner in neurologic or cognitively impaired patients undergoing primary THA. Clin Orthop Relat Res. 2010;468:3255–3262. doi: 10.1007/s11999-010-1340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howie CR, Ohly NE, Miller B. Cemented total hip arthroplasty with subtrochanteric osteotomy in dysplastic hips. Clin Orthop Relat Res. 2010;468:3240–3247. doi: 10.1007/s11999-010-1367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai H, Mashima N, Takahashi T, Yamamoto H. The relationship between increased hip range of motion, wear, and locking mechanism failure in the Harris-Galante acetabular component. J Arthroplasty. 2009;24:892–897. doi: 10.1016/j.arth.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Kessler O, Patil S, Stefan W, Mayr E, Colwell CW, Jr, D’Lima DD. Bony impingement affects range of motion after total hip arthroplasty: a subject-specific approach. J Orthop Res. 2008;26:443–452. doi: 10.1002/jor.20541. [DOI] [PubMed] [Google Scholar]
- 23.Kubiak-Langer M, Tannast M, Murphy SB, Siebenrock KA, Langlotz F. Range of motion in anterior femoroacetabular impingement. Clin Orthop Relat Res. 2007;458:117–124. doi: 10.1097/BLO.0b013e318031c595. [DOI] [PubMed] [Google Scholar]
- 24.Le Duff MJ, Wisk LE, Amstutz HC. Range of motion after stemmed total hip arthroplasty and hip resurfacing—a clinical study. Bull NYU Hosp Jt Dis. 2009;67:177–181. [PubMed] [Google Scholar]
- 25.Lubbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57:327–334. doi: 10.1002/art.22542. [DOI] [PubMed] [Google Scholar]
- 26.Mai K, Hardwick ME, Walker RH, Copp SN, Ezzet KA, Colwell CW., Jr Early dislocation rate in ceramic-on-ceramic total hip arthroplasty. HSS J. 2008;4:10–13. doi: 10.1007/s11420-007-9060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallory TH, Lombardi AV, Jr, Fada RA, Herrington SM, Eberle RW. Dislocation after total hip arthroplasty using the anterolateral abductor split approach. Clin Orthop Relat Res. 1999;358:166–172. doi: 10.1097/00003086-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita I, Morita Y, Ito Y, Gejo R, Kimura T. Activities of daily living after total hip arthroplasty: is a 32-mm femoral head superior to a 26-mm head for improving daily activities? Int Orthop. 2011;35:25–29. doi: 10.1007/s00264-009-0909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCollum DE, Gray WJ. Dislocation after total hip arthroplasty: causes and prevention. Clin Orthop Relat Res. 1990;261:159–170. [PubMed] [Google Scholar]
- 30.McGrory BJ, Freiberg AA, Shinar AA, Harris WH. Correlation of measured range of hip motion following total hip arthroplasty and responses to a questionnaire. J Arthroplasty. 1996;11:565–571. doi: 10.1016/S0883-5403(96)80111-2. [DOI] [PubMed] [Google Scholar]
- 31.Miki H, Yamanashi W, Nishii T, Sato Y, Yoshikawa H, Sugano N. Anatomic hip range of motion after implantation during total hip arthroplasty as measured by a navigation system. J Arthroplasty. 2007;22:946–952. doi: 10.1016/j.arth.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23:237–248. [PubMed] [Google Scholar]
- 33.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. doi: 10.1016/S0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 34.Padgett DE, Lipman J, Robie B, Nestor BJ. Influence of total hip design on dislocation: a computer model and clinical analysis. Clin Orthop Relat Res. 2006;447:48–52. doi: 10.1097/01.blo.0000218748.30236.40. [DOI] [PubMed] [Google Scholar]
- 35.Paterno SA, Lachiewicz PF, Kelley SS. The influence of patient-related factors and the position of the acetabular component on the rate of dislocation after total hip replacement. J Bone Joint Surg Am. 1997;79:1202–1210. doi: 10.2106/00004623-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Petrella AJ, Stowe JQ, D’Lima DD, Rullkoetter PJ, Laz PJ. Computer-assisted versus manual alignment in THA: a probabilistic approach to range of motion. Clin Orthop Relat Res. 2009;467:50–55. doi: 10.1007/s11999-008-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seel MJ, Hafez MA, Eckman K, Jaramaz B, Davidson D, DiGioia AM., 3rd Three-dimensional planning and virtual radiographs in revision total hip arthroplasty for instability. Clin Orthop Relat Res. 2006;442:35–38. [PubMed] [Google Scholar]
- 38.Seki M, Yuasa N, Ohkuni K. Analysis of optimal range of socket orientations in total hip arthroplasty with use of computer-aided design simulation. J Orthop Res. 1998;16:513–517. doi: 10.1002/jor.1100160418. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Inaoka H, Fukuoka Y, Masuda T, Ishida A, Morita S. Range of motion measurement of an artificial hip joint using CT images. Med Biol Eng Comput. 2007;45:1229–1235. doi: 10.1007/s11517-007-0258-y. [DOI] [PubMed] [Google Scholar]
- 40.Thillemann TM, Pedersen AB, Johnsen SP, Soballe K. Implant survival after primary total hip arthroplasty due to childhood hip disorders: results from the Danish Hip Arthroplasty Registry. Acta Orthop. 2008;79:769–776. doi: 10.1080/17453670810016830. [DOI] [PubMed] [Google Scholar]
- 41.Trudelle-Jackson E, Emerson R, Smith S. Outcomes of total hip arthroplasty: a study of patients one year postsurgery. J Orthop Sports Phys Ther. 2002;32:260–267. doi: 10.2519/jospt.2002.32.6.260. [DOI] [PubMed] [Google Scholar]
- 42.Turner RS. Postoperative total hip prosthetic femoral head dislocations: incidence, etiologic factors, and management. Clin Orthop Relat Res. 1994;301:196–204. [PubMed] [Google Scholar]
- 43.Weeden SH, Paprosky WG, Bowling JW. The early dislocation rate in primary total hip arthroplasty following the posterior approach with posterior soft-tissue repair. J Arthroplasty. 2003;18:709–713. doi: 10.1016/S0883-5403(03)00254-7. [DOI] [PubMed] [Google Scholar]
- 44.White RE, Jr, Forness TJ, Allman JK, Junick DW. Effect of posterior capsular repair on early dislocation in primary total hip replacement. Clin Orthop Relat Res. 2001;393:163–167. doi: 10.1097/00003086-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22:815–821. doi: 10.1016/j.orthres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed] [Google Scholar]
- 47.Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, Whittle M, D’Lima DD, Cristofolini L, Witte H, Schmid O, Stokes I, Standardization and Terminology Committee of the International Society of Biomechanics ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion. Part I. Ankle, hip, and spine. International Society of Biomechanics. J Biomech. 2002;35:543–548. doi: 10.1016/S0021-9290(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 48.Yuan L, Shih C. Dislocation after total hip arthroplasty. Arch Orthop Trauma Surg. 1999;119:263–266. doi: 10.1007/s004020050406. [DOI] [PubMed] [Google Scholar]
- 49.Zijlstra WP, van den Akker-Scheek I, Zee MJ, van Raay JJ. No clinical difference between large metal-on-metal total hip arthroplasty and 28-mm-head total hip arthroplasty? Int Orthop. 2011 March 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]