Abstract
Background
Large bone loss and frequently irradiated existing bone make reconstructing metastatic and other nonprimary periacetabular tumors challenging. Although existing methods are initially successful, they may fail with time. Given the low failure rates of porous tantalum acetabular implants in other conditions with large bone loss or irradiated bone, we developed a technique to use these implants in these neoplastic cases where others might fail.
Description of Technique
After local tumor curettage, a large uncemented tantalum shell (sometimes with tantalum augments) was fixed to remaining bone using numerous screws. When substantial medial bone loss was present, an antiprotrusio cage was placed over the top of the cup and secured to remaining ilium and ischium.
Patients and Methods
We retrospectively reviewed 20 patients who underwent THAs for neoplastic bone destruction with the described technique. Their mean age was 60 years (range, 22–80 years). We recorded pain and ambulatory status, pain medication use, and Harris hip scores. We assessed for progressive radiolucent lines and component migration on followup radiographs. Eleven of the 20 patients died at a mean of 17 months after surgery. The minimum followup for surviving patients was 26 months (mean, 56 months; range, 26–85 months).
Results
Harris hip scores improved from a mean 32 preoperatively to a mean 74 postoperatively. We observed no cases of progressive radiolucent lines or component migration. Complications included one perioperative death, two superficial infections, one deep vein thrombosis, and one dislocation.
Conclusion
Our initial experience has made tantalum reconstruction our preferred method for dealing with major periacetabular neoplastic bone loss. Additional studies comparing this technique with alternatives are required.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metastatic processes commonly involve the periacetabular region with substantial morbidity for patients. As the mechanical integrity of the bone weakens, patients frequently experience increasing pain, protrusio deformities, and disability. Present and/or impending pathologic fractures influence patients’ function and ability to receive chemotherapy [17, 21]. In the absence of frank or imminent fracture, the initial treatment for these patients usually includes analgesics and protected weightbearing combined with systemic therapy, radiotherapy, and/or percutaneous cementoplasty [9, 10, 21]. However, many patients eventually undergo surgery because of failure of symptoms to abate with nonoperative management, the development of a fracture, or the need to treat an ipsilateral femoral lesion [21].
The classic reconstruction of periacetabular lesions involves the Harrington construct [8], in which a conventional acetabular implant is supported with cement and metal pins driven through the lesion to obtain support on the nondiseased proximal ilium. Various modifications to the technique include the use of screws instead of pins (sometimes introduced in an antegrade fashion) [16], the use of antiprotrusio rings [25], and the use of bulk allograft combined with a cemented reconstruction [1]. These constructs have a high initial success rate but may fail with time primarily owing to disease progression and cup loosening or migration, with the two largest published series [8, 16] each having a failure rate of approximately 9% (range, 0%–9%).
Porous tantalum implants have an established and expanding role in hip arthroplasties for nonneoplastic indications [2, 14, 15, 18]. For example, at early clinical followup, porous tantalum constructions used for severe acetabular bone loss encountered during (nonneoplastic) revision hip surgery have very low rates of failure secondary to loosening [18, 24]. Additionally, these implants showed no loosening at early (minimum 2 years) followup in patients undergoing THAs who had prior pelvic radiation for nonosseous malignancies [22].
Owing to these low rates of failure in the context of severe bone loss or irradiated bone, we developed a technique that uses porous tantalum acetabular implants for treatment of periacetabular lesions from neoplastic processes where others might fail.
Description of Technique
The surgical approach was at the preference of one of the four surgeons performing the reconstructions (14 anterolateral, four posterior, two transtrochanteric). Eighteen of 20 cases were performed by two of the authors in this study (DGL, FHS). The surgeons removed all accessible gross tumor in the periacetabular region by curettage and then accessed the acetabular deficiency.
All reconstructions used a porous tantalum acetabular shell (Revision Shell; Zimmer Corp, Warsaw, IN, USA). Similar to the Harrington-type reconstructions, the primary goal of the reconstruction was to provide the patient with a secure construct that had immediate mechanical stability. Although the surgeon used preoperative imaging (which included radiographs and/or CT or MR scans) to assess the deficiency and factored this into the type of reconstruction, the extent of tumor and status of the bone quality observed intraoperatively ultimately determined the exact method of reconstruction.
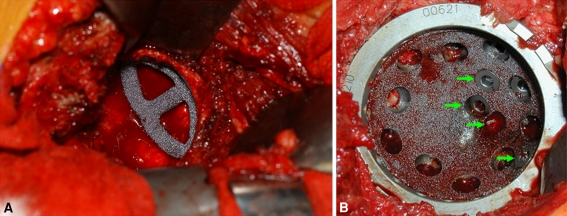
The acetabular cavity was prepared via reamers to accept as large a porous tantalum shell as the local anatomy would allow, typically limited by the AP dimension of the acetabulum. A trial fenestrated cup then was placed in the cavity. If there were residual large defects behind the proposed location of the shell, metal augments (also made of porous tantalum) were used. A round burr was applied to the edges of the defect to make the augment fit as snugly as possible. Once satisfied with the overall configuration in the trials, a real augment was impacted into the defect (Fig. 1A). The augments were oriented in such a fashion as to allow them to naturally accept an overlying shell. A small amount of cement was dispersed via a syringe along the inner lining of the augment to enhance its fixation to the overlying shell, and then a porous tantalum shell was firmly impacted into the prepared cavity/implanted augment.
Fig. 1A–B.
(A) Porous tantalum augments were used to reconstruct bony deficiencies. (B) The porous shell was secured with multiple screws, including extra holes drilled through the shell to access areas of remaining bone stock.
The use of a large shell and porous tantalum augments to fill defects served to maximize the overall contact area between the residual host bone (not destroyed by tumor) and the implant. This increased area of contact in turn enhanced the immediate stability of the construct by providing a more stable platform for the implant to rest on and by increasing the antishear frictional force between implant and bone. The characteristically high coefficient of friction that porous tantalum has with bone enhanced this effect. Additionally, the increased overall contact area between residual host bone and implant served to maximize the chances of obtaining bone ingrowth into the porous tantalum construct to help achieve long-term fixation.
Multiple screws were used in all cases to help fix the shell to the bone. In the more difficult cases with massive bone loss, as many screws as possible were used, each one having the maximum allowable length (without exiting the pelvis). A helicoidal burr was used to create additional screw holes in the implant, focusing on the areas where the bone quality was the greatest. Multiple screws then were placed to optimize purchase into nondiseased bone (Fig. 1B).
In situations where the tumor destroyed the medial wall of the acetabulum, an over-the-top cup-cage technique was used (Fig. 2). In this configuration, a large antiprotrusio cage (DePuy, Warsaw, IN, USA, or Zimmer Corp.) was placed over-the-top, rather than behind the porous tantalum shell. Thus, this did not disturb the fixation already achieved by the above methods between the implant shell (and augment) and bone. The cage had a large proximal plate that was fixed to the lateral ilium via screws. These screws were placed at near-90°-angles to some of the screws previously placed through the implant (ie, the acetabular screws headed in a proximal direction), sometimes nearly interdigitating with them. The ischial flange of the cage was placed along the outer cortex of the ilium (and fixed with screws) or driven into the substance of the ischium through a slot that was created with a pencil-tip burr. The acetabular reconstruction was completed by cementing a nonconstrained polyethylene liner into the construct.
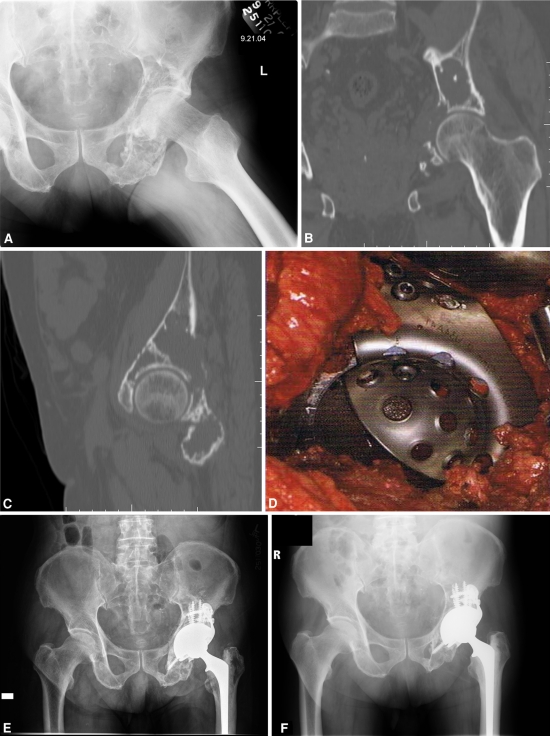
Fig. 2A–F.
(A) The radiograph shows a destructive lesion in the left periacetabular region secondary to metastatic prostate carcinoma in a 76-year-old man. Nonoperative treatment, including radiation with 45 Gy, failed and the patient was treated with surgical reconstruction. (B) Coronal and (C) sagittal CT scans show destruction of bone superiorly and medially. (D) An intraoperative photograph shows circumferential acetabular exposure and reconstruction with an over-the-top cup-cage construct. With this reconstruction technique, an antiprotrusio cage is placed over the top, rather than behind, a porous tantalum cup, allowing the back surface of the tantalum cup to directly interdigitate with any remaining host bone. Although in this case the ischial flange of the antiprotrusio cage was not used, an ischial flange that was driven into the ischium to maximize rotational stability of the acetabular reconstruction was used frequently in the reconstructions in this series. (E) Immediate postoperative and (F) 4½ year postoperative radiographs show stable implant fixation with no signs of loosening. This patient was pain-free and ambulating without gait aids at the time of last followup.
Different types of reconstructions were used for different cases, depending on the extent of bone destruction and underlying diagnosis. In seven cases, the construct consisted only of a porous tantalum shell that was impacted into the acetabulum and fixed with multiple screws. In four cases, one or two porous tantalum augments were used to fill a focal deficiency before placement of the primary revision shell. Seven cases used the over-the-top cup-cage technique (in one of these cases, an additional tantalum augment was performed for a focal cavitary deficiency). In two cases, a pelvic reconstruction plate was used in combination with a tantalum shell fixed with multiple screws to address a pelvic discontinuity (one of these also consisted of an additional tantalum augment for a focal cavitary deficiency). Overall, the mean number of screws used to fix the porous tantalum acetabular shell to the pelvis was 5.8 (range, 3–9); in cases involving cages, augments, and/or plates, additional screws typically were used to fix the cage, augment, and/or plate to the pelvis. The mean size of the acetabular shell was 58.6 mm (range, 48 mm–70 mm).
Mean operative time was 308 minutes (range, 199–422 minutes). Mean blood loss was 766 mL (range, 350–1300 mL).
Patients and Materials
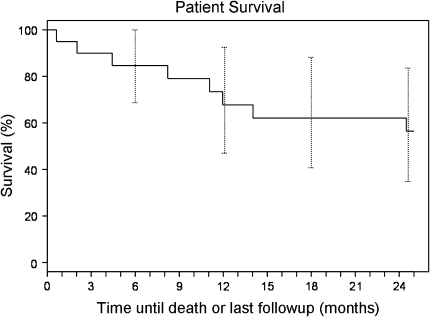
Using an institutional joint registry and surgical database, we identified 20 consecutive patients who had THAs with porous tantalum acetabular implants for treatment of neoplastic processes other than primary sarcoma in the periacetabular region. The THAs were performed between 2001 (when these implants became available) and 2008. No patient was treated with a Harrington-type procedure during this time. No attempted porous tantalum acetabular reconstruction was abandoned intraoperatively. Every patient who the treating surgeon thought required surgical acetabular reconstruction during this period was treated with this technique. The mean age of the patients at surgery was 60 years (range, 22–80 years). Eight patients had metastatic carcinoma (two with breast carcinoma, one with prostate carcinoma, two with lung carcinoma, one with renal cell carcinoma, and two with unknown primary carcinoma), seven had multiple myeloma, three had lymphoma, one had Langerhans cell histiocytosis, and one had Rosai-Dorfman syndrome (these last two patients each had a large area of neoplastic tumor destruction along the weightbearing superior dome of the acetabulum resulting in substantial mechanical symptoms and impending roof fracture). Eleven of 20 patients died at a mean of 17 months postoperatively (range, 0.6–58 months) (Fig. 3). The minimum followup for the nine surviving patients was 26 months (mean, 56 months; range, 26–85 months). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. Our institutional review board approved this study.
Fig. 3.
A Kaplan-Meier curve for patient survival using death or loss to followup as an endpoint is shown.
Eighteen procedures were primary surgeries and two were revisions of prior constructs. Fifteen patients had received prior radiotherapy, with a mean dose of 3550 cGy (range, 2000–5500 cGy). Each of the four patients who underwent surgery without prior radiation had either frank acetabular fracture or obvious impending acetabular fracture. Two patients with metastatic renal cell carcinoma underwent preoperative embolization to help reduce surgical bleeding because of the known (macro) vascularity of these tumors.
We classified acetabular deficiencies with preoperative imaging and intraoperative findings using the system described by Harrington [8] for the 18 primary arthroplasties and the AAOS classification [4] for the two revision surgeries. For the primary arthroplasties, seven patients had Harrington Class I deficiencies, three had Class II, and eight had Class III. One patient undergoing revision had an AAOS type II deficiency and the other had an AAOS type III deficiency (Table 1).
Table 1.
Classification of acetabular deficiencies in patients undergoing primary arthroplasties
| Class/type | Description | Number of patients |
|---|---|---|
| Harrington classification [8] | ||
| I | Small cavitary lesions but intact lateral cortices, medial, and superior walls | 7 |
| II | Intact lateral cortices, posterior, and superior walls, deficient medial wall | 3 |
| III | Deficient lateral cortices, medial and superior walls, deficient anterior and posterior columns, deficient superior dome | 8 |
| AAOS classification [4] | ||
| I | Segmental deficiency | 0 |
| II | Cavitary (contained) deficiency | 1 |
| III | Combined segmental and cavitary deficiency | 1 |
| IV | Pelvic discontinuity | 0 |
We assessed the clinical status at all followups and by using the joint registry at our institution per an established protocol at 3, 6, and 12 months, then yearly thereafter. Evaluation included pain characterization, narcotic use, Harris hip score (HHS), ambulatory status, and the need for gait aids. At each visit we obtained a nonweightbearing AP radiograph of the pelvis centered on the pubis, a nonweightbearing AP radiograph of the hip, and a nonweightbearing crosstable lateral radiograph of the hip. Clinical followups were available for the 18 patients who survived 3 months or more.
One of the nontreating surgeons (FAK) evaluated all radiographs for radiolucent lines and component migration. The immediate postoperative radiograph was considered the reference radiograph, and all subsequent radiographic measurements for evaluation of radiolucent lines or migration were compared with the measurements on this radiograph. Magnification of the radiographs was corrected for by using a known femoral head size. AP and lateral radiographs were analyzed for the presence and progression of any radiolucent lines using the system of DeLee and Charnley [5]. The acetabular reconstruction was divided into Zones I, II, and III. Any radiolucent line was recorded by zone. The width of the radiolucent line at each zone was measured. Radiolucent lines were recorded as progressive if, on serial radiographs, the number of radiolucent lines increased or if the width of any radiolucent line increased by greater than 1 mm. AP radiographs were used to measure the migration of acetabular components. Migration of the acetabular component was defined as translational or rotational. Migration was determined by comparing sequential films and measuring the distances between the acetabular component and Kohler’s line and the teardrop according to the technique of Callaghan et al. [3]. According to their criteria, a difference in the serial measurements of 2 mm or greater or a change in the angle of the cup of 3° or greater was considered to indicate migration. The reported intraobserver variability for component migration measurement using this technique is 0.5 mm [6].
Unless otherwise specified, we summarized the data as counts (percent) for discrete data, and mean (standard deviation) for continuous data. We estimated survival using the method of Kaplan and Meier [12] and death or loss to followup as an endpoint.
Results
At the time of last available followup, 12 of the 18 patients who survived more than 3 months reported no hip pain, four reported mild pain, one reported moderate pain, and one reported severe pain. Nine patients were taking less pain medicines than preoperatively, and nine were taking a similar amount; no patients had escalation of their narcotic requirements. All patients were ambulatory at last followup. Seven patients required no gait aids, six used a cane for long walks only, two used a cane full-time, and three used a walker or two crutches. HHS at last followup changed from a mean of 32 preoperatively to a mean of 74 postoperatively. One patient had a worsened HHS postoperatively, and this clinically appeared to result from lumbosacral radiculopathy from epidural tumor compression that developed from progression of disease postoperatively.
We observed no complete radiolucent lines. Nine of 20 patients showed an incomplete radiolucent line on their initial postoperative radiographs. Two of these resolved with followup. There were no cases of progressive radiolucent lines or component migration. No patients underwent revision for any reason.
We observed several complications. There were three major complications and four minor complications; three orthopaedic-related surgical complications and four nonorthopaedic complications occurred. One 74-year-old man with multiple myeloma and a Harrington Class III acetabular defect died 20 days postoperatively from disseminated intravascular coagulation and multiorgan failure. Although no deep infections occurred, two patients were treated for perioperative cellulitis and one for pneumonia. One patient had deep vein thrombosis (DVT) without pulmonary embolism, and another being treated with warfarin prophylactically for a history of DVT had gastrointestinal bleeding requiring transfusion and hospitalization. We observed dislocation in one patient who had three distinct episodes treated with closed reduction within a period of 2 months after surgery; he was scheduled to undergo revision surgery with implantation of a constrained liner but died of disease progression before it was performed. There were no signs of implant loosening observed on his latest radiographs. Overall seven of 20 patients experienced a complication. Aside from the dislocation, we believe none of the other nonfatal complications affected the ultimate clinical outcome.
Discussion
Reconstruction of periacetabular defects secondary to metastatic carcinomas and other nonsarcoma neoplastic disorders poses a formidable surgical challenge. Large areas of bone destruction, common use of local radiation, and concern for localized progression of disease are some of the factors making these reconstructions at risk for failure. The Harrington technique, which is the most commonly used method for these reconstructions, consists of a cemented socket supported with additional pins or screws designed to transfer load to nondiseased bone. It provides an excellent solution in the short-term, but may fail with time. As medical treatments for metastatic disease and myeloma continue to improve, the life expectancy for many of these patients is expected to increase. For example, advances in chemotherapy and stem cell transplantation have substantially improved the prognosis for many patients with multiple myeloma [20]. The limited durability of nonbiologic cemented acetabular revision techniques might become an increasing problem as such medical advances continue to improve the life expectancies of this patient population. We describe the use of uncemented porous tantalum components to reconstruct such defects. The use of these implants allows substantial intraoperative flexibility and enables the surgeon to create constructs with immediate and apparently sound mechanical stability. For example, the surgeon can (1) directly rest a large tantalum shell and tantalum augments (each having a high coefficient of friction with bone) on a relatively large area of remaining bone, (2) place multiple screws through the tantalum shell and augments in strategic locations corresponding to good remaining bone stock, and (3) supplement the entire construct with an additional antiprotrusio cage that provides substantial additional fixation to bone. Moreover, the use of a highly porous substance with substantial potential for bone ingrowth (even in the context of irradiated bone), gives this construct (in contrast to the Harrington technique) the potential for longer-term fixation. Thus, in addition to achieving sound immediate mechanical stability, we hoped to achieve biologic integration of the implants to minimize longer-term construct failure and need for revision.
Our study has several limitations. First, we report on a modest number of patients with short-term followup. This reflects the relatively low number of patients presenting to our institution who required surgical reconstruction for destructive nonprimary periacetabular tumors since the development of this new technique and the relatively limited expectancy of this patient population. Further followup is necessary to evaluate the durability of this technique in this patient population. Second, although we followed all patients through our joint registry in a prospective manner, we analyzed their results retrospectively. We did not systematically gather patient information to calculate performance status or other oncologic variables that might influence outcome. Our technique of assessing component migration and radiolucent lines has known limitations [11]. It is possible that subtle migration occurred and was not detected. We had no reoperations or autopsy retrievals to assess whether in vivo osseointegration actually occurred. Although all patients were treated with tantalum-based reconstructions, the reconstruction was individualized for each patient (shell alone, shell + augments, use of antiprotrusio cage) as dictated by the extent of tumor. No attempt was made to subclassify patients based on the specific method of reconstruction. The porous tantalum implants used in this study may be more expensive than the cemented implants used in the classic Harrington reconstruction depending on institutional resources. Surgeons should consider this in selecting reconstructions for patients with limited life expectancy. Finally, we did not attempt to identify factors that might be associated with prolonged prognosis. Although factors associated with shorter overall survival have been identified (eg, visceral metastases [16]), there are difficulties and inaccuracies associated with attempts to predict survival in this patient population [17]. Advanced mathematical modeling techniques are being developed to help predict the survival of patients with metastatic bone disease [7]. This information will be helpful in selecting patients who might benefit from more aggressive treatments for metastatic bone disease.
In this initial report, we observed no loosening in 20 patients with neoplastic periacetabular destruction despite poor bone quality and frequent prior radiation. Patients experienced good relief of pain, improvement in ambulatory status, and improvements in HHS. Our observations are similar to those of others who used the Harrington technique and its modifications (Table 2) [1, 8, 13, 16, 19, 25, 26]. The largest reported series is from Marco et al. [16]. This technique showed loosening in five of 55 cases at a mean 12 months postoperatively. Although we observed no loosening or component migration, we had fewer patients than did Marco et al. However, the mean followup in our series was 56 months for surviving patients, so the construct appears to be durable in the short- to intermediate-term. Even with advances in adjuvant treatments, it appears likely that construct survival will exceed life expectancy in this patient population.
Table 2.
Studies of Harrington-type and modified Harrington-type periacetabular reconstructions
| Study | Number of patients undergoing Harrington type reconstruction | Mean patient survival (months) | Followup of surviving patients (months) | Complications | Mechanical failure rate (cup loosening) |
|---|---|---|---|---|---|
| Allan et al. [1] | 12 | N/R | 14.4 | 1 perioperative death, 3 PEs, 2 dislocations (1 requiring open resection arthroplasty), 1 hematoma requiring surgical evacuation | 1/12 (8.3%) |
| Harrington [8] | 58 | 19 | N/R | 2 deaths, 2 superficial wound infections, 1 PE, 1 femoral nerve palsy | 8.6% |
| Kunisada and Choong [13] | 40 | N/R | N/R | 1 dislocation, 2 PEs, 1 death | 0% |
| Marco et al. [16] | 55 | 9 | N/R | 1 perioperative death, 5 DVTs, 3 superficial wound infections, 1 DIC, 1 sacral decubitus ulcer, 1 hematoma, 1 Ogilvie syndrome, 1 subluxation requiring revision | 9.1% |
| Nilsson et al. [19] | 32 | 11 | N/R | 2 deaths, 2 dislocations, 1 deep infection, 2 DVTs | 0% |
| Vena et al. [25] | 21 | 14.5 | 21.4 | 3 perioperative deaths, 2 dislocations, 2 deep infections, 1 femoral nerve palsy, 1 foot drop | 0% |
| Walker [26] | 4 | 15 | N/R (all patients died) | 1 pin migration requiring surgical removal | 0% |
| Current study | 20 | 36 | 56 | 1 perioperative death, 2 superficial infections, 1 deep vein thrombosis, and 1 recurrent dislocation | 0% (1 patient died before revision for instability) |
PE = pulmonary embolism; N/R = not reported; DVT = deep vein thrombosis; DIC = disseminated intravascular coagulation.
Similar to other studies [1, 8, 13, 16, 19, 23, 25, 26], we observed frequent complications, and one patient died perioperatively. One patient had early recurrent instability and was considered for revision surgery with implantation of a constrained liner, but he died of disease progression before surgery was performed. An additional five patients experienced nonoperative complications. These results highlight the difficulties and risks inherent in surgery in this patient population.
The porous tantalum-based technique reported here and the traditional Harrington-type reconstructions share the goal of obtaining immediate mechanical stability. A potential advantage of the porous tantalum construct over traditional cement-based Harrington-type reconstructions is the potential for bone ingrowth. Although longer-term studies are needed to document this effect and to compare it with Harrington-type reconstructions, the use of porous tantalum as opposed to cemented reconstructions makes the possibility of true bone ingrowth and thus longer-term fixation seem more likely. The success of tantalum reconstructions in the context of pelvic irradiation (without periacetabular metastases) [22] gives more credence to this possibility.
We present an initial study of the use of porous tantalum implants for reconstruction of neoplastic periacetabular defects. Patients showed consistent improvements in HHS and pain status with no radiographic evidence of loosening or impending construct failure. Our observations warrant additional studies comparing this technique with the traditional Harrington method of reconstruction. Studies with larger numbers and longer followup are needed to determine the ultimate utility and outcome of this new technique of reconstruction. Our findings have made reconstruction with porous tantalum implants our preferred method for dealing with major periacetabular bone loss from tumors.
Acknowledgment
We thank Dirk Larson for his contribution to the statistical analysis of the manuscript.
Footnotes
One of the authors (DGL) receives royalties from Orthosonics, Osteotech, and Zimmer (including for a product reported in this study); all other authors certify that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Mayo Clinic, Rochester, MN, USA.
References
- 1.Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10:301–306. doi: 10.1016/S0883-5403(05)80178-0. [DOI] [PubMed] [Google Scholar]
- 2.Boscainos PJ, Kellett CF, Maury AC, Backstein D, Gross AE. Management of periacetabular bone loss in revision hip arthroplasty. Clin Orthop Relat Res. 2007;465:159–165. doi: 10.1097/BLO.0b013e3181560c6c. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD, Jr, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982: a two to five-year follow-up. J Bone Joint Surg Am. 1985;67:1074–1085. [PubMed] [Google Scholar]
- 4.D’Antonio JA, Capello WN, Borden LS, Bargar WL, Bierbaum BF, Boettcher WG, Steinberg ME, Stulberg SD, Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–137. [PubMed] [Google Scholar]
- 5.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 6.Dorr LD, Wan Z. Ten years of experience with porous acetabular components for revision surgery. Clin Orthop Relat Res. 1995;319:191–200. [PubMed] [Google Scholar]
- 7.Forsberg JA, Eberhardt J, Boland PJ, Wedin R, Healey JH. Estimating survival in patients with operable skeletal metastases: an application of a bayesian belief network. PLoS One. 2011;6:e19956. doi: 10.1371/journal.pone.0019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63:653–664. [PubMed] [Google Scholar]
- 9.Harris K, Pugash R, David E, Yee A, Sinclair E, Myers J, Chow E. Bone Metastases Site Group. Percutaneous cementoplasty of lytic metastasis in left acetabulum. Current Oncol. 2007;14:4–8. doi: 10.3747/co.2007.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harty JA, Brennan D, Eustace S, O’Byrne J. Percutaneous cementoplasty of acetabular bony metastasis. Surgeon. 2003;1:48–50. doi: 10.1016/S1479-666X(03)80010-0. [DOI] [PubMed] [Google Scholar]
- 11.Ilchmann T. Radiographic assessment of cup migration and wear after hip replacement. Acta Orthop Scand Suppl. 1997;276:1–26. doi: 10.1080/17453674.1997.11744768. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 13.Kunisada T, Choong PF. Major reconstruction for periacetabular metastasis: early complications and outcome following surgical treatment in 40 hips. Acta Orthop Scand. 2000;71:585–590. doi: 10.1080/000164700317362217. [DOI] [PubMed] [Google Scholar]
- 14.Levine B, Della Valle CJ, Jacobs JJ. Applications of porous tantalum in total hip arthroplasty. J Am Acad Orthop Surg. 2006;14:646–655. doi: 10.5435/00124635-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671–4681. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82:642–651. doi: 10.2106/00004623-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Nathan SS, Healey JH, Mellano D, Hoang B, Lewis I, Morris CD, Athanasian EA, Boland PJ. Survival in patients operated on for pathologic fracture: implications for end-of-life orthopedic care. J Clin Oncol. 2005;23:6072–6082. doi: 10.1200/JCO.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 18.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–208. doi: 10.1097/01.blo.0000150133.88271.80. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson J, Gustafson P, Fornander P, Ornstein E. The Harrington reconstruction for advanced periacetabular metastatic destruction: good outcome in 32 patients. Acta Orthop Scand. 2000;71:591–596. doi: 10.1080/000164700317362226. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 21.Papagelopoulos PJ, Savvidou OD, Galanis EC, Mavrogenis AF, Jacofsky DJ, Frassica FJ, Sim FH. Advances and challenges in diagnosis and management of skeletal metastases. Orthopedics. 2006;7:609–620. doi: 10.3928/01477447-20060701-01. [DOI] [PubMed] [Google Scholar]
- 22.Rose PS, Halasy M, Trousdale RT, Hanssen AD, Sim FH, Berry DJ, Lewallen DG. Preliminary results of tantalum acetabular components for THA after pelvic radiation. Clin Orthop Relat Res. 2006;453:195–198. doi: 10.1097/01.blo.0000238854.16121.a3. [DOI] [PubMed] [Google Scholar]
- 23.Schneiderbauer MM, Knoch M, Schleck CD, Harmsen WS, Sim FH, Scully SP. Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am. 2004;86:1684–1689. doi: 10.2106/00004623-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Siegmeth A, Duncan CP, Masri BA, Kim WY, Garbuz DS. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res. 2009;467:199–205. doi: 10.1007/s11999-008-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vena VE, Hsu J, Rosier RN, O’Keefe RJ. Pelvic reconstruction for severe periacetabular metastatic disease. Clin Orthop Relat Res. 1999;362:171–180. doi: 10.1097/00003086-199905000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop Relat Res. 1993;294:170–175. [PubMed] [Google Scholar]