Abstract
Background
Limitations of conventional uncemented femoral stems persist, including proximal-distal mismatch, nonideal load transfer, loss of bone, and difficulties with minimally invasive surgery. Metaphyseal-engaging short-stem implants have been designed to address these issues in THA. While these devices have been studied in younger patients, it is unclear whether they offer advantages in older patients.
Questions/purposes
We asked whether the stability and bony ingrowth of an off-the-shelf short stem in patients 70 years and older were similar to those achieved in patients younger than 70 years at 2-year followup. Furthermore, we asked whether pain and function scores were affected by age, bone quality, or varus alignment.
Patients and Methods
We retrospectively reviewed 60 patients (65 hips) 70 years and older (mean, 75 years; range, 70–86 years) treated with an uncemented short stem (range, 90–105 mm). We compared radiographic alignment, stability, and bony ingrowth, as well as Harris hip scores and WOMAC pain scores, to a cohort of 89 patients (91 hips) younger than 70 years. Minimum followup was 24 months (mean, 35 months; range, 24–60 months).
Results
Radiographs showed proximal bony ingrowth and stable fixation of all implants. Average Harris hip score at last followup was 88 (range, 70–100) for the 70 years and older cohort and 93 (range, 70–100) for younger than 70 years cohort; no patients reported thigh pain. Postoperative WOMAC scores averaged 6 (range, 0–43) and 5 (range, 0–25), respectively.
Conclusions
Short-stem implants provide solid, dependable fixation in osteoporotic bone at minimum 2-year followup, while meeting some of the limitations in conventional primary THA.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The subpopulation of patients 70 years and older remains a substantial portion of the patient population who undergo THA [29]. Patients 70 years and older typically have a greater proportion of Dorr Type B and C [13] bone compared to younger patients [43]. Dorr Type C bone exhibits osteoporotic cortical degradation, as well as intramedullary canal widening [13], disproportionately widening the diaphysis. Proximal-distal mismatch can lead to intraoperative fractures and inability to obtain a tight proximal fit required by conventional uncemented stems, which achieve fixation either through a porous diaphyseal fit or porous metaphyseal/nonporous diaphyseal fit [5, 16, 21, 40, 41]. In addition, optimizing bone remodeling and decreasing stress shielding after THA may be more important in this older population secondary to diminished baseline bone quality [3, 5, 8]. This population also has increased risk of intraoperative and postoperative periprosthetic fractures, which range from 2% to 20% in the general population depending on the implant [5, 16, 24, 27, 36].
Successful THA requires stable initial and long-term axial and rotational fixation. A metaphyseal-engaging short-stem implant is one possible solution to the challenges in achieving stable initial fixation in varying proximal femoral morphology and optimizing proximal load transfer, while providing dependable fixation [46]. Pain relief, improved function, and radiographic stability of short stems of various designs have been documented in followup of 2 to 8 years [44, 46]. These studies report improvement in pain and function scores at minimum 2-year followup after THA with custom-made short-stem implants. They also primarily include patients younger than 70 years. It is unclear whether older patients who might benefit from the theoretical advantages of these devices would achieve the same pain relief and function from an off-the-shelf design.
Therefore, we asked whether (1) the stability and bony ingrowth of an off-the-shelf metaphyseal-engaging femoral stem in patients 70 years and older were similar to those achieved in patients younger than 70 years with the same implant design at minimum 2-year followup, (2) hip function and pain scores were similar in both cohorts, (3) bone quality affected function and pain scores in the 70 years and older cohort, and (4) the frequency of varus positioning was similar and was not associated with negative outcomes.
Patients and Methods
We retrospectively reviewed the prospectively collected data of all 181 patients who underwent 194 primary THAs with an off-the-shelf short-stem implant between December 2004 and July 2006. During that same time, we treated 183 patients with 196 primary THAs with all designs. Any patient who needed a primary THA without femoral deformity that would make an anatomic stem impossible was a candidate for this stem—our standard of care at the time. The indications for the short-stem implants were (1) osteoarthritis, (2) inflammatory arthritis (ie, rheumatoid), (3) avascular necrosis, and (4) traumatic arthritis. The contraindication for this stem was that of any anatomic implant: a femoral deformity that precluded fit and fill in the metaphysis, for example: (1) dysplastic hips with high offset/severe valgus or (2) metaphyseal deformity secondary to fracture. In such cases, we used specialized or modular implants to achieve stable bony and soft tissue anatomic reconstruction. Of these 181 patients, 15 died secondary to causes unrelated to our THA; five declined to participate in followup for various reasons unrelated to the THA; and we excluded two patients based on extensive medical comorbidities that impeded appropriate clinical and radiographic followup. Ten patients (10 THAs) were lost to followup. These 32 exclusions left 149 patients (160 THAs; two patients excluded had bilateral off-the-shelf implants). Sixty patients (65 THAs) were 70 years and older at the time of surgery with an average age of 75 years (range, 70–86 years) and average body mass index of 27 (range, 20–39). The minimum followup was 24 months (mean, 35 months; range, 24–60 months). The remaining 89 patients (95 THAs) who were younger than 70 years comprised the control group. In this group, the average age was 58 years (range, 29–69 years), average body mass index was 28 (range, 19–56), and minimum followup was 24 months (mean, 36 months; range, 24–62 months).
We compared the two groups in terms of preoperative demographic variables and preoperative Harris hip score (HHS) and WOMAC score (Table 1). The primary surgeon (SDS) examined all patients on preoperative and postoperative visits. All data were obtained from medical records, including prospectively collected patient-filled questionnaires providing information for pain and function scores and radiographs. We had prior institutional review board approval. The mean preoperative WOMAC scores were similar for the older and younger group: 46 (range, 9–82) versus 50 (range, 12–91), respectively.
Table 1.
Demographics of the two cohorts
| Variable | < 70 years old | > 70 years old | p Value |
|---|---|---|---|
| Total number of patients | 60 | 89 | |
| Men | 27 | 42 | 0.924 |
| Women | 33 | 47 | |
| Total number of THAs | 65 | 91 | |
| Right hips | 40 | 51 | 0.602 |
| Left hips | 25 | 40 | |
| Age at surgery (years)* | 75.3 (70–86) | 57.5 (29–69) | |
| Body mass index* | 27.3 (20.2–38.6) | 27.9 (19.2–55.7) | |
| Followup (months)* | 34.7 (21–60) | 35.5 (21–62) | 0.640 |
| Dorr bone quality | |||
| Type A | 12 | 58 | < 0.001 |
| Type B | 23 | 22 | 0.179 |
| Type C | 30 | 11 | < 0.001 |
| Preoperative Harris hip score* | 47 (20–75) | 55 (5–100) | 0.003 |
| Preoperative WOMAC* | 46 (9–82) | 50 (12–91) | 0.223 |
* Values are expressed as mean, with range in parentheses.
Using a 95% significance level (Type I error probability = 0.05) and 90% power level (Type II error probability = 0.1), our study population was sufficient to distinguish differences between the two cohorts in change in WOMAC score of 9.36 or more and in change in HHS of 8.75 or more using a two-sample t test with a two-sided alternative hypothesis.
The same surgeon (SDS) performed all of the arthroplasties with a standardized operative technique through a less invasive posterolateral approach. The implant was designed with the rationale of optimizing circumferential fit. The surgeon prepared the femur in a broach-only fashion and impacted the prosthesis until he obtained a tight metaphyseal fit. Thus, the stem size that gave the most contact in the metaphysis, regardless of which plane (anterior-posterior, medial-lateral, or along the calcar), was chosen. The anatomic implant was a commercially available shortened version of a widely used anatomic implant (Citation®; Stryker Orthopaedics, Mahwah, NJ, USA). The implant used in all patients was designed to fit and fill the metaphysis with a fixed medial flare at the metaphysis. The distal portion consisted of a cylindrical stem with a tapered tip. The stem length was proportional to the metaphysis fill with the goal to engage the diaphysis just beyond the metaphyseal-diaphyseal junction. The stem lengths varied between 90 and 105 mm. The femoral stem was made of a polished titanium alloy, with a hydroxyapatite coating on a titanium plasma spray in the proximal 1/3 of the stem (Stryker) (Fig. 1). There were no modular components as the implant had a fixed horizontal offset of 132°; however, the implant had 16 different sizes (eight right, eight left). All patients received a porous-coated acetabular component. The average femoral head size was 32 mm. Of the 60 patients 70 years and older, 17 had bilateral THAs, with at least one hip with an off-the-shelf short-stem implant. Five patients had bilateral off-the-shelf stems, with similar bone remodeling observed in each hip (Fig. 2). Ten patients had a conventional-length stem (five uncemented, five cemented) in one hip and an off-the-shelf short-stem in the contralateral hip. The proximal femurs with the short stem tended to have greater endosteal condensation in the proximal metaphysis compared to the uncemented conventional-stem hip. Of note, most of the conventional-length stems were the longer version of the off-the-shelf design (Fig. 3).
Fig. 1.

The off-the-shelf implant consisted of a polished titanium alloy with a hydroxyapatite coating on a titanium plasma spray in the proximal 1/3 of the stem.
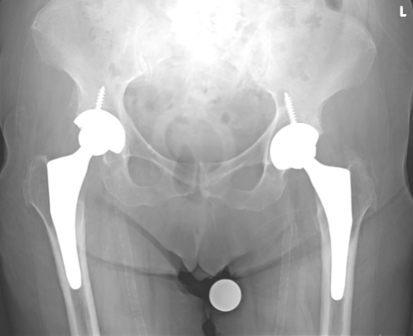
Fig. 2.
The radiograph of an 88-year-old woman 2 and 3 years shows right and left THAs, respectively, with off-the-shelf short-stem implants.
Fig. 3.
The radiograph of a 76-year-old man shows a metaphyseal-engaging short stem 4 years after surgery. On the left hip is an uncemented conventional-length version of the same implant as on the right hip.
All patients in both cohorts underwent the same postoperative protocol. The surgeon allowed full weightbearing immediately after surgery and all patients were mobilized on Postoperative Day 1 with hospital inpatient physical therapy. Twice daily physiotherapy consisted of gradual progression from up-to-chair tolerance to ambulation to stair climbing under the supervision of a certified physical therapist. Patients who cleared inpatient physical therapy for independent ambulation did not require further therapy; those patients who did not clear required further physiotherapy, ranging from home therapy to acute inpatient rehabilitation.
All patients returned to a prescheduled outpatient clinic appointment 4 weeks after surgery for clinical and radiographic examination, which included an AP pelvis and frog leg lateral of the operative hip. Clinical examination performed by the primary surgeon (SDS) included inspection of the wound, observation of gait, and evaluation of ROM and strength in the operative extremity. All patients independently completed a standardized questionnaire that provided HHS [18, 19] and WOMAC [2] pain subscale score at each visit. Subsequent routine followup examinations occurred at 3, 6, and 12 months and then annually thereafter. From the medical records, we obtained clinical data from preoperative, first postoperative, and minimum 2-year postoperative visits. Data gathered during review of patient charts, operative note records, and radiographs included basic demographic information, side of surgery, unilateral versus bilateral, size of femoral and acetabular components, intraoperative and postoperative complications, need for revision surgery, preoperative and postoperative clinical data, and analysis of AP and lateral radiographs. Those patients who did not have minimum 2-year followup at the time of data collection were called and the reason was documented as listed previously in the exclusions. We interviewed two patients (two THAs) by telephone who were unable to attend an examination and asked them to provide current radiographs; all other patients were seen as part of the routine followup protocol for the primary surgeon.
We digitized all radiographs and imported them into an online database that allowed digital calibration and subsequent analysis. Preoperative radiographs were assigned a Dorr classification [13]. We calibrated postoperative radiographs using the known diameter of the prosthetic femoral head. Two of us (SDS, RMP) examined all postoperative radiographs for implant alignment and stability [14, 15, 22, 39]. We measured varus/valgus positioning (≥ 5° from neutral) of the implant by measuring the angulation along the stem relative to the femoral shaft. To assess stability, we compared length measurements from the superior tip of the greater trochanter to the distal tip of the implant between immediate postoperative and long-term followup visits and defined subsidence as 2 mm or greater [15, 22, 39]. We analyzed the seven zones of Gruen et al. [17] for bony ingrowth via bone bridging or endosteal condensation and documented the presence of a fracture or a bony shelf at the tip of the component. We assessed for loosening by looking at varus/valgus positioning over time as well as any lucency greater than 2 mm around the stem.
We compared the two population means for body mass index, months of followup, preoperative and postoperative HHS and WOMAC scores, and changes in HHS and WOMAC scores using two-sample t tests with a two-sided alternative hypothesis (SPSS® Version 19.0.0.1; IBM Statistics, Chicago, IL, USA). We compared demographic ratios and rates of patient sex, Dorr bone quality classification, and varus alignment using two-sample chi square tests for equality of proportions with one degree of freedom (R Version 2.11.1; R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a statistical confidence level of 95% was selected.
Results
One implant subsided early postoperatively when radiographs at the 4-week followup were compared to the immediate postoperative radiograph (> 5-mm migration). The patient denied sense of instability or thigh pain. The implant was stable on all subsequent followups, including 2- and 3-month visits, and there was no evidence of loosening (at 4-year followup) with bone remodeling, suggesting ingrowth had occurred. There was one intraoperative nondisplaced fracture in a patient with Dorr Type C femur. We treated the fracture with cerclage wires and it was stable immediately and at long-term followup. All implants had radiographic evidence of bony ingrowth, as seen by bone bridging and endosteal condensation, including those placed in varus. Gruen Zones 6 and 7 most consistently showed this pattern, followed by Zones 2 and 3 (Fig. 4). All stems were radiographically stable with no reactive lines of greater than 2 mm or loosening identified. There was no evidence of distal pedestal formation.
Fig. 4A–B.
Radiographs show the hip of a 90-year-old man with Dorr Type C bone (A) before and (B) 2 years after a left THA; there is evidence of bony ingrowth.
At minimum 2-year followup, the mean HHS was 88 (range, 70–100) for the 70 years and older group and 93 (range, 70–100) for the younger than 70 years group. The mean net improvements were similar (p = 0.244) in the older (41) and the younger cohort (38). The mean followup WOMAC scores were similar (p = 0.376) in the older and younger groups: 6 (range, 0–43) versus 5 (range, 0–25), respectively.
We observed no difference in the HHS (p = 0.448) or WOMAC score (p = 0.100) between patients with DORR Type C bone and Dorr Type A and B bone. The followup HHS and WOMAC scores in the 30 hips with Dorr Type C bone were 90 and 4, respectively. For Dorr Type A and B bone, the followup HHS and WOMAC scores were 87 and 8, respectively.
We found three implants (5%) placed in varus (range, 5.2°–10°). The HSS and WOMAC scores for these stems were similar to those of implants placed in neutral or slight valgus (Table 2).
Table 2.
Varus position of implants
| Score | Three patients in varus | All others > 70 years old | p Value (two-sided t test) |
|---|---|---|---|
| Preoperative Harris hip score | 52 | 47 | 0.516 |
| Postoperative Harris hip score | 100 | 88 | 0.137 |
| Preoperative WOMAC | 37 | 45 | 0.480 |
| Postoperative WOMAC | 5 | 6 | 0.871 |
Discussion
Stems of various designs provide stable initial and long-term fixation in patients who undergo THA [3, 4, 7, 9, 20, 32, 33, 38]. Metaphyseal-engaging short stems provide theoretical benefits compared to conventional uncemented stems, including avoiding proximal-distal mismatch, decreasing proximal stress shielding, and limiting perioperative periprosthetic fractures. Several studies show custom short-stem designs provide short-term fixation [44, 46], specifically in patients younger than 60 years. Therefore, we asked whether (1) the stability and bony ingrowth of metaphyseal-engaging femoral stem in patients 70 years and older were similar to those achieved in patients younger than 70 years, (2) hip function and pain scores were similar, (3) bone quality affected function in the 70 years and older cohort, and (4) the frequency of varus positioning was similar and not associated with negative outcomes.
We acknowledge limitations to our study. First, the data reflect a single surgeon’s experience and one with a particular interest in short stems. Unlike hip resurfacing, the procedure for implanting short-stem devices is identical to that for inserting stems of conventional length. Thus, despite surgeon preference and experience, the technique and outcomes can be expected to be replicable. Second, we lacked an age-matched control group who underwent THA with uncemented stems of conventional length. However, a number of historical reports indicate such stems can be successfully implanted in older patients [9, 10, 25, 32]. Third, radiographic analysis is inferior to roentgen stereophotogrammetric and dual-energy xray analysis in determining bone mass, remodeling, and component migration [11, 23]. Engh et al. [15] have reported successful systematic methods of measuring bone remodeling on radiography by confirming radiographic results of stress shielding with histologic examination. Thus, a qualitative assessment of bone remodeling from radiographs acknowledges overall changes without the quantitative accuracy of advanced imaging. Fourth, HHS and WOMAC scores are intuitively based on patient report and are subject to patient reporting bias; however, any bias effect would be no greater in our study than in other studies using the widely acknowledged hip pain and function scoring systems. Fifth, durability of the implant, particularly in relation to radiographic stability and pain and function scores, requires long-term (ie, > 10-year) followup. While conventional uncemented THA has greater than 10-year followup in the literature, our study evaluates a newer stem design in a subset of the general patient population. Longer followup is under way. Lastly, we did not measure for inter- and intraobserver variability of radiographic measurement but instead agreed on findings through consensus. In a review of the literature, we found similar studies referencing Johnston et al. [22] and Engh et al. [14] when addressing radiographic evaluation; however, no statistical analysis of reliability was performed in these studies [1, 12, 30, 31, 37].
Osteoporotic bone exhibits diminished cellular and structural characteristics, potentially compromising ingrowth/outgrowth of the implant. Thus, aseptic loosening remains a concern in uncemented stems in diminished bone. In our cohort, no femoral component underwent revision for aseptic loosening, migration, subsidence, or osteolysis. All implants were radiographically stable up to 5 years after surgery. Current literature demonstrates radiographic stability of conventional proximally coated tapered or cylindrical stems in patients of all ages and bone quality (Table 3) [3, 7, 9, 10, 20, 25, 32, 35, 42]. Santori and Santori [44] reported no subsidence or loosening in 129 custom-made uncemented high-femoral-neck resection short-stem implants. The indications for the use of this stem in this cohort were age of less than 60 years and good bone stock. Stulberg and Dolan [46] also had no cases of femoral instability (ie, no loosening or subsidence) in 65 custom-made short-stem femoral implants in 60 patients younger than 70 years. Our study demonstrates solid, dependable fixation of short-stem implants in osteoporotic bone while meeting current challenges in primary THA. Initial stable fixation of a femoral implant does not require a conventional-length cylindrical or tapered stem [35, 44–46]. In fact, the short-stem model supports the three-stage fixation of cementless femoral components, with rigid primary fixation and extensive metaphyseal contact for osteointegration [8].
Table 3.
Uncemented femoral implants of various designs examined in cohorts of various ages and bone quality
| Study | Implant design | Stem fixation type | Number of hips | Average age (years) | Average followup (years) | Stem revisions for aseptic loosening |
|---|---|---|---|---|---|---|
| Santori and Santori [44] | Custom high-neck resection short stem | Uncemented with hydroxyapatite | 129 | 51 | 8 | 0 (0%) |
| Stulberg and Dolan [46] | Custom short stem | Uncemented with hydroxyapatite | 65 | 56 | 2 | 0 (0%) |
| Morrey [35] | Short stem with high-valgus neck | Uncemented | 20 | 2 | 1 (5%) | |
| Meding et al. [32] | Conventional | Uncemented with hydroxyapatite | 127 | 63* | 5 | 0 (0%) |
| Berend et al. [3] | Conventional | Uncemented | 49 | 79 | 5 | 0 (0%) |
| Kelly et al. [25] | Conventional | Uncemented with hydroxyapatite | 15 | 54* | 11.5 | 0 (0%) |
| Patel et al. | Off-the-shelf short stem | Uncemented with hydroxyapatite | 65 | 75 | 2 | 0 (0%) |
* All patients were classified as Dorr Type C bone.
There were no observable differences in HHS and WOMAC scores in the 70 years and older cohort when compared to the younger than 70 years group. Berend et al. [3] evaluated 49 hips in patients 75 years and older with an uncemented double-tapered implant at an average 5 years postoperatively and found a mean HHS of 84. There was no control for comparison. Nevertheless, they observed a mean increase from preoperative and postoperative HHS scores of 39, comparable to the 41 and 38 in our older and younger cohorts, respectively. They also reported 87% of their cohort had “minimal to no” pain, though no quantifiable data were given. Five percent of the patients did, however, complain of thigh pain. Konstantoulakis et al. [28] also had five of 132 patients (4%) who underwent THA with a conventional uncemented implant complain of thigh pain at 4-year followup. No patients in our 70 years and older cohort complained of thigh pain. This could be attributed to the shorter stem in our design and less potential for distal micromotion leading to thigh pain [6].
Meding et al. [32] observed no difference in HHS and pain scores when stratifying patients based on Dorr classification. They found 127 patients with Dorr Type C bone to have a HHS of 94.5 compared to 94.9 in 625 patients with Type A bone. These results were also confirmed by Kelly et al. [25] who noted an average HHS of 95 at 9-year followup in 15 patients with Dorr Type C bone. However, Dorr et al. [13] concluded increased incidence of thigh pain in patients with Dorr Type C bone was secondary to delayed remodeling. Our results at minimum 2-year followup refute this and instead support stable initial and durable fixation in short-stem metaphyseal-engaging implants. Maintaining proximal metaphyseal cortical density appears to be fundamental in long-term fixation of femoral implants in aging bone [33]. Longer followup in this cohort will be required to evaluate proximal bone stock in the elderly subpopulation.
While a short stem may be prone to varus malalignment, our rates are similar to rates in conventional uncemented stems [26, 34, 46]. Our 70 years and older group had a varus (angulation > 5°) alignment rate of 4.6% compared to 5.3% in the younger than 70 years group. Furthermore, though some controversy exists, varus stem positioning may not predispose to subsidence, loosening, or fracture as long as extensive metaphyseal fixation is achieved [26, 34]. Osteoporotic bone, with widened intramedullary canals, would theoretically be predisposed to varus positioning in implants that rely on the distal stem for positioning and fixation. Furthermore, these findings emphasize the importance of developing instrumentation and surgical techniques that minimize varus alignment when short stems are used.
The advantages of a short-stem implant include avoiding proximal-distal mismatch, particularly in osteoporotic patients with disproportionately widened intramedullary canals. Furthermore, given weakened cortices in this patient population, the risk of femoral perforation reduces with a shortened stem length. Additionally, a short stem lends itself to minimally invasive surgery, particularly the anterior approach, which is gaining popularity among surgeons and patients.
With growing interest in bone preservation techniques, further investigation through long-term and randomized prospective studies and commitment into short-stem designs can proceed in patients of all ages and bone quality.
Acknowledgments
The authors thank Max Cayo for his statistical analysis; Mark Dolan, MD, for his critical analysis; and Gina Bart, PA-C, for her research coordination.
Footnotes
One or more of the authors (SDS) has received royalties from Aesculap, Inc (Center Valley, PA, USA); stock or stock options from Stryker Orthopaedics (Mahwah, NJ, USA) and Johnson & Johnson (New Brunswick, NJ, USA); and serves as a paid consultant to Aesculap, Innomed, Inc (Savannah, GA, USA), Stryker, and Zimmer, Inc (Warsaw, IN, USA). The remaining authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his/her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Baker PN, McMurtry IA, Chuter G, Port A, Anderson J. THA with the ABG I prosthesis at 15 years: excellent survival with minimal osteolysis. Clin Orthop Relat Res. 2010;468:1855–1861. doi: 10.1007/s11999-009-1066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 3.Berend KR, Lombardi AV, Mallory TH, Dodds KL, Adams JB. Cementless double-tapered total hip arthroplasty in patients 75 years of age and older. J Arthroplasty. 2004;19:288–295. doi: 10.1016/j.arth.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Berend ME, Smith A, Meding JB, Ritter MA, Lynch T, Davis K. Long-term outcome and risk factors of proximal femoral fracture in uncemented and cemented total hip arthroplasty in 2551 hips. J Arthroplasty. 2006;21(6 Suppl 2):53–59. doi: 10.1016/j.arth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30:183–190. doi: 10.1016/S0030-5898(05)70073-0. [DOI] [PubMed] [Google Scholar]
- 6.Brown TE, Larson B, Shen F, Moskal JT. Thigh pain after cementless total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2002;10:385–392. doi: 10.5435/00124635-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Burt CF, Garvin KL, Otterberg ET, Jardon OM. A femoral component inserted without cement in total hip arthroplasty: a study of the Tri-Lock component with an average ten-year duration of follow-up. J Bone Joint Surg Am. 1998;80:952–960. doi: 10.2106/00004623-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan JJ, Rosenberg AG, Rubash HE. The Adult Hip. 2. Philadephia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 9.Capello WN, D’Antonio JA, Jaffe WL, Geesink RG, Manley MT, Feinberg JR. Hydroxyapatite-coated femoral components: 15-year minimum followup. Clin Orthop Relat Res. 2006;453:75–80. doi: 10.1097/01.blo.0000246534.44629.b2. [DOI] [PubMed] [Google Scholar]
- 10.Capello WN, D’Antonio JA, Manley MT, Feinberg JR. Hydroxyapatite in total hip arthroplasty: clinical results and critical issues. Clin Orthop Relat Res. 1998;355:200–211. doi: 10.1097/00003086-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Cohen B, Rushton N. Accuracy of DEXA measurement of bone mineral density after total hip arthroplasty. J Bone Joint Surg Br. 1995;77:479–483. [PubMed] [Google Scholar]
- 12.Corten K, Bourne RB, Charron KD, Au K, Rorabeck CH. What works best, a cemented or cementless primary total hip arthroplasty? Minimum 17-year followup of a randomized controlled trial. Clin Orthop Relat Res. 2011;469:209–217. doi: 10.1007/s11999-010-1459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242. doi: 10.1016/8756-3282(93)90146-2. [DOI] [PubMed] [Google Scholar]
- 14.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants: the femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 15.Engh CA, Jr, McAuley JP, Sychterz CJ, Sacco ME, Engh CA., Sr The accuracy and reproducibility of radiographic assessment of stress-shielding: a postmortem analysis. J Bone Joint Surg Am. 2000;82:1414–1420. doi: 10.2106/00004623-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald RH, Jr, Brindley GW, Kavanagh BF. The uncemented total hip arthroplasty: intraoperative femoral fractures. Clin Orthop Relat Res. 1988;235:61–66. [PubMed] [Google Scholar]
- 17.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 18.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation . J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 19.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;213:20–33. [PubMed] [Google Scholar]
- 20.Hozack WJ, Rothman RH, Eng K, Mesa J. Primary cementless hip arthroplasty with a titanium plasma sprayed prosthesis. Clin Orthop Relat Res. 1996;333:217–225. doi: 10.1097/00003086-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Jasty M, Henshaw RM, O’Connor DO, Harris WH. High assembly strains and femoral fractures produced during insertion of uncemented femoral components: a cadaver study. J Arthroplasty. 1993;8:479–487. doi: 10.1016/S0883-5403(06)80213-5. [DOI] [PubMed] [Google Scholar]
- 22.Johnston RC, Fitzgerald RH, Jr, Harris WH, Poss R, Muller ME, Sledge CB. Clinical and radiographic evaluation of total hip replacement: a standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–168. [PubMed] [Google Scholar]
- 23.Karrholm J. Roentgen stereophotogrammetry: review of orthopedic applications. Acta Orthop Scand. 1989;60:491–503. doi: 10.3109/17453678909149328. [DOI] [PubMed] [Google Scholar]
- 24.Keisu KS, Orozco F, Sharkey PF, Hozack WJ, Rothman RH, McGuigan FX. Primary cementless total hip arthroplasty in octogenarians: two to eleven-year follow-up. J Bone Joint Surg Am. 2001;83:359–363. doi: 10.1302/0301-620X.83B3.11006. [DOI] [PubMed] [Google Scholar]
- 25.Kelly SJ, Robbins CE, Bierbaum BE, Bono JV, Ward DM. Use of a hydroxyapatite-coated stem in patients with Dorr Type C femoral bone. Clin Orthop Relat Res. 2007;465:112–116. doi: 10.1097/BLO.0b013e318156bf96. [DOI] [PubMed] [Google Scholar]
- 26.Khalily C, Lester DK. Results of a tapered cementless femoral stem implanted in varus. J Arthroplasty. 2002;17:463–466. doi: 10.1054/arth.2002.32171. [DOI] [PubMed] [Google Scholar]
- 27.Kirsh G, Roffman M, Kligman M. Hydroxyapatite-coated total hip replacements in patients 65 years of age and over. Bull Hosp Jt Dis. 2001;60:5–9. [PubMed] [Google Scholar]
- 28.Konstantoulakis C, Anastopoulos G, Papaeliou A, Tsoutsanis A, Asimakopoulos A. Uncemented total hip arthroplasty in the elderly. Int Orthop. 1999;23:334–336. doi: 10.1007/s002640050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 30.Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468:1310–1315. doi: 10.1007/s11999-009-0937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le D, Smith K, Tanzer D, Tanzer M. Modular femoral sleeve and stem implant provides long-term total hip survivorship. Clin Orthop Relat Res. 2011;469:508–513. doi: 10.1007/s11999-010-1524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meding JB, Galley MR, Ritter MA. High survival of uncemented proximally porous-coated titanium alloy femoral stems in osteoporotic bone. Clin Orthop Relat Res. 2010;468:441–447. doi: 10.1007/s11999-009-1035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meding JB, Keating EM, Ritter MA, Faris PM, Berend ME. Minimum ten-year follow-up of a straight-stemmed, plasma-sprayed, titanium-alloy, uncemented femoral component in primary total hip arthroplasty. J Bone Joint Surg Am. 2004;86:92–97. doi: 10.2106/00004623-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Min BW, Song KS, Bae KC, Cho CH, Kang CH, Kim SY. The effect of stem alignment on results of total hip arthroplasty with a cementless tapered-wedge femoral component. J Arthroplasty. 2008;23:418–423. doi: 10.1016/j.arth.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Morrey BF. Short-stemmed uncemented femoral component for primary hip arthroplasty. Clin Orthop Relat Res. 1989;249:169–175. [PubMed] [Google Scholar]
- 36.Morrey BF. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991. [Google Scholar]
- 37.Moyer JA, Metz CM, Callaghan JJ, Hennessy DW, Liu SS. Durability of second-generation extensively porous-coated stems in patients age 50 and younger. Clin Orthop Relat Res. 2010;468:448–453. doi: 10.1007/s11999-009-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muirhead-Allwood SK, Sandiford N, Skinner JA, Hua J, Kabir C, Walker PS. Uncemented custom computer-assisted design and manufacture of hydroxyapatite-coated femoral components: survival at 10 to 17 years. J Bone Joint Surg Br. 2010;92:1079–1084. doi: 10.1302/0301-620X.92B8.23123. [DOI] [PubMed] [Google Scholar]
- 39.Mulliken BD, Bourne RB, Rorabeck CH, Nayak N. A tapered titanium femoral stem inserted without cement in a total hip arthroplasty: radiographic evaluation and stability. J Bone Joint Surg Am. 1996;78:1214–1225. doi: 10.2106/00004623-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS. The anatomic basis of femoral component design. Clin Orthop Relat Res. 1988;235:148–165. [PubMed] [Google Scholar]
- 41.Otani T, Whiteside LA. Failure of cementless fixation of the femoral component in total hip arthroplasty. Orthop Clin North Am. 1992;23:335–346. [PubMed] [Google Scholar]
- 42.Parvizi J, Keisu KS, Hozack WJ, Sharkey PF, Rothman RH. Primary total hip arthroplasty with an uncemented femoral component: a long-term study of the Taperloc stem. J Arthroplasty. 2004;19:151–156. doi: 10.1016/j.arth.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Sah AP, Thornhill TS, Leboff MS, Glowacki J. Correlation of plain radiographic indices of the hip with quantitative bone mineral density. Osteoporos Int. 2007;18:1119–1126. doi: 10.1007/s00198-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santori FS, Santori N. Mid-term results of a custom-made short proximal loading femoral component. J Bone Joint Surg Br. 2010;92:1231–1237. doi: 10.1302/0301-620X.92B9.24605. [DOI] [PubMed] [Google Scholar]
- 45.Santori N, Albanese CV, Learmonth ID, Santori FS. Bone preservation with a conservative metaphyseal loading implant. Hip Int. 2006;16(Suppl 3):16–21. doi: 10.1177/112070000601603S04. [DOI] [PubMed] [Google Scholar]
- 46.Stulberg SD, Dolan M. The short stem: a thinking man’s alternative to surface replacement. Orthopedics. 2008;31:885–886. doi: 10.3928/01477447-20080901-37. [DOI] [PubMed] [Google Scholar]