Abstract
Background
Currently more than 200,000 THAs are performed annually in the United States. In patients with bilateral disease, the chance of subsequent contralateral THA reportedly ranges from 16% to 85%. Factors influencing contralateral THA are not completely understood.
Questions/Purposes
We therefore determined (1) the 10-year probability free of progression to contralateral THA after index THA, (2) whether demographics differed between those who did and did not ultimately undergo contralateral THA, and (3) whether initial clinical symptoms and/or degree of radiographic osteoarthritis affects progression.
Patients and Methods
We retrospectively identified 332 patients with minimum 24-month followup and primary osteoarthritis who underwent unilateral THA between 2001 and 2008. There were 150 men and 182 women with a mean age of 61 years (range, 27–93 years) and a mean BMI of 29.6 kg/m2 (range, 17.6–49.2 kg/m2). We reviewed clinical, radiographic, and demographic data at index THA and last followup and classified patients as low, indeterminate, or high risk of undergoing contralateral THA.
Results
Seventy-four of the 332 patients (22%) underwent contralateral THA, resulting in an 83% 10-year probability free of progression to the contralateral hip. Low-risk patients had a less than 1% chance of progression, indeterminate-risk patients had a 16% to 24% chance of progression, and high-risk patients had a 97% chance of progression.
Conclusions
Indeterminate-risk patients may be managed nonoperatively and deserve further study with a larger multicenter analysis. We defined high- and low-risk patients who may be candidates for bilateral THA or may rarely need a contralateral THA.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The prevalence of symptomatic hip osteoarthritis (OA) is estimated to be 4.4% of adults older than 55 years [17], and there are more than 70 million people in the United States older than 55 years [23]. Given the large number of patients affected by this disease, the number of arthroplasties per year is expected to increase. Currently surgeons are performing more than 200,000 THAs each year in the United States [15, 24]. Over the next 20 years, this number is projected to increase to more than 500,000 [24]. Ten- to 25-year studies show prosthesis survivorship ranging from 85% to 96% [4, 6, 8, 9].
For all patients with hip OA, the incidence of those with bilateral end-stage disease ultimately having subsequent THA has ranged from 16% to 35% in the literature [14, 17, 21, 24]. In patients with bilateral disease, the chance of subsequent contralateral THA after unilateral THA is anywhere from 16% to 85% [10, 12, 19–22]. Factors related to subsequent joint arthroplasty in the contralateral joint are described as the radiographic grade of the joint [10, 21] and patient age [17, 21] at index arthroplasty. Although there is a study regarding the degree of radiographic OA and patient age [21], it does not evaluate symptoms in the contralateral hip and correlate this with radiographic grading in the contralateral hip at index THA.
To characterize the progression of contralateral hip disease after unilateral THA for osteoarthritis, we determined (1) the 10-year probability free of progression to contralateral THA after index unilateral THA, (2) whether there was a demographic difference between those who did and did not ultimately undergo contralateral THA, (3) whether clinical symptom severity in the contralateral hip at the time of index THA affects the progression of contralateral hip disease and leads to subsequent THA, (4) whether the degree of radiographic OA in the contralateral hip at the time of index THA affects the progression of contralateral hip disease and leads to subsequent THA, and (5) whether the combined radiographic and clinical degree of OA predicts the progression of contralateral hip disease.
Patients and Materials
We retrospectively identified 2008 patients who had 2008 hip arthroplasties performed between 2001 and 2008. From these, we excluded 1576 patients (1576 arthroplasties) for one of the following reasons: resurfacing arthroplasties (n = 1191); bilateral THAs (defined as having the second THA within 3 months of the first; n = 34); THAs that ultimately had revision (n = 9); and arthroplasties performed for diagnoses of osteonecrosis (n = 187), slipped capital femoral epiphysis (n = 27), developmental dysplasia of the hip (n = 24), prior septic arthritis (n = 8), posttraumatic arthritis (n = 39), synovial chondromatosis (n = 3), genetic inheritable hip disorders (eg, Marfan syndrome, osteopetrosis, etc) (n = 16), inflammatory arthritides (eg, rheumatoid arthritis, systemic lupus erythematosus, etc) (n = 21), and Legg-Calvé-Perthes disease (n = 17). We additionally excluded another 43 hips because, after review of the radiographs, we observed evidence of prior slipped capital femoral epiphysis, developmental dysplasia of the hip, or Legg-Calvé-Perthes disease that was not clinically diagnosed. Of the remaining 389 patients (389 THAs), 24 died and five were lost to followup before the minimum 2 years. Twenty-eight patients did not have the appropriate radiographs for analysis (lack of an AP pelvis radiograph at index arthroplasty). This left 332 patients who had a minimum 2-year followup (mean, 67 months; range, 24–115 months) and radiographs available for review at both index THA and at latest clinical followup. There were 150 men and 182 women with a mean age of 61 years (range, 27–93 years) and a mean BMI of 29.6 kg/m2 (range, 17.6–49.2 kg/m2). There were 172 right hips and 160 left hips. We did not recall any patients for this study and obtained all data from medical records and radiographs. We received institutional review board approval for the study of these patients.
All operations were performed by a single surgeon (MAM) through an anterolateral approach.
After index THA, patients returned to the clinic at 1, 3, and 6 months and yearly thereafter. At each visit, we performed a history and physical examination, as well as a radiographic examination consisting of weightbearing AP pelvic radiographs. We evaluated both hips for patient-reported symptoms (pain, decreased functionality, use of a walking aid, or prevention of performing activities of daily living directly related to the hip) and observed signs (flexion-extension, internal-external rotation, flexion contractures, weakness, or any gait abnormalities). We contacted each of the 332 patients via telephone to confirm they had not undergone a second procedure at another facility. The following demographic data were collected: age, sex, time from index THA to contralateral THA, and BMI. We evaluated the preoperative and postoperative pain level in the index hip and any resultant ambulatory limitations. Pain levels were scored on a scale of 1 to 10 points, based on clinical notes and patient questionnaires using a 10-cm VAS. Clinical severity in the contralateral hip was categorized into four categories: asymptomatic, mild, moderate, or severe. We defined asymptomatic as having no complaints with no effect on activities of daily living; mild as some pain (< 5 points on the VAS) with no limitation of activities of daily living and unlimited ambulation; moderate when two of three conditions were met (pain > 5 on the VAS, the use of an ambulatory assistance device, or any ambulatory limitation and decreased ability to perform activities of daily living); and severe when all three of the previous conditions were met. We recorded ambulatory limitation in number of blocks or as unlimited, based on patient report, as recorded in clinical notes and patient questionnaires (eg, Harris hip scores). Of the 332 patients, 153 (46%) had no symptoms, 99 (30%) had mild symptoms, 51 (15%) had moderate symptoms, and 29 (9%) had severe symptoms.
Two authors (AJJ, DEJ) independently reviewed standard weightbearing preoperative AP pelvis radiographs before the index procedure and at last followup, with a third author (SAS) adjudicating any discrepancies between reads of the other two authors. They used the Kellgren-Lawrence grading scale [14] to classify hip OA in the contralateral hip of each patient at index THA and at last followup. Kellgren-Lawrence Grade 0 was the absence of radiographic findings, Grade 1 was minimal joint space narrowing, Grade 2 was some osteophytes with some joint space narrowing, Grade 3 was multiple osteophytes with joint space narrowing accompanied by bony sclerosis, and Grade 4 was large osteophytes with marked joint space narrowing and severe bony sclerosis accompanied by deformity [14]. Radiographic characterization of the patient cohort revealed 25 (8%) had Grade 0, 87 (26%) had Grade 1, 113 (34%) had Grade 2, 30 (9%) had Grade 3, and 77 (23%) had Grade 4. For the combined clinical and radiographic analysis, we grouped Kellgren-Lawrence Grades 0 to 2 together as mild radiographic disease and Grades 3 and 4 together for advanced radiographic disease. The reviewers were blinded as to the side of index THA and the subsequent surgery that patients underwent.
We placed each contralateral hip into one of 20 categories based on initial clinical symptoms (four categories) and radiographic arthritis (five categories) (Table 1). We analyzed the hips to determine the percentage of patients in each grouping who later underwent contralateral THA and then condensed this information into four categories based on clinical symptoms (two categories) and radiographic arthritis (two categories).
Table 1.
Correlation of radiographic grade and clinical symptoms*
| Kellgren-Lawrence grade | Asymptomatic | Mild | Moderate | Severe |
|---|---|---|---|---|
| 0 | 0/25 | 0 | 0 | 0 |
| 1 | 0/61 | 0/25 | 1/1 | 0 |
| 2 | 0/57 | 2/40 | 3/16 | 0 |
| 3 | 0/10 | 5/18 | 2/2 | 0 |
| 4 | 0 | 2/16 | 30/32 | 29/29 |
* Each cell represents the number of hips that progressed to contralateral THA over the total number of hips in that category.
We then classified patients as low, indeterminate, or high risk for performance of a contralateral THA after index THA based on the above. We considered patients low risk if they had radiographic Grade 0 to 2 changes and were clinically asymptomatic or mild, indeterminate risk if they had radiographic Grade 0 to 2 changes with clinical severity of moderate to severe or had radiographic Grade 3 to 4 changes and clinical severity of asymptomatic or mild, and high risk if they had Grade 3 and 4 radiographic changes and clinically displayed moderate or severe symptoms.
We calculated 95% CIs for all data and used Kaplan-Meier survivorship analysis [13] to evaluate time from index THA to progression of contralateral THA. The Kaplan-Meier survivorship curve was the percentage of patients free of progression to contralateral THA. To determine whether there were any demographic effects on progression of contralateral disease, we used a multivariate Cox proportional-hazards model to determine the effect of age, sex, and BMI on survival of the contralateral THA. To evaluate the effect of clinical symptom severity and radiographic severity on the progression of disease in the contralateral hip, we calculated the incidence of contralateral THA for each cohort, as described above. Finally, clinical and radiographic severity of disease were combined from the 20 different permutations (Table 1) into low-, indeterminate-, and high-risk cohorts (Table 2) to determine the combined effect on the ultimate outcome of the contralateral hip. SPSS® Version 13.0 for Windows® (SPSS, Inc, Chicago, IL, USA) was used for all statistical analyses.
Table 2.
Patient risk categories
| Kellgren-Lawrence grade | Clinical severity | |
|---|---|---|
| Asymptomatic and mild | Moderate and severe | |
| 0–2 | Low risk | Medium risk |
| 2/208 (< 1%) | 4/17 (24%) | |
| 3–4 | Medium risk | High risk |
| 7/44 (16%) | 61/63 (97%) | |
Results
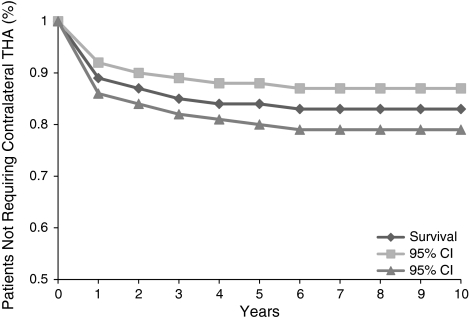
Of the 332 patients, 74 (22%) eventually underwent contralateral THA, resulting in a 10-year probability free of progression to contralateral hip of 83% (95% CI: 79%–87%) (Fig. 1). The mean time from index THA to contralateral THA was 20 months (range, 7–75 months). Time interval to the second operation influenced progression of the contralateral hip, while age, sex, and BMI did not (Table 3). Patients not operated on between 3 months and 1 year had a longer time interval (p < 0.001) to contralateral THA (mean, 34 months; range, 26–75 months).
Fig. 1.
A graph shows a Kaplan-Meier survivorship curve representing an 83% 10-year probability free from incidence of contralateral THA for the overall patient population
Table 3.
Demographics of patients stratified by whether they progressed to contralateral THA and by risk level
| Demographic | Those who progressed to contralateral THA | Those who did not progress to contralateral THA | p Value | Hazard ratio (95% CI) | Low risk | Indeterminate risk | High risk |
|---|---|---|---|---|---|---|---|
| Number of patients | 74 | 258 | 208 | 61 | 63 | ||
| Age (years)* | 59 (38–87) | 62 (27–93) | 0.07 | 1.4 (1.0–1.6) | 61 (30–91) | 58 (36–84) | 57 (26–88) |
| Sex (% women) | 58 | 54 | 0.59 | 1.1 (0.9–1.3) | 54 | 49 | 65 |
| BMI (kg/m2)* | 30 (20–47) | 30 (18–53) | 1.0 | 1.3 (1.0–1.5) | 30 (18–52) | 29 (20–48) | 30 (20–49) |
* Values are expressed as mean, with range in parentheses.
Patients who had a higher degree of clinical symptoms in the contralateral hip at the time of index THA were more likely to progress to subsequent THA. Of the 258 patients who did not undergo contralateral THA, 153 (59%) were completely asymptomatic, 90 (35%) had mild symptoms, and 15 (6%) had moderate symptoms. For the entire patient cohort, patients who were asymptomatic never developed symptoms requiring contralateral THA. Of the patients with clinically mild symptoms, nine (8%) later underwent contralateral THA. In the moderately and severely symptomatic groups, 36 (71%) and 29 (100%) eventually underwent contralateral THA.
Patients who had a higher degree of radiographic symptoms in the contralateral hip at the time of index THA were more likely to progress to subsequent THA. Of the 258 patients who did not have contralateral THA, 25 (10%) had Grade 0 radiographic changes, 86 (33%) had Grade 1 radiographic changes, 108 (42%) had Grade 2 radiographic changes, 23 (9%) had Grade 3 radiographic changes, and 16 (6%) had Grade 4 radiographic changes. For the entire patient cohort, patients with Grade 0 radiographic changes never progressed to the point where they had a contralateral THA. Of the patients with Grade 1 and 2 changes, one (1%) and five (4%), respectively, subsequently had a contralateral THA. Of the patients with Grade 3 and 4 changes, seven (23%) and 61 (79%), respectively, eventually underwent contralateral THA.
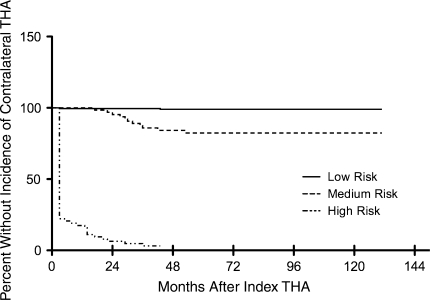
Patients who fell into the combined high-risk clinical and radiographic category had a higher risk for progression of the contralateral hip. Patients who fell into the low-risk category had a less than 1% incidence of subsequently having contralateral THA, while patients who fell into the high-risk category had a 97% chance of progressing to subsequent contralateral THA (Table 2). The 10-year probabilities free of progression to contralateral THA were 99% (95% CI: 97%–100%) and 82% (95% CI: 72%–91%) for the low- and medium-risk cohorts, respectively, whereas the 3-year probability free of progression to contralateral THA was only 3% (95% CI: 0% –7%) for the high-risk group (Fig. 2).
Fig. 2.
A graph shows Kaplan-Meier survivorship curves stratified by risk categories. The 10-year probabilities free from incidence of contralateral THA are 99% (95% CI: 97%–100%) for the low-risk cohort and 82% (95% CI: 72%–91%) for the medium-risk cohort. The 3-year probability free from incidence of contralateral THA in the high-risk group is 3% (95% CI: 0%–7%).
Discussion
In the United States, OA in the hip affects more than 3 million adults who are older than 55 years [16, 23]. This debilitating disease affects patients’ quality of life and overall activity level, leaving some homebound and others in wheelchairs. THA has become the gold standard for treating end-stage disease that does not improve with nonsurgical treatments. The number of THAs performed in the United States is projected to increase by more than 300,000 primary THAs over the next 20 years [15]. Since the disease affects a great number of adults in the United States, it is important to understand and characterize the risk factors leading to disease progression. Therefore, we examined the probability of having a contralateral THA after primary unilateral THA for OA and the clinical, radiographic, and demographic factors that might lead to progression of OA for which subsequent contralateral THA would be performed.
There were several limitations to our study. First, there was the potential for a patient to have had their index THA performed at our institution and then have a subsequent contralateral THA performed at another institution without our knowledge. To minimize its effect on our data, we contacted each patient to confirm they did not have a contralateral THA performed at another institution. Second, with regard to radiographic findings, Hart and Spector [11] criticized the Kellgren-Lawrence grading scale because of its subjective nature and its overemphasis on osteophytes. However, the Kellgren-Lawrence radiographic criteria are generally accepted as the standard for radiographically evaluating OA [2, 5, 7], and the World Health Organization and the American Rheumatism Association accept this grading scale for the classification of OA [2]. Kellgren and Lawrence [14] emphasized effective control of the intraobserver and interobserver error rate requires one observer. Although we had three authors read radiographs, there was a single author (SAS) who evaluated films when there were discrepancies between the other two observers (AJJ, DEJ). Third, we excluded patients who underwent THA on their contralateral hip within 3 months of index THA. These patients had sufficiently severe symptoms in both hips at their presentation to undergo THA, and we believed there was no need to include them.
Our observations supported previous studies evaluating the history and disease progression of hip OA. In one study evaluating the probability of contralateral THAs in 99 patients with a mean followup of 104 months (range, 12–149 months), after excluding patients undergoing contralateral THA within 12 months of index THA, the risk of contralateral THA was reportedly 21% [10].
A previous study showed clinical symptoms correlated well with contralateral disease progression and subsequent arthroplasty [17]. We confirmed patients with minimal symptoms were less likely to progress to contralateral THA than those with severe symptoms. However, more information was needed on patients with moderate symptoms in the contralateral hip, as some of these patients progressed to arthroplasty, while others did not. Further studies should work to address these indeterminate-risk patients. If this cohort of patients can be better characterized, it might be possible to focus nonoperative treatments on those who are more likely to progress to contralateral THA in an attempt to halt or slow the progression of the disease in the contralateral hip.
Ritter et al. [19] found patients with no radiographic signs of OA in the contralateral hip at the time of index THA had an 8.7% chance of progression to contralateral hip within 10 years. We also found patients with Grade 0 to 2 radiographic changes had rates of progression to the contralateral hip of 0% to 4%. One important difference between our study and previous studies was our correlation of radiographic grading and clinical symptoms (Table 3). These two criteria were key factors for predicting progression to contralateral THA [18]. It was not surprising there was an increased incidence in the subsequent contralateral THA in patients with Grade 3 or 4 radiographic changes who clinically displayed moderate to severe symptoms (high-risk patients).
Mont et al. [17] previously combined clinical symptom severity and radiographic stage of disease to assess the likelihood of subsequent contralateral TKA after index TKA. Using similar methods, they found, when combining clinical and radiographic criteria of severity, the majority of patients were low-risk patients who had an extremely low likelihood of progressing to subsequent contralateral TKA. This result should increase the confidence of the treating surgeon when reassuring the patient that they will not need a second procedure on the other hip. The high-risk patients who had a high incidence of contralateral arthroplasty had end-stage radiographic disease and severe symptoms at initial presentation. In both studies, there was a proportion of patients (18% in the current study) who had indeterminate risk for progression, for whom it was difficult to predict subsequent contralateral THA. This cohort included a large proportion of younger patients and a higher percentage of men than the other cohorts. We did not factor into our study the effect of activity on progression of contralateral hip. Perhaps, in future studies, this indeterminate-risk cohort could be examined to determine whether perhaps a younger, more active subset of the population might be at risk for progression of disease. The likelihood that high-risk patients would undergo contralateral THA was 97%. For these patients, simultaneous bilateral THAs may offer decreased complication rates [1, 3, 18] and be more cost-effective [18]. Parvizi et al. [18] reported on 98 patients who received simultaneous bilateral THAs versus 98 patients who received staged bilateral THAs, with a minimum 6-month followup of perioperative complications. They found decreased complication rate, anemia, and wound drainage in the simultaneous bilateral THA cohort compared to the staged bilateral THA cohort. Also, the mean hospital stay was shorter in the simultaneous bilateral THA cohort (mean, 4.3 days; range, 3–11 days) than in the staged bilateral THA cohort (mean, 8.1 days; range, 4–39 days). To determine the appropriateness for a given patient, surgeons must consider symptoms and general health, the risk of anesthesia, and medical problems, such as obesity, diabetes, and compromised cardiovascular or respiratory status.
In summary, we reported an overall 10-year probability free of progression to contralateral THA after index THA for OA of 83%. Although little can be done to reverse the effects of the disease in the high-risk cohort, we emphasize indeterminate-risk patients warrant further study since these patients may respond to nonoperative treatment in an effort to delay THA and preserve the native joint. As the numbers of patients in this indeterminate-risk category are low, a larger multicenter study may be warranted. However, a majority of patients (64%) fell into the low-risk category, indicating, if there is minimal radiographic evidence and clinical symptom severity at initial presentation, there is an extremely low incidence of contralateral THA.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. MAM has received royalties from Stryker Orthopaedics (Mahwah, NJ, USA); is a consultant for Johnson & Johnson (New Brunswick, NJ, USA), Joint Active Systems Inc (Effingham, IL, USA), Salient Surgical Technologies Inc (Portsmouth, NH, USA), Stryker, and TissueGene, Inc (Rockville, MD, USA); and has received institutional support from National Institutes of Health (NIAMS and NICHD), Stryker, TissueGene, and Wright Medical Technology, Inc (Arlington, TN, USA). The other authors have no disclosures.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aghayev E, Beck A, Staub LP, Dietrich D, Melloh M, Orljanski W, Roder C. Simultaneous bilateral hip replacement reveals superior outcome and fewer complications than two-stage procedures: a prospective study including 1819 patients and 5801 follow-ups from a total joint replacement registry. BMC Musculoskelet Disord. 2010;11:245. doi: 10.1186/1471-2474-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 3.Bhan S, Pankaj A, Malhotra R. One- or two-stage bilateral total hip arthroplasty: a prospective, randomised, controlled study in an Asian population. J Bone Joint Surg Br. 2006;88:298–303. doi: 10.1302/0301-620X.88B3.17048. [DOI] [PubMed] [Google Scholar]
- 4.Birtwistle SJ, Wilson K, Porter ML. Long-term survival analysis of total hip replacement. Ann R Coll Surg Engl. 1996;78:180–183. [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34:1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JJ, Albright JC, Goetz DD, Olejniczak JP, Johnston RC. Charnley total hip arthroplasty with cement: minimum twenty-five-year follow-up. J Bone Joint Surg Am. 2000;82:487–497. doi: 10.2106/00004623-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Cushnaghan J, Kirwan JR, Dieppe PA, Rogers J, McAlindon T, McCrae F. Radiographic assessment of the knee joint in osteoarthritis. Ann Rheum Dis. 1992;51:80–82. doi: 10.1136/ard.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery D, Britton A, Clarke H, Grover M. The Stanmore total hip arthroplasty: a 15- to 20-year follow-up study. J Arthroplasty. 1997;12:728–735. doi: 10.1016/S0883-5403(97)90001-2. [DOI] [PubMed] [Google Scholar]
- 9.Garellick G, Malchau H, Herberts P. Survival of hip replacements: a comparison of a randomized trial and a registry. Clin Orthop Relat Res. 2000;375:157–167. doi: 10.1097/00003086-200006000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip, longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43:988–994. doi: 10.1002/1529-0131(200005)43:5<988::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Hart DJ, Spector TD. Radiographic criteria for epidemiologic studies of osteoarthritis. J Rheumatol Suppl. 1995;43:46–48. [PubMed] [Google Scholar]
- 12.Husted H, Overgaard S, Laursen JO, Hindso K, Hansen LN, Knudsen HM, Mossing NB. Need for bilateral arthroplasty for coxarthrosis: 1, 477 replacements in 1, 199 patients followed for 0–14 years. Acta Orthop Scand. 1996;67:421–423. doi: 10.3109/17453679608996660. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mont MA, Mitzner DL, Jones LC, Hungerford DS. History of the contralateral knee after primary knee arthroplasty for osteoarthritis. Clin Orthop Relat Res. 1995;321:145–150. [PubMed] [Google Scholar]
- 18.Parvizi J, Tarity TD, Sheikh E, Sharkey PF, Hozack WJ, Rothman RH. Bilateral total hip arthroplasty: one-stage versus two-stage procedures. Clin Orthop Relat Res. 2006;453:137–141. doi: 10.1097/01.blo.0000246529.14135.2b. [DOI] [PubMed] [Google Scholar]
- 19.Ritter MA, Carr K, Herbst SA, Eizember LE, Keating EM, Faris PM, Meding JB. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11:242–246. doi: 10.1016/S0883-5403(96)80073-8. [DOI] [PubMed] [Google Scholar]
- 20.Roos NP, Lyttle D. Hip arthroplasty surgery in Manitoba: 1973–1978. Clin Orthop Relat Res. 1985;199:248–255. [PubMed] [Google Scholar]
- 21.Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38:E141–E143. [PubMed] [Google Scholar]
- 22.Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end-stage osteoarthritis of the lower limbs. Arthritis Rheum. 2002;46:3185–3189. doi: 10.1002/art.10649. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau. S0101. Age and Sex: 2006–2008 American Community Survey 3-Year Estimates. Available at: http://factfinder.census.gov/servlet/STTable?_bm=y&-geo_id=01000US&-qr_name=ACS_2008_3YR_G00_S0101&-ds_name=ACS_2008_3YR_G00_. Accessed December 31, 2010.
- 24.US Department of Health and Human Services, National Center for Health Statistics. National Hospital Discharge Survey, 2003. Available at: http://www.cdc.gov/nchs/data/series/sr_13/sr13_160.pdf. Accessed December 31, 2010.