Abstract
Background
While short-stem design is not a new concept, interest has surged with increasing utilization of less invasive techniques. Short stems are easier to insert through small incisions. Reliable long-term results including functional improvement, pain relief, and implant survival have been reported with standard tapered stems, but will a short taper perform as well?
Questions/purposes
We compared short, flat-wedge, tapered, broach-only femoral stems to standard-length, double-tapered, ream and broach femoral stems in terms of intraoperative complications, short-term survivorship, and pain and function scores.
Patients and Methods
We retrospectively reviewed the records of 606 patients who had 658 THAs using a less invasive direct lateral approach from January 2006 to March 2008. Three hundred sixty patients (389 hips) had standard-length stems and 246 (269 hips) had short stems. Age averaged 63 years, and body mass index averaged 30.7 kg/m2. We recorded complications and pain and function scores and computed short-term survival. Minimum followup was 0.8 months (mean, 29.2 months; range, 0.8–62.2 months).
Results
We observed a higher rate of intraoperative complications with the standard-length stems (3.1%; three trochanteric avulsions, nine femoral fractures) compared with the shorter stems (0.4%; one femoral fracture) and managed all complications with application of one or more cerclage cables. There were no differences in implant survival, Harris hip score, and Lower Extremity Activity Scale score between groups.
Conclusions
Fewer intraoperative complications occurred with the short stems, attesting to the easier insertion of these devices. While longer followup is required, our early results suggest shortened stems can be used with low complication rates and do not compromise the survival and functional outcome of cementless THA.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Although THA is continually noted as one of the most successful procedures performed by orthopaedic surgeons worldwide, current research and development continue to strive to improve on the biologic and mechanical designs of modern total hip prostheses. A multitude of published studies report overall survivorship of several standard-length tapered femoral components ranging from 94% to 100% at up to 20 years’ followup [10–12, 17–20, 25, 27–29, 33, 35, 42, 43, 47, 52–55, 58–60]. We recently reported femoral implant survival rates of 98.6% at 5 years, 98.4% at 10 years, 97.1% at 15 years, and 95.5% at 20 years in 2000 standard-length tapered titanium porous plasma-sprayed femoral components (Mallory-Head® Porous [MHP]; Biomet, Inc, Warsaw, IN, USA) [43]. Cementless femoral fixation with tapered-geometry designs has evolved over the past several decades with a recent surge of interest in shorter stems.
Short stems are thought to preserve more native host bone and optimize proximal load transfer, and while not a novel concept, they have become increasingly utilized with the advent of less invasive surgery and rapid-recovery protocols [15, 24, 40, 56, 62, 66]. There are several proposed advantages of short stems, including easier insertion through smaller incisions and less invasive techniques, simpler femoral preparation with a “broach only” system, and their basic inherent bone-conserving nature allowing for more favorable conditions in the potential revision setting. Several studies have demonstrated overall survivorship of short stems ranging from 94% to 100% at up to 18 years’ followup and incidences of thigh pain ranging from 0% to 4% [13, 15, 28, 33, 52, 54]. To justify using shorter tapered stems when more traditional standard-length tapered stems provide pain relief, restore function, and have high survivorship [9–11, 17–19, 25, 42, 43, 58, 60], it is important to show advantages with equal long-term survivorship and demonstrate similar intraoperative reproducibility with equivalent and/or decreased perioperative complications.
We therefore compared short, flat-wedge, tapered, broach-only femoral stems to standard-length, double-tapered, ream and broach femoral stems in terms of intraoperative and perioperative complications, short-term survivorship, and pain and function scores.
Patients and Materials
Institutional review board approved informed consent for historical prospective clinical study was obtained from all patients undergoing THA. We performed a retrospective analysis from a query of our electronic medical record system (DocuMed, Inc, Ann Arbor, MI), revealing 879 consecutive patients (953 hips) who underwent primary THA from January 2006 through March 2008 by two surgeons (AVL, KRB). All patients were managed with a rapid-recovery protocol [8, 41]. The majority of cases (70%; 671 hips) were performed via a less invasive direct lateral approach (LIDL) [6], while a standard direct lateral approach was used in 10% (100 hips), and one surgeon (KRB) beginning in February 2007 used an anterior supine intermuscular approach in 182 hips (19%). To eliminate the possible confounding variable of different surgical approaches as an influence on complication incidence and functional outcome, only cases performed via the LIDL approach were included. Thirteen LIDL cases were excluded in which other femoral stem types were implanted: four cemented stems, six hydroxyapatite-coated stems, two modular stems with a porous proximal sleeve, and one device which was part of a US Food and Drug Administration (FDA) investigational feasibility study. Therefore, our cohort was 606 patients (658 hips) who underwent primary THA using tapered, titanium, porous plasma-sprayed femoral components inserted via a single LIDL approach (Table 1). There were a total of 51 staged bilateral procedures and one simultaneous bilateral procedure. In terms of operative side, 357 (54%) were right hips and 301 (46%) were left hips. A standard-length taper, the MHP femoral component, was implanted in 389 hips (59%), with 110 (28%) being a lateralized offset option. The MHP was introduced and has been used in our practice since 1984. The TaperLoc® stem (Biomet) was introduced in 1982, and a shortened version, the TaperLoc® Microplasty™ femoral component received FDA approval in 2005 and was introduced into clinical use in our practice in January 2006. The lateralized option became available in May 2007. The short stem was implanted in 269 hips (41%), with 173 (64%) being a lateralized offset option. The stem type for patients during the study period was chosen by the surgeons in a nonsystematic manner consistent with the typical process of trialing and implementing any newly available approved device into clinical use. The two groups were similar in age, body mass index, sex, and underlying diagnosis including rheumatoid arthritis. One surgeon (AVL) performed a higher proportion of arthroplasties within the short-stem group, because while the other surgeon (KRB) continued to use the short stem, he began to use the anterior supine intermuscular approach. More ceramic heads and fewer metal-on-metal bearings were used in the short stem group as ceramic heads became available in larger sizes and awareness increased regarding adverse reactions to metal-on-metal. More lateralized option stems were required in the short-stem group because the standard offset neck shaft angle of that device is more valgus (138° versus 136.5°) than the standard-length taper standard offset neck. The minimum followup for the standard-length group was 0.8 months (average, 30.9 months; range, 0.8–61.2 months) and the minimum followup for the short-stem group was 0.9 months (average, 26.9 months; range, 0.9–62.2 months). Twenty-six patients (28 hips) died within the study period at an average of 22 months postoperatively, with none related to the arthroplasty procedure and no THA failures. Two of the patients who died had not returned for a postoperative evaluation. Of the surviving patients, 74% (469) had minimum 2-year followup. Four living patients, with two from each group, did not return for clinical evaluation and could not be located. Clinical data from all patients were included. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Characteristics of standard-length taper and short taper stem groups
| Characteristic | Standard-length stem group | Short-stem group | p value |
|---|---|---|---|
| Patients (number) | 360 | 246 | |
| Primary hips (number) | 389 | 269 | |
| Time period | January 2006 to March 2008 | January 2006 to March 2008 | |
| Average age (years) | 63 (20–89) | 63 (27–91) | 0.532 |
| Average body mass index (kg/m2) | 31.0 (17–66) | 30.1 (19–60) | 0.101 |
| Sex | 0.130 | ||
| Male | 185 (51%) | 111 (45%) | |
| Female | 175 (49%) | 135 (55%) | |
| Preoperative diagnosis | 0.119 | ||
| Osteoarthritis | 323 (83%) | 209 (78%) | |
| Avascular necrosis | 21 (5%) | 27 (10%) | |
| Developmental dysplasia | 16 (4%) | 12 (5%) | |
| Posttraumatic arthritis | 8 (2%) | 6 (2%) | |
| Rheumatoid arthritis | 10 (3%) | 3 (1%) | |
| Legg-Calvé-Perthes disease | 7 (2%) | 1 | |
| Acute fracture | 3 (1%) | 4 (2%) | |
| Slipped capital femoral epiphysis | 1 | 3 (1%) | |
| Psoriatic arthritis | 0 | 2 (1%) | |
| Failed ORIF (degeneration) | 0 | 1 | |
| Failure resurfacing (cup migration) | 0 | 1 | |
| Surgeon | 0.000 | ||
| AVL | 250 (64%) | 215 (80%) | |
| KRB | 139 (36%) | 54 (20%) | |
| Head material | 0.027 | ||
| Cobalt-chromium | 381 (98%) | 233 (95%) | |
| BIOLOX® delta ceramic | 8 (2%) | 13 (5%) | |
| Acetabular component* | 0.023 | ||
| Magnum™ (Biomet) | 261 (67%) | 158 (59%) | |
| RingLoc® (Biomet) | 105 (18%) | 99 (37%) | |
| Regenerex® (Biomet) | 5 (1%) | 10 (4%) | |
| Trabecular Metal® (Zimmer) | 12 (3%) | 2 (1%) | |
| Trident® Tritanium® (Stryker) | 2 | 0 | |
| C2a-Taper™ (Biomet) | 4 (1%) | 0 | |
| Femoral component offset | 0.044 | ||
| Standard offset | 279 (72%) | 173 (64%) | |
| Lateralized offset | 110 (28%) | 96 (36%) | |
* Manufacturers included: Biomet, Inc, Warsaw, IN, USA; Zimmer, Inc, Warsaw, IN, USA; and Stryker Orthopaedics, Mahwah, NJ, USA; ORIF = open reduction and internal fixation; AVL = Adolph V. Lombardi, Jr; KRB = Keith R. Berend.
The MHP femoral component is a titanium collarless stem with a proximal-to-distal taper in both the coronal and sagittal planes (double taper) and three separate surface finishes (Fig. 1). The proximal 1/3 is plasma-sprayed with titanium alloy, the midsection is grit-blasted, and the distal surface is a smooth satin finish. The system requires both reaming and broaching. The design features fins proximally that engage the cortical-cancellous junction to resist rotational moments about the implant in the proximal femur. There are seven different neck length options available and a lateralized neck offset option that allows for increased abductor tension and hip stability without lengthening of the limb.
Fig. 1.

The MHP femoral component, introduced in 1984, is a double-tapered titanium stem with a proximal porous plasma-sprayed surface applied circumferentially around the stem. The middle section is grit-blasted and the distal section has a matte finish. The device is available in 14 standard offset sizes with a neck angle of 136.5°, diameters from 6 to 19 mm, and lengths from 135 to 180 mm, and 10 lateralized offset sizes with a neck angle of 131.5°, diameters from 8 to 17 mm, and lengths from 145 to 180 mm. (Reproduced with permission of Joint Implant Surgeons, Inc, New Albany, OH.)
The TaperLoc® Microplasty™ femoral component is a titanium collarless stem with a flat, tapered-wedge geometry (3° medial to lateral taper) with a proximal, titanium, porous plasma-sprayed surface (Fig. 2). This is a broach-only system. The collarless feature allows for optimal component seating and enhanced rotational stability. There are seven neck length options, and a lateralized neck offset option is available.
Fig. 2.

The TaperLoc® Microplasty™ femoral component is a tapered, titanium, porous plasma-sprayed device available with either standard or lateralized offset and in 13 sizes, with diameters ranging from 5 to 25 mm and lengths ranging from 95 to 135 mm. The neck shaft angle is 138° for both standard and lateralized offset options, with lateralization achieved by shifting the trunnion medially. (Reproduced with permission of Joint Implant Surgeons, Inc, New Albany, OH.)
We used the same surgical approach for every case in this series: the LIDL approach [6], a less invasive modification of the direct lateral approach previously described by Frndak et al. [23] (Fig. 3). We performed preoperative templating in each case to assist in determining component size (both femoral and acetabular) and the appropriate neck resection level. The LIDL approach utilized a more oblique skin incision centered over the tip of the greater trochanter and angled from anterodistally to posteroproximally. The surgeons then incised the fascia lata in line with the skin incision, with minimal undermining between the subcutaneous fat and fascia lata to minimize dead space. The assistants then simultaneously abducted and slowly externally rotated the leg as the surgeon began taking down the anterior-most insertional fibers of the gluteus medius from distal to proximal beginning at the vastus ridge. At this stage, dissection was performed with electrocautery to allow for hemostasis. The anterior aspect of the vastus lateralis was incised in line with its fibers to allow for one continuous sleeve to be elevated off of the anterior femur. Working proximally toward the anterior-proximal-most tip of the greater trochanter, the fibers of the gluteus medius were split at an angle of 45° and bluntly dissected in an anterior and proximal direction for a few centimeters, directly in line with the femoral neck. A blunt Homan-type retractor was then placed deep to the gluteus medius, exposing the anterior-most fibers of the gluteus minimus and anterior hip capsule. The capsule was incised proximally along the superior aspect of the femoral neck, possibly including a small portion of the gluteus minimus tendon. This generally corresponded to a 1 o’clock position in a right hip. Then, the femoral head was resected and standard acetabular and femoral preparation was performed with either the standard-length or short tapered instrumentation and implants. We encountered a femoral or trochanteric fracture during femoral preparation in 13 patients; in these patients, we stabilized the fracture with one or more cerclage cables or wires [9].
Fig. 3.
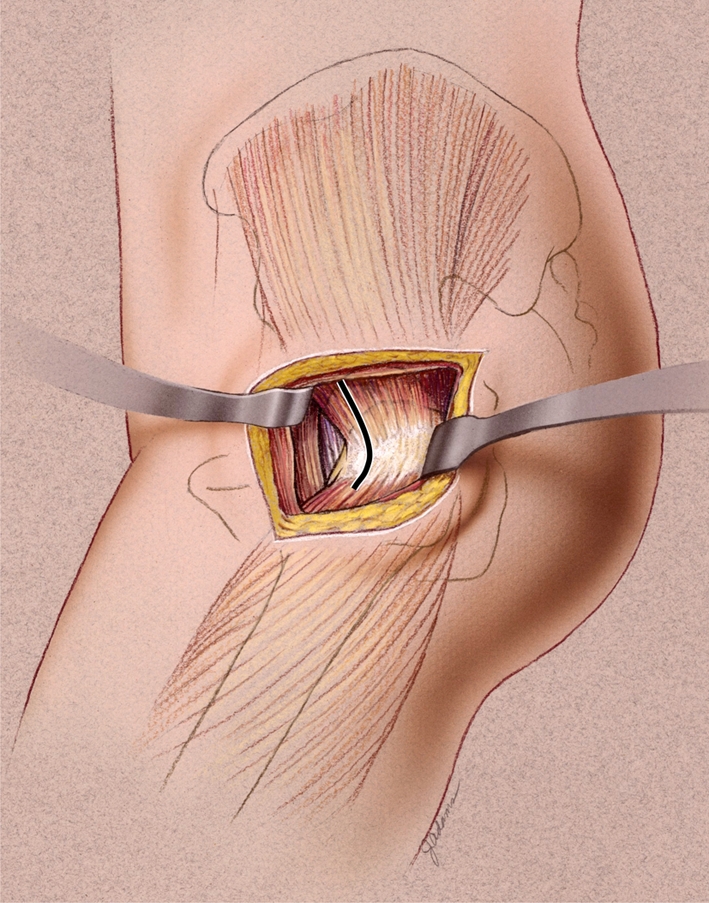
The LIDL approach to the hip is performed by elevating the gluteus medius insertion along with the capsule in one continuous soft tissue sleeve from the anterior aspect of the trochanter. The origin of the vastus lateralis is left intact and dissection proceeds anterior and medial below the vastus muscle. (Reproduced with permission of Joint Implant Surgeons, Inc, New Albany, OH.)
We allowed patients full weightbearing as tolerated with a walker or crutches immediately postoperatively and advised them to progress to a cane and eventually without any ambulatory assistance once they were pain-free and without a limp.
We saw patients at approximately 6 weeks postoperatively and yearly thereafter, unless a problem arose in which case patients were seen as soon as possible. At each clinical examination, radiographs were obtained, including an AP view of the pelvis and a lateral view of the involved hip. In addition, a standard physical examination was performed, including evaluation of the wound and strength assessment of the involved hip. Pain, deformity, ROM, and function were rated using the Harris hip score (HHS) [26] and activity level rated using the Lower Extremity Activity Scale (LEAS) of Saleh et al. [61]. We reviewed perioperative records for incidence of intraoperative complications, operative times, and estimated blood loss; and discharge and office notes for hemoglobin level at discharge, length of acute stay, discharge disposition, implant-related complications, and need for further surgery. Intraoperative records were available for all THAs. Discharge notes were available for 95%.
We used chi square analysis to compare difference in incidence of intraoperative complications between the two groups. We used a two-tailed Student’s t test to compare differences in mean HHSs, pain scores, and LEAS scores between the two groups.
Results
More intraoperative complications (fractures) occurred when the standard-length tapers (n = 12; 3.1%) were implanted compared with the short tapers (n = 1; 0.4%). The location of these fractures utilizing the Mallory classification [9, 46] were three trochanteric avulsions, seven Type I femoral fractures, and two Type III fractures within the standard-length stem group. One Type I femoral and two of the trochanteric avulsions were in patients with rheumatoid arthritis. The fracture in the short-stem group was a Type I femoral fracture in a patient with rheumatoid arthritis (Table 2). Other complications within the standard-length stem group included four acetabular revisions (three for loosening, one for metal sensitivity) (1.0%), one temporary (< 12 hours) sciatic nerve palsy (0.2%), and one chronic sciatic nerve palsy (0.2%). Other complications in the short-stem group included two acetabular revisions (0.7%), one for cup migration and one for acetabular fracture/cup protrusion. There was no difference in incidence of complications between the two surgeons.
Table 2.
Results for standard-length taper and short taper stem groups
| Parameter | Standard-length stem group | Short-stem group | p value |
|---|---|---|---|
| Intraoperative complications (number) | 12 (3.1%) | 1 (0.4%) | 0.013 |
| Avulsion of trochanter | 3 | 0 | |
| Femoral fracture Type I | 7 | 1 | |
| Femoral fracture Type II | 0 | 0 | |
| Femoral fracture Type III | 2 | 0 | |
| Average operative time (minutes) | 69.5 | 67.4 | 0.162 |
| Average estimated blood loss (mL) | 146.8 | 140.9 | 0.332 |
| Average hemoglobin at discharge (g/dL) | 11.1 | 11.0 | 0.328 |
| Average length of acute stay (days) | 2.1 | 2.0 | 0.184 |
| Discharge disposition (number) | 0.181 | ||
| Not available | 21 (5.4%) | 15 (5.6%) | |
| Home | 263 (67.6%) | 189 (70.3%) | |
| Home with home health or therapy | 14 (3.6%) | 15 (5.6%) | |
| Home with outpatient therapy | 2 (0.5%) | 4 (1.5%) | |
| Skilled nursing facility | 87 (22.4%) | 44 (16.4%) | |
| Transferred to acute care | 2 (0.5%) | 2 (0.7%) | |
| Average clinical preoperative (points) | |||
| HHS pain (0–44) | 13.8 | 13.0 | 0.077 |
| HHS total (0–100) | 50.0 | 49.9 | 0.980 |
| LEAS score (1–18) | 9.1 | 9.3 | 0.772 |
| Average clinical at 6 weeks postoperatively (points) | |||
| HHS pain (0–44) | 37.6 | 38.5 | 0.181 |
| HHS total (0–100) | 75.8 | 75.4 | 0.674 |
| LEAS score (1–18) | 7.7 | 7.8 | 0.784 |
| Average clinical at most recent followup (points) | |||
| HHS pain (0–44) | 38.5 | 38.0 | 0.496 |
| HHS total (0–100) | 83.8 | 83.1 | 0.570 |
| LEAS score (1–18) | 10.1 | 9.8 | 0.384 |
| Reoperations (number) | 5 (1.3%) | 6 (2.2%) | 0.156 |
| Incision and débridement, wound issue | 0 | 3 (1.1%) | 0.068 |
| Cup revision, loosening | 3 (0.8%) | 2 (0.7%) | 0.347 |
| Cup revision, metal sensitivity | 1 | 0 | 0.591 |
| Stem revision, fracture | 1 | 0 | 0.591 |
| Full revision, sepsis | 0 | 1 | 0.409 |
HHS = Harris hip score; LEAS = Lower Extremity Activity Scale.
We observed two stem failures requiring revision, one from each group for an overall incidence of stem failure for any reason of 0.26% in the standard-length stem group and 0.37% in the short-stem group. There were no patients with aseptic loosening in either group. The first failure was a periprosthetic femur fracture in the standard-length stem group, which we identified 2 weeks after the primary procedure (Fig. 4). We revised the patient to a larger MHP stem with cerclage cables and she was doing well at latest followup. The second patient was in the short-stem group and had continued pain, radiolucencies around the femoral component on plain radiographs, and a bone scan with increased uptake, suggesting aseptic loosening versus infection. Laboratory analysis demonstrated elevated erythrocyte sedimentation rate and C-reactive protein 19 months after the index procedure and we performed a radical débridement with antibiotic spacer placement. The patient subsequently underwent reimplantation with a standard-length stem and revision acetabular component and was doing well at most recent followup.
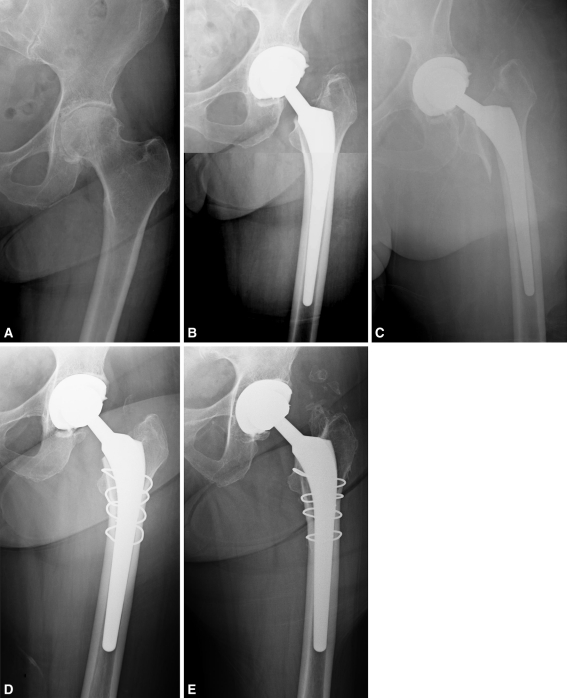
Fig. 4A–E.
(A) A preoperative radiograph of the left hip of a 79-year-old female patient who presented with severe pain and discomfort secondary to osteoarthritis shows severe joint space narrowing, sclerosis, and osteophyte and cyst formation. (B) An immediate postoperative radiograph shows treatment of cementless primary left THA with an 11- by 160-mm standard-length taper stem with lateralized offset and a 36-mm cobalt-chromium head with +6-mm neck articulated against highly crosslinked polyethylene. (C) At 2 weeks postoperative, the patient presented emergently with severe pain. A radiograph reveals a periprosthetic femoral fracture. (D) The patient’s femoral component was revised to a 14- by 175-mm standard-length tapered stem with lateralized offset, and the bone was secured with five cerclage cables. (E) A radiograph at 3 years after revision shows well-fixed components in satisfactory position and alignment. The patient had a HHS of 94 at latest followup.
Between groups, there were no differences in preoperative or postoperative HHSs or LEAS scores at 6 weeks and most recent followup (Table 2).
Discussion
There has been much evidence demonstrating standard-length, tapered, titanium, porous plasma-sprayed components perform well in the short, mid, and long term [10–12, 17–20, 25, 27–29, 33, 35, 42, 43, 47, 52–55, 58–60]. With recent interest in less invasive surgery through smaller incisions, shorter cementless stems have been popularized and utilized to achieve femoral fixation [15, 24, 40, 56, 62, 66]. We therefore compared the intraoperative and perioperative complication rate, short-term survival, and preoperative and postoperative HHSs and LEAS scores for pain and function between groups receiving standard-length double-tapered stems and shorter, flat-wedge, “microplasty” tapered stems.
Our study does contain several limitations. First, although both stems are titanium, porous plasma-sprayed, tapered designs, they are two completely different stems, not just a shorter version of the standard-length stem. The standard-length stem requires both reaming and broaching, while the short stem is a broach-only system. This extra-preparation and geometric design may influence the tendency for the standard-length stem to have a higher fracture risk [9, 40, 59, 63]. Second, the short-term followup for this study was a limiting factor in terms of comparing differences in survivorship and functional outcomes. Third, although this was not the intent of this study, there was no specific analysis of radiographs with regard to positioning and sizing of stems, although studies have shown stem position is not correlated with increased incidence of early component failure [11, 34].
We found a higher incidence of intraoperative femoral and trochanteric fracture with standard-length stems (3.1%) than with short stems (0.4%), which are both within the range of findings reported in other studies of primary cementless THA (Table 3). While these numbers are lower than previous reports of intraoperative fracture with tapered designs, which range from 1.6% to 7% [9, 59, 63], our study demonstrates this particular design of shorter stem does have a lower incidence of intraoperative femur fracture when compared with a standard-length stem. The literature is a bit conflicting when looking at overall outcomes of THAs after intraoperative fractures. Thillemann et al. [67] have shown an overall cumulative failure rate of 0.9% within the first 6 months for patients without intraoperative femoral fractures versus 3.4% for patients with associated intraoperative femoral fractures. The highest cumulative failure rates in that study were 5.7% for the patients when they had osteosynthesis compared to 1.5% in the intraoperative fractures treated nonoperatively [67]. The decision to treat certain intraoperative fractures without osteosynthesis is likely due to less severe fracture morphology with more inherent stability with distal stem fixation [67]. In contrast, we previously reported excellent long-term survival of MHP femoral components after intraoperative fracture with immediate cerclage wire or cable fixation (100% femoral component survival at up to 16 years) [9]. In another experimental study comparing a standard-length, cementless, tapered stem (CLS® Spotorno®; Zimmer, Inc, Warsaw, IN, USA) with a shorter, tapered stem (Mayo®; Zimmer), the authors observed a nonsignificant trend toward lower fracture resistance with the short-stemmed design [30]. This same study showed a much lower average amount of subsidence before fracture in the short stem (7.9 mm) versus the standard-length stem (13.9 mm) [30]. Although these reports show conflicting data, our experience with these two particular stem designs suggests the shorter stems are an equally efficacious alternative to the standard-length stems. While the shorter stems may make the procedure technically easier and more amenable to less invasive approaches, our data also demonstrate a decreased risk of intraoperative fracture when shorter stems are used.
Table 3.
Published studies reporting incidence of intraoperative femoral, trochanteric, and ankle fractures during primary THA
| Study | Number of patients | Number of hips | Cohort description* | Number of intraoperative fractures |
|---|---|---|---|---|
| Andrew et al. [1] (1986) | 400 | 400 | Isoelastic cementless stem (Mathys) of polyacetyl resin | 92 femoral (23%) |
| 19 trochanteric (4.8%) | ||||
| Jensen and Repta [31] (1987) | 36 | 36 | Judet cementless stem (Stryker) | 10 femoral (27.8%) |
| 3 trochanteric (8.3%) | ||||
| Fitzgerald et al. [22] (1988) | NA | 499 | PCA® (Stryker), Harris-Galante® (Zimmer), BIAS® (Zimmer), Omnifit® (Stryker) | 17 (3.5%) |
| Mallory et al. [46] (1989) | 267 | 309 | Cementless 1982 to 1986, MHP (Biomet), PCA® (Stryker), AML® (DePuy), Autophor (Smith and Nephew), CDH Tri-Lock® (DePuy) | 34 (11.0%) |
| Schwartz et al. [64] (1989) | NA | 1318 | AML® cementless stem (DePuy) | 39 (3.0%) |
| Stuchin [65] (1990) | 72 | 79 | Cementless | 16 (20.3%) |
| Cracchiolo et al. [16] (1992) | 34 | 40 | Cementless, rheumatoid arthritis | 3 (8.1%) |
| Martell et al. [48] (1992) | 111 | 121 | Harris-Galante® cementless stem (Zimmer) | 10 (8.3%) |
| Au [3] (1994) | 23 | 25 | Isoelastic cementless stem (Mathys) | 7 (28%) |
| Toni et al. [68] (1994) | NA | 61 | Lord straight-stemmed cementless (Benoist Girard) | 11 (18%) |
| 111 | AnCA™ standard cementless (Wright) | 2 (1.8%) | ||
| 223 | AnCA™ short cementless (Wright) | 3 (1.3%) | ||
| Bargar et al. [4] (1998) | 62 | 62 | Cementless, performed without computer guidance | 3 (4.8%) |
| 65 | 65 | Cementless, performed with ROBODOC® (Curexo) | 1 (0%) | |
| Liu et al. [38] (1998) | NA | 493 | Primary, performed 1972 to 1996 | 12 (2.4%) |
| Loehr et al. [39] (1999) | 15 | 21 | Cementless, rheumatoid arthritis | 1 (4.8%) |
| Schramm et al. [63] (2000) | 94 | 107 | CLS® (Zimmer) | 8 (7.5%) |
| Berend et al. [9] (2004) | NA | 1320 | MHP cementless stem (Biomet), standard direct lateral approach | 58 (4.4%) |
| Cameron [14] (2004) | NA | 679 | S-ROM® cementless stem (Depuy) | 47 (6.9%) |
| Parvizi et al. [59] (2004) | 121 | 129 | TaperLoc® cementless stem (Biomet) | 3 (2.3%) |
| Matta et al. [50] (2005) | 437 | 494 | Minimally invasive single-incision anterior approach on orthopaedic table, cementless stems | 6 femoral (1.2%) |
| 3 trochanteric (0.6%) | ||||
| 3 ankle (0.3%) | ||||
| Asayama et al. [2] (2006) | 50 | 50 | Cementless, standard direct lateral approach | 0 (0%) |
| 52 | 52 | Cementless, limited direct lateral approach | 2 (3.8%) | |
| Laffosse et al. [36] (2006) | NA | 42 | Minimally invasive anterolateral approach, large-diameter femoral heads | 4 femoral (9.5%) |
| 4 trochanteric (9.5%) | ||||
| NA | 58 | Standard posterior approach, 28-mm heads | ||
| 1 femoral (1.7%) | ||||
| Lerch et al. [37] (2007) | NA | 1216 | Bicontact® cementless stem (B Braun) | 42 (3.5%) |
| Lu et al. [44] (2007) | 60 | 63 | Minimally invasive two-incision approach | 2 (3.2%) |
| Fernandez-Fernandez et al. [21] (2008) | NA | 117 | Meridian® cementless stem (Stryker) | 13 (11.1%) |
| Mainard [45] (2008) | 42 | 42 | Cementless straight stem, nonnavigated | 1 (2.4%) |
| 42 | 42 | Cementless straight stem, navigated | 0 (0%) | |
| Masonis et al. [49] (2008) | NA | 300 | Minimally invasive direct anterior approach with fluoroscopic assistance | 3 (1.0%) |
| McGrory et al. [51] (2008) | 115 | 115 | Minimally invasive posterior approach | 1 (0.9%) |
| Thilleman et al. [67] (2008) | NA | 39,478 | Primary osteoarthritis, Danish registry 1995 to 2005 | 519 (1.3%) |
| Ghera and Pavan [24] (2009) | 65 | 65 | Proxima® cementless stem (DePuy UK) | 1 (1.5%) |
| Benum and Aamodt [5] (2010) | 191 | 191 | Custom CAD-CAM cementless stem (SCP), direct lateral approach | 2 (1.0%) |
| Berend and Lombardi [7] (2010) | NA | 439 | Monoblock, cementless, broach only, tapered-wedge stem, prepared with low-profile cutting blade calcar mill | 0 (0%) |
| NA | 18 | Modular, cementless, broach only, tapered-wedge stem prepared with deep-toothed cutting calcar mill | 2 (11.1%) | |
| Palutsis et al. [57] (2010) | 181 | 200 | Minimally invasive two-incision approach, cementless VerSys® MidCoat (Zimmer), M/L Taper (Zimmer) | 4 femoral (2%)† |
| 14 trochanteric ≤ 2 cm (7%) | ||||
| 2 trochanteric > 2 cm (1%) | ||||
| Jewett and Collis [32] (2011) | NA | 800 | Minimally invasive anterior approach with aid of fracture table | 19 trochanteric (2.4%) |
| 4 femoral (0.5%) | ||||
| Molli et al. (2011) | 360 | 389 | MHP (Biomet), less invasive direct lateral approach | 9 femoral (2.3%) |
| 3 trochanteric (0.8%) | ||||
| 246 | 269 | TaperLoc® Microplasty™ (Biomet), less invasive direct lateral approach | 1 femoral (0.4%) | |
| Total | 50,538 | 1064 (2.1%) |
* Manufacturers included: Mathys Ltd Bettlach, Bettlach, Switzerland; Stryker Orthopaedics, Mahwah, NJ; Zimmer, Inc, Warsaw, IN; Smith and Nephew, Inc, Memphis TN; DePuy Orthopaedics, Inc, Warsaw, IN; Benoist Girard, Bagneux, France; Wright Medical Technology, Inc, Arlington, TN; Curexo Technology Corp, Sacramento, CA; B Braun, Melsungen, Germany; DePuy International Ltd, Leeds, UK; Scandinavian Customized Prostheses (SCP), Trondheim, Norway; †there were four additional early postoperative femoral fractures in this series; NA = not available; PCA® = Porous Coated Anatomic; BIAS® = Biologic Ingrowth Anatomic System; MHP = Mallory-Head® porous; AML® = Anatomic Medullary Locking; CDH = congenital dysplasia of the hip; AnCA = Anatomic Ceramic Arthroplasty; CLS® = Cementless Self-locking; S-ROM® = Sivash Range of Motion; CAD-CAM = computer-aided design and computer-aided manufacture.
The overall stem survivorship at most recent followup with stem revision for any reason as the end point was 99.7%, with only two of 658 stems needing revision for any reason. We performed no revisions for aseptic loosening or failure of ingrowth, for an early rate of stable fixation of 100%. Although this study was predominately designed to compare the intraoperative complication rates between standard-length and short tapered stems, our short-term survival is encouraging. We previously reported a 98.6% overall stem survivorship at 5 years (98.4% at 10 years, 97.1% at 15 years, and 95.5% at 20 years) with the MHP femoral prosthesis, all performed via a direct lateral approach, with stem revision for any reason as the end point [43]. In the same study, stem survivorship, when looking only at aseptic loosening as reason for failure, was 99.4% at 5 years and 99.3% at 10, 15, and 20 years [43]. Further followup is needed to compare the long-term durability in clinical practice of these two devices in terms of survival and patient function and activity level.
With regard to postoperative pain and function scores, no differences in the HHS pain and total scores and LEAS scores were seen between the two groups at the 6-week or most recent followup visit. Both groups saw relatively equivalent improvements in their HHS pain and total scores postoperatively when compared with preoperative levels (Table 2).
In conclusion, the TaperLoc® Microplasty™ femoral stem appears to be a safe and reliable alternative to a more traditional standard-length double-tapered stem (Fig. 5). The risk of major intraoperative complications, particularly iatrogenic femoral fracture, was less with this short stem, making it an attractive alternative to more traditional stems. Although overall stem survivorship in the literature with traditional stems is exceptional, the nearly eight times higher risk of intraoperative fracture associated with these stems in this study certainly lends to more fracture-friendly stem options being sought. Short stems do not appear to compromise either femoral fixation or short-term clinical results and will likely continue to be utilized as many more surgeons and patients seek less invasive techniques for THA. Future studies focused on long-term survivorship and durability of these “short stems” will be important in determining their continued use in THA.
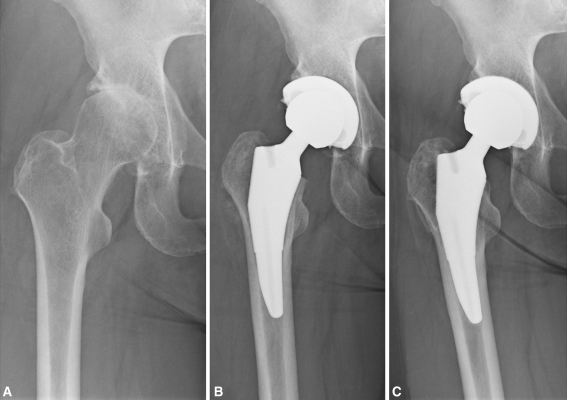
Fig. 5A–C.
(A) A preoperative radiograph of the left hip of a 64-year-old female patient who presented with severe pain and discomfort secondary to osteoarthritis shows severe joint space narrowing, sclerosis, and osteophyte and cyst formation. (B) A postoperative radiograph at 6 weeks shows treatment of cementless primary left THA with a 10- by 105-mm short taper stem and a 36-mm BIOLOX® delta ceramic-on-highly-crosslinked polyethylene articulation (Ceramtec AG, Plochingen, Germany). (C) A radiograph at 4 years postoperatively shows well-fixed components in satisfactory position and alignment. The patient had a HHS of 96.5 at latest followup.
Footnotes
One or more of the authors (AVL, KRB) receive royalties and institutional research support from and have consulting agreements with Biomet, Inc (Warsaw, IN, USA). One author (KRB) has consulting agreements with Salient Surgical Technologies (Portsmouth, NH, USA). One author (AVL) receives royalties from Innomed, Inc (Savannah, GA, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Joint Implant Surgeons, Inc.
References
- 1.Andrew TA, Flanagan JP, Gerundini M, Bombelli R. The isoelastic, noncemented total hip arthroplasty: preliminary experience with 400 cases. Clin Orthop Relat Res. 1986;206:127–138. [PubMed] [Google Scholar]
- 2.Asayama I, Kinsey TL, Mahoney OM. Two-year experience using a limited-incision direct lateral approach in total hip arthroplasty. J Arthroplasty. 2006;21:1083–1091. doi: 10.1016/j.arth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Au MK. Isoelastic total hip replacement: clinical evaluation of prosthetic isoelasticity. J Formos Med Assoc. 1994;93:497–502. [PubMed] [Google Scholar]
- 4.Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res. 1998;354:82–91. doi: 10.1097/00003086-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Benum P, Aamodt A. Uncemented custom femoral components in hip arthroplasty: a prospective clinical study of 191 hips followed for at least 7 years. Acta Orthop. 2010;81:427–435. doi: 10.3109/17453674.2010.501748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berend KR, Lombardi AV., Jr Total hip arthroplasty via the less invasive anterolateral abductor splitting approach. Semin Arthroplasty. 2004;15:87–93. doi: 10.1053/j.sart.2004.08.004. [DOI] [Google Scholar]
- 7.Berend KR, Lombardi AV., Jr Intraoperative femur fracture is associated with stem and instrument design in primary total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2377–2381. doi: 10.1007/s11999-010-1314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berend KR, Lombardi AV, Jr, Mallory TH. Rapid recovery protocol for peri-operative care of total hip and total knee arthroplasty patients. Surg Technol Int. 2004;13:239–247. [PubMed] [Google Scholar]
- 9.Berend KR, Lombardi AV, Jr, Mallory TH, Chonko DJ, Dodds KL, Adams JB. Cerclage wires or cables for the management of intraoperative fracture associated with a cementless, tapered femoral prosthesis: results at 2 to 16 years. J Arthroplasty. 2004;19(Suppl 2):17–21. doi: 10.1016/j.arth.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Berend KR, Lombardi AV, Mallory TH, Dodds KL, Adams JB. Cementless double-tapered total hip arthroplasty in patients 75 years of age and older. J Arthroplasty. 2004;19:288–295. doi: 10.1016/j.arth.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Berend KR, Mallory TH, Lombardi AV, Jr, Dodds KL, Adams JB. Tapered cementless femoral stem: difficult to place in varus but performs well in those rare cases. Orthopedics. 2007;30:295–297. doi: 10.3928/01477447-20070401-16. [DOI] [PubMed] [Google Scholar]
- 12.Bourne RB, Rorabeck CH. A critical look at cementless stems: taper designs and when to use alternatives. Clin Orthop Relat Res. 1998;355:212–223. doi: 10.1097/00003086-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Burt CF, Garvin KL, Otterberg ET, Jardon OM. A femoral component inserted without cement in total hip arthroplasty: a study of the Tri-Lock component with an average ten-year duration of follow-up. J Bone Joint Surg Am. 1998;80:952–960. doi: 10.2106/00004623-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Cameron HU. Intraoperative hip fractures: ruining your day. J Arthroplasty. 2004;19(4 Suppl 1):99–103. doi: 10.1016/j.arth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Chen HH, Morrey BF, An KN, Luo ZP. Bone remodeling characteristics of a short-stemmed total hip replacement. J Arthroplasty. 2009;24:945–950. doi: 10.1016/j.arth.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Cracchiolo A, 3rd, Severt R, Moreland J. Uncemented total hip arthroplasty in rheumatoid arthritis diseases: a two- to six-year follow-up study. Clin Orthop Relat Res. 1992;277:166–174. [PubMed] [Google Scholar]
- 17.Ellison B, Berend KR, Lombardi AV, Jr, Mallory TH. Tapered titanium porous plasma-sprayed femoral component in patients aged 40 years and younger. J Arthroplasty. 2006;21(Suppl 2):32–37. doi: 10.1016/j.arth.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Emerson RH, Jr, Head WC, Emerson CB, Rosenfeldt W, Higgins LL. A comparison of cemented and cementless titanium femoral components used for primary total hip arthroplasty: a radiographic and survivorship study. J Arthroplasty. 2002;17:584–591. doi: 10.1054/arth.2002.32696. [DOI] [PubMed] [Google Scholar]
- 19.Emerson RH, Jr, Head WC, Higgins LL. Clinical and radiographic analysis of the Mallory-Head femoral component in revision total hip arthroplasty: a minimum 8.8-year and average eleven-year follow-up study. J Bone Joint Surg Am. 2003;85:1921–1926. doi: 10.2106/00004623-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Emerson RH, Jr, Sanders SB, Head WC, Higgins L. Effect of circumferential plasma-spray porous coating on the rate of femoral osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1291–1298. doi: 10.2106/00004623-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Fernandez R, García-Elias E, Gil-Garay E. Peroperative fractures in uncemented total hip arthroplasty: results with a single design of stem implant. Int Orthop. 2008;32:307–313. doi: 10.1007/s00264-006-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald RH, Jr, Brindley GW, Kavanagh BF. The uncemented total hip arthroplasty: intraoperative femoral fractures. Clin Orthop Relat Res. 1988;235:61–66. [PubMed] [Google Scholar]
- 23.Frndak PA, Mallory TH, Lombardi AV., Jr Translateral surgical approach to the hip: the abductor muscle “split”. Clin Orthop Relat Res. 1993;295:135–141. [PubMed] [Google Scholar]
- 24.Ghera S, Pavan L. The DePuy Proxima hip: a short stem for total hip arthroplasty. Early experience and technical considerations. Hip Int. 2009;19:215–220. doi: 10.1177/112070000901900305. [DOI] [PubMed] [Google Scholar]
- 25.Gosens T, Langelaan EJ, Tonino AJ. Cementless Mallory-Head HA-coated hip arthroplasty for osteoarthritis in hip dysplasia. J Arthroplasty. 2003;18:401–410. doi: 10.1016/S0883-5403(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 26.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 27.Head WC, Mallory TH, Emerson RH., Jr The proximal porous coating alternative for primary total hip arthroplasty. Orthopedics. 1999;22:813–815. doi: 10.3928/0147-7447-19990901-08. [DOI] [PubMed] [Google Scholar]
- 28.Hozack W, Gardiner R, Hearn S, Eng K, Rothman R. Taperloc femoral component: a 2–6-year study of the first 100 consecutive cases. J Arthroplasty. 1994;9:489–493. doi: 10.1016/0883-5403(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 29.Hozack WJ, Rothman RH, Eng K, Mesa J. Primary cementless hip arthroplasty with a titanium plasma sprayed prosthesis. Clin Orthop Relat Res. 1996;333:217–225. doi: 10.1097/00003086-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Jakubowitz E, Seeger JB, Lee C, Heisel C, Kretzer JP, Thomsen MN. Do short-stemmed-prostheses induce periprosthetic fractures earlier than standard hip stems? A biomechanical ex-vivo study of two different stem designs. Arch Orthop Trauma Surg. 2009;129:849–855. doi: 10.1007/s00402-008-0676-9. [DOI] [PubMed] [Google Scholar]
- 31.Jensen JS, Retpen JB. Failures with the Judet noncemented total hip. Acta Orthop Scand. 1987;58:23–26. doi: 10.3109/17453678709146337. [DOI] [PubMed] [Google Scholar]
- 32.Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503–507. doi: 10.1007/s11999-010-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keisu KS, Orozco F, Sharkey PF, Hozack WJ, Rothman RH, McGuigan FX. Primary cementless total hip arthroplasty in octogenarians: two to eleven-year follow-up. J Bone Joint Surg Am. 2001;83:359–363. doi: 10.1302/0301-620X.83B3.11006. [DOI] [PubMed] [Google Scholar]
- 34.Khalily C, Lester DK. Results of a tapered cementless femoral stem implanted in varus. J Arthroplasty. 2002;17:463–466. doi: 10.1054/arth.2002.32171. [DOI] [PubMed] [Google Scholar]
- 35.Kim YH. Long-term results of the cementless porous-coated anatomic total hip prosthesis. J Bone Joint Surg Br. 2005;87:623–627. doi: 10.1302/0301-620X.87B5.15554. [DOI] [PubMed] [Google Scholar]
- 36.Laffosse JM, Chiron P, Accadbled F, Molinier F, Tricoire JL, Puget J. Learning curve for a modified Watson-Jones minimally invasive approach in primary total hip replacement: analysis of complications and early results versus the standard-incision posterior approach. Acta Orthop Belg. 2006;72:693–701. [PubMed] [Google Scholar]
- 37.Lerch M, Windhagen H, Lewinski G, Thorey F. Intraoperative femoral fractures during the implantation of the cementless BiCONTACT stem: a matched-pair analysis of 84 patients] [in German. Z Orthop Unfall. 2007;145:574–578. doi: 10.1055/s-2007-965617. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Lu S, Liu B. The reason and management of intraoperative femur fracture during hip arthroplasty] [in Chinese. Zhonghua Wai Ke Za Zhi. 1998;36:93–95. [PubMed] [Google Scholar]
- 39.Loehr JF, Munzinger U, Tibesku C. Uncemented total hip arthroplasty in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;366:31–38. doi: 10.1097/00003086-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Lombardi AV Jr, Berend KR, Adams JB. A short stem solution: through small portals. Orthopedics. 2009;32(9). doi: 10.3928/01477447-20090728-09. [DOI] [PubMed]
- 41.Lombardi AV, Jr, Berend KR, Adams JB. A rapid recovery program: early home and pain free. Orthopedics. 2010;33(9):656. doi: 10.3928/01477447-20100722-38. [DOI] [PubMed] [Google Scholar]
- 42.Lombardi AV, Jr, Berend KR, Mallory TH. Hydroxyapatite-coated titanium porous plasma spray tapered stem: experience at 15 to 18 years. Clin Orthop Relat Res. 2006;453:81–85. doi: 10.1097/01.blo.0000238872.01767.09. [DOI] [PubMed] [Google Scholar]
- 43.Lombardi AV, Jr, Berend KR, Mallory TH, Skeels MD, Adams JB. Survivorship of 2000 tapered titanium porous plasma-sprayed femoral components. Clin Orthop Relat Res. 2009;467:146–154. doi: 10.1007/s11999-008-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu ML, Chou SW, Yang WE, Senan V, Hsieh PH, Shih HN, Lee MS. Hospital course and early clinical outcomes of two-incision total hip arthroplasty. Chang Gung Med J. 2007;30:513–520. [PubMed] [Google Scholar]
- 45.Mainard D. Navigated and nonnavigated total hip arthroplasty: results of two consecutive series using a cementless straight hip stem. Orthopedics. 2008;31(10 Suppl 1). [PubMed]
- 46.Mallory TH, Kraus TJ, Vaughn BK. Intraoperative femoral fractures associated with cementless total hip arthroplasty. Orthopedics. 1989;12:231–239. doi: 10.3928/0147-7447-19890201-06. [DOI] [PubMed] [Google Scholar]
- 47.Marshall AD, Mokris JG, Reitman RD, Dandar A, Mauerhan DR. Cementless titanium tapered-wedge femoral stem: 10- to 15-year follow-up. J Arthroplasty. 2004;19:546–552. doi: 10.1016/j.arth.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Martell JH, Galante JO, Pierson RH, Jacobs JJ, Rosenberg AG, Maley M. Clinical experience with primary cementless total hip arthroplasty. Chir Organi Mov. 1992;77:383–396. [PubMed] [Google Scholar]
- 49.Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12 Suppl 2). [PubMed]
- 50.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–124. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 51.McGrory BJ, Finch ME, Furlong PJ, Ruterbories J. Incision length correlates with patient weight, height, and gender when using a minimal-incision technique in total hip arthroplasty. J Surg Orthop Adv. 2008;17:77–81. [PubMed] [Google Scholar]
- 52.McLaughlin JR, Lee KR. Total hip arthroplasty with an uncemented femoral component: excellent results at ten-year follow-up. J Bone Joint Surg Br. 1997;79:900–907. doi: 10.1302/0301-620X.79B6.7482. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin JR, Lee KR. Total hip arthroplasty in young patients: 8- to 13-year results using an uncemented stem. Clin Orthop Relat Res. 2000;373:153–163. doi: 10.1097/00003086-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292. doi: 10.1302/0301-620X.88B10.17660. [DOI] [PubMed] [Google Scholar]
- 55.McLaughlin JR, Lee KR. Total hip arthroplasty with an uncemented tapered femoral component. J Bone Joint Surg Am. 2008;90:1290–1296. doi: 10.2106/JBJS.G.00771. [DOI] [PubMed] [Google Scholar]
- 56.Morrey BF. Short-stemmed uncemented femoral component for primary hip arthroplasty. Clin Orthop Relat Res. 1989;249:169–175. [PubMed] [Google Scholar]
- 57.Palutsis RS, Sheridan KC, Wasielewski RC. One surgeon’s experience with the 2-incision technique for total hip arthroplasty. J Arthroplasty. 2010;25:71–75. doi: 10.1016/j.arth.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Park MS, Choi BW, Kim SJ, Park JH. Plasma spray-coated Ti femoral component for cementless total hip arthroplasty. J Arthroplasty. 2003;18:626–630. doi: 10.1016/S0883-5403(03)00203-1. [DOI] [PubMed] [Google Scholar]
- 59.Parvizi J, Keisu KS, Hozack WJ, Sharkey PF, Rothman RH. Primary total hip arthroplasty with an uncemented femoral component: a long-term study of the Taperloc stem. J Arthroplasty. 2004;19:151–156. doi: 10.1016/j.arth.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Reitman RD, Emerson R, Higgins L, Head W. Thirteen year results of total hip arthroplasty using a tapered titanium femoral component inserted without cement in patients with type C bone. J Arthroplasty. 2003;18(Suppl 1):116–121. doi: 10.1016/S0883-5403(03)00344-9. [DOI] [PubMed] [Google Scholar]
- 61.Saleh KJ, Mulhall KJ, Bershadsky B, Ghomrawi HM, White LE, Buyea CM, Krackow KA. Development and validation of a lower-extremity activity scale: use for patients treated with revision total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1985–1994. doi: 10.2106/JBJS.D.02564. [DOI] [PubMed] [Google Scholar]
- 62.Santori FS, Santori N. Mid-term results of a custom-made short proximal loading femoral component. J Bone Joint Surg Br. 2010;92:1231–1237. doi: 10.1302/0301-620X.92B9.24605. [DOI] [PubMed] [Google Scholar]
- 63.Schramm M, Keck F, Hohmann D, Pitto RP. Total hip arthroplasty using an uncemented femoral component with taper design: outcome at 10-year follow-up. Arch Orthop Trauma Surg. 2000;120:407–412. doi: 10.1007/PL00013771. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz JT, Jr, Mayer JG, Engh CA. Femoral fracture during non-cemented total hip arthroplasty. J Bone Joint Surg Am. 1989;71:1135–1142. [PubMed] [Google Scholar]
- 65.Stuchin SA. Femoral shaft fracture in porous and press-fit total hip arthroplasty. Orthop Rev. 1990;19:153–159. [PubMed] [Google Scholar]
- 66.Stulberg SD, Dolan M. The short stem: a thinking man’s alternative to surface replacement. Orthopedics. 2008;31:885–886. doi: 10.3928/01477447-20080901-37. [DOI] [PubMed] [Google Scholar]
- 67.Thillemann TM, Pedersen AB, Johnsen SP, Søballe K. Inferior outcome after intraoperative femoral fracture in total hip arthroplasty: outcome in 519 patients from the Danish Hip Arthroplasty Registry. Acta Orthop. 2008;79:327–334. doi: 10.1080/17453670710015210. [DOI] [PubMed] [Google Scholar]
- 68.Toni A, Ciaroni D, Sudanese A, Femino F, Marraro MD, Bueno Lozano AL, Giunti A. Incidence of intraoperative femoral fracture: straight-stemmed versus anatomic cementless total hip arthroplasty. Acta Orthop Belg. 1994;60:43–54. [PubMed] [Google Scholar]