Abstract
Background
Ceramic liner fracture is a concern in THA. However, it is unclear what factors influence the risk of facture. To study these factors under controlled conditions, we created a laboratory model to avoid fractures in vitro.
Questions/purposes
We determined (1) whether misaligned liner insertion, acetabular shell deformation, entrapment of soft tissue within the locking taper area, and damage to the taper during engagement of the ceramic liner on the locking taper influenced fracture at light and medium impaction forces; and (2) whether the number and force of impactions affect the locking taper force between the ceramic liner and acetabular shell and fracture of the ceramic liner.
Methods
Impaction and pushout tests were performed with each of five ceramic inserts in titanium shells per test to simulate clinical intraoperative situations of misaligned inserts (Test 1), deformed shells (Test 2), soft tissue within the locking taper area (Test 3), simulated cup taper damage (Test 4), and a combination of misaligned insert, deformed shells, and simulated taper damage to create an overall worst-case condition (Test 5).
Results
Higher pushout forces occurred with increased impact force and an increased number of strikes. Insert fractures only occurred where inserts were misaligned in the shell. No fractures occurred with deformed shells, soft tissue in the taper, or with simulated taper damage in the absence of misaligned inserts.
Conclusion
The data suggest a misaligned ceramic insert in an acetabular increases the potential for insert fracture. Shell deformation, soft tissue in the taper, or simulated taper damage seemed well tolerated even with very forceful impaction. Forceful and repetitive impaction is favorable for engagement of the taper and improving pullout strength.
Introduction
Ceramic-on-ceramic (COC) THA has a long history of clinical use, initially introduced by Boutin in 1970 [3]. Studies of modern COC bearings suggest higher survivorship and decreased osteolysis when compared with metal-on-polyethylene articulations [4, 6]. Alumina liners have been in use for several years, and the most common design in use is the direct taper-lock of the alumina liner into the metal shell. In the United States, more than 2400 liners have been implanted as part of COC clinical studies, and no fractures of these liners have been reported to date [22].
Numerous advantages have been reported with the use of ceramic THA designs, including extremely low wear resulting from the very low surface roughness (Ra = 0.02 μm) and increased wettability of the ceramic material [8]. In vivo linear wear rates as low as 0.016 to 0.025 mm per year have been observed [5, 6, 11], which is approximately 4000 times less than with historical metal-on-polyethylene devices. Furthermore, the wear particles generated in a COC coupling are relatively bioinert [17, 21]. Nizard et al. [16] compared the macrophage response to alumina and polyethylene microparticulate debris of identical size and volume by measuring production of tumor necrosis factor alpha (TNF-alpha), a cytokine known to induce osteolysis. They observed levels of TNF-alpha release eight to 10 times higher in the presence of polyethylene than with exposure to alumina debris. These data are supported by numerous long-term clinical studies, which show a very low incidence of osteolysis associated with use of a ceramic THA design [2, 6, 8, 13, 16].
Problems associated with use of ceramic THA bearing materials include fracture [3, 8, 9, 13, 25], audible squeaking [19, 24, 27], a risk of premature bearing wear in components revised for ceramic fracture resulting from remaining third-body microparticulate debris [1], increased cost, and a reduced positional range of error during component implantation. Early ceramic fracture rates as high as 7.5% [3] have resulted in multiple mechanical property improvements, including clean room processing, improved sintering techniques to reduce grain size and increase material strength, hot isostatic pressing to increase density and improve surface finish, proof testing, and laser marking to reduce stress raisers within the ceramic material [10, 20, 23, 26]. These changes have resulted in substantial increases in material strength and hardness as well as a reduction in grain size and the incidence of ceramic fracture. Despite ceramic material improvements, ceramic fracture still infrequently occurs. Willman [25] reported a ceramic fracture rate of 0.02% based on implantation of more than 1.5 million ceramic femoral heads since 1974, whereas Hannouche et al. [9] observed 13 ceramic fractures in a group of 5500 (0.23%) ceramic THA subjects. Modular ceramic acetabular component designs also introduce potential complications such as incomplete liner seating, liner dissociation, and chipping of the modular ceramic liner during insertion [7]. We suspect that surgeons may be tentative when impacting ceramic liners because of the brittle nature of the material, and this hesitancy may increase the risk of incomplete seating and subsequent liner fracture. Thus, there is a need for guidelines on the force of impaction and a knowledge of which insertion factors might relate to complications.
We simulated several different clinical scenarios in the laboratory to evaluate how each situation would contribute to fracture of a ceramic liner. We therefore determined the likelihood of fracture with four surgical variables on the locking taper force between the ceramic liner and acetabular shell and fracture of the ceramic liner; the four study variables include: (1) misaligned liner insertion; (2) acetabular shell deformation; 3) entrapment of soft tissue within the locking taper area; and 4) damage to the taper during engagement of the modular acetabular liner. We then determined whether the number and force of impactions during taper engagement influenced liner seating and fracture.
Materials and Methods
This study evaluated the effects of four study variables on the locking taper force between the ceramic liner and acetabular shell and fracture of the ceramic liner under simulated intraoperative conditions. The four study variables included: (1) misaligned liner insertion; (2) acetabular shell deformation; (3) entrapment of soft tissue within the locking taper area; and (4) damage to the taper during engagement of the modular acetabular liner. We also evaluated whether the number and force of impactions affected the locking taper force between the ceramic liner and acetabular shell and fracture of the ceramic liner. To isolate and effectively analyze the study variables, a total of 10 tests were conducted with each test comprising multiple impaction forces. Each test condition used a sample size of five for a total of 25 parts used in the test. Tests 6 through 9 reused test samples from previous Tests 1 through 5 (Table 1).
Table 1.
Test study design
| Test | Impaction test description | Impact force (lbs) | Number of strikes | Sample size |
|---|---|---|---|---|
| 1 | Misaligned inserts (2–4 mm) | a) 1000 | 2 | n = 5 |
| b) 2000 | 2 | |||
| 2 | Deformed shells from 1-mm oblong underream | a) 1000 | 2 | n = 5 |
| b) 2000 | 2 | |||
| 3 | Taper with soft tissue impingement | a) 1000 | 2 | n = 5 |
| b) 2000 | 2 | |||
| 4 | Taper damage using a 0.25-mm diameter wire | a) 1000 | 2 | n = 5 |
| b) 2000 | 2 | |||
| 5 | Misaligned inserts (2–4 mm), deformed shells from 1-mm press-fit, taper damage using a 0.25-mm diameter wire | a) 1000 | 2 | n = 5 |
| b) 2000 | 2 | |||
| 6 | Misaligned inserts (2–4 mm) | a) 2000 | 10 | n = 5 (from Test 1) |
| b) 3000 | 10 | |||
| c) 4000 | 10 | |||
| d) 5000 | 10 | |||
| 7 | Deformed shells from 1-mm oblong underream | a) 2000 | 10 | n = 5 (from Test 2) |
| b) 3000 | 10 | |||
| c) 4000 | 10 | |||
| d) 5000 | 10 | |||
| 8 | Taper damage using a 0.25-mm diameter wire | a) 2000 | 10 | n = 5 (from Test 4) |
| b) 3000 | 10 | |||
| c) 4000 | 10 | |||
| d) 5000 | 10 | |||
| 9 | Misaligned inserts (2–4 mm), deformed shells from 1-mm press-fit, taper damage using a 0.25-mm diameter wire | a) 2000 | 10 | n = 5 (from Test 5) |
| b) 3000 | 10 | |||
| c) 4000 | 10 | |||
| d) 5000 | 10 |
We performed impact and pushout testing on 28-mm inner diameter (ID) ceramic inserts manufactured from an alumina matrix composite ceramic (BIOLOX® delta) material supplied by CeramTec (Plochingen, Germany). Sample size selection was determined per CeramTec test procedure VA 02 04 4123 (CeramTec), which is widely used by the industry and has been reviewed by the FDA for many modular ceramic acetabular devices. The test procedure requires a sample size of five for pushout testing; therefore, a sample size of five was chosen for each test condition in this test for a total of 25 test samples used in the test. Sample size also depended on the particular test: burst testing (n = 7), postfatigue burst (n = 3), and pushout (n = 3). Impaction and pushout tests were performed with the BIOLOX® delta inserts assembled within 48-mm titanium shells (Pinnacle; DePuy Orthopaedics, Inc, Warsaw, IN, USA). A 48-mm shell size with 28-mm ID ceramic inserts was used for testing to compare with previous standard mechanical testing with this assembly. Smaller shell sizes generally provide less taper locking force compared with larger shell sizes. The 28-mm ID ceramic insert in a 48-mm shell previously was used as a worst-case for burst testing; therefore, it is considered a reasonable worst-case testing construct. The tests were designed to simulate clinical intraoperative conditions or potential misuse and include misaligned inserts (Test 1, n = 5), deformed shells (Test 2, n = 5), soft tissue within the locking taper area (Test 3, n = 5), simulated cup taper damage (Test 4, n = 5), and a combination of misaligned insert, deformed shells, and simulated taper damage (Test 5, n = 5). In Test 1, the ceramic inserts were misaligned into the Pinnacle shells by 2 to 4 mm as determined by a micrometer (Fig. 1). For Test 2, acetabular shells were impacted into oblong cavities created from Sawbones foam block material to deform the shells. In Test 3, soft tissue consisting of a mixture of bovine serum and bovine marrow was placed in the locking taper region between the shell and insert. Test 4 was similar to Test 3 but used a 0.25-mm diameter titanium wire to simulate shell taper damage (Fig. 2) that could be unintentionally caused from instrumentation while implanting the shell into the acetabulum. The titanium wire was not used to scratch the surface of the Morse taper. The wire was placed on the Morse taper to simulate damage that could occur from a significant scratch or gouge in this locking taper region. The wire was left in place during impaction of the ceramic liner. Test 5 was a combination of Tests 1, 2, and 4 to create an overall worst-case condition.
Fig. 1.
Insert is shown misaligned 2 to 4 mm above the shell face.
Fig. 2.
A 0.25-mm diameter titanium wire was used to simulate taper damage.
The Pinnacle shells were assembled into Sawbones foam block (ASTM F-1839-08) spherical cavities, representing cortical bone density, underreamed by 1 mm to provide a press-fit between the Pinnacle shell and foam block cavity to simulate standard clinical press-fit reaming conditions. The shells for Tests 2 and 5 were assembled into Sawbones foam block using oblong cavities underreamed by 1 mm in one axis and 0 mm in the perpendicular axis to create a pinching effect to visually deform the shells. Deformation of the shell in the taper region was evident although not specifically measured. The result of this deformation caused substantial toggle of the insert about the minor axis (axis of compressive load) in the shell. The amount of toggle appeared representative of clinical descriptions of ceramic or metal insert toggling that can occur with press-fit conditions in hard bone. The test condition may not represent a worst-case for shell deflection, but it is representative of the clinically described issue of insert toggling. The shells were pressed into Sawbones foam block cavities using a MTS electromechanical test frame (MTS, Eden Prairie, MN, USA) until the shells were fully seated in the Sawbones cavities with no apparent space remaining between the apex of the shell and the reamed cavity. The ceramic inserts were impacted with two strikes into the shells using a Pneumatic impact test system (DePuy Orthopaedics Inc) with a PCB Piezotronics load sensor (PCB Piezotronics, Inc, Depew, NY, USA) equipped with a straight cup impactor. The first set of testing used a 1000-pound impact force to simulate a light to medium surgeon impaction force [14]. The ceramic inserts were pushed out of the shells using the MTS electromechanical test frame and the pushout force was recorded. The tests were repeated using an increased impaction force of 2000 pounds to represent a medium impaction force [14].
Further impaction and pushout testing (Tests 6-9) was performed by increasing the impaction force and number of impact strikes with the test conditions from Tests 1 to 5 with the exception of Test 3 (Table 1). Essentially Tests 6 to 9 were extensions of the previous test conditions with greater impaction forces and number of strikes where the test conditions of Test 6 = Test 1, Test 7 = Test 2, Test 8 = Test 4, and Test 9 = Test 5.
Test 3 (soft tissue) was not continued with greater impaction forces and number of strikes because the pushout forces were greatest in this condition and was less likely to cause failure compared with Test 4 (simulated taper damage). The strength of the ceramic inserts was tested by impacting them 10 times with a 2000-pound impaction force using the same test method as previously performed in Tests 1 to 5 with the exception of the modified impaction force and number of strikes. If no fractures occurred, the inserts were pressed out of the shells and the pushout force was recorded. The test was repeated with 3000-pound, 4000-pound, and 5000-pound impaction forces or until fracture. A 5000-pound impaction force was near the pneumatic impact test system capacity; therefore, higher impaction forces were not achievable. A 5000-pound impaction force is considered very high and typically above what surgeons would achieve under hip arthroplasty surgical impaction [14].
The slope from a simple linear regression model of pushout force versus impact force was tested to see if pushout force increased substantially with increased impact force. Specifically, the linear regression model was used to analyze inserts with deformed shells (Test 7) and inserts with simulated taper damage (Test 8). A t-test was used to compare pushout force versus the number of impact strikes. t-tests were carried out with the Satterthwaite method to accomodate potentially unequal variances. Data for each respective test were assumed to be normally distributed because data were nearly symmetrical within each test. Test conditions with correctly aligned inserts, deformed shells (Test 2 versus Test 7), and simulated taper damage (Test 4 versus Test 8) compared pushout force of inserts impacted at 2000 pounds with two and 10 impact strikes. Statisica analyses were carried out with SAS® version 9.2 (Cary, NC, USA).
Results
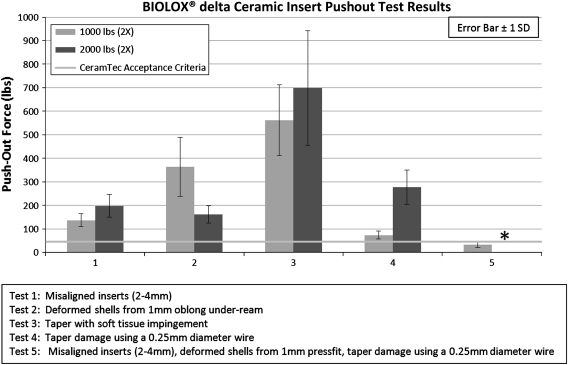
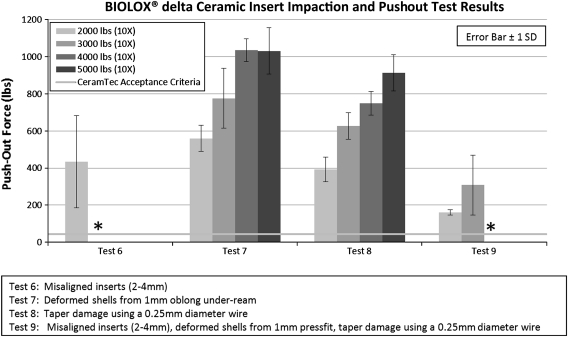
Pushout force for properly aligned inserts was above the CeramTec pushout acceptance criteria and did not fracture under any loading conditions. The pushout forces for inserts impacted twice at 1000 pounds and 2000 pounds were above the minimum CeramTec pushout criteria in each study variable (Tests 1–4). Pushout forces were below the CeramTec acceptance criteria where misaligned inserts, deformed shell, and taper damage variables were combined (Test 5a). Pushout test results were lost for Test 5b as a result of a computer malfunction. Pushout force increased with greater impaction force from 1000 pounds to 2000 pounds except with deformed shells (Test 2) (Fig. 3). No ceramic insert fractures were observed in any of these test conditions (Tests 1–5). Further testing with greater impaction forces and increased number of strikes provided evidence that pushout force was affected by impaction force and inserts failed when misaligned in the shell. Pushout force increased for greater impaction force in both deformed shells (Test 7) (R2 = 0.685, p < 0.001) and simulated taper damage (Test 8) (R2 = 0.873, p < 0.001). Pushout results from Tests 6 to 9 were above the CeramTec insert pushout acceptance criteria (Fig. 4) where insert fracture did not occur. Inserts under misaligned inserts conditions (Tests 6 and 9) fractured at 3000 pounds and 4000 pounds, respectively. These were rim fractures only. The pushout force was the highest for soft tissue impingement (Test 3); therefore, no further testing was pursued with higher impaction forces and increased number of strikes.
Fig. 3.
Ceramic insert pushout test results (Tests 1–5) demonstrating combined malalignment, taper damage, and cup deformation resulted in unacceptable results. * Data were lost as a result of computer malfunction.
Fig. 4.
Pushout results from Tests 6–9 were above the CeramTec insert pushout acceptance criteria (Fig. 4) where insert fracture did not occur. * Inserts fractured during this test condition.
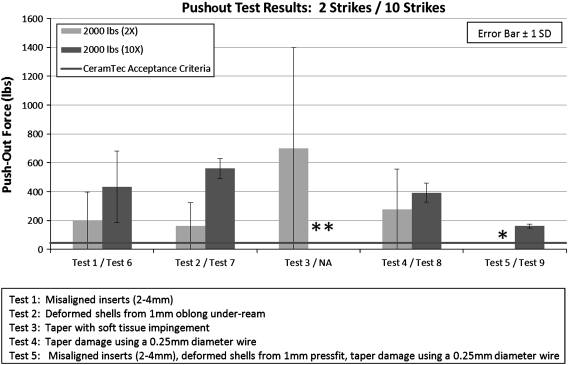
Pushout forces for properly aligned inserts improved by increasing the number of strikes from two to 10 with a 2000-pound impaction force. Comparing the number of strikes with deformed shells (Test 2 versus Test 7) and simulated taper damage (Test 4 versus Test 8) at 2000-pound impaction force (Fig. 5), a greater number of impaction strikes increased pushout force (Test 2 = 162 ± 37 lbs, Test 7 = 560 ± 70 lbs, p = 0.001; Test 4 = 278 ± 73 lbs, Test 8 = 393 ± 66, p = 0.036).
Fig. 5.
Pushout force comparison demonstrated benefit of multiple (10 versus two) strikes of a 2000-pound impact force. * Data lost as a result of computer malfunction. ** Not tested.
Discussion
The extremely low wear of ceramic bearings has been documented extensively [5, 6, 8, 11] and needs to be weighed against well-described disadvantages including fracture [3, 8, 9, 13], audible squeaking [19, 24], increased cost, and a reduced positional range of error during component implantation. Early ceramic fracture rates as high as 7.5% [3] have resulted in multiple mechanical property improvements, resulting in substantial increases in material strength and hardness and subsequent reduction in the incidence of ceramic fracture. Despite these improvements, ceramic fracture still infrequently occurs, including a 1.1% incidence of postoperative liner fractures seen when using the liner studied in this article [7]. We hypothesized that the liner fractures seen in that earlier study were the result of improper initial seating of the liner and failure to properly impact and seat this particular liner. We therefore determined (1) whether misaligned liner insertion, acetabular shell deformation, entrapment of soft tissue within the locking taper area, and damage to the taper during engagement of the ceramic liner on the locking taper influenced fracture at light and medium impaction forces; and (2) whether the number and force of impactions affected the locking taper force between the ceramic liner and acetabular shell and fracture of the ceramic liner.
We recognize limitations to our study. First, the experimental design mandated that we perform this study in the laboratory setting. We decided to perform these tests in a Sawbones model to control for variations seen in cadaveric specimens. This model may not completely reproduce the exact mechanical environment seen in the live patient. However, the model we devised provided a reproducible external environment and allowed us to study the internal environment of the cup with multiple similar specimens, which was the primary goal of the study. Second, one may argue that an increased number of specimens would improve the power of the study. We believed this number of specimens adequately addressed the questions we posed. Lastly, the results from this study are specific to one commercially available design that has a unique locking mechanism and taper angle for the ceramic insert. It is unclear if these results can be extrapolated to implants made by other manufacturers, but this study will help to elucidate potential problems with this design and provide guidance for surgeons using this newly available device.
The insertion of ceramic liners into metal shells must be precise to avoid complications. If the liner is not inserted precisely at the time of surgery, postoperative catastrophic failure of the liner is possible [7, 15]. In our experience with an alumina matrix composite ceramic material (BIOLOX® delta on BIOLOX® delta ceramic product; CeramTec, Plochingen, Germany), two postoperative liner fractures were encountered, and both were believed to result from eccentrically or incompletely placed liners at the time of surgery [7]. Experience with the Trident ceramic-on-ceramic THA system (Stryker, Mahwah, NJ, USA) with a metal-backed ceramic liner has also demonstrated problems with complete seating of the liner. In one report of 117 hips in 113 patients in which this liner was used [12], 16.4% were noted to have incomplete seating of the liner seen on postoperative radiographs. The authors suggested technical difficulties, combined with shell deformation, may prevent complete seating [12].
Other ceramic implants have seen in vivo failures leading to the manufacturers discontinuing their use [18]. A cementless cup with a polyethylene liner and ceramic inlay (Hedrocel ceramic bearing cup; Implex, Allendale, NJ, USA) was discontinued after 14 of 315 devices failed at the ceramic-polyethylene interface. The company performed biomechanical testing on unimplanted cups to determine the lever-out force of the ceramic liner. The strength of the assembly (defined as the resistance to torsional dislodgment of the ceramic liner) at body temperature averaged 33.4 ± 3.8 Nm with a range of 24.9 to 41.2 Nm [18]. Finite element calculation of maximum principal tensile stress within the ceramic liner indicated a value of 100 MPa for a 5-kN load. In contrast, the manufacturer of the alumina ceramic liner (BIOLOX® forte) has reported the four-point strength to be 580 MPa (84,000 psi), far more than the maximum load predicted by the finite element analysis [10].
We recently reported our experience with the delta-delta ceramic articulation [7]. Of 157 ceramic liners inserted, there were three intraoperative events involving the ceramic liner. In the first patient, the surgeon had difficulty symmetrically seating the ceramic liner. On impaction, the liner fractured and was removed. The cup was retained and a 32-mm polyethylene liner and a ceramic femoral head were implanted. In the second patient, a surgeon at a different site had difficulty seating the acetabular liner and on initial impaction found the liner was not symmetrically seated in the cup. The surgeon attempted to remove the liner by tamping the edge of the metal cup, but the process of doing so fractured the ceramic liner. The cup, fractured liner, and ceramic fragments were removed and replaced with a new cup and ceramic liner without difficulty. In the last patient, the same surgeon had difficulty seating the ceramic liner. The cup and liner were removed and replaced with a new cup and ceramic liner without difficulty. The liner did not fracture in this case.
Our findings reinforce the importance of meticulous seating of a ceramic insert in an acetabular shell before impaction. Insert fractures only occurred in test conditions in which inserts were misaligned in the shell. Conversely, shell deformation, soft tissue in the taper, or simulated taper damage seemed well tolerated even with very forceful impaction. Surgeons may be reluctant to forcefully impact a ceramic liner into an acetabular shell because of concerns of fracturing the implant. Our data suggest forceful and repetitive impaction is actually favorable for engagement of the taper and improving pullout performance. It is likely that undetected suboptimal engagement of the taper would be reduced with forceful impaction. It is important to stress that we studied a single ceramic composite matrix material, and these results may not translate to other ceramic materials or acetabular hip ceramic hip systems. Additionally, the testing in this report was not exhaustive in each test condition; thus, changing the variables in the test could substantially affect the results. These test variables include insert misalignment angle, shell deformation, soft tissue, taper damage, and impact load limitations. The test variables and simulated clinical conditions attempted to represent reasonable worst-case scenarios; however, it is conceivable that clinical experiences could achieve conditions beyond the extent of testing in this report, including surgical impact force. Surgeons should carefully align the inserts and encourage forceful impaction to ensure engagement and resistance to pullout.
Footnotes
One or more of the authors (JPM, DAD, WGH) are consultants for DePuy (Warsaw, IN, USA) and have a product development agreement with DePuy. One of the authors (JG) is an employee of DePuy Orthopaedics, who funded the material expenses for the study.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Allain J, Roudot-Thoraval F, Delecrin J, Anract P, Migaud H, Goutallier D. Revision total hip arthroplasty performed after fracture of a ceramic femoral head. A multicenter survivorship study. J Bone Joint Surg Am. 2003;85:825–830. doi: 10.2106/00004623-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bizot P, Nizard R, Hamadouche M, Hannouche D, Sedel L. Prevention of wear and osteolysis: alumina-on-alumina bearing. Clin Orthop Relat Res. 2001;393:85–93. doi: 10.1097/00003086-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Boutin P. Total arthroplasty of the hip by fritted aluminum prosthesis. Experimental study and 1st clinical applications [in French] Rev Chir Orthop Reparatrice Appar Mot. 1972;58:229–246. [PubMed] [Google Scholar]
- 4.D’Antonio J, Capello W, Manley M, Naughton M, Sutton K. Alumina ceramic bearings for total hip arthroplasty: five-year results of a prospective randomized study. Clin Orthop Relat Res. 2005;436:164–171. doi: 10.1097/01.blo.0000162995.50971.39. [DOI] [PubMed] [Google Scholar]
- 5.Dorlot JM, Christel P, Meunier A. Wear analysis of retrieved alumina heads and sockets of hip prostheses. J Biomed Mater Res. 1989;23:299–310. doi: 10.1002/jbm.820231405. [DOI] [PubMed] [Google Scholar]
- 6.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed] [Google Scholar]
- 7.Hamilton WG, McAuley JP, Dennis DA, Murphy JA, Blumenfeld TJ, Politi J. THA with Delta ceramic on ceramic: results of a multicenter investigational device exemption trial. Clin Orthop Relat Res. 2010;468:358–366. doi: 10.1007/s11999-009-1091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannouche D, Hamadouche M, Nizard R, Bizot P, Meunier A, Sedel L. Ceramics in total hip replacement. Clin Orthop Relat Res. 2005;430:62–71. doi: 10.1097/01.blo.0000149996.91974.83. [DOI] [PubMed] [Google Scholar]
- 9.Hannouche D, Nich C, Bizot P, Meunier A, Nizard R, Sedel L. Fractures of ceramic bearings: history and present status. Clin Orthop Relat Res. 2003;417:19–26. doi: 10.1097/01.blo.0000096806.78689.50. [DOI] [PubMed] [Google Scholar]
- 10.Heros R, Willman G. Ceramics in total hip arthroplasty: history, mechanical properties, clinical results, and current manufacturing state of the art. Semin Arthroplasty. 1998;9:114. [Google Scholar]
- 11.Jazrawi LM, Bogner E, Della Valle CJ, Chen FS, Pak KI, Stuchin SA, Frankel VH, Di Cesare PE. Wear rates of ceramic-on-ceramic bearing surfaces in total hip implants: a 12-year follow-up study. J Arthroplasty. 1999;14:781–787. doi: 10.1016/S0883-5403(99)90025-6. [DOI] [PubMed] [Google Scholar]
- 12.Langdown AJ, Pickard RJ, Hobbs CM, Clarke HJ, Dalton DJ, Grover ML. Incomplete seating of the liner with the Trident acetabular system: a cause for concern? J Bone Joint Surg Br. 2007;89:291–295. doi: 10.1302/0301-620X.89B3.18473. [DOI] [PubMed] [Google Scholar]
- 13.Lusty PJ, Tai CC, Sew-Hoy RP, Walter WL, Walter WK, Zicat BA. Third-generation alumina-on-alumina ceramic bearings in cementless total hip arthroplasty. J Bone Joint Surg Am. 2007;89:2676–2683. doi: 10.2106/JBJS.F.01466. [DOI] [PubMed] [Google Scholar]
- 14.Maharaj G, Jamison RD. Intraoperative impact: characterization and laboratory simulation on composite hip prostheses. In: Jamison R, Gilbertson LN, editors. Composite Materials for Implant Applications in the Human Body: Characterization and Testing. Philadelphia, PA, USA: American Society for Testing and Materials; 1993. pp. 98–108. [Google Scholar]
- 15.McCarthy MJ, Halawa M. Lining up the liner: 2 case reports of early ceramic liner fragmentation. J Arthroplasty. 2007;22:1217–1222. doi: 10.1016/j.arth.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Nizard R, Pourreyron D, Raould A, Hannouche D, Sedel L. Alumina-on-alumina hip arthroplasty in patients younger than 30 years old. Clin Orthop Relat Res. 2008;466:317–323. doi: 10.1007/s11999-007-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petit A, Catelas I, Antoniou J, Zukor DJ, Huk OL. Differential apoptotic response of J774 macrophages to alumina and ultra-high-molecular-weight polyethylene particles. J Orthop Res. 2002;20:9–15. doi: 10.1016/S0736-0266(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 18.Poggie RA, Turgeon TR, Coutts RD. Failure analysis of a ceramic bearing acetabular component. J Bone Joint Surg Am. 2007;89:367–375. doi: 10.2106/JBJS.F.00148. [DOI] [PubMed] [Google Scholar]
- 19.Ranawat AS, Ranawat CS. The squeaking hip: a cause for concern—agrees. Orthopedics. 2007;30:738–743. doi: 10.3928/01477447-20070901-32. [DOI] [PubMed] [Google Scholar]
- 20.Sedel L. The Tribology of Hip Replacement. London, England: European Instructional Course Lectures. 1997:22–25.
- 21.Sedel L, Simeon J, Meunier A, Villette JM, Launay SM. Prostaglandin E2 level in tissue surrounding aseptic failed total hips. Effects of materials. Arch Orthop Trauma Surg. 1992;111:255–258. doi: 10.1007/BF00571519. [DOI] [PubMed] [Google Scholar]
- 22.Tateiwa T, Clarke IC, Williams P, Garino J, Manaka M, Shishido T, Yamamoto K, Imakiire A. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop. 2008;37:E26–E31. [PubMed] [Google Scholar]
- 23.Walter A. On the material and the tribology of alumina-alumina couplings for hip joint prostheses. Clin Orthop Relat Res. 1992;282:31–46. [PubMed] [Google Scholar]
- 24.Walter WL, O’Toole GC, Walter WK, Ellis A, Zicat BA. Squeaking in ceramic-on-ceramic hips: the importance of acetabular component orientation. J Arthroplasty. 2007;22:496–503. doi: 10.1016/j.arth.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Willmann G. Ceramics for total hip replacement—what a surgeon should know. Orthopedics. 1998;21:173–177. doi: 10.3928/0147-7447-19980201-11. [DOI] [PubMed] [Google Scholar]
- 26.Wu C. Grain Size dependence of wear in ceramics. Ceramic Engineering Science Proceedings. 1985;6:995–1011. doi: 10.1002/9780470320280.ch52. [DOI] [Google Scholar]
- 27.Yang CC, Kim RH, Dennis DA. The squeaking hip: a cause for concern-disagrees. Orthopedics. 2007;30:739–742. doi: 10.3928/01477447-20070901-33. [DOI] [PubMed] [Google Scholar]