Abstract
Background
Decision-making regarding nonoperative versus operative treatment of patients with thoracolumbar burst fractures in the absence of neurologic deficits is controversial. Lack of evidence-based practice may result in patients being treated inappropriately and being exposed to unnecessary adverse consequences.
Purpose
Using meta-analysis, we therefore compared pain (VAS) and function (Roland Morris Disability Questionnaire) in patients with thoracolumbar burst fractures without neurologic deficit treated nonoperatively and operatively. Secondary outcomes included return to work, radiographic progression of kyphosis, radiographic progression of spinal canal stenosis, complications, cost, and length of hospitalization.
Methods
We searched MEDLINE, EMBASE®, and the Cochrane Central Register of Controlled Trials for ‘thoracic fractures’, ‘lumbar fractures’, ‘non-operative’, ‘operative’ and ‘controlled clinical trials’. We established five criteria for inclusion. Data extraction and quality assessment were in accordance with Cochrane Collaboration guidelines. The main analyses were performed on individual patient data from randomized controlled trials. Sensitivity analyses were performed on VAS pain, Roland Morris Disability Questionnaire score, kyphosis, and return to work, including data from nonrandomized controlled trials and using fixed effects meta-analysis. We identified four trials, including two randomized controlled trials consisting of 79 patients (41 with operative treatment and 38 with nonoperative treatment). The mean followups ranged from 24 to 118 months.
Results
We found no between-group differences in baseline pain, kyphosis, and Roland Morris Disability Questionnaire scores. At last followup, there were no between-group differences in pain, Roland Morris Disability Questionnaire scores, and return to work rates. We found an improvement in kyphosis ranging from means of 12.8º to 11º in the operative group, but surgery was associated with higher complication rates and costs.
Conclusions
Operative management of thoracolumbar burst fractures without neurologic deficit may improve residual kyphosis, but does not appear to improve pain or function at an average of 4 years after injury and is associated with higher complication rates and costs.
Level of Evidence
Level II, therapeutic study. See the Guidelines for Authors for a complete description of level of evidence.
Introduction
The majority of spinal fractures occur in the thoracolumbar region (T10-L3) presumably as a result of transition from the relatively immobile thoracic spine to the mobile lumbar spine. Spinal fractures include compression, burst, flexion-distraction, and fracture-dislocation injuries, with burst fractures accounting for 10% to 20% [22, 27, 29]. The mechanism of injury is usually a fall from a height or a motor vehicle accident, occurring in 34% to 59% and 25% to 43% of cases, respectively [47, 50]. Burst fractures are characterized by failure of the middle and anterior spinal columns secondary to axial compression [20, 36]. Patients with such fractures may be treated with either nonoperative or operative modalities. Nonoperative treatment may include the use of a brace, cast, bed rest, and analgesics [7, 11, 12, 14, 34, 46, 47]. Operative treatment usually involves instrumented intervertebral fusion, with or without spinal decompression. The three major surgical approaches for stabilization are posterior, anterior, or combined AP [26, 35, 37, 40, 49, 53].
Clinical features of burst fractures include acute back pain and restricted motion, which might be accompanied by neurologic deficits, including motor or sensory changes and sphincter disturbances [27]. Approximately 50% of thoracolumbar burst fractures have an associated neurologic deficit, with the deficit predominantly occurring at the time of injury [9, 20, 28]. Burst fractures are radiographically characterized by posterior vertebral body angle exceeding 100°, reduction in posterior vertebral height, widened interpedicle distance, posterior cortical line disruption, and posterior vertebral body break, which may be associated with varying degrees of canal stenosis [6, 25]. However evaluation of such fractures on plain radiographs alone can result in misdiagnosis, with associated ligamentous injuries being missed and approximately 25% of burst fractures being misdiagnosed as compression fractures [8, 17]. The treatment of burst fractures with neurologic deficits is controversial as decompression might not result in resolution of the deficits and neurologic status (and the degree of compression) might improve with time, regardless of decompression [30, 31].
Similarly, decision-making regarding nonoperative versus operative treatment of patients with burst fractures in the absence of neurologic deficits is contentious [21, 45]. Most studies have been observational, and although several relevant trials have been published, sample sizes have been small, ranging from 10 to 80 patients [1–5, 9–14, 27, 34, 41, 42, 44, 49]. Proponents of nonoperative management argue that avoiding surgery decreases associated costs and surgical complications including infection, hardware-related complications, and iatrogenic injury [11, 12, 14, 34, 38, 44]. Indications for operative treatment may include neurologic deficit, unstable fracture, severe kyphosis greater than 35°, canal compromise greater than 50%, or posterior ligamentous complex injury [18, 38]. Other arguments for surgery include decreased rates of neurologic deterioration, improved kyphosis correction, and facilitation of early mobilization that may decrease complications from prolonged bed rest [1, 5, 45].
Observational studies generally have yielded comparable pain relief, function, and deformity correction in nonoperative and operative modalities in patients who initially are neurologically intact [1–5, 9–14, 27, 34, 41, 42, 44, 49]. The majority of nonoperative trials show that patients experience little or no pain and good functional outcomes (as assessed by return to work, Denis work scale [20], Greenough Low Back Outcome Score [23], and Roland Morris Disability Questionnaire (RMDQ) [39]) at followup, with between 75% to 100% returning to work. This is associated with kyphosis progression of 1° to 8.3°, spontaneous spinal canal remodeling (from an average of 26% to 36% compression at the time of injury to 12% to 18% at last followup) [3, 4, 10–14, 27, 41, 44], and rare cases of neurologic deterioration [21, 34]. Observational studies of operative treatment suggest the majority of patients experience satisfactory pain and functional outcomes, kyphosis correction of 0.5° to 10.2°, reduction in canal stenosis, and no neurologic deterioration at last followup [1, 2, 5, 10, 11, 21, 27, 41, 42, 52].
Several systematic reviews of nonoperative versus operative treatment for thoracolumbar burst fractures without a neurologic deficit have been published [18, 46, 48, 55]. However, Dai et al. [18], Thomas et al. [46], and van der Roer et al. [48], based their conclusions primarily on descriptive summaries, included observational studies, and did not perform meta-analyses. Similarly, the review by Yi et al. [55] included only one randomized control trial (RCT), thus limiting its power and clinical utility. They also did not use available supplemental data [51] detailing individual patient outcomes, radiographic features, and baseline characteristics.
We therefore performed a systematic review and meta-analysis of prospective controlled clinical trials to establish the best evidence. Our primary outcomes were pain (VAS) and function (RMDQ). Secondary outcomes were return to work, radiographic progression of kyphosis (degrees) (and its association with pain and function), radiographic progression of spinal canal stenosis, complications, cost, and length of hospitalization (days).
Search Strategy and Criteria
We used the Cochrane Collaboration guidelines [15] to develop the following methods, with results reported according to the QUOROM checklist [33]. Electronic searches of MEDLINE (1950 - present), EMBASE® (1980–present), and the Cochrane Central Register of Controlled Trials (most recent edition) were performed in April 2009, to identify trials (Table 1). Trials with the following characteristics were included: (1) randomized, quasirandomized, or controlled clinical trials; (2) patients with confirmed nonpathologic thoracolumbar (T10-L3) burst fractures based on CT and plain AP and lateral radiographs; (3) patients with no neurologic deficit; (4) comparison of nonoperative and operative management (regardless of the type of treatment); (5) participants 18 years and older; and (6) full text. Trials including patient groups with neurologic deficits or examining multiple interventions were included if examined separately. There were no restrictions on language or publication date. Articles were assessed independently by two authors (SRG, SA). When inclusion was unclear based on abstracts, full text articles were retrieved. Disagreements were resolved by discussion, and a third investigator (IAH) acted as arbitrator when necessary.
Table 1.
Search strategy
| MEDLINE | EMBASE® | Cochrane Central Register |
|---|---|---|
| 1. thoracic vertebrae/or thoracic$.mp. | 1. Thoracolumbar Spine/ | 1. thoracic vertebrae/or thoracic$.mp. |
| 2. lumbar vertebrae/or lumbar$.mp. | 2. (thoracolumbar or thoraco-lumbar).mp. | 2. lumbar vertebrae/or lumbar$.mp. |
| 3. 1 and 2 | 3. (thoracic$ and lumbar$).mp. | 3. 1 and 2 |
| 4. (thoracolumbar or thoraco-lumbar).mp. | 4. or/1-3 | 4. (thoracolumbar or thoraco-lumbar).mp. |
| 5. 3 or 4 | 5. spine fracture/or vertebra fracture/ | 5. 3 or 4 |
| 6. spinal fractures/or fractur$.mp. | 6. fractur$.mp. | 6. spinal fractures/or fractur$.mp. |
| 7. 5 and 6 | 7. 5 or 6 | 7. 5 and 6 |
| 8. 7 and burst$.mp. | 8. 4 and 7 | 8. (nonoperat$ or non-operat$).mp. |
| 9. (nonoperat$ or non-operat$).mp. | 9. 8 and burst$.mp. | 9. (nonsurg$ or non-surg$).mp. |
| 10. (nonsurg$ or non-surg$).mp. | 10. (nonoperat$ or non-operat$).mp. | 10. conservativ$.mp. |
| 11. conservativ$.mp. | 11. (nonsurg$ or non-surg$).mp. | 11. expectant$.mp. |
| 12. expectant$.mp. | 12. conservativ$.mp. | 12. (medical$ adj2 manag$).mp. |
| 13. (medical$ adj2 manag$).mp. | 13. expectant$.mp. | 13. (dt or rh or th).fs. |
| 14. (dt or rh or th).fs. | 14. (medical$ adj2 manag$).mp. | 14. su.fs. |
| 15. su.fs. | 15. (dt or rh or th).fs. | 15. 13 and 14 |
| 16. 14 and 15 | 16. su.fs. | 16. or/8-12,15 |
| 17. or/9-13,16 | 17. 15 and 16 | 17. 7 and 16 |
| 18. 8 and 17 | 18. or/10-14,17 | |
| 19. 7 and 17 | 19. 9 and 18 | |
| 20. exp Controlled Clinical Trials as Topic/ | 20. 8 and 18 | |
| 21. Random Allocation/ | 21. exp controlled clinical trial/ | |
| 22. Double-Blind Method/ | 22. randomization/ | |
| 23. single blind method/ | 23. double blind procedure/ | |
| 24. controlled clinical trial/or randomized controlled trial/ | 24. single blind procedure/ | |
| 25. Comparative Study/ | 25. Crossover Procedure/ | |
| 26. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).mp. | 26. exp case control study/ | |
| 27. (randomi?ed control$ trial$ or rct$).mp. | 27. comparative study/or intermethod comparison/ | |
| 28. (random adj2 (allocat$ or assign$)).mp. | 28. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).mp. | |
| 29. placebos/or placebo$.mp. | 29. (randomi?ed control$ trial$ or rct$).mp. | |
| 30. or/20-29 | 30. (random adj2 (allocat$ or assign$)).mp. | |
| 31. 19 and 30 | 31. placebo/or placebo$.mp. | |
| 32. limit 19 to “therapy (optimized)” | 32. or/21-31 | |
| 33. limit 19 to (controlled clinical trial or randomized controlled trial) | 33. 20 and 32 | |
| 34. or/31-33 | 34. limit 20 to “treatment (2 or more terms min difference)” | |
| 35. 18 or 34 | 35. 33 or 34 | |
| 36. 9 or 35 |
The following outcomes were extracted at baseline and last followup where available: Primary outcomes: (1) pain, measured using subjective scales, eg, VAS (0–100, 0 = no pain, 100 = worst pain); (2) function and quality of life, measured using validated indices eg, RMDQ (0 = no disability, 24 = severe disability) [39] and Greenough Low Back Outcome Score (0 = severe disability, 75 = no disability) [23]. Secondary outcomes assessed were: (1) return to work; (2) kyphosis progression, measured in degrees based on radiographic evaluation; (3) spinal canal stenosis progression determined by CT evaluation of the percentage of canal compromise at the midsagittal spinal canal diameter at the fracture level; (4) complications, divided into general (eg, thromboembolism, pneumonia, wound infection, urinary tract infection), neurologic deterioration, and need for later surgery; (5) costs, and (6) length of hospital stay (days).
Data extraction was performed independently by two authors (SRG, SA), and included data regarding study type, participants, methods, interventions, and outcome measures. Data were managed using Review Manager (RevMan) 5 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). If reported data were inadequate, we attempted to contact authors for supplementary information. We contacted Shen et al. [43] and Hitchon et al. [26] to obtain individual patient data, and Siebenga et al. [45] regarding the outcomes of patients who had neurologic complications in the nonoperative group, but we received data from only the latter. Data were extracted using an intention-to-treat basis to include original study participants, where possible.
A quality assessment of each trial was performed independently by two authors (SRG, SA), with disagreements resolved by discussion. This included evaluation of allocation sequence, allocation concealment, blinding, loss to followup, and completeness of outcome reporting [15] (Table 2).
Table 2.
Characteristics of trials
| Characteristic | Siebenga et al. [45] | Wood et al. [51] | Shen et al. [43] | Hitchon et al. [26] |
|---|---|---|---|---|
| Study design | Multicenter prospective randomized control trial | Multicenter prospective randomized control trial | Pseudorandomized controlled clinical trial | Prospective controlled clinical trial |
| Size | 34 patients | 53 patients | 83 patients | 31 |
| Location | Netherlands | Minnesota, MN, USA | Kaohsiung, Taiwan | Iowa University, Iowa City, IA, USA, & VA Medical Centers |
| Inclusion criteria | Traumatic fracture of T10-L4 | Isolated thoracolumbar burst fracture; CT scan revealing a burst-type fracture with retropulsion of vertebral body bone into the canal; no new neurologic abnormality; < 3 weeks after injury; age 18–66 years; no medical comorbidities precluding surgery; no ongoing cancer, infection, bleeding disorder, or skin disease | Neurologically intact; single-level closed burst fracture T11-L2; no dislocations, with pedicles and facet joints appearing intact, although the pedicles may have fractured from the vertebral body; age 18 to 65 years; no major organ or musculoskeletal injuries | Nonoperative treatment: angular deformity < 10°; residual spinal canal as measured on CT > 50%; anterior body height > 50% of the posterior height |
| AO Type A; no neurologic deficit; age 18 to 60 years; period between trauma and operative treatment < 10 days. | ||||
| Operative treatment: angular deformity generally measured > 10°; residual spinal canal > 50% of normal | ||||
| Baseline characteristics | ||||
| Operative | ||||
| Age (years) | 45.7 (mean) | 43.3 (mean) | 42 (median) | – |
| Gender M:F | 10:7 | 16:8 | 18:15 | – |
| Mechanism of injury | MVA = 18% (3/17); fall = 53% (9/17); sports = 18% (3/17); industrial = 12% (2/17) | MVA = 50% (12/24); fall = 21% (5/24); industrial = 25% (6/24); recreation = 4% (1/24) | MVA or fall = 98% (78/80) | – |
| Site | T1 = 12% (2/17) | T11 = 4% (1/24) | T12 = 30% (10/33) | |
| L1 = 65% (11/17) | T12 = 17% (4/24) | L1 = 42% (14/33) | ||
| L2 = 6% (1/17) | L1 = 54% (13/24) | L2 = 27% (9/33) | – | |
| L3 = 12% (2/17) | L2 = 25% (6/24) | |||
| L4 = 6% (1/17) | ||||
| Nonoperative | ||||
| Age (years) | 37.3 (mean) | 39.4 (mean) | 44 (median) | – |
| Gender M:F | 10:5 | 16:7 | 23:24 | – |
| Mechanism of injury | MVA = 33% (5/15); fall = 67% (10/15) | MVA = 35% (8/23); fall = 48% (11/23); recreational trauma = 13% (3/23); sports injury = 4% (1/23) | MVA or fall = 98% (78/80) | – |
| Site | T12 = 40% (6/15) | T12 = 17% (4/23) | T11 = 2% (1/47) | |
| L1 = 47% (7/15) | L1 = 65% (15/23) | T12 = 23% (11/47) | ||
| L2 = 7% (1/15) | L2 = 17% (4/23) | L1 = 49% (23/47) | – | |
| L3 = 7% (1/15) | L2 = 26% (12/47) | |||
| Outcomes | VAS pain; RMDQ-24; VAS spine; return to work; kyphosis; complications; and length of stay | VAS pain; RMDQ; Oswestry back pain questionnaire; Short form-36; return to work; kyphosis; percentage of canal compromise; complications; length of hospital stay; and cost of treatment | VAS pain; Greenough Low Back Outcome Score; patient satisfaction; return to work; kyphosis; percentage of canal compromise; complications; hospital cost; and length of hospital stay | Cost |
| Review authors’ judgments about each methodologic quality | ||||
| Adequate sequence generation | Unclear | Yes | No | No |
| Allocation concealment | Unclear | Unclear | Unclear | Unclear |
| Blinding | Unclear | Unclear | Unclear | Unclear |
| Free of other bias | No | No | No | No |
| Completeness of outcome reporting | Incomplete | Incomplete | Incomplete | Incomplete |
| Loss to followup | 6% (2/34) | 11% (6/53) | 4% (3/83) | Unclear |
MVA = motor vehicle accident; VAS = visual analog scale; RMDQ = Roland Morris Disability Questionnaire.
Descriptive statistics were used for baseline trial characteristics. VAS pain, RMDQ score, kyphosis, and return to work were pooled where data were available from at least two trials. When individual patient data were available, data were analyzed by intention-to-treat before pooling of outcomes was performed. Mean differences were used for continuous outcomes and odds ratios for dichotomous outcomes. Appropriate measures of precision were extracted for the purposes of this analysis including standard error, standard deviation, p value, or a 95% confidence interval. Meta-analysis was performed using the random effects model. The inverse variance method was used for continuous outcomes and the Mantel–Haenszel method for dichotomous outcomes. Our main analyses present data only from RCTs. We performed sensitivity analyses by including data from nonrandomized studies to explore whether our findings for pain, function, and deformity were robust. We also chose a priori to perform a sensitivity analysis using fixed-effects meta-analysis, which does not adjust for any statistical heterogeneity found. Heterogeneity (a measure of between-study differences that are not attributable to chance) was estimated using the I2 statistic. Results were reported with corresponding 95% confidence intervals and p values. STATA version 10.1 (StataCorp, College Station, TX, USA) was used for analyses.
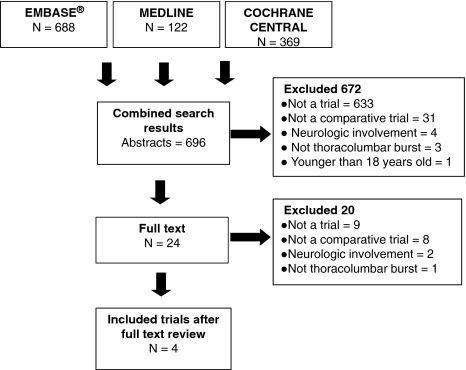
We identified four trials that satisfied our inclusion criteria: two RCTs [45, 51], one quasiRCT [45], and one controlled clinical trial [26] (Fig. 1). An overview of each study is provided, with sample sizes reflecting the number of patients at the start of each trial, including those lost to followup (Table 2).
Fig. 1.
The study criteria are shown.
A multicenter RCT [45] was performed of short-segmented posterior stabilization with pedicle screws inserted above and below the fracture level, in association with autogenous bone grafting, physiotherapy, and hyperextension orthoses (3 months) postoperatively. Autogenous bone was grafted from the posterior pelvic crista for transpedicle spongioplasty or posterolateral monosegmental fusion. Fifteen of 17 implants were removed at 9 to 12 months. This treatment was compared with bed rest (5 days minimum), hyperextension orthoses (3 months), and physiotherapy. However, this small trial was limited to a specific fracture (AO Type A3), with the unconventional inclusion of L3 (n = 3) and L4 (n = 1) fractures. The authors also admitted that failure to screen patients by MRI potentially led to missed detection of a posterior column injury in the nonoperative group, possibly skewing pain, function, and kyphosis results. Moreover, bias cannot be excluded as a result of unclear randomization, allocation concealment, and blinding. There was a small loss to followup (two of 34; 6%).
In another multicenter RCT [51], the mode of operation was at the discretion of the surgeon, and consisted of anterior or posterior arthrodesis and instrumentation, and autologous bone grafting, without formal attempt at decompression. The posterior operative approach involved two to five levels of posterolateral spinal arthrodesis with pedicle screw-hook instrumentation and autologous iliac crest bone grafting, whereas the anterior approach involved two-level fibular and rib-strut construct arthrodesis with local autologous bone grafting and instrumentation. The method of bone grafting was not specified. Nonoperative treatment consisted of either a body cast (8–12 weeks) followed by thoracolumbosacral orthoses (4–8 weeks) or thoracolumbosacral orthoses alone (12–16 weeks). Before either surgery, cast, or orthoses, there was a period of bed rest (2–5 days). Although randomization appears adequate (computer-generated), allocation concealment and blinding are unclear. There is risk of attrition bias, with 11% loss to followup. The study group also was heterogeneous, with patients having multiple-level and single-level fractures, and multiple treatment options.
In a pseudoRCT [43], short-segment posterior fixation (using pedicle screws above and below the fracture), autogenous bone grafting, and facetectomy were compared with a hyperextension brace (3 months). Autogenous bone was grafted from the posterior iliac crest and the bone inserted between the laminae and between adjacent transverse processes. The study was limited by a short followup (24 months) and inadequate sequence generation, with patient self-selection bias. Patients originally were assigned randomly, but seven were reassigned to nonoperative treatment after refusing surgery. Similarly, allocation concealment and blinding were unclear. There was a 4% loss to followup (three of 83) and intention-to-treat was not used in analysis. Furthermore, there was incomplete reporting of hospital charges, canal stenosis, and mechanism of injury.
In a prospective controlled clinical trial [26], patients (n = 31) were allocated according to clinical and radiographic parameters. Criteria for nonoperative treatment included angular deformity less than 10°, residual spinal canal greater than 50% as measured on CT, and anterior body height greater than 50% of the posterior height. Operative treatment was used when angular deformity measured greater than 10° and residual spinal canal was greater than 50% of normal. Operative treatment consisted of decompression, stabilization via pedicle screws, and autologous bone fusion, followed by ambulation using polyester or acrylic thoracolumbar orthoses (3–5 months). Decompression was via a transpedicle approach, costotransversectomy, or through a lateral extraperitoneal approach. Autologous bone fusion occasionally in conjunction with allogenic banked bone was performed, although the source and method of grafting were not specified. Nonoperative treatment included recumbency (1–6 weeks) followed by ambulation wearing thoracolumbar orthoses (3–5 months). However, the heterogeneous cohort included patients with neurologic deficits, and reporting did not differentiate between patients who were neurologically intact managed operatively and nonoperatively except in terms of management costs. The neurologic status in the operative and nonoperative groups was dissimilar (p = 0.0001), with only a small number of patients who were neurologically intact treated operatively (n = 5) compared with nonoperatively (n = 26). Followups also were dissimilar with means of 21 and 9 months for the operative and nonoperative groups, respectively. Moreover, allocation concealment and blinding were unclear.
Individual patient data were available from two RCTs [45, 51], in which 79 patients (41 operative, 38 nonoperative) were identified (Table 3). This does not include data for those lost to followup. The mean age of these 79 patients was 41.5 years, male to female ratio was 2:1, and mean followup was 47 months (range, 24–118 months). The majority of fractures were at T12-L1, accounting for 78% of fractures. Falls (44%) and motor vehicle accidents (35%) were the most common mechanisms of injury. Default quantitative analyses included data only from RCTs, whereas sensitivity analyses were performed using data from the quasiRCT.
Table 3.
Baseline characteristics of patients included in individual patient data meta-analysis
| Characteristic | Total | Operative | Nonoperative | p value |
|---|---|---|---|---|
| Sample size | 79 | 41 (52%) | 38 (48%) | |
| Mean age (years) | 41.5 | 44 | 39 | 0.046 |
| Gender | ||||
| Male | 52 (66%) | 26 (63%) | 26 (68%) | 0.64 |
| Female | 27 (34%) | 15 (37%) | 12 (32%) | |
| Mechanism of injury | ||||
| Fall | 35 (44%) | 14 (34%) | 21 (55%) | 0.06 |
| MVA | 28 (35%) | 15 (37%) | 13 (34%) | |
| Sports/recreation | 5 (6%) | 4 (10%) | 1 (3%) | |
| Work/industrial | 8 (10%) | 8 (20%) | 0 | |
| Other | 3 (4%) | 0 | 3 (8%) | |
| Level of injury | ||||
| T11 | 1 (1%) | 1 (2%) | 0 | 0.6 |
| T12 | 16 (20%) | 6 (15%) | 10 (26%) | |
| L1 | 46 (58%) | 24 (59%) | 22 (58%) | |
| L2 | 12 (15%) | 7 (17%) | 5 (13%) | |
| L3 | 3 (4%) | 2 (5%) | 1 (3%) | |
| L4 | 1 (1%) | 1 (2%) | 0 | |
| Mean followup (months) | 47 | 47 | 48 | 0.68 |
MVA = motor vehicle accident.
There was a difference in age but no between-group differences in gender, mechanism of injury, level of fracture, or length of followup (Table 3). There also were no differences in baseline measurements of the main outcome measures of pain (mean difference [MD] = 0.8; p = 0.77; 95% CI, −4.4–6.0), function using the RMDQ score (MD = 0.6; p = 0.13; 95% CI, −0.2–1.3), or degrees of kyphosis (MD = 0.21; p = 0.91; 95% CI, -3.5–3.9).
Results
We found no between-group differences in mean VAS pain at last followup between nonoperative (22 points) and operative groups (25 points) (MD = −1.0; p = 0.95; 95% CI, −29.0 to 27.1; I2 = 88%). Sensitivity analysis including pain and functional results from the pseudoRCT [43] also showed no between-group difference in pain (MD = −0.2; p = 0.97; 95% CI, −22.1–21.7; I2 = 77%).
There were no differences (p = 0.89) in mean RMDQ scores between groups at last followup (5.8 in the nonoperative group versus 6.1 in the operative group; MD = −0.7; 95% CI, −10.7 to 9.2; I2 = 92%). There also were no changes (p = 0.70) in RMDQ scores from baseline (5.3 in the nonoperative group versus 4.8 in the operative group). Pooling of data from the pseudoRCT [43] using the Greenough Low Back Outcome Score also found no difference (MD = −0.06; p = 0.89; 95% CI, −0.88 to 0.76; I2 = 83%).
Of the cases where work status was reported in the RCTs [45, 51], return to work rates were 67% for the nonoperative group and 70% in the operative group. Pooled results from RCTs showed no difference (p = 0.76) in total return to work rates (odds ratio [OR] = 1.6; 95% CI, 0.07–38.7; I2 = 87%).
Mean kyphosis at approximately 4 years was 16° in the nonoperative group and 11° in the operative group, with a reduction from baseline of −3.3° in the nonoperative group versus 1.8° in the operative group. No between-group difference (p = 0.23) was found in degrees of kyphosis (MD = –6.2; 95% CI, −16.3 to 3.95; I2 = 87%). A sensitivity analysis was performed using fixed-effects meta-analysis (instead of the random-effects method, which adjusts for between-study heterogeneity) which showed improvement (p < 0.001) in kyphosis in the operative group from baseline to last followup (MD = −7.5; 95% CI, −11.0–4.1; I2 = 87%). Addition of data from the pseudoRCT [43] did not alter long-term kyphosis.
Our meta-analysis showed no association (r = −0.04; p = 0.71) between degree of kyphosis and pain (VAS pain) at last followup. Similarly, there was no association (r = −0.03; p = 0.81) between degree of kyphosis and function (RMDQ) at last followup.
There was a higher (p = 0.007) pooled complication rate (Table 4), including general complications, neurologic deterioration, and need for further surgery, in the operative group compared with the nonoperative group (OR = 6.4; 95% CI, 1.7–24.6; I2 = 0%). However, analysis including only complications that resulted in neurologic deterioration or required surgery did not show any differences (OR = 3.3; p = 0.44; 95% CI, 0.16–68.5; I2 = 65%).
Table 4.
Outcomes
| Outcome | Siebenga et al. [45] | Wood et al. [51] | Shen et al. [43] | Hitchon et al. [26] | ||
|---|---|---|---|---|---|---|
| Total return to work | ||||||
| Operative | 85% | 63% | 73% | – | ||
| Nonoperative | 38% | 83% | 67% | – | ||
| Time to return to work | ||||||
| Operative | Mean time postinjury = 6.7 months (1–18 months) | Within 6 months after discharge = 42%; within 24 months = 58% | – | – | ||
| 24 months or more = 63% | ||||||
| Nonoperative | Mean time = 13.6 months (6–33 months) | Within 6 months = 74%; within 24 months = 83% | – | – | ||
| Workload at return to work | ||||||
| Operative | Same profession = 100% | Similar job = 53% | Heavy work = 63%; light work = 90%; | – | ||
| less demanding = 47% | ||||||
| Nonoperative | Same profession = 60%; less demanding job = 40% | Similar job = 79%; less demanding = 21% | Heavy work = 56%; light work = 86% | – | ||
| Complications | ||||||
| General | ||||||
| Operative | 3 | 11 | 1 | – | ||
| Nonoperative | 1 | 2 | 0 | – | ||
| Further surgery owing to complications | ||||||
| Operative | 2 | 6 | 5 | – | ||
| Nonoperative | 0 | 0 | 0 | – | ||
| Neurologic deterioration | ||||||
| Operative | 0 | 0 | 0 | – | ||
| Nonoperative | 2 | 0 | 0 | – | ||
| Mean canal stenosis | Time of injury | Followup | Time of injury | Followup | ||
| Operative | – | 39% (13–63) | 22% (0–58) | 32% (10–70) | Unavailable | – |
| Nonoperative | – | 34% (5–75) | 19% (0–46) | 34% (10–70) | 15% | – |
| Cost | ||||||
| Operative | – | $49,063 ($26,517–$102,583) | Operative treatment four times the cost of nonoperative treatment | $45,300 ± $12,400 | ||
| Nonoperative | – | $11,264 ($4686–$20,891) | $13,900 ± $5400 | |||
| Mean duration of hospitalization (days) | ||||||
| Operative | 14.6 (9–21) | 10.7 (6–27) | 10.4 | – | ||
| Nonoperative | 12.2 (6–25) | 7.9 (2–17) | 9.2 | – | ||
| Patient satisfaction | ||||||
| Operative | – | – | Very satisfied = 30%; satisfied = 55%; unsatisfied = 9%; very unsatisfied = 6% | – | ||
| Nonoperative | – | – | Very satisfied = 38%; satisfied = 49%; unsatisfied = 13% | – | ||
| Mean followup (months) | ||||||
| Operative | 51.6 | 43 | 24 | 21 | ||
| Nonoperative | 51.9 | 46 | 24 | 9 | ||
Canal stenosis improved at followup in the operative and nonoperative groups. One RCT [51] reported improvement from a mean of 39% (range, 13%–63%) at the time of injury to 22% (range, 0%–58%) at followup in the operative group (p = 0.0001) and from 34% (range, 5%–75%) to 19% (range, 0%–46%) in the nonoperative group (p < 0.0001). Similarly, the pseudoRCT [43] reported canal stenosis improved in the nonoperative group from a mean baseline of 34% (range, 10%–70%) to 15% at 1 year followup, although values were unavailable for the operative group (Table 4).
Costs were consistently higher for patients treated operatively compared with nonoperatively (Table 4) [26, 43, 51]. One RCT [51] found that the mean cost of operative management was higher (p < 0.01) than that for nonoperative treatment: $49,063 (US dollars)(range, $26,517–$102,583) versus $11,264 (range, $4686–$20,891), respectively. This was confirmed by the pseudoRCT [43], which reported that operative costs were four times (p < 0.01) that of nonoperative costs. Similarly, the controlled clinical trial [26] reported increased costs associated with surgery ($45,300 ± $12,400 US dollars) compared with nonsurgical management ($13,900 ± $5400) (p value not reported).
There was no difference in length of hospitalization between nonoperative and operative groups. Mean length of stay in the nonoperative group ranged from 7.9 to 12.2 days, compared with a mean stay of 10.4 to 14.6 days in patients treated operatively [43, 45, 51]. These differences reportedly were not significant [43, 51], although one RCT did not perform analyses for differences [45].
Discussion
Decision-making regarding nonoperative versus operative treatment of patients with thoracolumbar burst fractures in the absence of neurologic deficits is controversial, and evidence from trials is sparse [21, 45]. The limited high-quality evidence available has impeded informed decision-making, potentially resulting in patients being treated inappropriately and being exposed to unnecessary adverse consequences. We therefore performed a systematic review incorporating meta-analysis to evaluate pain, function, return to work, kyphosis progression, spinal canal stenosis progression, complications, cost, and length of stay between treatment groups.
We acknowledge limitations of the literature and our review. First, although several relevant trials have been published, the majority are small, retrospective, and of low quality [1–5, 9–14, 27, 34, 41, 42, 44, 49]. As a result, few comparative trials satisfied our inclusion criteria, including two RCTs that yielded contrasting results [45, 51]. Although the moderate sample-size was appropriate to address project-aims, larger cohorts might better detect clinically important differences. We could not perform subgroup analysis based on burst fracture type as the information required was unavailable. Such analysis also might not be valid as included trials were small, and further subclassification would have resulted in reduced reliability. Second, the heterogeneity of study populations in terms of neurologic status and therapeutic options poses additional challenges in evaluating the individual therapeutic options. This clinical heterogeneity, combined with the small sample sizes of the included studies, resulted in high I2 values for our pooled results. However, we believe the study population represents the general population of patients with thoracolumbar burst fractures in terms of baseline characteristics. Third, the use of variable outcome measures and suboptimal reporting, often at nonstandardized intervals, further undermines informed decision-making Fourth, included studies did not differentiate between major and minor complications, thus limiting our analysis and ability to interpret the findings for clinical purposes. Despite these reservations, our review incorporates an individual patient data meta-analysis of RCTs comparing operative and nonoperative management of patients with thoracolumbar burst fractures without a neurologic deficit, and we believe reflects the best evidence currently available. As such, this review hopefully will facilitate greater evidence-based practice and quality management.
Our meta-analysis showed no differences at baseline and at last followup in VAS pain between patients managed nonoperatively and operatively. Data from Shen et al. [43] suggested that surgery resulted in earlier improvement of VAS pain compared with nonoperative treatment at 1 month (3.8 versus 5.5; p = 0.02), although there were no between-group differences at 6 months after injury.
Similarly, our review showed no difference in functional outcomes including RMDQ scores and return to work between operative and nonoperative groups at last followup. This is similar to functional recovery seen in observational trials [1–5, 9–14, 27, 34, 41, 42, 44, 49], although admittedly the wide variety of functional scales used in such trials often makes comparisons problematic.
Our study suggests that although there may be a difference (using sensitivity analyses) in kyphosis between operative and nonoperative groups at last followup, progression of kyphosis occurs in both groups regardless of treatment. Reid et al. [38] reported that much of this progression also appears to occur in the initial period after injury, with relative stabilization of kyphosis within 12 to 18 months. Furthermore, our meta-analysis showed no association between degree of kyphosis and pain and function, which is consistent with findings from other studies [10, 12, 13, 27, 34, 47, 51]. There is limited evidence that pain is more common with a kyphotic angle greater than 30° [22, 49], a level of deformity that vastly exceeded the mean kyphosis observed at last followup in operatively and nonoperatively treated patients in our analysis. Therefore although kyphosis is a common outcome measure in the literature, the importance attributed to this anatomic parameter in studies is difficult to justify, as its clinical importance is questionable [27, 43, 50].
Our review showed a similar decrease in canal stenosis in both groups. Such remodeling has been reported for operatively and nonoperatively treated patients, occurring as a result of resorption of intracanal bone fragments [19, 24, 32, 34, 42, 50, 52, 54]. Although concerns have been raised regarding inadequate remodeling in an observational study [32], Mumford et al. [34] reported substantial remodeling in effectively all canals with greater than 50% compromise in nonoperative trials. This was supported by Dai [16], who reported that there was no difference in percentage of remodeling between operative and nonoperative groups. Despite such findings, there has been no evident association between the percentage of canal stenosis and clinical symptoms in patients who are neurologically intact.
There was a higher rate of complications in the operative group compared with the nonoperative group. Although our analysis did not have the power to show differences in neurologic deterioration, there were two such cases in the nonoperative group [45]. One patient had signs of a conus medullaris syndrome and another had scoliosis of 14° with late signs of nerve root compression, but whether such deterioration was amenable to surgical correction cannot be determined as both patients declined surgery. The literature shows that the majority of such deficits are surgically correctable [21, 34]. Seven cases of neurologic deterioration have been reported in observational studies of nonoperative treatment [21, 34]. Denis et al. [21] reported neurologic manifestations in six patients initially treated nonoperatively (17%). Three patients later recovered neurologic function after undergoing surgery, one patient did not achieve full recovery after incomplete surgical decompression, whereas the other two patients refused surgery for the neurologic deficit [20]. The one case of single-root radiculopathy reported by Mumford et al. [34] was reversed by surgery.
Such results raise questions regarding the need for operative treatment, subjecting patients to the substantial risks associated with surgery without any proven long-term clinical and functional benefits [43, 51]. Similarly, the low risk of neurologic deterioration in nonoperatively treated patients further raises questions regarding whether such risks are overestimated in clinical practice.
Our review showed there is insufficient evidence that operative management is superior to nonoperative management in treating patients with thoracolumbar burst fractures without a neurologic deficit. Operative treatment may result in improved kyphosis correction, but given the limitations of our study, it does not appear to be associated with substantial benefits in long-term pain and function, and is associated with increased costs and greater risk of complications.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Akbarnia BA, Crandall DG, Burkus K, Matthews T. Use of long rods and a short arthrodesis for burst fractures of the thoracolumbar spine: a long-term follow-up study. J Bone Joint Surg Am. 1994;76:1629–1635. doi: 10.2106/00004623-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Alanay A, Acaroglu E, Yazici M, Oznur A, Surat A. Short-segment pedicle instrumentation of thoracolumbar burst fractures: does transpedicular intracorporeal grafting prevent early failure? Spine. 2001;26:213–217. doi: 10.1097/00007632-200101150-00017. [DOI] [PubMed] [Google Scholar]
- 3.Aligizakis A, Katonis P, Stergiopoulos K, Galanakis I, Karabekios S, Hadjipavlou A. Functional outcome of burst fractures of the thoracolumbar spine managed non-operatively, with early ambulation, evaluated using the load sharing classification. Acta Orthop Belg. 2002;68:279–287. [PubMed] [Google Scholar]
- 4.Aligizakis AC, Katonis PG, Sapkas G, Papagelopoulos PJ, Galanakis I, Hadjipavlou A. Gertzbein and load sharing classifications for unstable thoracolumbar fractures. Clin Orthop Relat Res. 2003;411:77–85. doi: 10.1097/01.blo.0000068187.83581.5d. [DOI] [PubMed] [Google Scholar]
- 5.Andress HJ, Braun H, Helmberger T, Schurmann M, Hertlein H, Hartl WH. Long-term results after posterior fixation of thoraco-lumbar burst fractures. Injury. 2002;33:357–365. doi: 10.1016/S0020-1383(02)00030-X. [DOI] [PubMed] [Google Scholar]
- 6.Atlas SW, Regenbogen V, Rogers LF, Kim KS. The radiographic characterization of burst fractures of the spine. AJR Am J Roentgenol. 1986;147:575–582. doi: 10.2214/ajr.147.3.575. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CS, Dvorak MF, Thomas KC, Boyd MC, Paquett S, Kwon BK, France J, Gurr KR, Bailey SI, Fisher CG. Comparison of thoracolumbosacral orthosis and no orthosis for the treatment of thoracolumbar burst fractures: interim analysis of a multicenter randomized clinical equivalence trial. J Neurosurg Spine. 2009;11:295–303. doi: 10.3171/2009.3.SPINE08312. [DOI] [PubMed] [Google Scholar]
- 8.Ballock RT, Mackersie R, Abitbol JJ, Cervilla V, Resnick D, Garfin SR. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg Br. 1992;74:147–150. doi: 10.1302/0301-620X.74B1.1732246. [DOI] [PubMed] [Google Scholar]
- 9.Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg Br. 2000;82:629–635. doi: 10.1302/0301-620X.82B5.11321. [DOI] [PubMed] [Google Scholar]
- 10.Briem D, Lehmann W, Rueckner AH, Windolf J, Rueger JM, Linhart W. Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch Orthop Trauma Surg. 2004;124:461–468. doi: 10.1007/s00402-004-0710-5. [DOI] [PubMed] [Google Scholar]
- 11.Butler JS, Walsh A, O’Byrne JO. Functional outcome of burst fractures of the first lumbar vertebrae managed surgically and conservatively. Int Orthop. 2005;29:51–54. doi: 10.1007/s00264-004-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976) 1993;18:971–976. doi: 10.1097/00007632-199306150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Celebi L, Muratli HH, Doğan O, Yağmurlu MF, Aktekin CN, Biçimoğlu A. The efficacy of non-operative treatment of burst fractures of the thoracolumbar vertebrae][in Turkish. Acta Orthop Traumatol Turc. 2004;38:16–22. [PubMed] [Google Scholar]
- 14.Chow GH, Nelson BJ, Gebhard JS, Brugman JL, Brown CW, Donaldson DH. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine (Phila Pa 1976) 1996;21:2170–2175. doi: 10.1097/00007632-199609150-00022. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. In Higgins JP, Green S, eds. West Sussex, England: The Cochrane Collaboration; 2008.
- 16.Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;382:119–123. doi: 10.1097/00003086-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Dai LY. Imaging diagnosis of thoracolumbar burst fractures. Chin Med Sci J. 2004;19:142–144. [PubMed] [Google Scholar]
- 18.Dai LY, Jiang SD, Wang XY, Jiang LS. A review of the management of thoracolumbar burst fractures. Surg Neurol. 2007;67:221–231. doi: 10.1016/j.surneu.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 19.Klerk LW, Fontijne WP, Stijnen T, Braakman R, Tanghe HL, Linge B. Spontaneous remodeling of the spinal canal after conservative management of thoracolumbar burst fractures. Spine (Phila Pa 1976) 1998;23:1057–1060. doi: 10.1097/00007632-199805010-00018. [DOI] [PubMed] [Google Scholar]
- 20.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Denis F, Armstrong GW, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit: a comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142–149. [PubMed] [Google Scholar]
- 22.Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine (Phila Pa 1976) 1992;17:528–540. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Greenough CG. Recovery from low back pain: 1–5 year follow-up of 287 injury-related cases. Acta Orthop Scand Suppl. 1993;254:1–34. [PubMed] [Google Scholar]
- 24.Ha KI, Han SH, Chung M, Yang BK, Youn GH. A clinical study of the natural remodeling of burst fractures of the lumbar spine. Clin Orthop Relat Res. 1996;323:210–214. doi: 10.1097/00003086-199602000-00029. [DOI] [PubMed] [Google Scholar]
- 25.Harris JR., Jr Radiographic evaluation of spinal trauma. Orthop Clin North Am. 1986;17:75–86. [PubMed] [Google Scholar]
- 26.Hitchon PW, Torner JC, Haddad SF, Follett KA. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49:619–626. doi: 10.1016/S0090-3019(97)00527-2. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer WJ, Schemitsch EH, Lever J, McBroom RJ, McKee MD, Waddell JP. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma. 1996;10:541–544. doi: 10.1097/00005131-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Limb D, Shaw DL, Dickson RA. Neurological injury in thoracolumbar burst fractures. J Bone Joint Surg Br. 1995;77:774–777. [PubMed] [Google Scholar]
- 29.Magel F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 30.McAfee PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89–104. [PubMed] [Google Scholar]
- 31.McDonough PW, Davis R, Tribus C, Zdeblick TA. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine (Phila Pa 1976) 2004;29:1901–1908. doi: 10.1097/01.brs.0000137059.03557.1d. [DOI] [PubMed] [Google Scholar]
- 32.McNamara MJ, Stephens GC, Spengler DM. Transpedicular short-segment fusions of treatment for lumbar burst fractures. J Spinal Disord. 1992;5:183–187. doi: 10.1097/00002517-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 34.Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures: the clinical efficacy and outcome of nonoperative management. Spine (Phila Pa 1976) 1993;18:955–970. doi: 10.1097/00007632-199306150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ni WF, Huang YX, Chi YL, Xu HZ, Lin Y, Wang XY, Huang QS, Mao FM. Percutaneous pedicle screw fixation for neurologic intact thoracolumbar burst fractures. J Spinal Disord Tech. 2010;23:530–537. doi: 10.1097/BSD.0b013e3181c72d4c. [DOI] [PubMed] [Google Scholar]
- 36.Oner FC, Ramos LM, Simmermacher RK, Kingma PT, Diekerhof CH, Dhert WJ, Verbout AJ. Classification of thoracic and lumbar spine fractures: problems of reproducibility. A study of 53 patients using CT and MRI. Eur Spine J. 2002;11:235–245. doi: 10.1007/s00586-001-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oprel PP, Tuinebreijer WE, Patka P, Hartog D. Combined anterior-posterior surgery versus posterior surgery for thoracolumbar burst fractures: a systematic review of the literature. Open Orthop J. 2010;4:93–100. doi: 10.2174/1874325001004010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid DC, Hu R, Davis LA, Saboe LA. The nonoperative treatment of burst fractures of the thoracolumbar junction. J Trauma. 1988;28:1188–1194. doi: 10.1097/00005373-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine (Phila Pa 1976) 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 40.Sanderson PL, Fraser RD, Hall DJ, Cain CM, Osti OL, Potter GR. Short segment fixation of thoracolumbar burst fractures without fusion. Eur Spine J. 1999;8:495–500. doi: 10.1007/s005860050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine: a comparative study of three fixation devices in 70 patients. Spine (Phila Pa 1976) 1993;18:450–460. [PubMed] [Google Scholar]
- 42.Scapinelli R, Candiotto S. Spontaneous remodeling of the spinal canal after burst fractures of the low thoracic and lumbar region. J Spinal Disord. 1995;8:486–493. doi: 10.1097/00002517-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Shen WJ, Liu TJ, Shen YS. Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine (Phila Pa 1976) 2001;26:1038–1045. doi: 10.1097/00007632-200105010-00010. [DOI] [PubMed] [Google Scholar]
- 44.Shen WJ, Shen YS. Nonsurgical treatment of three-column thoracolumbar junction burst fractures without neurologic deficit. Spine (Phila Pa 1976) 1999;24:412–415. doi: 10.1097/00007632-199902150-00024. [DOI] [PubMed] [Google Scholar]
- 45.Siebenga J, Leferink VJ, Segers MJ, Elzinga MJ, Bakker FC, Haarman HJ, Rommens PM, ten Duis HJ, Patka P. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine (Phila Pa 1976) 2006;31:2881–2890. doi: 10.1097/01.brs.0000247804.91869.1e. [DOI] [PubMed] [Google Scholar]
- 46.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4:351–358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 47.Tropiano P, Huang RC, Louis CA, Poitout DG, Louis RP. Functional and radiographic outcome of thoracolumbar burst fractures managed by closed orthopaedic reduction and casting. Spine (Phila Pa 1976) 2003;28:2459–2465. doi: 10.1097/01.BRS.0000090834.36061.DD. [DOI] [PubMed] [Google Scholar]
- 48.Roer N, Lange ES, Bakker FC, Vet HC, Tulder MW. Management of traumatic thoracolumbar fractures: a systematic review of the literature. Eur Spine J. 2005;14:527–534. doi: 10.1007/s00586-004-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively a long-term follow-up. Spine (Phila Pa 1976) 1988;13:33–38. doi: 10.1097/00007632-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox RK, Boerger TO, Allen DJ, Barton DC, Limb D, Dickson RA, Hall RM. A dynamic study of thoracolumbar burst fractures. J Bone Joint Surg Am. 2003;85:2184–2189. doi: 10.2106/00004623-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V, Butterman G. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective, randomized study. J Bone Joint Surg Am. 2003;85:773–781. doi: 10.1302/0301-620X.85B3.13849. [DOI] [PubMed] [Google Scholar]
- 52.Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech. 2005;18(suppl):15–23. doi: 10.1097/01.bsd.0000132287.65702.8a. [DOI] [PubMed] [Google Scholar]
- 53.Wood KB, Khanna G, Vaccaro AR, Arnold PM, Harris MB, Mehbod AA. Assessment of two thoracolumbar fracture classification systems as used by multiple surgeons. J Bone Joint Surg Am. 2005;87:1423–1429. doi: 10.2106/JBJS.C.01530. [DOI] [PubMed] [Google Scholar]
- 54.Yazici M, Atilla B, Tepe S, Calisir A. Spinal canal remodeling in burst fractures of the thoracolumbar spine: a computerized tomographic comparison between operative and nonoperative treatment. J Spinal Disord. 1996;9:409–413. doi: 10.1097/00002517-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Yi L, Jingping B, Gele J, Baoleri X, Taixiang W. Operative versus non-operative treatment for thoracolumbar burst fractures without neurological deficit. Cochrane Database Syst Rev. 2006;18(4):CD005079. doi: 10.1002/14651858.CD005079.pub2. [DOI] [PubMed] [Google Scholar]