Abstract
Background
Large-diameter metal-on-metal articulations reportedly improve stability and wear in THAs. However, some reports suggest some patients have unexplained hip and early failures with these implants. Thus, the potential benefits may be offset by these concerns. However, the incidence of these problems is not clearly established.
Questions/purposes
We therefore assessed hip pain, function, osteolysis, and complications in patients with large-diameter metal-on-metal THA.
Patients and Methods
We retrospectively reviewed 611 patients who had 681 large-diameter metal-on-metal THAs with the same cup and head design. The average age at operation was 62 years, 53% of the THAs were in men, and the average body mass index was 32 kg/m2. The diagnosis was osteoarthritis in 92% of the THAs. The minimum followup was 24 months (mean, 37 months; range, 24–60 months).
Results
Nine of the 611 patients (1.5%) experienced moderate or severe pain in the hip region that we considered to be coming from an extraarticular source in each case. Harris hip scores for pain averaged 42 points. Total Harris hip scores averaged 93 points. Cup abduction averaged 42°, and cup anteversion averaged 26°. There were no infections. Three cups (0.4%) were considered radiographically loose. All were secondary to inadequate seating of the shell.
Conclusion
Our observations suggest with this implant the concerns of higher incidences of groin pain, early failures, and adverse tissue reactions were not confirmed. Early successes or failures with large-diameter metal-on-metal articulations may be implant specific.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
In recent years, metal-on-metal (MOM) THA has become a commonly used alternative to conventional metal-on-polyethylene THA due to the potential for less wear and increased stability afforded with large-diameter (LD) femoral heads [6, 7, 22, 24, 28, 43, 48–51]. Indeed, MOM bearings, including surface replacement arthroplasties, account for 35% of all bearing surfaces used in the United States between 2005 and 2007 [6]. A 2010 American Association of Hip and Knee Surgeons survey demonstrated MOM bearings were routinely used in 68% of the society’s respondents [4]. However, this enthusiasm is tempered by concerns over metal ion release [25, 32, 42, 43], pseudotumors [10] in the form of adverse local tissue reactions (ALTRs) [24] or aseptic lymphocyte-dominated vasculitis-associated lesions [9], hypersensitivity [30], metallosis [38, 53], osteolysis [15, 47], and pain [3, 45].
Observations on MOM THAs with 28- and 32-mm articulations have been extensively reported [13, 18, 19, 21, 29, 43, 46, 47]. Some authors have noted intermediate results “equivalent” to metal-on-polyethylene THA [13] and reported survival rates of 86% at 5 years [21], 94% at 10 years [46], 98% at 10 years [29], 99% at 2 years [47], 99% at 6 years [18], and 100% at 7 years [19]. LD-MOM THA articulations (≥ 36 mm [14, 30, 43]) offer the potential advantages of less wear than smaller MOM heads [1, 2, 12, 16, 22, 50] and reduced risk of dislocation [33, 43] and may be a reasonable alternative for younger, more active patients [33, 37, 41]. Therefore, it is important to distinguish between smaller-diameter MOM THA and LD-MOM THA. Furthermore, it is axiomatic that not all MOM articulations are necessarily the same due to differences not only in head sizes but also in geometry, sphericity, metallurgy, surface finish, method of fabrication, and acetabular design [1, 2, 15, 22, 27, 36, 43, 56]. Thus, the potential benefits of MOM THA may be offset by the above concerns. Because of this variability, however, the incidences of these problems are not clearly established.
Thus, we determined the incidence of (1) hip pain, (2) function, (3) osteolysis, and (4) complications in patients with LD-MOM THA.
Patients and Materials
Between January 2005 and March 2008, we treated (1026 patients) with 1276 primary THAs at our institution. Of these, 10 THAs were implanted with cemented cups and 1266 THAs were implanted with uncemented cups. Of the uncemented THAs, 629 patients had 699 THAs using the same LD-MOM articulation design. In general, the indications for the use of a LD-MOM articulation were (1) younger or more active patients, (2) patients at increased risk of instability, and/or (3) adequate bone stock with the ability to achieve adequate fixation at operation without supplemental screw fixation. The contraindications were (1) inability to achieve adequate fixation with this cup, (2) lower-demand patients, and/or (3) patients unable to be compliant with protected weightbearing. Eight patients (eight hips) died before the 2-year followup, and 10 patients (10 hips) refused followup beyond 6 months. This left 611 patients with 681 primary LD-MOM THAs. Of these 681 THAs, 631 (53%) were in men. The average age was 62 years, and the average body mass index was 32 kg/m2 (Table 1). The diagnosis was osteoarthritis for 627 of the THAs (92%). All 611 patients had a minimum 2-year followup (mean, 36.5 months; range, 24–60 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Demographic data
| Demographic | Value |
|---|---|
| Number of patients | 611 |
| Number of THAs | 681 |
| Uncemented stems | 662 (97%) |
| Cemented stems | 19 (3%) |
| Diagnosis (number of hips) | |
| Osteoarthritis | 627 (92%) |
| Osteonecrosis | 34 (5%) |
| Femoral neck fracture | 16 (2%) |
| Rheumatoid arthritis | 3 (< 1%) |
| Legg-Calvé-Perthes | 1 (< 1%) |
| Sex (number of hips) | |
| Women | 320 (47%) |
| Men | 361 (53%) |
| Age at operation (years) | |
| Average | 62 |
| Range | 29–92 |
| SD | 11 |
| Body mass index (kg/m2) | |
| Average | 32 |
| Range | 17–59 |
| SD | 6.9 |
| Followup (months) | |
| Average | 36.5 |
| Range | 24–60 |
| SD | 1.1 |
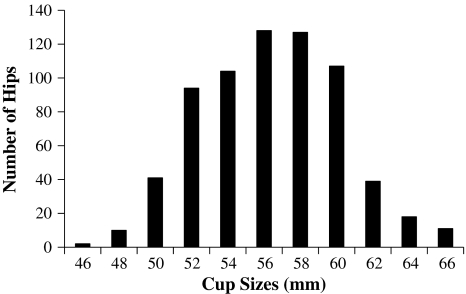
Five surgeons (EMK, PMF, JBM, MEB, RAM) implanted all THAs, three through a posterior approach (398 THAs) and two through an anterolateral approach (283 THAs). The surgeons used a cemented stem in 19 hips and an uncemented stem in the remaining 662 hips. They used the same monoblock cups and metal head design in all cases (Magnum™; Biomet, Inc, Warsaw, IN, USA). The cup was manufactured from high-carbon (0.23%–0.28%) CoCrMo alloy ‘as cast,’ without hot isostatic pressing or solution annealing. The outer diameter of the cup was a full hemisphere (180°). The outer press-fit surface was a titanium plasma spray with a surface roughness of 3090.5 μm. The spherical tolerance of the surface finish was 200 μm. The closed-back femoral head was manufactured with the same metallurgy and sphericity, yielding a radial clearance between the cup and head of between 150 and 300 μm (Fig. 1). The outside diameter of the cup ranged from 46 to 66 mm (Fig. 2). The most common sizes implanted were 54 mm (104 THAs), 56 mm (128 THAs), 58 mm (126 THAs), and 60 mm (107 THAs). Femoral head sizes were a commensurate 6 mm smaller in each case. Thus, the most common head sizes were 48 mm (104 THAs), 50 mm (128 THAs), 52 mm (126 THAs), and 64 mm (107 THAs). The cups were press fit in all cases, and surgeons intraoperatively recorded, measured, and referenced cup anteversion using the frontal plane of the pelvis.
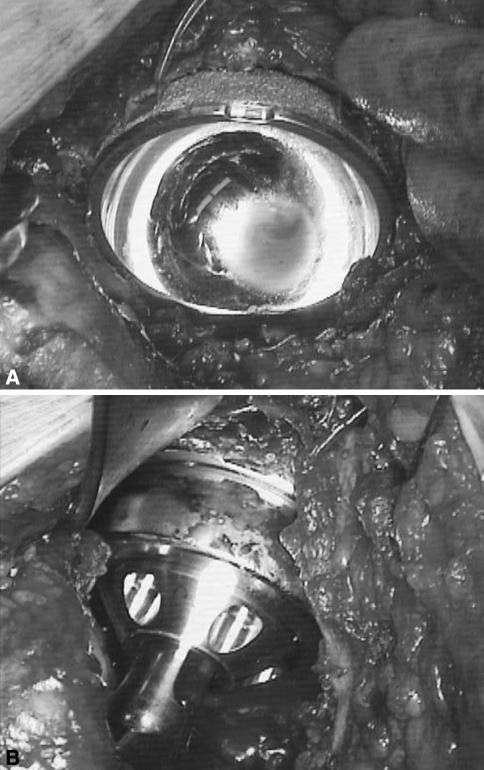
Fig. 1A–B.
Intraoperative photographs of the Magnum™ cup and head show (A) placement of the cup just before seating and (B) after the cup is fully seated and the hip is reduced.
Fig. 2.
A histogram depicts distribution of cup sizes. Femoral head sizes would be a corresponding 6 mm smaller for each cup size.
All patients received the same deep vein thrombosis prophylaxis using 1000 to 1500 U intravenous heparin sodium (Elkins-Sinn, Cherry Hill, NJ, USA) intraoperatively. All patients received either a cephalosporin or vancomycin perioperatively. According to surgeon preference, patients started either full or partial weightbearing on the first postoperative day. All patients received supervised in-hospital physiotherapy, walking assisted for 4 to 8 weeks. Supervised outpatient physiotherapy varied among patients according to need. Surgeons did not allow active hip flexion beyond 70° for 8 weeks.
The operative surgeon evaluated patients for pain and function at 8 weeks, 6 months, 1 year, 3 years, and 5 years using the Harris hip score (HHS) [31]. At each visit, they obtained supine AP pelvis and frog lateral radiographs. Each surgeon collected data on his own patients at each visit and each measured cup abduction [26]. We defined osteolysis as any sharp demarcated area adjacent to the acetabular component [44] and recorded radiolucencies according to the zonal distribution of DeLee and Charnley [20]. All data were collected prospectively.
Results
The average HHS for pain improved from 12 points (range, 0–40 points; SD, 6.4 points) preoperatively to 42 points (range, 20–44 points; SD, 6.1 points) at final followup. Nine patients (nine hips) complained of either continuous or occasional moderate or severe hip pain (pain score ≤ 30 points). None of these patients had pain localized to the groin or pain with flexion and internal rotation of the hip. Three of the nine patients had relief of symptoms with corticosteroid injections into the trochanteric bursa. The other six patients localized their pain to the low back or buttock region. We judged the hip pain to be extraarticular in all nine patients.
The average total HHS improved from 51 points (range, 13–96 points; SD, 11.7 points) preoperatively to 93 points (range, 34–100 points; SD, 10.2 points) at final followup.
There were no cases of radiographic osteolysis in the unrevised cups. At last followup, radiolucencies were seen in one patient in Zone 1 and only in two patients in Zone 3. The average postoperative cup abduction measured 42° (range, 15°–60°; SD, 12.1°). Cup anteversion averaged 26° (range, 10°–50°; SD, 6.3°).
Two of the three loose cups were revised at 6 months and 14 months. One patient had a radiographically loose cup at 9 months; at the time of followup, the hip had not been revised. One dislocation (posterior) occurred 9 months postoperatively. The head was revised, leaving the monoblock cup in place. There were no infections or stem failures. We identified three loose cups (0.4%), all within the first year after surgery. The etiology in all three cases was due to inadequate seating and not achieving adequate intraoperative stability of the shell at the time of operation (Fig. 3).
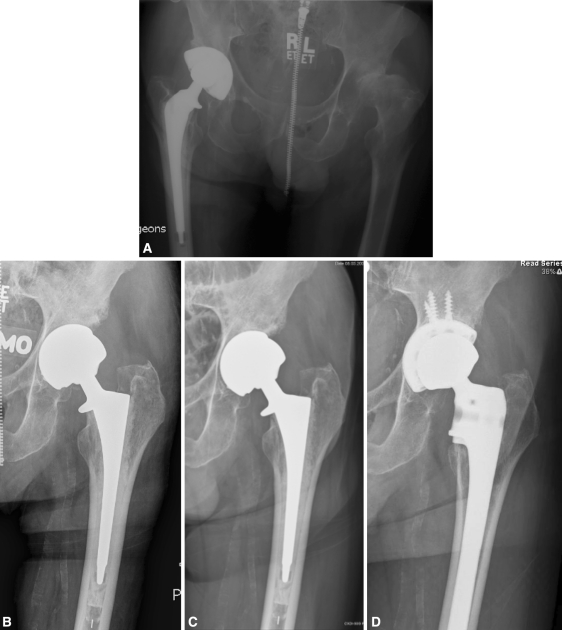
Fig. 3A–D.
Radiographs illustrate the case of a 68-year-old man who underwent LD-MOM THA of the left hip 17 years after THA of the right hip. (A) A preoperative radiograph shows avascular necrosis and superolateral subluxation of the left hip. (B) The initial 2-month radiograph of the Magnum™ THA shows incomplete seating of the shell against the medial wall. (C) The patient lived with pain for 12 months before revision THA. Note inferior osteolysis and progressive loosening of the cup. (D) At revision, the cemented stem was also loose. Workup for infection was unremarkable.
Discussion
Unexplained hip pain and ALTR after MOM THA can result in an unacceptably high rate of early cup loosening and revision [3]. In response to these concerns, both the Medicines and Healthcare products Regulatory Agency in the United Kingdom and the US Food and Drug Administration have issued warnings that patients with unexplained hip pain or prosthetic malposition after MOM THA should be monitored closely with clinical and radiographic evaluation and metal ion testing. Due to these early failures, we evaluated LD-MOM THA by determining the incidence of (1) hip pain, (2) function, (3) osteolysis, and (4) complications in patients with LD-MOM THA.
There are limitations to this study. First, the followup is still relatively early. However, in a review of 39 failed MOM THAs, Browne et al. [9] noted 20 failures (51%) occurred before 24 months. No failures were evident at 24 months in our study. Second, we did not use advanced imaging techniques, such as ultrasound, MRI, or CT, to establish or rule out the presence of a soft tissue mass. While the inclusion of these studies would be of substantial scientific interest, routine use of these studies in asymptomatic patients would be questionable at this time. Third, we did not obtain serum or whole-blood metal ion levels. Although the etiology and diagnosis of the nine patients with regional hip pain may have been accurate, one cannot rule out with 100% certainty that each of these patients did not have an intraarticular hip problem. Although such laboratory information is useful in symptomatic hips, we believe it premature to routinely measure metal ion levels in asymptomatic patients with MOM THAs [35]. Fourth, surgeons did not implant all patients with this particular prosthesis during this time interval. Indications varied with the surgeon’s confidence to obtain adequate fixation of the cup at the time of implantation. In general, however, this implant is not used in women of childbearing age and patients with end-stage renal disease, known metal allergy, and inadequate bone stock to support a press-fit cup with no screw supplementation. Fifth, we measured cup anteversion intraoperatively. Although accurate intraoperative determination of the frontal plane of the pelvis can be difficult, we did not obtain CT scans to measure or validate the accuracy of the anteversion. This variable would be of extreme importance when considering the relationship between cup malposition and failure in MOM THA [8, 17, 21, 42]. Finally, while we cannot definitely state the reasons for groin pain, the incidence was low.
The reasons for our successful early results may be both surgeon and implant related. Surgeon factors include cup positioning and seating. Early failures of MOM articulations are associated with cup malposition, including cup abduction of 50° to 55° and cup anteversion of greater than 45° to 50° [8, 17, 18, 40, 53]. The average abduction and anteversion in this study are not as extreme and may be contributing factors. While the adequacy of cup seating is not specifically measured in this report, it is noteworthy the seating and initial press fit of all three loose cups were determined to be inadequate. When using a monoblock shell with no screw holes, we recognize the native acetabular anatomy may preclude the use of this design due to bone stock deficiency. In some cases, this scenario may not be recognized until cup insertion. Implant factors associated with less wear in MOM articulations include surface finish [27, 36], clearance (affected by head size tolerance, sphericity, and cup deformation) [23, 54], carbon content [8, 14, 16], and casting process [11]. Additionally, closed backing of the femoral head can diminish metal ion release compared to an open-head design [55].
In contrast to 28- and 32-mm MOM THAs, there are relatively few studies reporting clinical results of LD-MOM THA (Table 2). Malviya et al. [43] reviewed 28 small-diameter MOM THA studies and specifically noted the importance in differentiating between large- and small-diameter heads. Unfortunately, of nine published LD-MOM studies, only two reported results beyond 24 months [5, 48]. The other six reported results at 13 months or less.
Table 2.
Small- and large-diameter metal-on-metal THA
| Study | Prosthesis | Number of hips | Average age (years) | Average followup (months) | Femoral head size (mm) | Number of loose/failed cups |
|---|---|---|---|---|---|---|
| Delaunay [18] | Metasul | 98 | 60 | 72 | 28 | 1 (1%) |
| Delaunay et al. [19] | Metasul | 83 | 41 | 87 | 28 | None |
| Donell et al. [21] | Ultima | 652 | 51 | NR | 28 | 90 (14%) |
| Neumann et al. [46] | Lubrimet | 94 | 57 | 126 | 32 | 6 (6%) |
| Park et al. [47] | Ultima | 169 | 55 | 27 | 28 | 2 (1%) |
| Cuckler et al. [14] | M2a | 616 | NR | 13 | 38 | None |
| Smith et al. [51] | M2a | 337 | 56 | 4 | 38 | None |
| Peters et al. [48] | Magnum | 99 | 54 | 36 | 46–56 | 1 (1%) |
| Stuchin [52] | Birmingham | 40 | 57 | 12 | 38–58 | None |
| Lavigne et al. [39] | Durom | 24 | 50 | 14 | NR | None |
| Garbuz et al. [28] | Durom | 56 | 52 | 13 | NR | None |
| Illgen et al. [34] | Durom | 29 | 50. | 12 | 42–56 | None |
| Berton et al. [5] | Durom | 92 | 50 | 43 | NR | 5 (5%) |
| Vendittoli et al. [55] | Durom | 29 | 50 | 12 | NR | None |
| Meding et al. | Magnum | 681 | 62 | 36 | 40–60 | 3 (0.4%) |
Prostheses included: Metasul® (Zimmer, Inc, Warsaw, IN, USA); Ultima® (DePuy Orthopaedics, Inc, Warsaw, IN, USA); Lubrimet™ (Smith and Nephew, Rotkreuz, Switzerland); M2a- Magnum™ (Biomet, Inc, Warsaw, IN, USA); Magnum™ (Biomet, Inc, Warsaw, IN, USA); Birmingham Hip™ (Smith and Nephew, Inc, Memphis, TN, USA); Durom® (Zimmer, Inc, Warsaw, IN, USA); NR = not reported.
In our study, there were no cases of regional hip pain that could be considered intraarticular in nature. Of the MOM THA studies reporting pain results [5, 18, 19, 21, 28, 34, 39, 48, 52], only Lavigne et al. [39], Peters et al. [48], and Stuchin [52] reported no cases of pain attributed to the hip.
Most authors reported functional results as well [5, 18, 19, 21, 28, 34, 39, 46–48, 52, 55]. Average HHSs of at least 90 have been noted [5, 34, 46, 48]. Stuchin [52] reported an average HHS of 88 in 40 LD-MOM THAs. Delaunay [18] and Delaunay et al. [19] reported 97% and 98% good or excellent results, respectively.
Several authors reported dislocation rates in their MOM THA series [5, 14, 18, 19, 21, 34, 48, 51, 52]. Of the LD-MOM series, only Peters et al. [48] reported any dislocations (two of 99 THAs). Not unexpectedly, the dislocation rate was higher in the MOM series using smaller head sizes: two of 98 THAs (2%) [18], one of 83 THAs (1.2%) [19], 19 of 652 (3%) THAs [21], and four of 94 (4%) THAs [46].
Of the seven studies reporting radiographic cup osteolysis [5, 14, 34, 46, 47, 51, 52], only Neumann et al. [46] and Park et al. [47] reported cases of osteolysis (4% and 17%, respectively).
The highest rates of loose or failed cups were noted in the 28-mm Ultima® articulations [21, 47] (Table 2). Of the LD-MOM cups, Berton et al. [5] reported on 92 Durom® LD-MOM THAs at an average followup of 43 months and noted loosening in five of 92 cups (5%). Peters et al. [48] reported on 99 Magnum™ LD-MOM THAs with an average followup of 36 months and noted one loose cup. Specifically, these authors noted no cases of unexplained hip pain or synovitis.
In conclusion, although we remain concerned over the issues of metal ion release, ALTR, and unexplained hip pain, the clinical manifestations of these reported problems are not clinically evident in our study. Further study is needed to determine whether our early observations will be confirmed.
Acknowledgments
We thank Philip M. Faris, MD, and Robert A. Malinzak, MD, who along with E. Michael Keating, MD, John B. Meding, MD, and Michael E. Berend, MD, were the orthopaedic surgeons for the patient population included in this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institutional approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Affatato S, Leardini W, Jedenmalm A, Ruggeri O, Toni A. Larger diameter bearings reduce wear in metal-on-metal hip implants. Clin Orthop Relat Res. 2006;456:153–158. doi: 10.1097/01.blo.0000246561.73338.68. [DOI] [PubMed] [Google Scholar]
- 2.Barnes CL, DeBoer D, Corpe RS, Nambu S, Carroll M, Timmerman I. Wear performance of larger-diameter differential-hardness hip bearings. J Arthroplasty. 2008;23:56–60. doi: 10.1016/j.arth.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468:2346–2356. doi: 10.1007/s11999-010-1356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry DJ, Bozic KJ. Current practice patterns in primary hip and knee and knee arthroplasty among members of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25:2–4. doi: 10.1016/j.arth.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Berton C, Girard J, Krantz N, Migaud H. The Durum large diameter head acetabular component: early results with a large-diameter metal-on-metal bearing. J Bone Joint Surg Br. 2010;92:202–208. doi: 10.1302/0301-620X.92B2.22653. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Ong K, Lau E, Kurtz SM, Vail TP, Rubash HE, Berry DJ. Risk of complication and revision total hip arthroplasty among Medicare patients with different bearing surfaces. Clin Orthop Relat Res. 2010;468:2357–2362. doi: 10.1007/s11999-010-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic KJ, Pui CM, Ludeman MJ, Vail TP, Silverstein MD. Do the potential benefits of metal-on-metal hip resurfacing justify the increased cost and risk of complications? Clin Orthop Relat Res. 2010;468:2301–2312. doi: 10.1007/s11999-010-1301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodner W, Grübl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19:66–70. doi: 10.1016/j.arth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Browne JA, Bechtold CD, Berry DJ, Hanssen AD, Lewallen DG. Failed metal-on-metal hip arthroplasties. Clin Orthop Relat Res. 2010;468:2313–2320. doi: 10.1007/s11999-010-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell P, Ebramzadeh E, Nelson S, Takamura K, Smet K, Amstutz HC. Histological features of pseudomotor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawley J, Metcalf JE, Jones AH, Band TJ, Skupien DS. A tribological study of cobalt chromium molybdenum alloys used in metal-on-metal resurfacing hip arthroplasty. Wear. 2003;255:999–1006. doi: 10.1016/S0043-1648(03)00046-2. [DOI] [Google Scholar]
- 12.Clarke MT, Lee PT, Arora LA, Villar RN. Levels of metal ions after small- and large-diameter metal-on-metal hip arthroplasty. J Bone Joint Surg Br. 2003;85:913–917. [PubMed] [Google Scholar]
- 13.Cuckler JM. The rationale for metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2005;441:132–136. doi: 10.1097/01.blo.0000193809.85587.f8. [DOI] [PubMed] [Google Scholar]
- 14.Cuckler JM, Moore KD, Lombardi AV, Jr, McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):41–44. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24:837–845. doi: 10.1016/j.arth.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Renal clearance of cobalt in relation to the use of metal-on-metal bearings in hip arthroplasty. J Bone Joint Surg Am. 2010;92:840–845. doi: 10.2106/JBJS.H.01821. [DOI] [PubMed] [Google Scholar]
- 17.Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 18.Delaunay CP. Metal-on-metal bearings in cementless primary total hip arthroplasty. J Arthroplasty. 2004;19:35–40. doi: 10.1016/j.arth.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Delaunay CP, Bonnomet F, Clavert P, Laffargue P, Migaud H. THA using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop Relat Res. 2008;466:340–346. doi: 10.1007/s11999-007-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 21.Donell ST, Darrah C, Nolan JF, Wimhurst J, Toms A, Barker TH, Case CP, Tucker JK, Norwich Metal-on-Metal Study Group Early failure of the Ultima metal-on-metal total hip replacement in the presence of normal plain radiographs. J Bone Joint Surg Br. 2010;92:1501–1508. doi: 10.1302/0301-620X.92B11.24504. [DOI] [PubMed] [Google Scholar]
- 22.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints. J Arthroplasty. 2004;19:118–130. doi: 10.1016/j.arth.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Dumbleton JH, Manley MT. Metal-on-metal total hip replacement: what does the literature say? J Arthroplasty. 2005;20:174–188. doi: 10.1016/j.arth.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Engh CA, Jr, Ho H, Engh CA. Metal-on-metal hip arthroplasty: does early clinical outcome justify the chance of an adverse local tissue reaction? Clin Orthop Relat Res. 2010;468:406–412. doi: 10.1007/s11999-009-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engh CA, Jr, MacDonald SJ, Sritulanondha S, Thompson A, Naudie D, Engh CA. Metal ion levels after metal-on-metal total hip arthroplasty: a randomized trial. Clin Orthop Relat Res. 2009;467:101–111. doi: 10.1007/s11999-008-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein NJ, Woolson ST, Giori NJ. Acetabular component positioning using the transverse acetabular ligament: can you find it and does it help? Clin Orthop Relat Res. 2010;469:412–416. doi: 10.1007/s11999-010-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher J, Jin Z, Tipper J, Stone M, Ingham E. Tribology of alternative bearings. Clin Orthop Relat Res. 2006;453:25–34. doi: 10.1097/01.blo.0000238871.07604.49. [DOI] [PubMed] [Google Scholar]
- 28.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25:841–848. doi: 10.1002/jor.20381. [DOI] [PubMed] [Google Scholar]
- 30.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. doi: 10.1302/0301-620X.83B3.9674. [DOI] [PubMed] [Google Scholar]
- 31.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 32.Hart AJ, Skinner JA, Winship P, Faria N, Kulinskaya E, Webster D, Muirhead-Allwood S, Aldam CH, Anwar H, Powell JJ. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg Br. 2009;91:835–842. doi: 10.1302/0301-620X.91B6.21844. [DOI] [PubMed] [Google Scholar]
- 33.Huo MH, Parvizi J, Bal BS, Mont MA. What’s new in total hip arthroplasty? J Bone Joint Surg Am. 2009;91:2522–2534. doi: 10.2106/JBJS.I.00801. [DOI] [PubMed] [Google Scholar]
- 34.Illgen RL, Heiner JP, Squire MW, Conrad DN. Large-head metal-on-metal total hip arthroplasty using the Durum acetabular component at minimum 1-year interval. J Arthroplasty. 2010;25:26–30. doi: 10.1016/j.arth.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19:59–65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Kamali A, Hussain A, Li C, Pamu J, Daniel J, Ziaee H, Daniel J, McMinn DJ. Tribological performance of various CoCr microstructures in metal-on-metal bearings: the development of a more physiological protocol in vitro. J Bone Joint Surg Br. 2010;92:717–725. doi: 10.1302/0301-620X.92B5.23320. [DOI] [PubMed] [Google Scholar]
- 37.Kim RH, Dennis DA, Carothers JT. Metal-on-metal total hip arthroplasty. J Arthroplasty. 2008;23:44–46. doi: 10.1016/j.arth.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty: five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 39.Lavigne M, Therrien M, Nantel J, Roy A, Prince F, Venittoli PA. The functional outcome of hip resurfacing and large-head THA is the same: a randomized, double-blind study. Clin Orthop Relat Res. 2010;468:326–336. doi: 10.1007/s11999-009-0938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop Relat Res. 2009;467:2259–2265. doi: 10.1007/s11999-009-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;19:12–16. doi: 10.1016/j.arth.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Maezawa K, Nozawa M, Matsuda K, Sugimoto M, Shitoto K, Kurosawa H. Serum chromium levels before and after revision surgery for loosened metal-on-metal total hip arthroplasty. J Arthroplasty. 2009;24:549–553. doi: 10.1016/j.arth.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Malviya A, Ramaskandhan J, Holland JP, Lingard EA. Metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2010;92:549–553. doi: 10.2106/JBJS.I.01426. [DOI] [PubMed] [Google Scholar]
- 44.Moen TC, Ghate R, Salaz N, Ghodasra J, Stulberg SD. A monoblock porous tantalum acetabular cup has no osteolysis on CT at 10 years. Clin Orthop Relat Res. 2011;469:382–386. doi: 10.1007/s11999-010-1500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasser AB, Beaulé PE, O’Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468:392–399. doi: 10.1007/s11999-009-1133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann DR, Thaler C, Hitzl W, Huber M, Hofstadter T, Dorn U. Long-term results of a contemporary metal-on-metal total hip arthroplasty: a 10-year follow-up study. J Arthroplasty. 2010;25:700–708. doi: 10.1016/j.arth.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–1521. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- 48.Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22:140–144. doi: 10.1016/j.arth.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Schmalzried TP. The optimal metal-metal arthroplasty is still a total hip arthroplasty: in opposition. J Arthroplasty. 2006;21:77–79. doi: 10.1016/j.arth.2006.02.089. [DOI] [PubMed] [Google Scholar]
- 50.Silva M, Heisel C, Schmalzried TP. Metal-on-metal total hip replacement. Clin Orthop Relat Res. 2005;430:53–61. doi: 10.1097/01.blo.0000149995.84350.d7. [DOI] [PubMed] [Google Scholar]
- 51.Smith TM, Berend KR, Lombardi AV, Jr, Emerson RH, Jr, Mallory TH. Metal-on-metal total hip arthroplasty with large heads may prevent early dislocation. Clin Orthop Relat Res. 2005;441:137–142. doi: 10.1097/01.blo.0000193810.23706.73. [DOI] [PubMed] [Google Scholar]
- 52.Stuchin SA. Anatomic diameter femoral heads in total hip arthroplasty: a preliminary report. J Bone Joint Surg Am. 2008;90:52–56. doi: 10.2106/JBJS.H.00690. [DOI] [PubMed] [Google Scholar]
- 53.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J. Bone Joint Surg Am. 2010;92:2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 54.Tuke MA, Scott G, Roques A, Hi XQ, Taylor A. Design considerations and life prediction of metal-on-metal bearings: the effect of clearance. J Bone Joint Surg Am. 2008;90(Suppl 3):134–141. doi: 10.2106/JBJS.H.00610. [DOI] [PubMed] [Google Scholar]
- 55.Vendittoli PA, Amzica T, Roy AG, Lusignan D, Girard J, Lavigne M. Metal ion release with large-diameter metal-on-metal hip arthroplasty. J Arthroplasty. 2011;26:282–288. doi: 10.1016/j.arth.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Williams S, Leslie I, Isaac G, Jin Z, Ingham E, Fisher J. Tribology and wear of metal-on-metal hip prostheses: influence of cup angle and head position. J Bone Joint Surg Am. 2008;90(Suppl 3):111–117. doi: 10.2106/JBJS.H.00485. [DOI] [PubMed] [Google Scholar]