Abstract
Background
The Bernese periacetabular osteotomy (PAO) is commonly used to surgically treat residual acetabular dysplasia. However, the degree to which function and radiographic deformity are corrected in patients with more severe deformities that have undergone previous reconstructive pelvic or femoral osteotomies is unclear.
Questions/purposes
We evaluated hip pain and function, radiographic deformity correction, complications, reoperations, and early failures (conversion to THA) associated with PAO in hips treated with previous reconstructive hip surgery.
Methods
We retrospectively reviewed 63 patients who had undergone 67 PAOs after a previous reconstructive hip procedure. We compared preoperative hip scores and radiographic parameters with postoperative values at most recent followup. We recorded complications, need for nonarthroplasty revision surgery, and failures. Minimum followup was 2 years.
Results
Five of the 67 hips (8%) were converted to THA between 24 and 118 months. The average followup for the remaining 62 hips was 60 months (range, 24–147 months). The average Harris hip score improved 11 points, and postoperatively, 83% of the hips had pain component scores of greater than 30 (none, slight, or mild pain). Radiographically, there were improvements in lateral center-edge angle (25°), anterior center-edge angle (23°), Tönnis angle (17°), and medialization of the hip center (8 mm). Complications occurred in 13 hips (19%). Seven hips (10%) underwent a subsequent surgical procedure to address residual pain or deformity.
Conclusions
PAO performed after previous reconstructive hip surgery improves hip function and corrects residual dysplasia deformities. These procedures are inherently more complex than primary PAO and are associated with a considerable risk of perioperative complications, reoperations, and early treatment failures.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Since its initial description by Ganz et al. [13], the Bernese periacetabular osteotomy (PAO) has been increasingly used to treat symptomatic acetabular dysplasia [8]. Numerous reports document the ability of this procedure to improve femoral head coverage, decrease acetabular inclination, and medialize the hip center of rotation [5, 7, 10, 20, 25, 30, 31]. Additionally, improvement in hip pain and function scores occurs in 73% to 97% of patients [5, 7, 11, 20, 25, 30, 31]. The indications for PAO continue to expand as the interest and utilization of this procedure continue to grow, with several authors combining PAO with other procedures such as surgical dislocation [1] and proximal femoral osteotomy (PFO) [9] for the treatment of complex structural hip deformities.
While PAOs in most series were performed in isolation for the correction of untreated acetabular dysplasia, some patients in these series had reconstructive hip surgery before their PAO [5, 7, 10, 20, 23–25, 31]. Mayo et al. [21] reported on 19 hips that underwent PAO after a previous reconstructive hip surgery and, compared with a control group of PAO-only patients, found no difference in improvement in hip scores or failures at followup times of 26 to 85 months. Hips with prior reconstructive procedures can be more challenging to treat due to retained hardware, scar tissue, osteotomy deformities, abductor compromise, and potential compromised blood supply from perivascular and muscular stripping during previous surgical procedures. However, current information is inadequate to determine the effect of previous reconstructive hip surgery on measurable outcome parameters in patients undergoing PAO.
We therefore analyzed (1) hip pain and function (Harris hip score [HHS]), (2) radiographic deformity correction, (3) complications, (4) need for subsequent surgery, and (5) early failures associated with PAO in hips treated with a previous reconstructive procedure (shelf, pelvic, and/or femoral osteotomy).
Patients and Methods
We performed a total of 741 PAOs in 712 patients for symptomatic acetabular dysplasia from October 1, 1997, to May 15, 2008. We reviewed the clinical records from these patients to identify all hips that had a previous reconstructive hip procedure before the index PAO. Of these 712 patients, we identified 74 patients (78 hips) who underwent a previous reconstructive hip surgery (shelf procedure, pelvic osteotomy, PFO, or combination thereof). Eleven patients (11 hips) with less than 2-year followup were not available for evaluation and could not be located despite extensive efforts. The average followup of these 11 patients was 13 months (range, 0–22 months). Two of these 11 patients never returned after their procedure; nine returned and none of these had failed treatment or had a complication at the time of last followup. Excluding these 11 patients, the remaining 63 patients (85%) (67 hips) had a minimum of 2 years of clinical and radiographic followup. One patient did not have a preoperative HHS, but this patient was included in the analysis. Four patients had staged bilateral PAO procedures. Twenty-nine procedures were performed at hospitals affiliated with Washington University School of Medicine (JCC, PLS), while 38 of these procedures were performed at Children’s Hospital Boston (YJK, MBM). There were 52 female patients and 11 male patients, and the average age at the time of surgery was 19.2 years (range, 10–40 years). The average BMI was 23 (range, 15–35). The minimum followup was 24 months (average, 59 months; range, 24–147 months). The review of the data included in this study was approved by the institutional review board at each institution (Washington University School of Medicine and Children’s Hospital Boston).
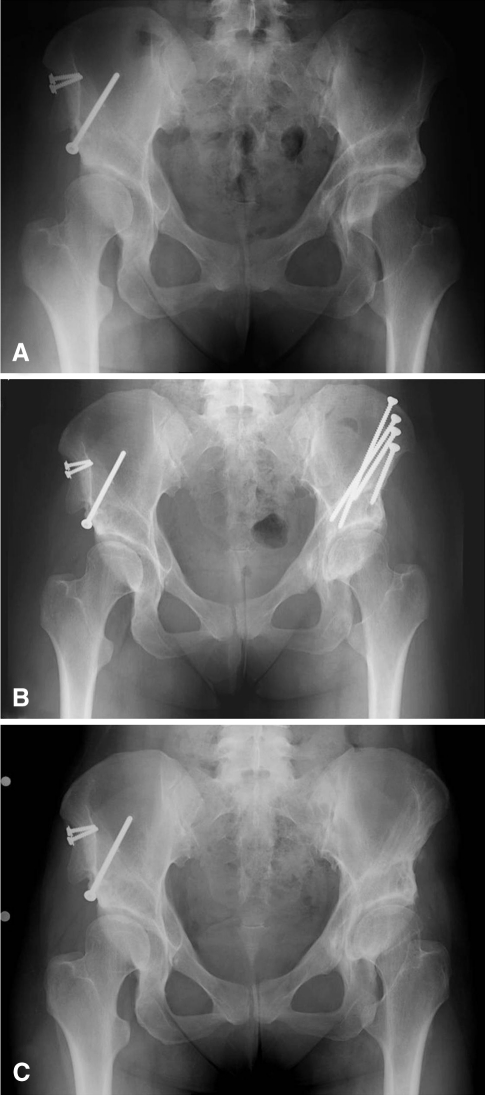
The preoperative diagnoses in the 67 cases performed included isolated hip dysplasia in 49 hips, while the remainder of cases involved hip dysplasia in addition to one of the following diagnoses: Charcot-Marie-Tooth disease (three hips), peripheral arthrogryposis (one hip), proximal femoral focal deficiency (one hip), multiple epiphyseal dysplasia (one hip), femoral growth arrest (one hip), and Legg-Calvé-Perthes disease (10 hips). One patient had femoroacetabular impingement secondary to a retroverted acetabulum after a previous PAO (Fig. 1).
Fig. 1A–C.
(A) An AP pelvic radiograph shows the hips of a 31-year-old woman who presented with left hip pain 10 years after PAO. Clinically, she was diagnosed with symptomatic femoroacetabular impingement from acetabular retroversion. (B) She was treated with an anteversion PAO. (C) She had an excellent clinical result 3 years after surgery with no pain and no limitation of activity.
Ninety previous procedures were performed on these 67 hips (Table 1). Eighteen hips had a prior pelvic osteotomy to correct for acetabular dysplasia, 21 hips had undergone a varus- or valgus-producing PFO, and 28 hips underwent a combined pelvic and PFO procedure. Three of the 18 prior pelvic-only procedures involved salvage osteotomies (Chiari osteotomy or shelf procedures), and four hips from the combined pelvic/PFO group included a salvage pelvic osteotomy. The reconstructive (nonsalvage) osteotomy procedures performed about the pelvis included Salter (13 hips), Dega (three hips), Pemberton (seven hips), Steele (three hips), and Ganz PAO (four hips) and unspecified osteotomies (10 hips). The time from each patient’s index reconstructive hip procedure to PAO at one of our institutions was not specified in enough cases to be included in this study.
Table 1.
Previous surgical procedures
| Previous procedure | Number of hips |
|---|---|
| Nonsalvage pelvic osteotomy | 15 |
| PFO | 21 |
| Combined pelvic and PFO | 24 |
| Salvage pelvic osteotomy | 3 |
| Salvage pelvic osteotomy and PFO | 4 |
| Total previous osteotomies | 90 |
PFO = proximal femoral osteotomy.
We performed a thorough history of ongoing symptoms and previous surgical procedures and examination of hip ROM in all patients. We also performed a radiographic analysis of AP pelvis and false-profile radiographs. Patients were considered candidates for PAO surgery if they had hip pain, radiographic evidence of acetabular dysplasia or retroversion, acetabular deformity that was correctible through the use of a PAO, Tönnis Grade 0 or 1 osteoarthritis (OA) [29], and adequate hip motion (hip flexion of at least 90°). Patients were not considered good surgical candidates if they had advanced Tönnis OA (Grade 3 and 4), if their previous reconstructive hip surgery resulted in a severely incongruent joint, or if they had poor hip motion. Mild preoperative joint incongruity was accepted in this more complex patient population.
We used a modified anterior [22] or Smith-Peterson [13] approach to perform the acetabular osteotomy procedure as previously described by Ganz et al. [6, 13]. Modifications in the surgical incision and approach were made on a case-by-case basis in hips that had undergone a previous pelvic osteotomy surgery when necessary. Previous hardware was removed if it would interfere with the osteotomy cuts or fixation of the osteotomy fragment. In no cases with previous pelvic osteotomy surgery was a separate surgical dissection performed to release or explore the sciatic nerve before PAO. A Cell Saver® device (Haemonetics, Braintree, MA, USA) was used for blood collection and reinfusion, and EMG peripheral nerve monitoring in the operative extremity was also utilized in all procedures at one of the institutions. We used intraoperative AP and false-profile views with fluoroscopic image intensification to monitor the osteotomy cuts, the reduction and correction of the osteotomy fragment, and screw fixation of the osteotomy fragment. Three, four, or five 4.5-mm cortical screws were used to fix the acetabular fragment. The goal for deformity correction was to improve femoral head coverage and to medialize the hip to help reduce the joint reaction force. When possible, we corrected the lateral center-edge angle (LCEA) and anterior center-edge angle (ACEA) to greater than 20° and the Tönnis angle to less than 10°, and we medialized the hip such that the most medial aspect of the femoral head was 5 to 10 mm lateral to the ilioischial line. In some hips with more severe deformity, this extent of correction was limited by the ability to obtain stable osteotomy fragment fixation because of deformity from previous surgery or because correction of the deformity would lead to an unacceptable limitation in postoperative ROM (< 90° hip flexion). In these hips, a balance was accomplished that allowed adequate hip motion at the expense of complete correction of the radiographic parameters described above. In cases of severe acetabular retroversion, the anterior margin of the pubic ramus osteotomy was resected with a high-speed burr to facilitate correction and avoid binding on the remnant medial pubic osteotomy segment if necessary. On completion of acetabular fragment fixation, an anterior arthrotomy was variably performed (32 of 67 hips, 48%), and the ROM of each hip was assessed in all cases for impingement or residual deformity that limited postcorrection motion. We made an intraoperative decision based on this assessment to perform additional reconstructive procedures if necessary. A total of 42 hips (63%) had PAO only, while 25 hips (37%) had an additional procedure performed to reduce hip impingement or improved residual deformity. Thirteen hips had an osteoplasty at the anterior head-neck junction for impingement. Five hips had a varus- or valgus-producing PFO performed to correct residual proximal femoral deformity, and three hips had a surgical hip dislocation procedure combined with advancement of the greater trochanter to functionally lengthen the femoral neck and improve hip offset. A total of four hips had both PFO and surgical hip dislocation with head-neck junction osteoplasty, labral repair, and trochanteric advancement to correct residual deformity. Two of the patients who underwent both PAO and PFO also had an adductor tenotomy to address an adductor contracture. In all these cases, the PAO was performed first, followed by the subsequent procedure on the femoral side of the joint if necessary. In cases with severe femoral deformity in which the deformity will not allow for the PAO correction to be performed, an initial femoral procedure to reduce this deformity may be necessary, but this situation did not occur in this series of cases.
An epidural catheter was used in most patients for 24 to 48 hours postoperatively for pain control, and patients were encouraged to ambulate with the assistance of crutches or a walker under the supervision of a physical therapist on Postoperative Day 2. Weightbearing was limited to 50% body weight in most cases for 4 weeks. Once independence with ambulation and adequate pain control were achieved, patients were discharged from the hospital with instructions to participate in a physical therapy routine until their gait normalized or plateaued.
Patients returned to their surgeon’s outpatient clinic at a minimum routine interval of 2 weeks, 6 weeks, 3 months, 6 months, and annually thereafter. We assessed hip function using the HHS [15]. Patients were assessed preoperatively and at subsequent postoperative followup clinic visits. One patient (two hips) in the cohort did not have a preoperative HHS, and this patient’s postoperative score was excluded from the analysis. We defined clinical failure as a patient having persistent hip pain and progression of hip arthrosis requiring conversion to a THA. These patients (n = 5) were analyzed separately, and their hip scores and radiographic analysis were excluded from the remainder of the cohort. The average followup for the remainder of the patients was 60 months (range, 24–147 months). We noted the need for and type of subsequent surgical procedures and recorded the presence of complications using a version of the Clavien-Dindo complication classification system for general surgery [4] that was modified for hip preservation procedures [26]. In this scheme, complications are graded from 1 to 5 in severity, with each grade based on the long-term morbidity of the complication and the treatment necessary to manage the complication. A Grade 1 complication needs no change in postoperative care, Grade 2 requires modification in outpatient care, Grade 3 involves an invasive surgical or radiographic intervention, Grade 4 includes potential life-threatening complications or those with high long-term morbidity, and a Grade 5 complication involves death.
Two orthopaedic joint reconstruction/hip preservation fellows (GGP, ENN) performed an unblinded radiographic analysis of preoperative and postoperative radiographic images for procedures performed at Washington University School of Medicine and Children’s Hospital Boston, respectively. From AP and false-profile radiographs obtained at each clinic visit, the following measurements were performed: LCEA [2, 32], ACEA [14, 18], Tönnis angle [29], medialization of hip center in millimeters, and Tönnis OA grade [29]. These measurements are widely accepted as reliable ways to quantify the extent of hip deformity and are useful to calculate the extent of correction [19, 27]. Preoperative and postoperative values at most recent followup for these measurements were compared to quantify the extent of deformity correction and to assess for the progression to OA. The presence of bridging bone across the osteotomy sites was used to determine radiographic evidence of osteotomy healing. One patient (one hip) did not have preoperative radiographs available for review, and her data were excluded from the analysis.
We used a paired Student’s t-test to compare preoperative and followup radiographic measurements and clinical hip scores (Microsoft Corp, Redmond, WA, USA).
Results
The average preoperative and postoperative HHSs were 70 points (range, 24–97 points) and 81 points (range, 39–100 points), respectively, in the 62 hips that did not go on to clinical failure (Table 2). Forty-four hips (71%) had an improved HHS; the average improvement in HHS was 11.4 points. Thirty-eight hips (61%) had a HHS of greater than 80 points. Thirty-four patients (36 hips) had greater than 10-point improvement in their HHS. The pain component of the HHS was available and isolated in 59 hips, and postoperatively, the pain scores improved such that 49 hips (83%) had pain component scores of greater than 30 (none, slight, or mild pain), and 10 hips (17%) had pain component scores of less than 30 (moderate, marked, or disabling pain).
Table 2.
Clinical results
| Variable | Value | p value |
|---|---|---|
| Preoperative HHS (points)* | 70 (24–97) | |
| Postoperative HHS (points)* | 81 (39–100) | < 0.001 |
| Change in HHS (points)* | 11 (−41–44) | |
| Postoperative HHS > 80 points (number of hips) | 38 (61%) | |
| > 10-point improvement in HHS (number of hips) | 36 (58%) | |
| Preoperative HHS pain component score > 30† (number of hips) | 22 (37%) | |
| Preoperative HHS pain component score < 30† (number of hips) | 37 (63%) | |
| Postoperative HHS pain component score > 30† (number of hips) | 49 (83%) | |
| Postoperative HHS pain component score < 30† (number of hips) | 10 (17%) |
* Values are expressed as mean, with range in parentheses; †pain component of HHS: < 30 points = none, slight, or mild pain; > 30 points = moderate, marked, or disabling pain; HHS = Harris hip score.
The radiographs suggested postoperative improvement in the correction of underlying hip deformity (Table 3). The average change was 25° in LCEA (p < 0.001), 23° in ACEA (p < 0.001), 17° in Tönnis angle (p < 0.001), and 8 mm in medialization of the hip center (p < 0.001). Twelve patients (19.6%) had progression of their Tönnis OA grade by one grade. No hips had Tönnis Grade 3 OA preoperatively or at most recent followup. Five hips had reduction in their Tönnis Grade from 1 to 0.
Table 3.
Radiographic outcome
| Variable | Preoperative | Postoperative | Change | p value |
|---|---|---|---|---|
| LCEA (°) | 2 | 26 | 25 | < 0.001 |
| ACEA (°) | 3 | 25 | 23 | < 0.001 |
| Tönnis angle (°) | 27 | 10 | 17 | < 0.001 |
| Medial translation of joint center (mm) | 16 | 8 | 8 | < 0.001 |
| Tönnis OA grade (number of hips) | ||||
| 0 | 29 | 33 | ||
| 1 | 28 | 16 | ||
| 2 | 3 | 11 | ||
| 3 | 0 | 0 | ||
LCEA = lateral center-edge angle; ACEA = anterior center-edge angle; OA = osteoarthritis.
In 54 of the 67 hips (81%), there were no complications, while 12 of the 67 hips had one complication and one had two, for an overall complication rate of 19% (Table 4). There was one Grade 1 complication, five Grade 2 complications, six Grade 3 complications, two Grade 4 complications, and no Grade 5 complications (Table 4).
Table 4.
Complications
| Complication | Treatment | Number | Clinical failure? |
|---|---|---|---|
| Grade 1 | |||
| Transient foot paresthesias | 1 | No | |
| Total | 1 | ||
| Grade 2 | |||
| Subcutaneous hematoma | 1 | No | |
| Transient lateral femoral cutaneous nerve dysesthesia | Gabapentin | 1 | No |
| Superficial wound dehiscence | Oral antibiotics | 1 | No |
| Transient femoral nerve palsy | 2 | Yes | |
| Total | 5 | ||
| Grade 3 | |||
| Superficial infection* | I&D | 1 | Yes* |
| Posterior column nonunion* | Bone grafting | 1 | Yes* |
| Superficial infection | Hospital readmission | 1 | Yes |
| Wound infection | I&D | 1 | Yes |
| Deep peroneal nerve palsy | Femoral shortening osteotomy + peroneal nerve release | 1 | Yes |
| PFO nonunion and hardware failure | ORIF of PFO + bone grafting | 1 | No |
| Total | 6 | ||
| Grade 4 | |||
| Sciatic nerve neuron optmesis | Nerve exploration + neurolysis + sural nerve cable grafting | 1 | No |
| Sciatic nerve palsy, Grade 4/5 ankle dorsiflexion/eversion strength | 1 | No | |
| Total | 2 | ||
* Same patient; I&D = irrigation and débridement; ORIF = open reduction and internal fixation; PFO = proximal femoral osteotomy.
Seven patients underwent a secondary surgical procedure for persistent pain and 45 patients had removal of their hardware (Table 5). These procedures were performed to address residual deformity that was not completely corrected with the PAO procedure (one patient underwent a PFO) or subsequent labral pathology or symptomatic hip impingement (treated with arthroscopy or an open surgical dislocation procedure in five hips). Overall, this subgroup of patients had a decrease in their hip scores after their subsequent surgical procedure, with scores changing from 79 points (range, 57–97 points) preoperatively to 64 points (range, 39–83 points) postoperatively.
Table 5.
Secondary surgical procedures
| Description | Number |
|---|---|
| Removal of hardware | 45 |
| Procedures to address residual deformity or impingement | |
| Hip arthroscopy | 3 |
| Surgical dislocation with femoral osteoplasty | 2 |
| PFO | 1 |
| Other procedures | |
| Hip arthrotomy, partial iliopsoas tendon and lateral femoral cutaneous nerve resection | 1 |
PFO = proximal femoral osteotomy.
Five patients had incomplete resolution of their pain, showed radiographic progression of hip arthrosis, and underwent THA at an average of 79 months (range, 25–118 months) after their PAO (Table 6). The average age of this group of patients was 18 years (range, 10–27 years). Of the failures, two of these patients had undergone a previous salvage procedure, one patient had undergone a combined pelvic and femoral procedure, one patient had undergone an isolated pelvic osteotomy, and one patient had undergone an isolated PFO.
Table 6.
Failures
| Patient | Age (years) | Diagnosis | Previous surgery | Current surgery | Reason for failure | Complications (grade/description) | Interval between PAO and THA (months) |
|---|---|---|---|---|---|---|---|
| 1 | 20.0 | DDH | PFO | PAO | Pain/radiographic progression of arthrosis | Grade 3/superficial infection requiring incision and drainage | 47 |
| 2 | 18.0 | DDH, Perthes’ disease | PFO, abductor tenotomy | PAO + PFO | Pain/radiographic progression of arthrosis | Grade 3/peroneal nerve palsy requiring femoral shortening osteotomy | 109 |
| 3 | 26.5 | DDH | Chiari osteotomy | PAO | Pain/radiographic progression of arthrosis | Grade 3/superficial infection requiring I&D; Grade 3/posterior column nonunion requiring ORIF | 118 |
| 4 | 14.5 | DDH | Pelvic osteotomy, not specified | PAO | Pain/radiographic progression of arthrosis | Grade 2/superficial wound infection requiring antibiotics | 25 |
| 5 | 10.4 | DDH | Pemberton osteotomy | PAO | Pain/radiographic progression of arthrosis | Grade 2/transient femoral nerve palsy | 97 |
| Average | 17.9 | 79.2 |
PAO = periacetabular osteotomy; DDH = developmental dysplasia of the hip; PFO = proximal femoral osteotomy; I&D = irrigation and débridement; ORIF = open reduction and internal fixation.
Discussion
PAO is commonly used to surgically treat residual acetabular dysplasia. The outcome of this procedure in patients with more severe deformities that have undergone previous reconstructive pelvic or femoral osteotomies is not well known. We therefore analyzed hip pain and function (HHS), radiographic deformity correction, complications, reoperations, and early failures associated with PAO in hips treated with a previous reconstructive procedure (shelf, pelvic, and/or femoral osteotomy).
There are limitations to this study. First, we did not have a comparable control group. Because of the more complex deformities present in this cohort of patients, though, it is difficult to compare these patients with a historical control group of patients in which no previous reconstructive hip surgery has been performed. Second, while the average followup in this study was approximately 5 years, many patients had shorter followup. This underscores the importance of longer-term followup on this group of patients as the survivorship of these reconstructions is not known. Third, the details of many of the patient’s previous hip operations were unknown to us at the time of their present evaluation and surgical intervention. Fourth, the patient population included in this study represents a heterogeneous group of patients with various diagnoses operated on for various indications, and therefore our findings may not be generalizable to all patients undergoing PAO after a previous reconstructive hip operation.
The clinical scores in our patients improved on average 11 points. There are limited data in the orthopaedic literature on similar patients who have undergone PAO after previous hip reconstruction (Table 7). Mayo et al. [21] compared 19 hips in 18 patients who underwent PAO after a previous hip operation with a group of patients (104 osteotomies) without previous hip surgery. At an average followup of 45.4 months (range, 26–85 months), these hips had an average improvement in HHS of 30 points, and two patients went subsequently had THA. The authors found no difference in HHS between the groups. In a more recent study analyzing complications after PAO in adolescents, Thawrani et al. [28] included a subset of patients who had previously undergone reconstructive hip surgery. Of the 83 hips included in their series, 56% had undergone either pelvic or femoral osteotomy before their PAO. The authors found no difference in complications or radiographic deformity correction compared with the remainder of patients in their series when this subset was analyzed separately. While the HHS may have improved less in our series than previously reported [21], it may be the case that the HHS underestimates the overall clinical improvement in this group of patients with complex deformities. Ten percent of the hips in our series were in patients with underlying medical conditions associated with their dysplasia (Charcot-Marie-Tooth, multiple epiphyseal dysplasia, proximal femoral focal deficiency, arthrogryposis, or femoral growth arrest), which may have affected their overall function, and thus their clinical hip score, in the absence of a painful hip.
Table 7.
Comparison of PAO studies highlighting cases after previous reconstructive hip surgery
| Study | Number of hips/patients | % patients with previous reconstructive hip surgery | Followup (months)* | Mean improvement in HHS | Conversion to THA |
|---|---|---|---|---|---|
| Mayo et al. [21] | 19/19 | 100% | 45 (26–85) | 30 | 2 |
| Thawrani et al. [28] | 83/76 | 56% | NA | NA | 0 |
| Current study | 67/63 | 100% | 59 (24–147) | 11 | 5 |
* Values are expressed as mean, with range in parentheses; HHS = Harris hip score; NA = not available.
Our data suggest residual dysplasia deformities can be corrected in patients who have undergone previous reconstructive hip surgery procedures (Fig. 2), and radiographic parameters indicative of appropriate deformity correction were achieved in most cases. The average improvement in LCEA, ACEA, and Tönnis angle of 25°, 23°, and 17°, respectively, is consistent with previous published results [3, 5, 7, 11, 17, 20, 25, 30, 31] that report ranges in improvement of 22° to 45° for LCEA, 16° to 44° for ACEA, and 5° to 26° for Tönnis angle in patients who underwent PAO. That the deformity correction achieved in our series of complex patients falls within the range accomplished in PAO-only patients is a testament to the power and versatility of this surgical procedure to correct residual acetabular deformity. Interestingly, the number of patients with Tönnis Grade 0 hips increased by five after PAO surgery. This likely does not represent actual regression in the degree of OA, but rather an improvement in the radiographic appearance of the joint space, which is better observed in the setting of less femoral head subluxation and improved femoral head coverage. This improved radiographic appearance results in a better Tönnis OA grade. Another consideration regarding the use of the Tönnis OA grade in joint-preserving surgery may be that it is less meaningful in lower grades of OA. Extensive focal articular cartilage damage can be seen in the absence of joint space narrowing, which would elevate a radiograph to a higher Tönnis grade. More modern imaging techniques, such as delayed gadolinium-enhanced MRI of cartilage, likely provide a more accurate assessment of the health of articular cartilage [16]. Analysis of complication rates after PAO is confounded by the variability in methods in which complications are reported in the literature. In a systematic review of the literature, major complications were noted to occur in 6% to 37% of PAO cases performed [8]. Davey and Santore [12] also described the effect that the learning curve has on complication rates, citing a reduction in major complications from 17% to 3% when the first 35 PAO procedures performed by a surgeon were compared with the subsequent 35 procedures. The complications noted in our series ranged from benign superficial wound infections and paresthesias to a major nerve palsy. The majority of complications required either observation for resolution of transient nerve palsies or oral antibiotics to treat superficial infection. Seven hips (10%) experienced a complication requiring an additional surgical intervention or hospitalization, and only one patient (1.4%) had a complication that resulted in a permanent disability. We attribute the high number of nerve lesions in this series (seven hips, 10%) to the complex nature of the deformities encountered, requiring a greater degree of correction and potential retraction of neurovascular structures, and to the presence of scar tissue and nerve adhesions related to previous surgery that may make the nerves more susceptible to stretch injuries and retraction.
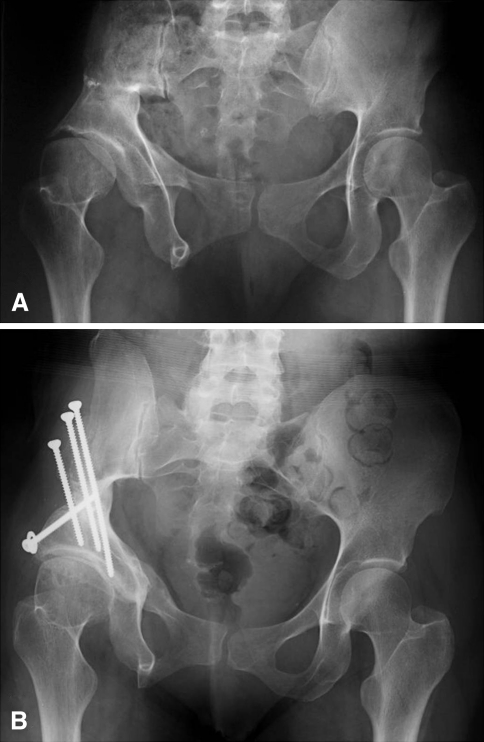
Fig. 2A–B.
(A) An AP pelvic radiograph shows the hips of a 46-year-old woman with a history of triple innominate osteotomy in adolescence. She presented with worsening symptoms related to the right hip. She had severe abductor insufficiency due to an abductor takedown at the time of her previous pelvic osteotomy. Clinically, she was diagnosed with symptomatic acetabular dysplasia with anterior instability. She was treated with a PAO, removal of previous shelf, and osteochondroplasty of the femoral head-neck junction. (B) Four years after surgery, the patient has an excellent clinical result. She has complete relief of pain but has limited hip function due to persistent abductor insufficiency related to her first surgery.
THA was performed in patients in our series who had persistent hip pain after their PAO with radiographic evidence of progression of their arthrosis. The clinical failures requiring conversion to THA of 7% in our series (five patients) are similar to data previously reported in other series of patients receiving PAO. In case series with average followup of at least 4 years, the THA conversion rates range from 0% [3, 5, 7] to 6% [20, 31] to 14% [30]. Our data represent the failure rate at short- to medium-term followup, and with additional time, greater numbers of THA procedures will likely become necessary. The number of patients undergoing PAO after previous reconstructive hip surgery represents a relatively small fraction (10%) of patients who have undergone this procedure at our institutions, which further underscores the importance that multicenter longitudinal cohort studies play in the future to evaluate the overall failure rates of such patients. Only when the pooled data from multiple sites are combined with a standardized method of clinical evaluation, outcome, and radiographic interpretation of images will the numbers of such patients be sufficient to identify characteristics for success or risk factors for failure.
Our data suggest PAO performed after previous reconstructive hip surgery can improve hip function and correct residual dysplasia deformities. However, these procedures are inherently more complex than primary PAO, and there is a substantial risk of perioperative complications, reoperations, and early treatment failures.
Acknowledgment
The authors thank Debbie Long for her assistance with the preparation of this manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Washington University School of Medicine and Children’s Hospital Boston.
References
- 1.Anderson LA, Crofoot CD, Erickson JA, Peters CL. Staged surgical dislocation and redirectional periacetabular osteotomy: a report of five cases. J Bone Joint Surg Am. 2009;91:2469–2476. doi: 10.2106/JBJS.H.00066. [DOI] [PubMed] [Google Scholar]
- 2.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 3.Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32:611–617. doi: 10.1007/s00264-007-0372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 5.Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87:254–259. doi: 10.2106/JBJS.D.02093. [DOI] [PubMed] [Google Scholar]
- 6.Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia: surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 pt 1):65–83. doi: 10.2106/JBJS.E.00887. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy JC, Nunley RM, Curry MC, Schoenecker PL. Periacetabular osteotomy for the treatment of acetabular dysplasia associated with major aspherical femoral head deformities. J Bone Joint Surg Am. 2007;89:1417–1423. doi: 10.2106/JBJS.F.00493. [DOI] [PubMed] [Google Scholar]
- 8.Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467:2041–2052. doi: 10.1007/s11999-009-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clohisy JC, St John LC, Nunley RM, Schutz AL, Schoenecker PL. Combined periacetabular and femoral osteotomies for severe hip deformities. Clin Orthop Relat Res. 2009;467:2221–2227. doi: 10.1007/s11999-009-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crockarell J, Jr, Trousdale RT, Cabanela ME, Berry DJ. Early experience and results with the periacetabular osteotomy: the Mayo Clinic experience. Clin Orthop Relat Res. 1999;363:45–53. doi: 10.1097/00003086-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham T, Jessel R, Zurakowski D, Millis MB, Kim YJ. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am. 2006;88:1540–1548. doi: 10.2106/JBJS.E.00572. [DOI] [PubMed] [Google Scholar]
- 12.Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:33–37. doi: 10.1097/00003086-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed] [Google Scholar]
- 14.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 15.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 16.Jessel RH, Zilkens C, Tiderius C, Dudda M, Mamisch TC, Kim YJ. Assessment of osteoarthritis in hips with femoroacetabular impingement using delayed gadolinium enhanced MRI of cartilage. J Magn Reson Imaging. 2009;30:1110–1115. doi: 10.1002/jmri.21830. [DOI] [PubMed] [Google Scholar]
- 17.Kralj M, Mavcic B, Antolic V, Iglic A, Kralj-Iglic V. The Bernese periacetabular osteotomy: clinical, radiographic and mechanical 7–15-year follow-up of 26 hips. Acta Orthop. 2005;76:833–840. doi: 10.1080/17453670510045453. [DOI] [PubMed] [Google Scholar]
- 18.Lequesne M. False profile of the pelvis. A new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies [in French] Rev Rhum Mal Osteoartic. 1961;28:643–652. [PubMed] [Google Scholar]
- 19.Mast JW, Brunner RL, Zebrack J. Recognizing acetabular version in the radiographic presentation of hip dysplasia. Clin Orthop Relat Res. 2004;418:48–53. doi: 10.1097/00003086-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Matta JM, Stover MD, Siebenrock K. Periacetabular osteotomy through the Smith-Petersen approach. Clin Orthop Relat Res. 1999;363:21–32. [PubMed] [Google Scholar]
- 21.Mayo KA, Trumble SJ, Mast JW. Results of periacetabular osteotomy in patients with previous surgery for hip dysplasia. Clin Orthop Relat Res. 1999;363:73–80. doi: 10.1097/00003086-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SB, Millis MB. Periacetabular osteotomy without abductor dissection using direct anterior exposure. Clin Orthop Relat Res. 1999;364:92–98. doi: 10.1097/00003086-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Peters CL, Erickson JA. Treatment of femoro-acetabular impingement with surgical dislocation and debridement in young adults. J Bone Joint Surg Am. 2006;88:1735–1741. doi: 10.2106/JBJS.E.00514. [DOI] [PubMed] [Google Scholar]
- 24.Sen C, Asik M, Tozun IR, Sener N, Cinar M. Kotz and Ganz osteotomies in the treatment of adult acetabular dysplasia. Int Orthop. 2003;27:78–84. doi: 10.1007/s00264-002-0417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebenrock KA, Scholl E, Lottenbach M, Ganz R. Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:9–20. doi: 10.1097/00003086-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sink EL, Beaule PE, Sucato D, Kim YJ, Millis MB, Dayton M, Trousdale RT, Sierra RJ, Zaltz I, Schoenecker P, Monreal A, Clohisy J. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93:1132–1136. doi: 10.2106/JBJS.J.00794. [DOI] [PubMed] [Google Scholar]
- 27.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188:1540–1552. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 28.Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92:1707–1714. doi: 10.2106/JBJS.I.00829. [DOI] [PubMed] [Google Scholar]
- 29.Tönnis D. Congenital Dysplasia and Dislocation of the Hip in Children and Adults. Berlin, Germany: Springer; 1987. [Google Scholar]
- 30.Trousdale RT, Ekkernkamp A, Ganz R, Wallrichs SL. Periacetabular and intertrochanteric osteotomy for the treatment of osteoarthrosis in dysplastic hips. J Bone Joint Surg Am. 1995;77:73–85. doi: 10.2106/00004623-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Trumble SJ, Mayo KA, Mast JW. The periacetabular osteotomy: minimum 2 year followup in more than 100 hips. Clin Orthop Relat Res. 1999;363:54–63. doi: 10.1097/00003086-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteoarthritis. Acta Chir Scand. 1939;83(suppl 58):5–135. [Google Scholar]