Abstract
Background
We observed a substantial increase in the incidence of pulmonary embolism (PE) after total joint arthroplasty (TJA) when multidetector computerized tomography (MDCT) replaced ventilation-perfusion (V/Q) scans as the diagnostic modality of choice. We questioned whether this resulted from the detection of clinically unimportant PE with the more sensitive MDCT and in 2007 instituted a hypoxia protocol to enhance the detection of PE.
Questions/purposes
We determined whether this new hypoxia protocol increased the specificity of our workups for suspected clinically important PE in the immediate postoperative period without affecting patient morbidity and mortality.
Patients and Methods
We compared the frequency of MDCT, V/Q scan and total investigations, incidence of PE, and overall mortality rates in the 3 years prior (January 2003 to December 2006) and 2 years after (January 2007 to November 2009) the implementation of the algorithm.
Results
After instituting the protocol, we observed a trend toward a decrease in the number of patients worked up for PE (4.6 to 4.0 per 100 TJAs, 13.5% decrease). At the same time, there was an increase in the percent of positive findings of PE per workup for PE (23–33 positive PEs per 100 patients, 40.5% increase). All-cause mortality rates decreased for the 30-day period (3.1 to 1.4 per 1000 TJAs, 53.5% decrease) and the 90-day period (5.0 to 2.6 per 1000 TJAs, 48.3% decrease).
Conclusions
With the implementation of this algorithm, the specificity of our management of postoperative hypoxia and suspected clinically important PE improved without affecting patient morbidity or mortality.
Introduction
Pulmonary embolism (PE) is one of the most serious complications after total joint arthroplasty (TJA). Correct identification of the cause of hypoxia and early diagnosis of PE is important as a result of the life-threatening potential if PE is left untreated [4, 6]. However, because the treatment for PE (normally a prolonged course of chemical anticoagulation) is associated with substantially increased risks of hemorrhage and drug interactions [5, 7, 12], it is important to distinguish at an early stage PEs that require treatment from those that do not.
Diagnosing PE clinically is problematic because its symptoms overlap with those of other conditions and because there are numerous causes of postoperative hypoxia (eg, hypoventilation, narcotic effects, fluid overload, and postoperative atelectasis) [1]. Moreover, the modality used to make this diagnosis has changed substantially over the years, transitioning from the invasive pulmonary angiogram [3] to the ventilation/perfusion (V/Q) scan. More recently, as enhanced technology has improved the sensitivity of new generations of contrast CT scans (from single-detector row CT scans to spiral CT scans to multidetector row spiral CT [MDCT]), MDCT scans have become the standard of care for diagnosing suspected PE [14, 17], because they allow for better visualization of small emboli in subsegmental branches of the pulmonary arteries [11, 15].
As highly sensitive MDCT imaging became the method of choice to detect PE at our institution, however, we noted a substantial increase in the incidence of radiographically diagnosed PE, whereas the perioperative mortality rate remained constant [10]. Three studies have suggested the increased use of MDCT scans has increased the detection of clinically irrelevant, incidental PEs [8, 9, 16]. However, there is no consensus regarding the clinical importance and treatment of isolated subsegmental PE [7], although there is evidence that enhanced visualization of emboli does not improve the clinical outcome [13]. Our concern is that enhanced visualization of the pulmonary arteries with MDCT detects clinically unimportant findings (eg, small PEs or fat emboli) that may lead to administration of prolonged anticoagulation. This response to a solely radiographic finding may place the patient at risk of developing complications such as bleeding and drug interactions. After a study at our institution demonstrated the implementation of sensitive imaging such as MDCT had resulted in overdiagnosis of “PE” [10], we instituted an algorithm for the investigation of hypoxia that was intended to prevent this problem. Our goal in implementing an algorithm for the patient suspected of PE was to reduce overall morbidity and costs by performing inexpensive tests that would identify etiologies for hypoxia other than PE. By increasing the threshold at which an MDCT scan was ordered, we hoped this algorithm would reduce the false-positive rate of MDCT and thus expose fewer patients to potentially harmful therapeutic anticoagulation.
We asked whether our hypoxia workup protocol altered (1) the rate of patients evaluated for PE; (2) the incidence of diagnosis of PE; and (3) PE-specific mortality rates.
Patients and Methods
A hypoxia algorithm was implemented at our institution in January 2007 in an effort to identify only those clinically important PEs after TJA (Fig. 1). Before implementation of this algorithm, patients were worked up for PE solely based on the clinician’s assessment and interpretation of a patient’s signs and symptoms. That is, the patient who had an asymptomatic drop in oxygen saturation by pulse oximetry received the same workup as the patient who had substantial oxygen desaturation unresponsive to oxygen administration, dyspnea, tachycardia, and hemoptysis. In contrast, the algorithm stratifies patients according to the nature of their presenting symptoms (PE-related symptoms/signs or an asymptomatic drop in oxygen saturation as measured by pulse oximetry and arterial blood gas) and sets the threshold for workup for PE higher than it was previously. Combinations of trials of oxygen administration, tests to rule out other etiologies for the observed symptoms, and careful monitoring are attempted first; only after patients appear unresponsive to these measures is the standard workup for PE initiated. Of note, routine pulse oximetry is used only when clinically indicated (ie, for patients using DepoDur [Pacira, Parsippany, NJ, USA] or requiring high doses of opioids or for patients with comorbidities such as morbid obesity, sleep apnea, or respiratory disease). Once the threshold for workup for PE has been achieved, MDCT and V/Q scans are used in a complementary fashion: patients are worked up with MDCT unless they have a potential allergy to the contrast used in this imaging modality, in which case a V/Q scan is used instead. If the V/Q scan is indeterminate and there remains a high clinical suspicion for PE, however, patients will receive a steroid preparation and subsequent CT scan if possible; otherwise, they will receive standard treatment for PE.
Fig. 1.
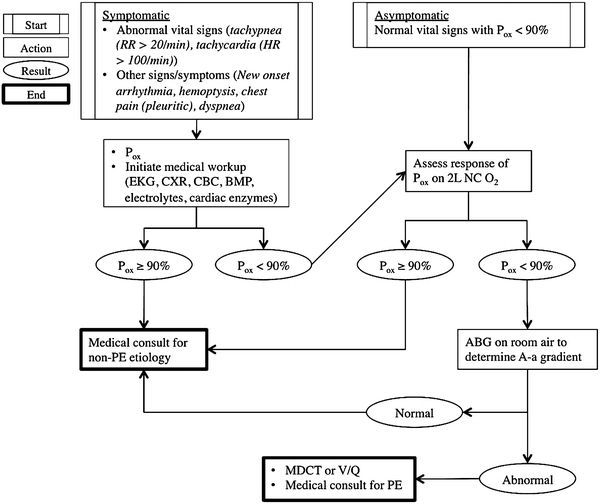
Hypoxia algorithm is shown. Note: Routine pulse oximetry only for patients with DepoDur [Pacira, Parsippany, NJ, USA] and when clinically indicated (ie, patients with morbid obesity, sleep apnea, respiratory disease, or patients who require high doses of opioids). RR = respiratory rate; HR = heart rate; Pox = percentage oxygen saturation; EKG = echocardiogram; CXR = chest xray; CBC = complete blood count; BMP = basic metabolic panel; PE = pulmonary embolism; NC = nasal cannula; ABG = arterial blood gas; A-a = alveolar-arterial; MDCT = multidetector computed tomography scan; V/Q = ventilation/perfusion scan.
The preprotocol study period for these variables was from January 1, 2003, to December 31, 2006; the postprotocol period was from January 1, 2007, to February 28, 2009. For mortality data, postprotocol data extended to November 30, 2009. Patients were excluded if they were either admitted or discharged by a service other than the orthopaedic surgery service at our hospital, because we could not confirm that these patients had been worked up for PE according to the hypoxia protocol under investigation. For the workup, incidence, and type of PE variables, there were 7650 patients (9416 TJAs; average, 6.45 per day of a 7-day work week) in the preprotocol period and 4416 patients (5216 TJAs) in the postprotocol period (average, 6.61 per day). We also assessed differences in demographics (age, gender distribution, body mass index [BMI], length of hospital stay, and joint operated on) between the two groups. Institutional Review Board approval was obtained before the collection of data for this project.
We determined the frequency of workup for PE by querying our hospital’s electronic orders and admissions databases. Patients who had undergone TJA at our institution and who had orders for contrast CT and V/Q scans during a hospital admission or after discharge from the hospital were identified. Individual patient charts were then verified to confirm that scans were actually performed during the 90 days after TJA.
To assess the incidence of PE before and after the protocol change, we reviewed all radiology reports for which we had electronic reports (all reports were dictated at the time of the scan) and classified patients as having been positive, negative, or indeterminate for PE. Positive and negative scans confirmed the presence or absence of PE, respectively; scans for which technical limitations (ie, poor bolus timing or patient positioning) made such confirmation impossible were classified as indeterminate. For patients with multiple postoperative scans, if a single positive scan existed, the patient was classified as positive; if not, the patient was classified according to the reading of the latest scan performed during the same hospital admission. Because we had assumed CT scans would be more sensitive in detecting subsegmental PEs, we further classified each positive PE as having been either segmental or subsegmental. We also examined the incidence of PE per workup for PE in each study period, ie, the “success” rate of the workup for PE.
To assess the effect of the protocol change on mortality rates, we first queried the Social Security Death Index to identify patients who died within 90 days of TJA. We then consulted the State Department of Vital Statistics and the Centers for Disease Control and Prevention’s (CDC’s) National Death Index for information on mortalities and causes of death for all patients who died before and after the protocol was initiated. As a result of backlogging of data, we were not able to obtain specific causes of death for patients who died in the postprotocol period. As a result, we are only able to report overall mortality rates before and after the protocol changes. Both 30- and 90-day mortality rates were assessed.
We determined differences among frequency of workup, incidence, type of PE, and mortality before and after the implementation of the protocol. We used the total number of joints performed in a given study period as the denominator for comparing pre- and postprotocol change values. All variables were normalized by dividing by the total number of joint arthroplasties performed before and after the protocol change. Additional analyses for further comparison of incidence of PE in the pre- and postprotocol periods were performed using the total number of workups for PE as the denominator. Comparisons were made using chi-squared tests for workup, incidence, and mortality data and Student’s t-test for demographic data. All analyses were performed with SPSS Version 16 (Chicago, IL, USA).
Results
We observed a trend (p = 0.08) toward a decrease in the number of patients worked up for PE after the protocol (4.63 versus 4.01 per 100 arthroplasties before and after, or a 13.5% decrease). We noted a decrease (p = 0.01) in the incidence of V/Q scans (1.19 versus 0.75 per 100 arthroplasties before and after, a 37.1% decrease) but no decrease in the number of CT scans ordered; the latter was similar during the two time periods (3.64 versus 3.34 per 100 arthroplasties before and after) (Table 1).
Table 1.
Summary of findings comparing diagnosis of pulmonary embolism (PE) in the pre- and postprotocol periods
| Variable | Percent of TJAs* | Percent positive scans of TJAs worked up | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | p value | Pre | Post | p value | ||
| Test | CT | 3.64 | 3.34 | 0.3356 | 26.24 | 37.36 | 0.0110† |
| VQ | 1.19 | 0.75 | 0.0113† | 10.71 | 10.26 | 1.0 | |
| Overall | 4.63 | 4.01 | 0.0784‡ | 23.17 | 32.54 | 0.0129† | |
| Type of PE | Segmental | 0.87 | 1.05 | 0.2694 | |||
| Subsegmental | 0.08 | 0.19 | 0.0776‡ | ||||
| Mortality | 30-day | 0.31 | 0.14 | 0.0470† | |||
| 90-day | 0.50 | 0.26 | 0.0206† | ||||
* We report percentage of total TJAs because the two timeframes differed in terms of total number of TJAs. There were 9416 TJAs in the preprotocol period and 5216 in the postprotocol period; †p < 0.05; ‡0.05 < p < 0.10; TJA = total joint arthroplasty; CT = computed tomography; VQ = ventilation-perfusion scan.
There was no difference in the incidence of PE diagnoses by V/Q scans before and after the protocol change (10.71 versus 10.26 per 100 arthroplasties worked up with V/Q before and after). We similarly observed no difference in the incidence of segmental PEs before and after initiation of the protocol (8.7 versus 10.5 per 1000 arthroplasties before and after); there was a trend (p = 0.08) toward an increase in the incidence of subsegmental PEs diagnosed after the protocol (0.8 versus 1.9 per 1000 arthroplasties before and after, a 125.7% increase). The ratio of positive PEs to number of patients worked up for PE increased (p = 0.01) after implementation of the protocol (23.17 PEs per 100 patients worked up before the protocol versus 32.54 PEs per 100 patients worked up after the implementation of the protocol); that is, there was a 40.5% increase in the success rate of the workup (Table 1).
We observed a decline in both 30- and 90-day rates after implementation of the protocol from 3.1 to 1.4 per 1000 TJAs, a 53.5% decrease (p = 0.05), and from 5.0 to 2.6 per 1000 TJAs, a 48.3% decrease (p = 0.02), respectively. We identified three deaths resulting from PE in the preprotocol period; backlogged CDC data rendered it impossible for us to definitively determine the number of PE-specific deaths after implementation of the protocol, however (Table 1).
Discussion
PE continues to be one of the most serious complications after TJA. If left untreated it can be life-threatening and therefore correct early diagnosis is imperative to ensure proper treatment is instituted [4, 6]. Overdiagnosis of PE on the other hand can impart potential for increased morbidity as a result of administration of prolonged anticoagulation [5, 7, 12]. We therefore instituted a hypoxia workup protocol to reduce and minimize the unnecessary administration of prolonged anticoagulation to patients with clinically irrelevant PEs and to reduce the costs associated with PE workup without altering morbidity or mortality rates. In this study, we asked whether our hypoxia workup protocol altered (1) the rate of patients evaluated for PE; (2) the incidence of diagnosis of PE; and (3) PE-specific mortality rates.
This study has some limitations. First, as a result of the backlog in the CDC database, we were not able to identify the cause of death for some patients who died during the latter 2 years of this study. So despite the fact that both 30- and 90-day mortality declined after introduction of the hypoxia protocol, we cannot be certain about the cause of death for all patients. Second, because we did not perform a multivariate analysis (since we were not able to quantify and standardize numerous variables that could have been responsible for differences before and after introduction of the protocol), we cannot claim that introduction of the hypoxia protocol was the sole reason for the decline in mortality rate. We also did not perform an a priori power analysis to determine expected differences before initiating the protocol, so although we can conclude statistically significant differences between pre- and postprotocol measurements of some variables, we cannot make any conclusions about nonsignificant findings. Third, this was a retrospective, cross-sectional study, and is therefore subject to the inherent limitations of such a study. Specifically, when we examined differences between the two groups, we found statistically significant differences (in BMI and length of hospital stay) between the pre- and postprotocol patient populations (Table 2). Although these differences may have influenced main study outcome variables, we do not believe they are clinically important, because their level of precision is greater than that which we can claim for either of these two variables. We did note that the percentage of hip (versus knee) arthroplasties was significantly lower in the post- than the preprotocol period, which again is a limitation of the cross-sectional study design. Finally, we are not certain that the introduced protocol was followed for every case in this study. It is possible that as a result of some circumstances, protocol deviations may have occurred that were not recorded in the medical records.
Table 2.
Patient demographics between pre- and postprotocol periods
| Variable (units) | Pre | Post | p value |
|---|---|---|---|
| Age at surgery (years) | 63 ± 12 | 63 ± 11 | 0.1171 |
| Body mass index (kg/m2) | 30 ± 6 | 30 ± 6 | 0.0256* |
| Length of hospital stay (days) | 4 ± 3 | 4 ± 2 | < 0.0001* |
| Gender (% male) | 42.6 | 43.5 | 0.3129 |
| Joint (% hips) | 50.6 | 45.8 | < 0.0001* |
* p < 0.05.
We found the institution of the protocol was accompanied by a decrease in the number of patients worked up for PE. We have previously reported that the introduction of “sensitive” imaging modalities, namely MDCT scan, has led to an increase in diagnosis of PE at our institution. This occurred despite improvements in surgical techniques (reduction in operative time), a tendency for increased use of regional anesthesia, and early ambulation of patients [10]. According to our hypothesis, this increase was the result of the overdetection of clinically insignificant PEs. The fact that implementation of our hypoxia protocol resulted in a reduced number of patients worked up for PE, then, fell in line with our hypothesis that the increase in PE diagnosis was not the result of an increased prevalence of PE but rather attributable to a difference in the sensitivity and administration of tests for diagnosing PE.
We also found the “success rate” for workup for PE increased over 40% after implementation of our hypoxia protocol. This decrease in the false-positive rate of PE workup has two benefits. First, it reduces risk exposure to the patient (ie, ionizing radiation, potential anticoagulation therapy, etc). The second benefit is the reduction of cost. This increase in success rate may have been the result of either an increase in the numerator (number of patients positive for PE) or a decrease in the denominator (number of patients worked up for PE). We do know the rate of findings of subsegmental PEs in the postprotocol period increased, but the clinical relevance of these findings is questionable. The study previously conducted at our institution identified that the majority of the “PEs” detected by MDCT were small subsegmental emboli that may even have been fat and marrow debris of little clinical relevance [10]. Additionally, Carrier et al. recently demonstrated the use of highly sensitive MDCT scanners was correlated with an increase in rates of diagnosis of subsegmental PEs that was not paralleled by a decrease in the risk of PE among patients with negative scans, suggesting the subsegmental PEs that were detected may not have been clinically relevant [2]. The increase in “success rate” of workup for PE may also have been the result of the trend toward a decrease in the rate of workup for PE after implementation of the protocol. Although further work should be conducted to determine the clinical relevance of subsegmental PEs, the fact that the false-positive rate of PE scan was reduced in the postprotocol period suggests the hypoxia protocol improves the accuracy of workups for PE at our institution.
Finally, we found implementation of the protocol did not result in an increase in overall mortality rates. In fact, the reverse was true: mortality rates decreased after implementation of the protocol. Because we were not able to identify PE-specific causes of death in the postprotocol period, the most important conclusion to draw from this finding is that the implementation of the protocol did not lead to an increase in mortality.
The decreased number of patients with negative workups for PE and the corresponding (presumed) reduced rate of unnecessary prolonged anticoagulation being administered and length of hospital stay have immense financial implications. Additionally, by including workups for alternative etiologies for hypoxia, our algorithm may also identify diagnoses that are not only more likely than PE, but may also require timely interventions. This is particularly important as we enter the era of cost-effective care and evidence-based practice of medicine. We believe not all patients exhibiting hypoxia require a workup for PE and there is a lack of evidence that mild and transient hypoxia is a clinically important event. Thus, patients exhibiting mild or transient hypoxia should be managed by other methods (ie, administration of oxygen, reversal of opiate-induced hypoxia, and identification of other causes of hypoxia) than by immediate workup for PE.
In conclusion, we believe continued effort to diagnose clinically relevant PE and implement appropriate treatment is critical for prevention of catastrophic fatal outcomes. However, it is equally important to avoid “overdiagnosis” of PE, which leads to administration of prolonged anticoagulation with all its associated costs and morbidities. Based on the findings of this study, it appears that our institutional algorithm is efficient and safe in the management of postoperative hypoxia.
Footnotes
Two authors (JP, RHR) are consultants for and one (RHR) has royalties with Stryker Orthopaedics [Mahwah, NJ, USA]. One author (JP) has Intellectual Properties on SmarTech.
Each author certifies that our institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Austin L, Pulido L, Ropiak R, Porat M, Parvizi J, Rothman RH. Hypoxemia after total joint arthroplasty: a problem on the rise. J Arthroplasty. 2008;23:1016–1021. doi: 10.1016/j.arth.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, Pleasance S, Le Gal G. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheely R, McCartney WH, Perry JR, Delany DJ, Bustad L, Wynia VH, Griggs TR. The role of noninvasive tests versus pulmonary angiography in the diagnosis of pulmonary embolism. Am J Med. 1981;70:17–22. doi: 10.1016/0002-9343(81)90406-X. [DOI] [PubMed] [Google Scholar]
- 4.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17:259–270. doi: 10.1016/S0033-0620(75)80017-X. [DOI] [PubMed] [Google Scholar]
- 5.Della Valle CJ, Jazrawi LM, Idjadi J, Hiebert RN, Stuchin SA, Steiger DJ, Di Cesare PE. Anticoagulant treatment of thromboembolism with intravenous heparin therapy in the early postoperative period following total joint arthroplasty. J Bone Joint Surg Am. 2000;82:207–212. doi: 10.2106/00004623-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ, Visani L, Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 7.Lawton RL, Morrey BF. The use of heparin in patients in whom a pulmonary embolism is suspected after total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1063–1072. doi: 10.2106/00004623-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Le Gal G, Righini M, Parent F, Strijen M, Couturaud F. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724–731. doi: 10.1111/j.1538-7836.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 9.Oser RF, Zuckerman DA, Gutierrez FR, Brink JA. Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology. 1996;199:31–35. doi: 10.1148/radiology.199.1.8633168. [DOI] [PubMed] [Google Scholar]
- 10.Parvizi J, Smith EB, Pulido L, Mamelak J, Morrison WB, Purtill JJ, Rothman RH. The rise in the incidence of pulmonary embolus after joint arthroplasty: is modern imaging to blame? Clin Orthop Relat Res. 2007;463:107–113. doi: 10.1097/BLO.0b013e318145af41. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, Kazerooni EA, Cascade PN. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. Radiology. 2003;227:455–460. doi: 10.1148/radiol.2272011139. [DOI] [PubMed] [Google Scholar]
- 12.Patterson BM, Marchand R, Ranawat C. Complications of heparin therapy after total joint arthroplasty. J Bone Joint Surg Am. 1989;71:1130–1134. [PubMed] [Google Scholar]
- 13.Prologo JD, Gilkeson RC, Diaz M, Cummings M. The effect of single-detector CT versus MDCT on clinical outcomes in patients with suspected acute pulmonary embolism and negative results on CT pulmonary angiography. AJR Am J Roentgenol. 2005;184:1231–1235. doi: 10.2214/ajr.184.4.01841231. [DOI] [PubMed] [Google Scholar]
- 14.Remy-Jardin M, Pistolesi M, Goodman LR, Gefter WB, Gottschalk A, Mayo JR, Sostman HD. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology. 2007;245:315–329. doi: 10.1148/radiol.2452070397. [DOI] [PubMed] [Google Scholar]
- 15.Schoepf UJ, Holzknecht N, Helmberger TK, Crispin A, Hong C, Becker CR, Reiser MF. Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology. 2002;222:483–490. doi: 10.1148/radiol.2222001802. [DOI] [PubMed] [Google Scholar]
- 16.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184:264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 17.Strashun AM. A reduced role of V/Q scintigraphy in the diagnosis of acute pulmonary embolism. J Nucl Med. 2007;48:1405–1407. doi: 10.2967/jnumed.107.041988. [DOI] [PubMed] [Google Scholar]


