Abstract
O-Specific polysaccharides of Gram-negative bacteria are synthesized by two different mechanisms: polymerization of the pre-formed O-repeating unit or sequential addition of the monosaccharides to the growing polysaccharide chain. In the second case, growth of the polymer can be further subdivided into two groups depending on the presence or absence of a special monosaccharide or non-sugar substituent that terminates the glycan. A family of polymannose O-polysaccharides provides prototypes for the chain terminating process. Polysaccharides of Klebsiella pneumoniae O3, Hafnia alvei PCM 1223, and Escherichia coli O9 have the same penta-mannose repeating unit. E. coli O9a has tetra-mannose repeat and this structure can be produced by mutants of E. coli O9. The mechanism of biosynthesis of H. alvei 1223 O-polysaccharide has not been reported. Here we show that all above polysaccharides contain the same modification at the non-reducing end; presence of a methyl phosphate group at O-3 of α-mannopyranose, that serves as the signal for termination of the chain elongation.
Keywords: Hafnia, Klebsiella, LPS, methyl phosphate, O-specific polysaccharide
Lipopolysaccharide (LPS) is a component of the outer membrane of Gram-negative bacteria. It consists of three distinct regions: lipid A, core oligosaccharide, and O-specific polysaccharide (O-polysaccharide, OPS). OPS defines serospecificity of many Gram-negative bacteria. Biosynthesis of almost all O-polysaccharides proceeds by one of two mechanisms (reviewed in 1). In the Wzy-dependent pathway, individual polyprenol-linked repeating units are synthesized at the cytosol:cytoplasmic membrane interface, exported by the Wzx flippase, and polymerized by the Wzy polymerase at the periplasmic face of the membrane. In ATP-binding cassette (ABC) transporter-dependent biosynthesis, the OPS is assembled at the cytoplasmic side of the membrane and transported to the periplasm. ABC transporter-dependent biosynthesis is employed in the production of E. coli O8, O9, O9a OPS, all Klebsiella pneumoniae OPSs, and the OPSs of several other bacteria.2 The LPS molecular species from a given isolate possess OPS that exhibit a characteristic “modal” range of chain lengths, revealed by SDS-PAGE profiles. The length of LPS is crucial for the biology of microorganisms because it contributes to the resistance to complement-mediated serum killing.3 In the ABC transporter pathway, the chain-length is determined prior to export and in some of these OPSs, a special residue added to the non-reducing terminus serves as a chain terminator. The structures of the terminal groups have been determined for a number of Klebsiella pneumoniae,4 Vibrio cholerae O1,5 Bordetella bronchiseptica and Bordetella parapertussis OPS.6 Methyl groups, Kdo, and complex monosaccharides were identified at the non-reducing end of these OPSs. The non-reducing end group can confer serological and immunological properties of LPS. In case of V. cholerae O1 Ogawa and Inaba, the serospecificity is due to the differences in methylation of the terminal perosamine moiety; in case of B. bronchiseptica, it is attributed to the presence of different N-acyl groups at the terminal 2,3,4-triamino-2,3,4-trideoxy-α-galacturonamide residue.
The polymannose OPS of E. coli O8, O9 and O9a (EcO8, EcO9, EcO9a) provide prototypes for systems where chain extension is terminated by the addition of a special residue. The K. pneumoniae O3 and O5 OPSs (KpO3, KpO5) are identical to those of EcO9 and EcO8, respectively, in respect to their OPSs structures 4,7–10 and the genetic loci required for OPS expression.11 EcO9a is a genetic variant of EcO9 and can be generated by a single substitution in one of the mannosyltransferases.12 The structure of the EcO8/KpO5 OPS, and its terminal 3-methyl mannose residue, have been established.4 In contrast, while terminal methyl groups were implicated in the termination of OPS from KpO3 (EcO9), the precise linkage of this residue to the glycan chain could not be determined.4 Biochemical studies of the chain-terminating enzyme WbdD from EcO9a, provided the first indication of a more complex terminal structure. WbdD possesses both kinase and methyltransferase domains13 and these domains are conserved in the terminator homolog from the assembly systems for OPSs from EcO9 and KpO3.11 In vitro biosynthesis studies established that phosphorylation of the glycan preceded methylation13 and, most recently, the termination reaction was reconstituted in vitro using a synthetic tetrasaccharide acceptor, comprising the EcO9a repeating unit, and a purified soluble form of WbdD.14 These studies suggested that terminal modification involve methyl phosphate residue but confirmatory data for a native polysaccharide were missing.
E. coli O9, K. pneumoniae O3, and Hafnia alvei PCM 122315 produce polysaccharides of the same structure, containing penta-mannose repeating units. The proton NMR spectra of these three OPSs are nearly identical and contain two characteristic small signals at 3.63 ppm, also present in the spectrum of E. coli O9a. These signals are visible in all published spectra. Here we describe the identification of the methyl phosphate group, responsible for these signals and situated at the non-reducing end of the mannose residue.
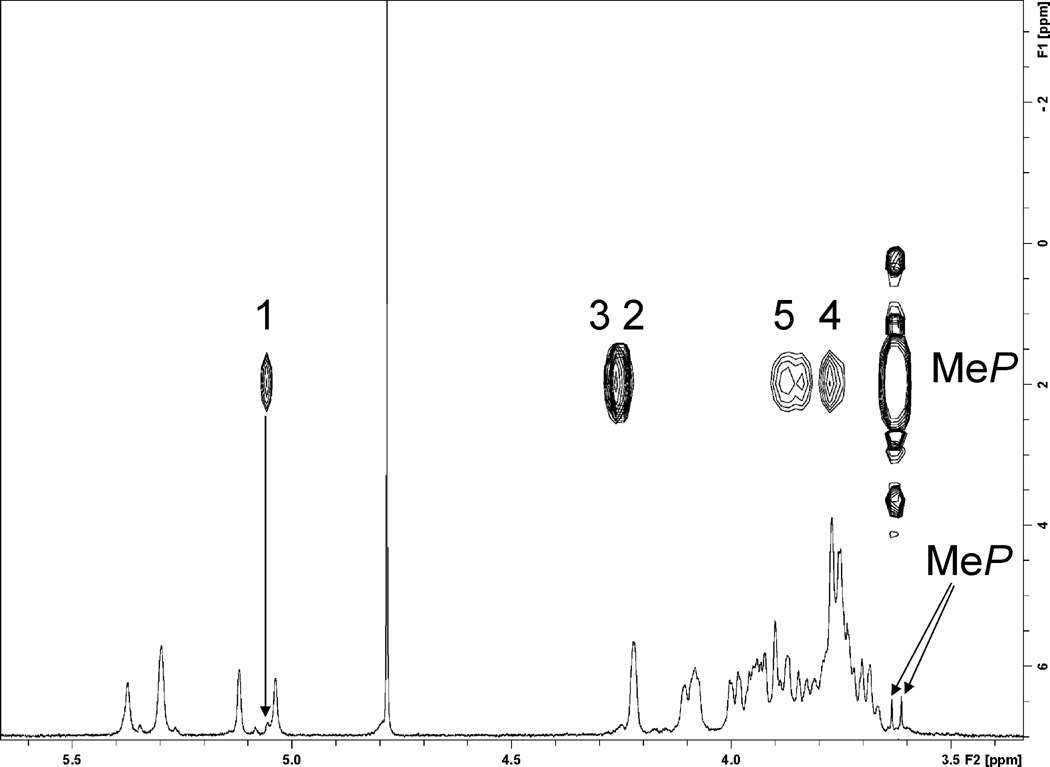
OPSs of E. coli O9, K. pneumoniae O3, and H. alvei PCM 1223 were isolated by mild acid hydrolysis of LPS and purified by gel chromatography. Additionally, polysaccharide obtained from E. coli mutant with a complementing gene CatOO1 expressing plasmid pLG91 with a tetrasaccharide repeating unit identical to that of E. coli O9a was used. Proton and 2D NMR (DQCOSY, TOCSY, NOESY, 1H-13C HSQC, 1H-13C HMBC, 1H-31P HMQC, 1H-31P HMQC-TOCSY) spectra for all polysaccharides were recorded and interpreted using Topspin program (Bruker). Assignment of the signals of pentasaccharide structures was essentially the same as published 4 (labelling of the residues was different in all publications), with the exception of the necessary correction of proton chemical shifts (by + 0.05–0.07 ppm) for the data presented in 4. Data for the tetrasaccharide structure are presented in Table 1. All spectra contained major spin systems of the repeating unit monosaccharides and two minor spin systems of α-mannopyranoses Z and Y (T and E' in 4), forming a disaccharide Z-2-Y. Man Y was linked to O-2 of the next mannose, identical to the repeating unit Man A, further tracing of the chain was not possible due to merging of signals with the that of the bulk of polymer repeating units. 1H-31P HMQC correlation indicated phosphorylation of the Man Z at position 3 (Fig. 1). Two sharp proton signals around 3.63 ppm were parts of the doublet with JP-H of 11 Hz, correlating with the same phosphate at O-3 of Man Z. 1H-31P HMQC-TOCSY contained correlations of protons H-1,2,3,4,5 of Man Z with methyl phosphate. Thus phosphate was a diester with methyl and Man Z C-3 substituents. This finding is in agreement with the data obtained by the study of biosynthesis.14 Interestingly, E. coli Cat001 (pLG91) also produces smaller amount of another polysaccharide of higher molecular mass with the same structure of repeating unit but without MeP substituent, which may point to the altered mechanism of chain termination in this mutant.
Table 1.
NMR data for E.coli pLG91 OPS (δ, ppm). PMe: H/C 3.63/54.1 ppm; 31P 2.08 ppm.
| Sugar | H/C 1 | H/C 2 | H/C 3 | H/C 4 | H/C 5 | H/C 6 | |
|---|---|---|---|---|---|---|---|
| Man3PMe Z | H | 5.06 | 4.25 | 4.26 | 3.77 | 3.85 | 3.75; 3.88 |
| C | 103.0 | 70.3 | 76.9 | 66.7 | 74.4 | 62.3 | |
| Man Y | H | 5.36 | 4.10 | 3.93 | 3.71 | 3.74 | 3.75; 3.88 |
| C | 101.8 | 80.4 | 71.2 | 67.9 | 74.2 | 62.3 | |
| Man D | H | 5.04 | 4.12 | 3.93 | 3.75 | 3.76 | 3.75; 3.88 |
| C | 103.3 | 70.8 | 79.2 | 67.3 | 74.4 | 62.3 | |
| Man B | H | 5.29 | 4.11 | 3.96 | 3.72 | 3.73 | 3.75; 3.88 |
| C | 101.8 | 79.7 | 71.2 | 68.1 | 74.4 | 62.3 | |
| Man A | H | 5.37 | 4.08 | 3.99 | 3.69 | 3.80 | 3.75; 3.88 |
| C | 101.8 | 79.9 | 71.2 | 68.2 | 74.6 | 62.3 | |
| Man C | H | 5.12 | 4.22 | 3.99 | 3.75 | 3.80 | 3.75; 3.88 |
| C | 103.2 | 70.8 | 79.6 | 67.3 | 74.6 | 62.3 |
Fig. 1.
1H and 1H-31P HMQC-TOCSY spectra of Hafnia alvei PCM 1223 OPS. Labels on cross peaks indicate numbers of hydrogens in the mannose residue Z. H-1 of Man Z and methyl phosphate signals are indicated by arrows on 1H spectrum.
E.coli pLG91 OPS
E. coli O9, K. pneumoniae O3, H. alvei PCM 1223:
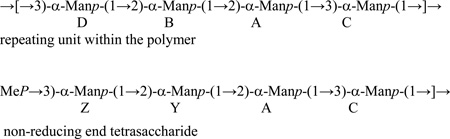
→[→3)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→3)-α-Manp-(1→]→repeating unit within the polymer
MeP→3)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→3)-α-Manp-(1→]→non-reducing end pentasaccharide
1. Experimental
1.1. Growth of bacteria, isolation of LPS and O-PS
The origin of bacterial strains and the conditions of their growth were described earlier.4, 8, 15, 16 The LPSs of E. coli O9,8 K. pneumoniae O3,4 E. coli Cat001 (pLG91) (prepared by L. Greenfield, C. Bouwman, C. Whitfield, unpublished data), and H. alvei PCM 122315 were extracted from bacterial masses by hot phenol method 16 and after dialysis recovered from water phase. The LPSs were treated with 2 % acetic acid at 100 °C for 3 h, precipitate of lipid A removed by centrifugation, soluble products separated by gel chromatography on Sephadex G-50 column to give polysaccharide and core fractions.
1.2. NMR spectroscopy
NMR spectra were recorded at 25 °C in D2O on a Varian UNITY INOVA 600, instrument, using acetone as reference for proton (2.225 ppm) and carbon (31.5 ppm) spectra. Varian standard programs DQCOSY, NOESY (mixing time of 400 ms), TOCSY (spinlock time 120 ms), HSQC, and gHMBC (long-range transfer delay 100 ms) were used. For P-H correlations non-decoupled HMQC and HMQC-TOCSY sequences with JPH of 11 Hz were used, TOCSY spin lock for 80 ms.
Acknowledgement
This research was supported in part by the Intramural Research Program of the NIH, NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raetz CR, Whitfield C. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbertson L, Kos V, Whitfield C. Microbiol. Mol. Biol. Rev. 2010;74:341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joiner KA. Annu. Rev. Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 4.Vinogradov E, Frirdich E, MacLean LL, Perry MB, Petersen BO, Duus JØ, Whitfield C. J. Biol. Chem. 2002;277:25070–25081. doi: 10.1074/jbc.M202683200. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovac P, Fournier JM, Glaudemans CP. J. Biol. Chem. 1998;273:2777–2783. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradov E, Peppler MS, Perry MB. Eur. J. Biochem. 2000;267:7230–7236. doi: 10.1046/j.1432-1327.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- 7.Parolis LAS, Parolis H, Dutton GGS. Carbohydr. Res. 1986;155:272–276. doi: 10.1016/s0008-6215(00)90158-7. [DOI] [PubMed] [Google Scholar]
- 8.Prehm P, Jann B, Jann K. Eur. J. Biochem. 1976;67:53–56. doi: 10.1111/j.1432-1033.1976.tb10631.x. [DOI] [PubMed] [Google Scholar]
- 9.Tada R, Nagi-Miura N, Adachi Y, Ohno N. Chem. Pharm. Bull. 2007;55:992–995. doi: 10.1248/cpb.55.992. [DOI] [PubMed] [Google Scholar]
- 10.Jansson PE, Lönngren J, Widmalm G, Leontein K, Slettengren K, Svenson SB, Wrangsell G, Dell A, Tiller PR. Carbohydr. Res. 1985;145:59–66. doi: 10.1016/s0008-6215(00)90412-9. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Kido N, Kato Y, Koide N, Yoshida T, Yokochi T. Gene. 1997;198:111–113. doi: 10.1016/s0378-1119(97)00300-4. [DOI] [PubMed] [Google Scholar]
- 12.Kido N, Kobayashi H. J. Bacteriol. 2000;182:2567–2573. doi: 10.1128/jb.182.9.2567-2573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke BR, Cuthbertson L, Whitfield C. J. Biol. Chem. 2004;279:35709–35718. doi: 10.1074/jbc.M404738200. [DOI] [PubMed] [Google Scholar]
- 14.Clarke BR, Richards MR, Greenfield LK, Hou D, Lowary TL, Whitfield C. J. Biol. Chem. 2011 Oct 11; doi: 10.1074/jbc.M111.295857. doi:10.1074/jbc.M111.295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzenellenbogen E, Kocharova NA, Zatonsky GV, Kubler-KieIb J, Gamian A, Shashkov AS, Knirel YA, Romanowska E. FEMS Immunol. Med. Microbiol. 2001;30:223–227. doi: 10.1111/j.1574-695X.2001.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 16.Westphal O, Jann K. Methods Carbohydr.Chem. 1965;5:83–91. [Google Scholar]