Abstract
A new PET ligand, 3-fluoro-5-(2-(2-18F-(fluoromethyl)-thiazol-4-yl)ethynyl)benzonitrile (18F-SP203) can quantify metabotropic glutamate subtype 5 receptors (mGluR5) in human brain by a bolus injection and kinetic modeling. As an alternative approach to a bolus injection, binding can simply be measured as a ratio of tissue to metabolite-corrected plasma at a single time point under equilibrium conditions achieved by administering the radioligand with a bolus injection followed by a constant infusion. The purpose of this study was to validate the equilibrium method as an alternative to the standard kinetic method for measuring 18F-SP203 binding in brain. Nine healthy subjects were injected with 18F-SP203 using a bolus plus constant infusion for 300 minutes. A single ratio of bolus-to-constant infusion (the activity of bolus equaled to that of infusion over 219 minutes) was applied to all subjects to achieve equilibrium in approximately 120 minutes. As a measure of ligand binding, we compared total distribution volume (VT) calculated by the equilibrium and kinetic methods in each scan. The equilibrium method calculated VT by the ratio of radioactivity in brain to the concentration of 18 F-SP203 in arterial plasma at 120 minutes, and the kinetic method calculated VT by a two-tissue compartment model using brain and plasma dynamic data from 0 to 120 minutes. VT obtained via the equilibrium method was highly correlated with VT obtained via kinetic modeling. Inter-subject variability of VT obtained via the equilibrium method was slightly smaller than VT obtained via the kinetic method. VT obtained via the equilibrium method was ~10% higher than VT obtained via the kinetic method, indicating a small difference between the measurements. Taken together, the results of this study show that using the equilibrium method is an acceptable alternative to the standard kinetic method when using 18F-SP203 to measure mGluR5. Although small differences in the measurements obtained via the equilibrium and kinetic methods exist, both methods consistently measured mGluR5 as indicated by the highly correlated VT values; the equilibrium method was slightly more precise, as indirectly measured by the smaller coefficient of variability across subjects. In addition, when using 18F-SP203, the equilibrium method is more efficient because it requires much less data.
Keywords: 18F-SP203, bolus plus constant infusion, equilibrium method, metabotropic glutamate subtype 5 receptor (mGluR5), positron emission tomography (PET)
1. Introduction
Evidence suggests that mGluR5 dysfunction may be implicated in several human disorders, including anxiety, schizophrenia, substance abuse, and fragile X syndromes (Slassi et al., 2005). Thus, developing a useful positron emission tomography (PET) radioligand for imaging mGluR5 in the human brain is key to assessing its role in the pathophysiology of these diseases, and to developing new therapeutic approaches for their treatment.
The new, promising PET ligand 3-fluoro-5-(2-(2-18F-(fluoromethyl)-thiazol-4-yl)ethynyl)benzonitrile (18F-SP203) was developed to image the metabotropic glutamate subtype 5 receptor (mGluR5) (Siméon et al., 2007). Initial evaluation of this ligand with a bolus injection and kinetic modeling in healthy humans showed that receptor binding measured as total distribution volume (VT) was well identified and had relatively small inter-subject variability, indicating that the measurement was precise (Brown et al., 2008). 18F-SP203 showed high uptake to, and relatively fast washout from, the brain.
PET measurement of ligand binding to receptors and transporters in brain is commonly performed at major research facilities with a bolus injection of the PET ligand followed by frequent and intense data acquisition from both brain and arterial blood, initially at several time points per minute. Such data acquisition is necessary to capture dynamic changes in activity over time. Notably, this type of data acquisition deviates markedly from common clinical PET or nuclear medicine practice, where one static image acquisition is performed (e.g., 18F-FDG and 99mTc-methylene diphosphonate bone scans). The need for such frequent and intense data acquisition makes the widespread application of PET imaging of brain receptors and transporters to various patient groups difficult.
The equilibrium method is a possible alternative approach for quantifying VT with one or a few static data acquisitions, and such a method would not deviate markedly from common clinical PET or nuclear medicine practice. Furthermore, the equilibrium method has several advantages over the more common method of a bolus injection and kinetic modeling. Typically, to achieve equilibrium rapidly in brain regions and plasma, a radioligand is administered as a bolus followed by constant infusion (Carson, 2000), and the ratio of bolus-to-infusion needs to be optimized using data obtained from bolus scans (Carson et al., 1993); in contrast, the equilibrium method requires markedly reduced brain and blood data sampling. The equilibrium method allows simple calculation of VT based on a concentration ratio of tissue to metabolite-corrected plasma at a single time point, which is somewhat similar to static image acquisition in clinical PET and nuclear medicine. Quantification is also simplified in the equilibrium method; instead of acquiring brain and blood data at multiple time points to capture the dynamic changes over time as required for the kinetic method, the equilibrium method requires only a small number of blood samplings and a brief brain scan, which allows several subjects to be scanned with a single camera. Finally, in the equilibrium method ligand binding can be measured simply by calculating the ratios of brain to metabolite-corrected plasma instead of using bolus data as required by the kinetic method.
The purpose of this study was to validate the equilibrium method as an alternative to the standard kinetic method for measuring 18F-SP203 binding in brain.
2. Material and Methods
2.1 Study Design
The equilibrium method has been successfully applied to several PET and single-photon emission computed tomography (SPECT) ligands and requires much less data to measure VT than the more common method of kinetic modeling. To investigate whether the equilibrium method was applicable to 18F-SP203, we studied whether this method provides similar measures to those obtained via kinetic modeling by calculating VT using both methods in each scan. In addition to comparing VT values, the precision of the two methods was indirectly compared based on inter-subject variability of VT values.
To achieve equilibrium in brain and plasma, the ratio of bolus to constant infusion of 18F-SP203 was optimized based on data obtained from bolus injections that we reported previously (Brown et al., 2008) and in vitro examination to measure radioligand adsorption to bags and tubes.
Before comparing VT values, we estimated optimal scan duration based on time-stability analyses on the kinetic method using a two-tissue compartment model and on the equilibrium method using brain to plasma ratios. The purpose of estimating optimal scan duration was two-fold; first, so that scan duration would be long enough to obtain necessary information regarding ligand kinetics and to achieve conditions close to equilibrium, and second for radiometabolites entering the brain to cause relatively small effects. A similar time-stability analysis had been conducted in our previous study (Brown et al., 2008).
Image and kinetic analyses were performed using PMOD 3.0 software (PMOD Technologies, Zurich, Switzerland).
2.2 Subjects
Three female and six male healthy volunteers were enrolled (body weight 78.6 ± 15.3 kg; age 37.8 ± 12.4 years). All subjects were free of medical or neuropsychiatric illnesses based on a medical history, physical examination, electrocardiogram, and standard blood and urine analyses, which included a complete blood count, serum chemistries, thyroid function test, urinalysis, urine drug screen, as well as syphilis, HIV, and hepatitis B screenings. Our use of 18F-SP203 in human subjects was approved by the Radiation Safety Committee of the National Institutes of Health (NIH) and the Institutional Review Board (IRB) of the National Institute of Mental Health (NIMH).
2.3 Radioligand Preparation
18F-SP203 was prepared from the bromomethyl analog of SP203 and 18F-fluoride ion, as previously described (Siméon et al., 2007). The preparation method is described in detail in our Investigational New Drug Application 78,260 (NIMH/SNIDD Tracer Database Initiative). Radiochemical purity was 100 ± 0 % (n = 9) and specific activity at the time of injection was 129 ± 51 GBq/μmol (n = 9), respectively, in nine preparations.
2.4 Administration of 18F-SP203
18F-SP203 was administered by bolus injection and constant infusion. An optimal ratio of radioactivity in the bolus to that in the constant infusion was calculated based on previously reported bolus injection data and was corrected for the amount of adsorption of the radioligand into the bag and tube. Just after the one-minute bolus injection of 18F-SP203, the infusion was started at a constant rate for 300 minutes using an infusion pump (GemStar® Infusion Systems, Hospira, Inc. Lake Forest, IL) and a low adsorbing tube (LifeShield® GemStar® Primary I.V. Pump Set with polyethylene-lined light-resistant tubing, Hospira, Inc. Lake Forest, IL).
For all subjects, we applied a single ratio of radioactivity in bolus (MBq) to that in constant infusion (MBq/min) defined as Kbolus (min). This Kbolus was calculated using previously reported bolus injection data (Brown et al., 2008) so that conditions close to equilibrium would be achieved in all subjects. Briefly, for each of the seven bolus scans in the previous study, the optimal Kbolus for achieving equilibrium was calculated using the method described by Carson and colleagues (Carson et al., 1993). The calculation was done for 10 brain regions (frontal cortex, parietal cortex, occipital cortex, temporal cortex, medial temporal cortex, caudate, putamen, cingulate, thalamus, and cerebellum) as well as for unmetabolized radiotracer in plasma. The average Kbolus value of 219 minutes obtained from the calculation was applied in all bolus plus constant infusion scans in the current study.
Adsorption of 18F-SP203 in infusion tubes and bags decreased the concentration of radioligand in solution. Before performing the current study of bolus plus constant infusion scans, we measured the magnitude of the adsorption by doing the experiments and then setting the infusion activity for the scans by taking the adsorption into account. Four experiments were performed to measure the recovery of 18F-SP203 after flowing 18F-SP203 solution through the polyethylene-lined low-adsorbing tubes. 18F-SP203 (5.3 ± 2.7 μmol (n = 4), 264 ± 70 MBq) was diluted in 10 mL of saline and injected into a 250 mL saline bag (Braun Medical Inc. Scarborough, OH). An infusion tube (LifeShield® GemStar® Primary I.V. Pump Set with polyethylene-lined light-resistant tubing, Hospira, Inc. Lake Forest, IL) with the saline bag was connected to a three-way stopcock on the downstream side. The solution was dripped for 300 minutes and sampled from the stopcock several times. Radio-concentration of the samples was measured with a dose calibrator and a scale. To check the stability of the radioligand, the composition of radioactivity in the samples was measured with a high-performance liquid chromatograph. In addition, the concentration of radioactivity in infusion solution was measured four times from 110 to 290 minutes and the actual Kbolus was calculated based on the residual activity in the bag and tube after the scans.
2.5 PET Scans
Three-dimensional dynamic PET images were acquired on an Advance camera (GE Healthcare, Waukesha, WI) for 300 minutes in 49 frames of increasing duration from 30 seconds to five minutes. Subjects had three rest periods (30 minutes each) outside the camera, beginning at approximately 120, 180, and 240 minutes after radioligand injection. All PET images were corrected for attenuation and scatter. Difference in head position among scans was corrected by realigning all images from each subject using Statistical Parametric Mapping after the scans (Version 8 for windows; Wellcome Department of Cognitive Neurology, UK).
2.6 Measurement of 18F-SP203 in Plasma
Blood samples (1.5 mL each) were drawn from the radial artery at 15-second intervals until 120 seconds, followed by 1.5-mL samples at 3, 5, 10, 20, 30, and 45 minutes; 3-mL samples were then drawn at 60, 90, 120, 150, 180, and 210 minutes. To determine the stability of 18F-SP203 in vitro, approximately 15 kBq of radioligand were incubated with blood and plasma from each subject for 30 minutes at room temperature. In blood and plasma, 97 ± 1% (n = 9) and 97 ± 1% (n = 9) of radioactivity was from unmetabolized 18F-SP203 after the incubation, respectively. Radioactivity in plasma and the in vitro standards were extracted into acetonitrile and analyzed by reverse-phase chromatography with a Novapak-C18 column (100 × 8 mm; Waters Corp., Milford, MA), using a radial compression module (RCM-100; Waters) with a sentry precolumn and a mobile phase of methanol:water:triethylamine (70:30:0.1 by volume) at a flow rate of 2.0 mL/min. Plasma-free fraction was measured by ultrafiltration as previously described (Gandelman et al., 1994). The plasma free fraction was 4.3 ± 0.7% (n = 9).
2.7 Image Analysis
Preset volumes of interest were positioned on each subject’s PET image after spatial normalization based on magnetic resonance (MR) images. The MR image of each subject was coregistered to the averaged PET image using Statistical Parametric Mapping (Version 8 for windows; Wellcome Department of Cognitive Neurology, UK). The averaged PET image was created by averaging frames 4 through 49 of the PET images, which showed good delineation of cerebral cortices. Axial MR images of 1-mm contiguous slices were obtained using a 3.0-T Achieva device (Philips Healthcarre, Andover, MA) with a repetition time of 20 milliseconds, an echo time of 4.9 milliseconds, and a flip angle of 30°. Both MR and all PET images were then spatially normalized to standard anatomic orientation (the MNI152 standard space, Montreal Neurological Institute, Montreal, QC, Canada) based on transformation parameters from the MR images. A template of preset volumes of interest (Sullivan et al., 2010) was applied to the spatially normalized PET images to extract time-activity curves for the following 10 regions: frontal (432 cm3), parietal (247 cm3), occipital (172 cm3), temporal (251 cm3), and medial temporal (36 cm3) cortices; caudate (16 cm3); putamen (17 cm3); cingulate (28 cm3); thalamus (17 cm3); and cerebellum (195 cm3).
2.8 Calculation of Distribution Volume
VT in the brain regions and in each voxel was calculated using both the equilibrium and kinetic methods. For the equilibrium method, radio-concentration in the brain regions was divided by the concentration of unmetabolized 18F-SP203 in the arterial plasma at a single time point rather than using dynamic data starting from time zero.For the kinetic method, VT was calculated with the standard unconstrained two-tissue compartment model using dynamic data of brain uptake relative to serial concentrations of parent radioligand in arterial plasma as an input function starting at time zero; in our previous study, this method showed better quantification than one-tissue and constrained two-tissue compartment models (Brown et al., 2008).
2.9 Time Stability Analysis on Measurement of Distribution Volume
In the previous bolus study (Brown et al., 2008), VT continued to increase with increasing scan duration, compatible with radiometabolites entering the brain. By examining changes in VT and identifiability with bolus data of various lengths (time stability analysis), we concluded that 120 minutes—but not the full-length data acquired for 300 minutes—was the appropriate length to calculate VT. In the current study of bolus plus constant infusion, similar time stability analyses were performed for both kinetic modeling and equilibrium methods, in order to define optimal scan duration necessary to calculate VT. We examined the dependency of VT values on data length and their inter-subject variability by truncating scan duration. In addition to these two parameters, in the kinetic method identifiability of VT was also taken into account. Because the equilibrium method gives VT by simply dividing brain activity by plasma 18 F-SP203, the identifiability of VT was not taken into account in the equilibrium method. The scan duration length that yielded the smallest dependence of VT on changes in scan duration, minimum inter-subject variability, and best identifiability of VT was chosen. The identifiability of VT by unconstrained two-tissue compartment fitting was calculated as the standard error, which itself reflected the diagonal of the covariance matrix (Carson, 1986). Identifiability was expressed as a percentage and equals the ratio of the standard error of the VT divided by the value of the VT itself. A lower percentage indicates better identifiability.
2.10 Statistical analysis
We analyzed the simple regression of VT values obtained via the equilibrium method to those obtained via the kinetic method (by the two-tissue compartment model) as well as the correlation between them. All analyses were done for VT values in the ten regions. To assess differences in VT values obtained using each method, we applied a repeated-measures analysis of variance for VT values in the regions. To assess differences in inter-subject variability of VT values between the equilibrium and kinetic methods, repeated-measures analyses of variance for the coefficient of variation of population in every region was applied. All statistical analyses were performed with SPSS (ver.17 for Windows, SPSS Inc. Chicago, IL). Group data are expressed as mean ± SD.
3. Results
3.1 Optimal ratio of bolus to infusion to achieve equilibrium
The optimal ratio of radioactivity in bolus to that in constant infusion (Kbolus) was 219 ± 26 min (n = 7), based on calculations conducted using previously reported bolus injection data (Brown et al., 2008). In our preliminary analysis, the average recovery of concentration of 18F-SP203 in the infusing solution was 67.9 ± 3.0 % (n = 4), and the radiochemical purity was still 100% at 300 minutes. We thus administered 137 ± 22 MBq 18F-SP203 as bolus followed by constant infusion at a rate of 0.92 ± 0.15 MBq/min over 300 minutes. The ratio after taking into account the adsorption—137 MBq / (0.92 MBq × 67.9 %)—gave an estimated Kbolus of 219 min. Therefore, in the current study, we administered 18F-SP203 by bolus plus constant infusion to achieve Kbolus of 219 minutes after subtracting the adsorption predicted from the non-scan experiments.
In the current PET scans, we measured the stability of concentration of radioactivity in infusion solution during the constant infusion and the actual Kbolus calculated based on the residual activity in the bag and tube after the scans. The concentration in infusion solution remained stable during infusion. The average ratio of the concentration in infusion solution to that in the prepared concentration at four time points was the same as in our preliminary analysis (67.9%). The Kbolus calculated based on the residual activity measured in each scan was 218 ± 21 minutes (n = 9), almost the same as the predicted Kbolus of 219 minutes.
3.2 Time course of radio-concentration in brain regions and plasma
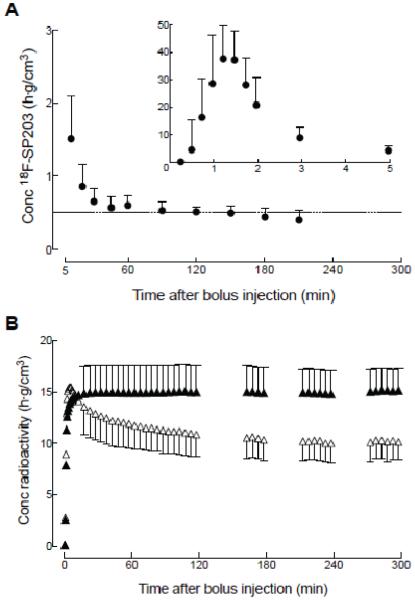
With bolus plus constant infusion under optimal Kbolus conditions, radioactivity in the brain became stable at 120 minutes. In the plasma, although the concentration of unmetabolized 18F-SP203 almost reached a stable level at 120 minutes, it decreased thereafter. Average changes in brain activity and plasma 18F-SP203 levels were −3.3 ± 1.9 and −15.4 ± 10.0 % per hour at 120 minutes, respectively (Fig. 1).
Figure 1. Time activity curves of unmetabolized 18F-SP203 in arterial plasma and radioactivity in brain regions.
(A) Time course for concentration of unmetabolized 18F-SP203 in arterial plasma after a bolus injection followed by constant infusion. The value at five minutes is shown on both graphs, which differ in range of y-axis. (B) Time course of radioactivity in brain after a bolus injection of 18F-SP203 followed by constant infusion. Radioactivity concentration is shown for brain regions with the highest (temporal cortex, ▲) and lowest (thalamus, △) concentrations. The concentration was corrected for individual body weight (g) as well as the constant infusion rate of radioactivity (MBq/h). Data represent mean ± SD of all nine subjects. Error bars are provided only after 10 minutes for graph (B) to avoid unnecessary clutter.
3.3 Time stability analysis for kinetic modeling
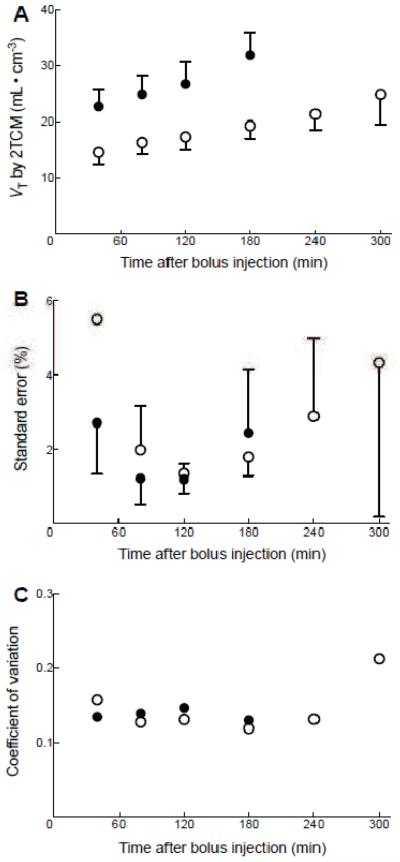
We performed a time stability analysis because results from our previous bolus study led us to suspect that radiometabolites of 18F-SP203 entered the brain, and that VT continued to increase with longer scan duration (Brown et al., 2008). In the present study, we found that a scan duration of 120 minutes was optimal for calculating VT rather than the full 300 minutes. We further found that VT obtained by the unconstrained two-tissue compartment model increased with longer scan duration (Fig 2A). Specifically, the identifiability (standard errors) of the two-tissue compartment fitting was good (less than 10%) with a scan duration of 40 to 180 minutes, and the average of the standard errors was smallest at for 120-minute scans (Fig 2B); inter-subject variability (coefficient of variation of population) of VT was also small in scans lasting 40 to 180 minutes. In contrast, VT was not identifiable in some regions—including the temporal cortex—in scans lasting between 240 and 300 minutes.
Figure 2. Time-stability analysis for the kinetic method.
Total distribution volume (VT) values (A), identifiability (B), and inter-subject variability (C) are plotted as a function of duration of image acquisition. VT was calculated for temporal cortex (●) and thalamus (○) using the two-tissue compartment model on brain data from time 0 to time specified on x-axis. Data represent mean ± SD of nine subjects for VT (A) and its standard error (B). For inter-subject variability (C), the coefficient of variance of VT was calculated by SD / mean VT of nine subjects.
Standard errors and inter-subject variability identified in the current study suggest that the kinetic analysis for the 120-minute bolus plus constant infusion data provided similarly precise measures as the same-length kinetic analysis in the previous study with bolus injection. In the 120-minute data from the present study, we observed a standard error of 1.4 ± 0.2% and an inter-subject variability of 14.8 ± 1.5%, compared with a standard error of 1.4 ± 0.2% and an inter-subject variability of 13.2 ± 4.3% observed with 120-minute data from the previous bolus study (Brown et al., 2008) indicating that the precision of the kinetic analysis for the bolus plus constant infusion data was similar to that of the kinetic analysis for the bolus data.
3.4 Time stability analysis for equilibrium method
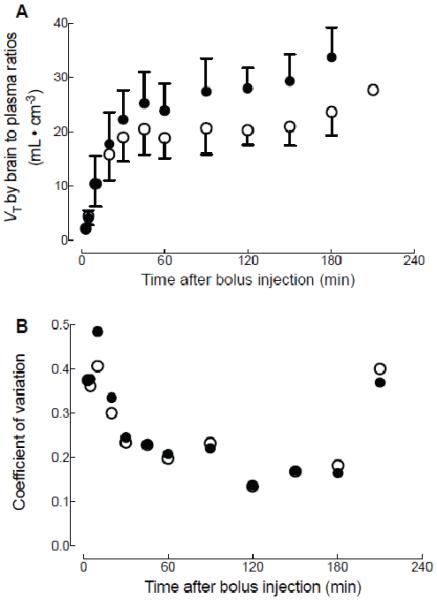
The time stability analysis for the equilibrium method indicated that the measure obtained at 120 minutes after bolus injection was optimal for calculating VT. As with the kinetic method, radiometabolites entering the brain may increase VT with long scan duration. We observed that at 120 minutes after constant infusion began, VT was stable (Fig 3A), and inter-subject variability was minimal (Fig 3B).
Figure 3. Time-stability analysis for the equilibrium method.
Total distribution volume (VT) values (A) and inter-subject variability (B) are plotted as a function of the time to measure brain-to-plasma ratios. Concentration of radioactivity in temporal cortex (●) and thalamus (○) at time specified on x-axis was divided by the concentration of 18F-SP203 in plasma at the same time. Data represent mean ± SD of nine subjects for VT values (A), and their inter-subject variability expressed as SD / mean of VT in nine subjects (B).
3.5 Comparison of VT obtained by kinetic modeling and equilibrium methods
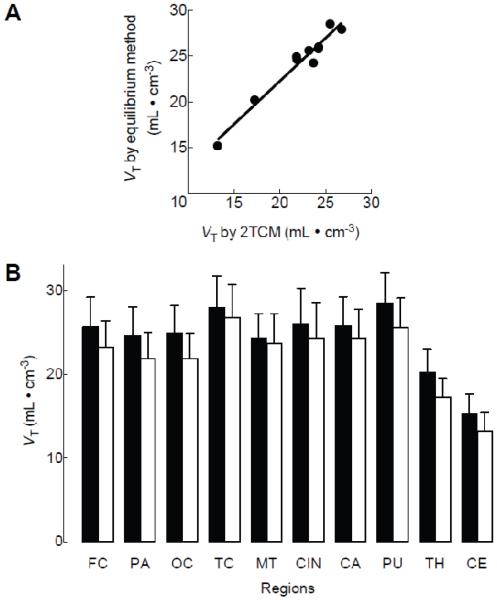
The simple linear regression analysis showed a strong linear correlation between VT values obtained via the equilibrium and kinetic methods (R2 = 0.96, p < 0.0001, Fig 4A). To test the robustness of the correlation, we conducted the same analysis but excluded the cerebellum and thalamus, which had low VT values. Even after these two regions were excluded, the correlation remained significant (R2 = 0.71, p < 0.01). Figure 5 shows the VT image created from PET images at 120 minutes divided by the concentration of 18F-SP203 in plasma at the same time, reflecting the distribution of mGluR5.
Figure 4. Comparison of total distribution volume (VT) in various brain regions.
VT values were calculated by the equilibrium method and an unconstrained two-tissue compartment model in nine subjects injected with 18F-SP203 as bolus plus constant infusion. Scatterplots compare mean VT values from each region measured by the equilibrium method vs. the two-tissue compartment model (2TCM) (A). The simple linear regression showed significant correlation (R2 = 0.95, p < 0.0001). (B) Mean VT values obtained via the equilibrium method (filled bars) were significantly higher than those obtained via the two-tissue compartment model (open bars) (p < 0.05 by repeated-measures analysis of variance). Data represent mean ± SD of all nine subjects. FC: frontal cortex, PA: parietal cortex, OC: occipital cortex, TC: temporal cortex, MT: medial temporal cortex, CIN: cingulate cortex, CA: caudate cortex, PU: putamen, TH: thalamus, CE: cerebellum.
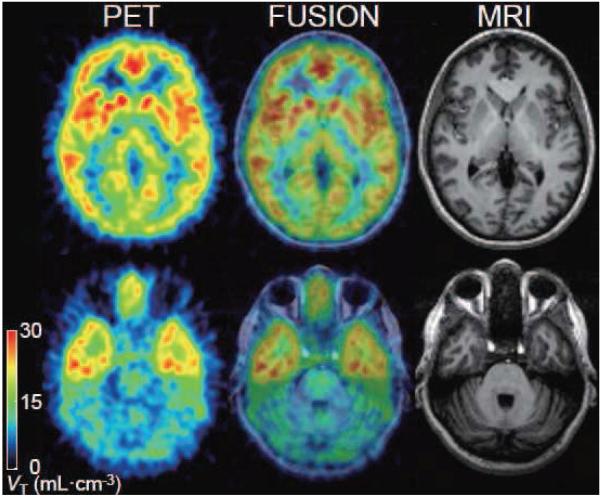
Figure 5. Transaxial PET images of total distribution volume of 18F-SP203.
One frame of PET image with five-minute duration at 120 minutes was divided by the concentration of 18F-SP203 in plasma at the same time. The pixel values represent the total distribution volume (VT) of 18F-SP203, reflecting the distribution of mGluR5. The subject was injected with 141 MBq 18F-SP203 as a bolus and 40 MBq/h as constant infusion. The coregistered MRI shows that the PET slices extended through caudate and putamen (top) and cerebellum (bottom).
Although VT obtained via both methods was highly correlated, VT obtained via the equilibrium method was significantly higher than that obtained by the kinetic method, indicating the presence of differences in the measurements. In all regions, VT obtained via the equilibrium method was higher than that obtained by the two-tissue compartment model (the mean regional difference was 9.6 ± 4.4 % and F(1, 8) = 7.53, p < 0.05 by repeated-measures ANOVA, Fig 4B).
However, the magnitude of errors in VT obtained by the equilibrium method was slightly but significantly smaller than that obtained via the kinetic method, reflecting lower inter-subject variability (equilibrium method: 13.8 ± 1.4%, two-tissue compartment model: 14.8 ± 1.5%, F(1, 9) = 10.6, P < 0.05 by repeated-measures ANOVA).
4. Discussion
This study demonstrated that, when measuring binding of 18F-SP203 in brain, the equilibrium method is a valid alternative to the standard kinetic method. VT obtained via the equilibrium method correlated well with VT obtained via the kinetic method, indicating that the former method measured 18F-SP203 binding in brain in a manner comparable to the latter. Inter-subject variability of VT obtained via the equilibrium was slightly but significantly smaller than that obtained via the kinetic method, indicating that the former provided a slightly more precise measure of inter-subject variability than the latter. However, VT obtained via the equilibrium method was ~10% higher than that obtained via the kinetic method, indicating the presence of small differences in the measurements. These findings have important clinical implications, precisely because the equilibrium method requires that data be acquired only at static phases, a practice somewhat similar to clinical PET and nuclear medicine methods; thus, it is possible that the equilibrium method could be used by clinicians who have limited experience with dynamic data acquisition after a bolus injection.
4.1 Advantages of using the equilibrium method when measuring 18F-SP203
Major research facilities typically use a bolus injection of PET ligand and continuous data acquisition during the entire scan period. In contrast, because the equilibrium method requires much less data at static phases, it has three particular advantages: lower level of invasion, higher level of efficiency, and greater ease of data acquisition. The present study demonstrated that, despite needing much less data, the equilibrium method measured different binding levels of 18F-SP203 across brain regions in a similar manner as the kinetic method, underscored by the highly significant correlation between the two methods (Fig. 4A). Furthermore, using inter-subject variability as an indirect measure of precision, we demonstrated that the equilibrium method measured 18F-SP203 binding slightly more accurately than the kinetic method.
Frequent arterial blood sampling required for kinetic methods is not necessary in the equilibrium method because this method does not require dynamic blood data over time. Instead, only one or a few blood samples at late time points are needed. Frequent blood sampling is the most invasive and labor-intensive part of PET studies using a bolus injection of a radioligand, which can therefore typically be conducted only at research-dedicated facilities such as major universities and national centers. Eliminating the need for that procedure makes scans much easier to perform in a larger number of facilities. It is important to note, however, that reference tissue models, which are usually used to avoid blood sampling, may not be applicable to mGluR5 ligands because there is no brain region without mGluR5. Cerebellum is not appropriate as a reference region in primates and humans because, unlike rodents with minimal receptor density, non-human primates and humans have significant levels of receptors in this region (Patel et al., 2007).
In addition, the equilibrium method can be performed with much shorter scans than the kinetic method. In the current study, subjects were infused with the radioligand for 300 minutes, but only a single image of five minutes duration obtained at 120 minutes was needed to calculate precise VT values. The short data acquisition associated with the equilibrium method thus allows scanning multiple subjects with a single synthesis of radioligands and a single camera.
4.2 Possible causes for difference in VT between methods
VT measured via the equilibrium method was approximately 10% higher than that measured via the kinetic method. Two possible reasons for this discrepancy exist: 1) radio-metabolites entering or developing in the brain; and 2) deviations from equilibrium.
With regard to the first possibility, the evidence suggests that radio-metabolites may have entered or developed in the brain, thereby increasing VT more markedly, when the equilibrium method was used. In our previous study with bolus injection of 18F-SP203 (Brown et al., 2008), the concentration of radioactivity in the brain at late time points was always higher than those calculated by the two-tissue compartment model, and VT continued to increase with longer scan duration. Relatedly, a previous in vivo study showed that 18F-SP203 decomposed slightly in human brain homogenates (Siméon et al., 2007). In the present study, we conducted a time-stability analysis to study the possible effects of metabolites on VT and found that VT values continuously increased with longer scan duration using both equilibrium and kinetic methods. However, it is possible that VT values obtained by the equilibrium method could have been more affected by the radio-metabolites than those obtained via the two-tissue compartment model; this is because the two-tissue compartment model using 120-minute data put lower weights based on noise equivalent counts to late time points when more radio-metabolites would have entered the brain, whereas the equilibrium method calculated VT using data at a single time point (120 minutes).
As regards the second point, it is possible that deviations from equilibrium for unmetabolized 18F-SP203 in plasma contributed to the disparity. The concentration of unmetabolized compound in plasma was not stable at 120 minutes but was slowly decreasing. Thus, measurements obtained via the equilibrium method may have been biased because VT was not measured under true equilibrium but rather under transient equilibrium. Under transient equilibrium, VT values obtained via the equilibrium method may be overestimated by the clearance effects (Carson, 2000).
Nevertheless, the evidence also suggests that the equilibrium method still provided a valid measure of 18F-SP203 binding. Specifically, VT values obtained via the equilibrium method were highly correlated with those obtained via the kinetic method. In addition, the inter-subject variability of VT values obtained via the equilibrium method was slightly but significantly smaller than that obtained via the kinetic method, indicating that the equilibrium method measured VT with slightly better precision than the kinetic method. Finally, the rank of VT values in the regions was almost the same as that observed for other mGluR5 radioligands, such as 11C-ABP688 (Treyer et al., 2007) and 18F-FPEB (Sullivan et al., 2010). VT values were high in striatum, frontal cortex, and temporal cortex, intermediate in parietal and occipital cortex, and low in thalamus and cerebellum.
4.3 Cause of deviation from equilibrium of unmetabolized 18F-SP203 in plasma
In the present study we observed less stable levels of brain activity, and particularly plasma 18F-SP203 levels, than predicted from data simulations conducted using data from the previous bolus study. Concentrations of unmetabolized 18F-SP203 in plasma almost reached a stable level before 120 minutes but decreased thereafter (Fig. 1A). In contrast, the simulation had suggested that concentrations of unmetabolized 18F-SP203 in plasma would reach stable levels after 120 minutes with the optimal Kbolus of 219 min. Plasma data indicated that the cause of the deviation from equilibrium was increased metabolism of 18F-SP203 in the middle of the scan, because no change was observed in radio-concentration of total plasma, which is a summation of 18F-SP203 and the radiometabolites. The cause of the higher than expected metabolism is not clear. However, one possibility is the effect of food on the metabolism of 18F-SP203. During the constant infusion, rested outside the camera at 120 minutes and ate lunch; notably, food intake affects the metabolism of some drugs (Singh, 1999).
4.4 Application of equilibrium method using 18F-SP203
The equilibrium method measures ligand binding under stable levels of the radioligand both in brain and plasma. Deviations from equilibrium can cause measurement errors. However, if a constant infusion is performed for a sufficient length of time, these errors will be small. Typically, the optimal ratio of radioactivity in bolus to that in constant infusion (Kbolus) is determined from parameters obtained in a pilot study of bolus injection scans in a small number of subjects. The Kbolus is then applied to larger studies (eg, a comparison between healthy subjects and patients). However, deviations from equilibrium can occur if the subjects used in the pilot study and those used in subsequent larger studies have markedly different receptor binding in brain or different clearance of radioligand in periphery. Nevertheless, even if such marked differences exist, equilibrium can be achieved by infusing the radioligand for a sufficient length of time.
In order to achieve equilibrium quickly in the present study, we administered a bolus injection of 18F-SP203 followed by constant infusion, as proposed by Carson and colleagues (Carson et al., 1993). It should be noted that, in theory, equilibrium can be achieved without having an optimal Kbolus via only the prolonged constant infusion of the radioligand (Lassen, 1992). Therefore, small deviations from equilibrium are generally not a problem when the constant infusion is performed for a sufficient length of time.
To compare receptor binding between groups when minor deviations from equilibrium are suspected, it is important ensure that the deviations do not cause artificial differences. The possibility of errors in the group comparison results can be examined by comparing the clearance of radioligand in plasma and changes in activity in brain and plasma. For example, if patients show increased brain activity over time and controls show decreased activity, but both groups show stable levels in plasma, it is likely that receptor binding was underestimated and overestimated in the patient and control groups, respectively.
5. Conclusions
This study demonstrated that, when using 18F-SP203 to measure mGluR5, the equilibrium method is an excellent alternative to the more widely used kinetic method. Despite small differences across brain regions with differing densities of mGluR5, the equilibrium method provided VT values similar to those obtained via the kinetic method. In addition, the equilibrium method was more precise, as indirectly measured by the coefficient of variation of population. Furthermore, the equilibrium method is associated with several key advantages, including lower levels of invasiveness and greater efficiency than the kinetic method, allowing multiple subjects to be scanned at the same time.
Acknowledgements
This research was supported by the Intramural Program of the National Institute of Mental Health (projects Z01-MH-002795-07 and Z01-MH-002852-04). We thank Kacey Anderson, Leah P. Dickstein, Maria D. Ferraris Araneta, Gerald L. Hodges, Kimberly Jenko, Nobuyo Kimura, William C. Kreisl, Barbara A. Scepura, Cheryl L. Wallisch, Jeih-San Liow, Robert L. Gladding, and the staff of the PET Department for successful completion of the studies, George J. Grimes, Judith M. Starling and Christine Y. Hon for their comments on adsorption of the ligand, David Luckenbaugh for his comments on the statistics, Ioline Henter for editorial assistance, and PMOD Technologies (Zurich, Switzerland) for providing its image analysis and modeling software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown AK, Kimura Y, Zoghbi SS, Siméon FG, Liow JS, Kreisl WC, Taku A, Fujita M, Pike VW, Innis RB. Metabotropic glutamate subtype 5 receptors are quantified in the human brain with a novel radioligand for PET. J Nucl Med. 2008;49(12):2042–2048. doi: 10.2967/jnumed.108.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE. Parameter estimation in positron emission tomography. In: Phelps ME, Mazziotta JC, Schelbert HR, editors. Positron Emission Tomography and Autoradiography: Principles and Applications for the Brain and Heart. Raven Press; New York: 1986. pp. 347–390. [Google Scholar]
- Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27(7):657–660. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, Herscovitch P. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13(1):24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83(7):1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Neuroreceptor quantitation in vivo by the steady-state principle using constant infusion or bolus injection of radioactive tracers. J Cereb Blood Flow Metab. 1992;12(5):709–716. doi: 10.1038/jcbfm.1992.101. [DOI] [PubMed] [Google Scholar]
- NIMH/SNIDD Tracer Database Initiative [Accessed March 5, 2010];Complete IND and FDA review, PET imaging of mGluR5 receptors with [18F]SP203. Available at: http://pdsp.med.unc.edu/snidd/IND/SP203.php.
- Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol. 2007;34(8):1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Siméon FG, Brown AK, Zoghbi SS, Patterson VM, Innis RB, Pike VW. Synthesis and simple 18F-labeling of 3-fluoro-5-(2-(2-(fluoromethyl)thiazol-4-yl)ethynyl)benzonitrile as a high affinity radioligand for imaging monkey brain metabotropic glutamate subtype-5 receptors with positron emission tomography. J Med Chem. 2007;50(14):3256–3266. doi: 10.1021/jm0701268. [DOI] [PubMed] [Google Scholar]
- Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–255. doi: 10.2165/00003088-199937030-00003. [DOI] [PubMed] [Google Scholar]
- Slassi A, Isaac M, Edwards L, Minidis A, Wensbo D, Mattsson J, Nilsson K, Raboisson P, McLeod D, Stormann TM, Hammerland LG, Johnson E. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr Top Med Chem. 2005;5(9):897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Lim K, Labree D, Lin S, McCarthy TJ, Seibyl J, Tamagnan G, Huang Y, Carson RE, Morris ED, Ding Y. Kinetic modeling of the mGluR5 tracer [18F]F-FPEB in humans. Neuroimage. 2010;52(S1):S169–170. [Google Scholar]
- Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, Schmidt M, Gasparini F, Hock C, Buck A. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med. 2007;48(7):1207–1215. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]