Abstract
Objective
The goal of the current study was to examine whether parenting an adult child with a serious mental illness (SMI) has a physiological impact on parents.
Method
Multiple samples of saliva were collected on four days from 61 parents (mean age = 60.07 years, SD = 10.01) of individuals with a SMI (bipolar disorder, schizophrenia, and major depression; mean age = 32.46 years, SD = 10.57) and a comparison group of 321 parents (mean age = 58.09 years, SD = 12.88) of individuals without a SMI (mean age = 32.36; SD = 13.87). Saliva samples were assayed for the hormone cortisol and group differences in diurnal cortisol patterns and their association with daily stress severity were explored.
Results
On days following elevated stress, a hypoactivation pattern of diurnal cortisol suggestive of chronic stress was evident for parents of individuals with a SMI. Following more stressful days, cortisol levels increased less from waking to 30 minutes after waking and declined less from 30 minutes after waking to bedtime for parents of individuals with a SMI.
Conclusions
The results of the current study add to a growing body of evidence that the long-term effects of parenting an adult with a disability has a biological impact on aging parents and support the need for family interventions across adulthood and into old age for parents of individuals with SMI.
Keywords: cortisol, serious mental illness, caregiving, midlife
Stress associated with caregiving takes a toll on physical health (Vitaliano, Zhang, & Scanlan, 2003). Although much research has examined the psychological impact of parenting an adult child with a serious mental illness (SMI), especially in terms of perceived burden (Satorius, Leff, Lopez-Ibor, Maj, & Okasha, 2005), little research has focused on the physiological impact of parenting an adult child with a SMI. The hormone cortisol is a biomarker of hypothalamus-pituitary-adrenal (HPA) axis activation that plays an important role in mediating the effects of life stressors on physical health. Over time, repeated or chronic stress exposure can lead to flatter profiles and persistently low levels of circulating cortisol, a pattern of hypoactivity that is symptomatic of wear and tear on the HPA axis (Miller, Chen, & Zhou, 2007).
Hypoactive cortisol profiles are linked to chronic stress exposure, including chronic caregiving stress in parents of individuals with developmental disabilities and SMIs. For example, among parents of adult children with a developmental disability or mental illness, cortisol declined less on days parents spent more time with their children, including the affected child (Seltzer et al., 2009). Likewise, in a sample of mothers of adults with an autism spectrum disorder, a flatter cortisol awakening response (CAR) was experienced on days following elevated behavior problems, especially for mothers whose adult child had a history of high levels of behavior problems (Seltzer et al., 2010). In a sample of 38 parents of individuals with schizophrenia, parents whose son or daughter was not institutionalized had a flatter CAR than parents whose son or daughter was institutionalized (Gonzolez-Bono, De Andres-Garcia, Moya-Albiol, 2010).
In the current study we examine the CAR and daily decline in cortisol in a sample of parents of adults with a SMI, and compare these parameters to those manifested by parents of non-disabled adult children. We expected that parents of individuals with an SMI would exhibit a flatter CAR and flatter daily decline in cortisol on days following more severe stress.
Method
Procedure
All participants completed an identical data collection protocol based on the methodology developed for the National Study of Daily Experiences (NSDE; Almeida, McGonagle, & King, 2009), one of the projects that comprise the National Survey of Midlife in the United States (MIDUS; Brim, Ryff, & Kessler, 2004). The NSDE data collection protocol included 8 days of telephone interviews and 4 days of saliva collection. Institutional Review Boards at both the University of Wisconsin and the Pennsylvania State University approved the data collection protocol and oral consent was obtained from all participants during telephone interviews.
Participants
Of the participants drawn from the MIDUS study who also participated in the NSDE 37 (22 mothers and 15 fathers) self-identified as having an adult child with a SMI (17 bipolar disorder, 8 schizophrenia, and 12 major depression). An additional 24 parents (11 mothers and 13 fathers) from the Wisconsin Longitudinal Study (WLS; Hauser & Warren, 1997) who were identified as having a son or daughter with a SMI (7 bipolar disorder, 6 schizophrenia, 11 major depression; see Aschbrenner, Greenberg, & Seltzer, 2009 for a detailed description) also participated in the identical NSDE protocol. A comparison sample (N = 321) of parents with at least one living child, but no child with a disability or chronic health condition, and who never provided care to a family member was also drawn from the MIDUS/NSDE.
Of the parents of individuals with a SMI, 46% were fathers, 72% were married, 59% had completed some post-secondary education, and all were White. On average they were 60.07 (SD= 10.01) years of age. Of the parents in the comparison group, 51% were fathers, 82% were married, 69% had completed some post-secondary education, and 93% were White. On average they were 58.09 (SD = 12.88) years of age. There were no significant differences between the SMI and comparison groups for any of the parent characteristics. The individuals with a SMI were 32.46 (SD = 10.57) years of age, on average, 39% lived in the family home, and 47.5% were male. The average age of all children of parents in the comparison group was 32.36 (SD = 13.87) years. Fifty percent of all comparison group children were male and 21% of all comparison group children lived in the family home.
Measures
Daily Stressors
Number and severity of daily stressors were assessed during the evening phone interviews with the Daily Inventory of Stressful Events (DISE; Almeida, Wethington, & Kessler, 2002). Following the scoring procedures outlined by Mroczk and Almeida (2004), a total daily stress score was calculated for each of the 8 days by summing the severity scores for all seven items. Possible scores ranged from 0 (experienced none of the stressful situations) to 28 (experiences all seven situations as a level that was very stressful). Higher scores indicated that more events were experienced that were considered highly stressful.
Cortisol
On days 2 through 5 of the 8-day diary period parents collected 4 saliva samples per day (upon wakening, 30 minutes after getting out of bed, before lunch, and at bed time) to be assayed for cortisol. We analyzed only the first two morning samples and the bed time sample because only these three samples are used in the calculation of the CAR and daily decline. Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL, Hamburg, Germany), with intra-assay and inter-assay coefficient variations below 5% (Dressendorfer, Kirschbaum, Rhode, Stahl, & Strasburger, 1992; Polk, Cohen, Doyle, Skoner, & Kirschbaum, 2005). To minimize the influence of extreme outliers, salivary cortisol values higher than 60 nmol/L were recoded as 61 (Dixson & Yuen, 1974; Wainer, 1976). Using the raw scores for absolute levels of salivary cortisol two parameters of diurnal rhythm were calculated: CAR and daily decline. CAR was calculated for each participant for each day of data collection by subtracting wake values from the 30 minutes after waking values. Likewise, daily decline was calculated by subtracting the bed time values from the 30 minutes after waking values.
Medication Use
On the last day of saliva collection, during the evening telephone interview, participants indicated whether they had taken allergy, steroid (oral and creams), hormonal (e.g., birth control), and anti-anxiety or anti-depression medications at any point during the 4-day cortisol collection period because these types of medication can affect cortisol levels (Granger, Hibel, Fortunato, & Kapelewski, 2009).
Results
Descriptive statistics are presented in Table 1. Parents of individuals with a SMI experienced more stressful events and had higher average ratings of stress severity compared to parents of individuals without a SMI, on average across the 8-day diary period. On average, cortisol declined less from out of bed to bed collection times for parents in the SMI group compared to parents of individuals without a SMI. Additionally, parents of individuals with a SMI were more likely to be taking anxiety/depression medication compared to parents of individuals without a SMI.
Table 1.
Descriptive Statistics and Mean-Level Comparisons
| SMI Parents N = 61 |
Comparison Parents N = 321 |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Stress | ||||||
| Number of Stressors | .65 | .56 | .42 | .37 | −3.08 | .00 |
| Stress Severity Sum | 1.89 | 1.63 | 1.16 | 1.10 | −3.30 | .00 |
| Cortisol (nmol/L) | ||||||
| Wake | 17.46 | 10.72 | 16.75 | 7.38 | −.14 | .90 |
| Out of bed | 22.83 | 11.48 | 24.19 | 10.59 | 1.13 | .26 |
| Bed | 5.72 | 8.56 | 4.22 | 5.57 | −1.86 | .06 |
| Cortisol Awakening Response | 5.62 | 9.70 | 7.48 | 8.34 | 1.55 | .12 |
| Decline from Out of Bed | 17.13 | 11.35 | 20.11 | 10.71 | 1.97 | .05 |
| Medication Use (proportion) | ||||||
| Allergy | .26 | .44 | .16 | .37 | −1.71 | .09 |
| Steroid | .20 | .40 | .12 | .33 | −1.32 | .19 |
| Hormone | .13 | .34 | .14 | .35 | .25 | .80 |
| Anxiety/Depression | .26 | .44 | .12 | .33 | −2.36 | .02 |
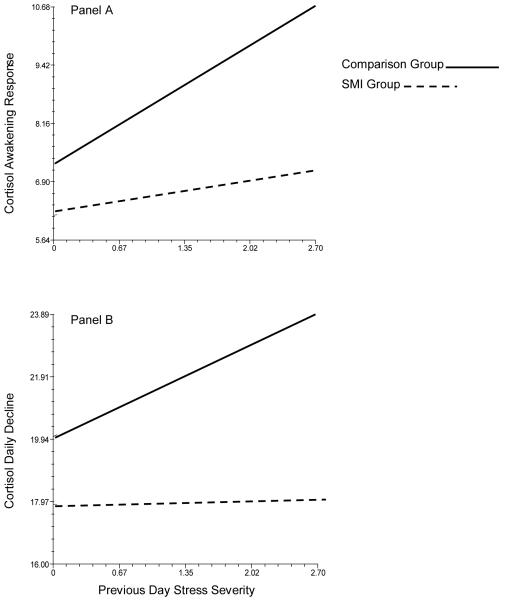
Multilevel modeling was used to determine whether within-person associations between daily reports of subjective experiences of stress and next-day cortisol expression differed for parents of individuals with a serious mental illness compared to parents of individuals without a serious mental illness (see Table 2). Data were analyzed with the hierarchical linear modeling (HLM) program (Raudenbush & Bryk, 2002). Between-persons effects of parent gender, parent age, and allergy, steroid, hormonal, and anxiety/depression medication were controlled. Additionally, within-person effects of saliva collection time and number of previous day stressors were controlled. Controlling for the number of stressors allowed us to more accurately model subjective experiences of stress severity. For all analyses, continuous variables were grand-mean centered. For both daily rhythm parameters, the between-persons by within-person interaction of previous-day subjective stress severity and parent group was significant. Compared to parents of individuals without an SMI, parents of individuals with a SMI had less pronounced CAR (see Figure 1 Panel A and less pronounced daily decline (see Figure 1 Panel B) on days that followed high stress days.
Table 2.
Group Comparison (MI parents vs. Comparison parents) Predicting Cortisol Awakening Response and Daily Decline (nmol/L) from Previous Day Stress
| Cortisol Awakening Response (Out of Bed - Wake) |
Daily Decline (Out of Bed - Bed) |
|||
|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | |
| Between-Persons Differences | ||||
| Intercept | 6.63* | .624 | 20.45* | .831 |
| Parent Gender (Mothers = 1) | .95 | .935 | .36 | 1.221 |
| Parent Age | .09* | .038 | .08 | .048 |
| Allergy Med (Yes = 1) | −1.53 | 1.235 | −3.54* | 1.573 |
| Steroid Med (Yes = 1) | −.96 | 1.172 | −1.57 | 1.908 |
| Hormone Med (Yes = 1) | 2.11 | 1.211 | −.35 | 1.990 |
| Anxiety/Depression Med (Yes = 1) | 3.37* | 1.269 | 3.53* | 1.547 |
| Parent Group (SMI = 1) | −1.11 | 1.214 | −2.14 | 1.666 |
| Within-Person Effects | ||||
| Wake Collection Time (fixed effect) | 4.07 | 2.664 | ||
| Out of Bed Collection Time (fixed effect) | −5.09 | 2.661 | −1.13* | .401 |
| Bedtime Collection Time (fixed) | .22 | .427 | ||
| Number of Stressors (fixed effect) | −2.92* | 1.465 | −3.22 | 1.841 |
| Stress Severity (random effect) | 1.26* | .526 | 1.43* | .660 |
| Between-Persons X Within- Person Interaction |
||||
| Stress Severity X Parent Group (SMI = 1) | −.92* | .333 | −1.40* | .434 |
| Variance Components (SD) | ||||
| Intercept | 34.368* | (5.862) | 76.408* | (8.741) |
| Stress Severity | .224* | (.473) | .214 | (.462) |
| Level-1 Effect | 110.153 | (10.495) | 120.409 | (10.973) |
p < .05.
Figure 1.
Parent Group Interactions with Previous Day Stress Severity Predicting Daily Cortisol Rhythms (nmol/L)
Discussion
The current study was the first to use a daily diary design to investigate whether parents of individuals with a SMI have dysregulated diurnal cortisol patterns indicative of chronic stress experiences. As hypothesized, on days following elevated stress we found a hypoactivation pattern of cortisol for parents of individuals with a SMI. Following more stressful days, cortisol in parents of individuals with a SMI increased less from waking to 30 minutes after waking and declined less from 30 minutes after waking to bedtime.
Our results add to a growing body of evidence that the long-term effects of parenting an adult with a disability has a biological impact on aging parents. The fact that a similar pattern of hypoactivated daily cortisol in response to stress has been found across studies of parents of individuals with different diagnoses (i.e., schizophrenia, autism, developmental disabilities, and in the present analysis, SMI) and that used different measures of stress (i.e., behavioral problems of the adult child with the diagnosis, time spent with the adult child, and in the present analysis, daily stress not necessarily associated with the adult child) provides strong converging evidence for this effect.
There are a few limitations of this study that warrant attention. First, our identification of the SMI sample is based on parental reports and not a clinical interview with the patient. It should be noted that parents were asked to report only those diagnoses that had been given by a medical or mental health professional. Second, we did not have available a direct measure of the presence of a pre-existing mental health disorder in the parents that might affect cortisol profiles and experiences of stress. Thirdly, our measure of stress exposure did not assess stress associated with caring for a child with a SMI. Thus, it is unclear whether elevated experiences of stress reported by the SMI parents were related directly to caring for the child with an SMI or whether stress associated with having a child with an SMI spills over to or increases sensitivity to stress in other areas of parents lives. Finally, it should be noted that the differences between the two groups of parents in terms of number of stressors reported and subjective experiences of stress, although statistically significant, were small in magnitude.
Despite these limitations, the results of the current study highlight the need for family interventions across adulthood and into old age for parents of individuals with SMI aimed at reducing stress exposure within and outside the caregiving roll and promoting adaptive coping skills to reduce reactivity to stress (Goodman, 2004). Future research on caregiving stress and cortisol in parents of individuals with SMI should examine how specific illness-related stressors and resulting perceptions of caregiving burden (Hjärthag, Helldin, Karilampi, & Norlander, 2010) contribute to dysregulated daily cortisol rhythms, and compromise the health and well-being of aging parents of individuals with a SMI.
Acknowledgments
Author Note This manuscript was prepared with support from the National Institute on Aging (P01 AG20166; Carol Ryff, PI; P01 AG021079; Robert Hauser, PI) and a Social Sciences and Humanities Research Council of Canada postdoctoral fellowship awarded to the first author. We would like to thank the parents who participated in this study for their support of our research.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea.
References
- Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbrenner KA, Greenberg JS, Seltzer MM. Parenting an adult child with bipolar disorder in later life. Journal of Nervous and Mental Disorders. 2009;197:298–304. doi: 10.1097/NMD.0b013e3181a206cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. The MIDUS National Survey: An overview. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we?: A national study of well-being at midlife. University of Chicago Press; Chicago, IL: 2004. pp. 1–36. [Google Scholar]
- Dixson WJ, Yuen KK. Trimming and winsorization: A review. Statistical Papers. 1974;15:157–170. [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rhode W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemical and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bono E, De Andres-Garcia S, Moya-Albiol L. The cortisol awakening response in caregivers of schizophrenic offspring show sensitivity to patient status. Anxiety Stress Coping. 2010;23:1–14. doi: 10.1080/10615806.2010.481792. doi 10.1080/10615806.2010.481792. [DOI] [PubMed] [Google Scholar]
- Goodman H. Elderly parents of adults with severe mental illness: Group work interventions. Journal of Gerontological Social Work. 2004;44:173–188. [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. Sociological Methodology. 1997;27:177–298. [Google Scholar]
- Hjärthag F, Helldin L, Karilampi U, Norlander T. Illness-related components for the family burden of relatives to patients with psychotic illness. Social Psychiatry and Psychiatric Epidemiology. 2010;45:275–283. doi: 10.1007/s00127-009-0065-x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Polk De. E., Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in health adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Satorius N, Leff J, Lopez-Ibor J, Maj M, Okasha A. Families and mental disorders: From burden to empowerment. John Wiley & Sons; Chichester, England: 2005. [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Taylor JL. Psychological and biological markers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and child behavior problems in families of adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer H. Robust statistics: A survey and some prescriptions. Journal of Educational Statistics. 1976;1:285–312. [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]