Abstract
Obesity is on the rise in all developed countries, and a large part of this epidemic has been attributed to excess caloric intake induced by ever present food cues and the easy availability of energy dense foods in an environment of plenty. Clearly, there are strong homeostatic regulatory mechanisms keeping body weight of many individuals exposed to this environment remarkably stable over their adult life. Other individuals, however, seem to eat not only because of metabolic need, but also because of excessive hedonic drive to make them feel better and relief stress. In the extreme, some individuals exhibit addiction-like behavior towards food, and parallels have been drawn to to drug and alcohol addiction. However, there is an important distinction in that unlike drugs and alcohol, food is a daily necessity. Considerable advances have been made recently in the identification of neural circuits that represent the interface between the metabolic and hedonic drives of eating. We will cover these new findings by focusing first on the capacity of metabolic signals to modulate processing of cognitive and reward functions in cortico-limbic systems (bottom-up) and then on pathways by which the cognitive and emotional brain may override homeostatic regulation (top-down).
Introduction
In our modern world, we no longer eat only when metabolically hungry. We often eat in the complete absence of hunger and in spite of large fat reserves. Eating when fuels are depleted and abstaining from eating when replete serves a “homeostatic” model for the regulation of energy balance. In contrast to this metabolically driven eating, all other eating can be considered “non-homeostatic”, implying that it is not regulated or compensated by some form of metabolic feedback. A more expressive term for “non-homeostatic” is “hedonic” eating, which refers to the involvement of cognitive, reward and emotional factors. Much progress has been made in identifying the metabolic feedback signals and neural systems, located mainly in brainstem and hypothalamus, that represent a “homeostatic regulator (Fig. 1). On the other hand, the neural pathways and functions principally located in cortico-limbic structures responsible for “hedonic” eating – eating that has parallels with addiction mechanisms - are much less understood. Importantly, it is necessary to understand how these metabolic and hedonic pathways interact with each other. The following is an attempt to delineate these potential pathways and mechanisms of such interaction by reviewing recent evidence.
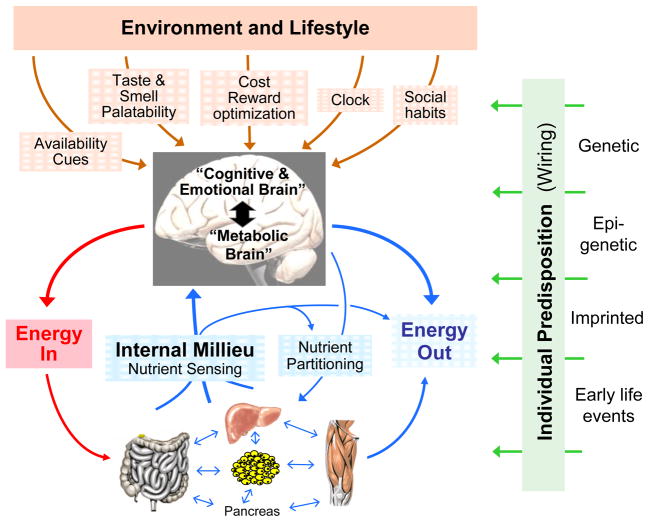
Fig. 1.
Schematic diagram showing the major factors determining neural control of appetite and regulation of energy balance. The brain monitors the internal milieu through a number of hormonal and neural nutrient sensing mechanisms and is under constant influence of the environment and lifestyle through the senses and mainly the cognitive and emotional brain. The two streams of information are integrated to generate adaptive behavioral (food intake) and autonomic/endocrine responsses determining nutrient partitioning, energy expenditure, and overall energy balance. All of the peripheral and central signaling steps are subject to individual predisposition either through genetic, epigenetic, or non-genetic early life imprinting mechanisms.
Bottom-up processes: Metabolic signals modulate higher brain functions
It has long been known that food deprivation or restriction increases the reinforcement value of a food reward [1]. This basic observation has been confirmed in numerous studies, and modern neuroimaging techniques have begun to identify the specific neural systems involved in humans. A recent study in fasting healthy human subjects viewing pictures of high-calorie versus low-calorie foods showed that high-calorie foods selectively increased neural activity in reward related areas such as the orbitofrontal cortex, ventral striatum, amygdala, and anterior insula. These increases were positively correlated with subjective liking of the foods represented by the images [2]. Together, the findings suggest that some fasting-related signal, a signal conveying “caloric need”, modulates the hedonic value assigned to specific familiar foods triggered by looking at pictures, and that these foods will be preferred if available for eating. The process of fasting-induced heightened motivation has been termed incentive salience attribution [3]. In an important paper, Tindell, Berridge and colleagues recently demonstrated that prior experience with the specific nutrient stimulus is not necessary for making it more attractive under deprivation conditions [4]. In salt-depleted rats, increased wanting of salt was accompanied by cue-induced firing of ventral pallidal neurons even before the intense, and normally disliked, saltiness had ever been tasted and liked, suggesting that a cue’s incentive salience can be recomputed adaptively [4].
Although these studies show that the metabolic state can strongly modulate hedonic effects of food and food-related stimuli, they do not identify the specific neural pathways and mechanisms. In the sections that follow, I discuss the putative mechanisms of how the metabolic state influences neural function and thereby ingestive behavior.
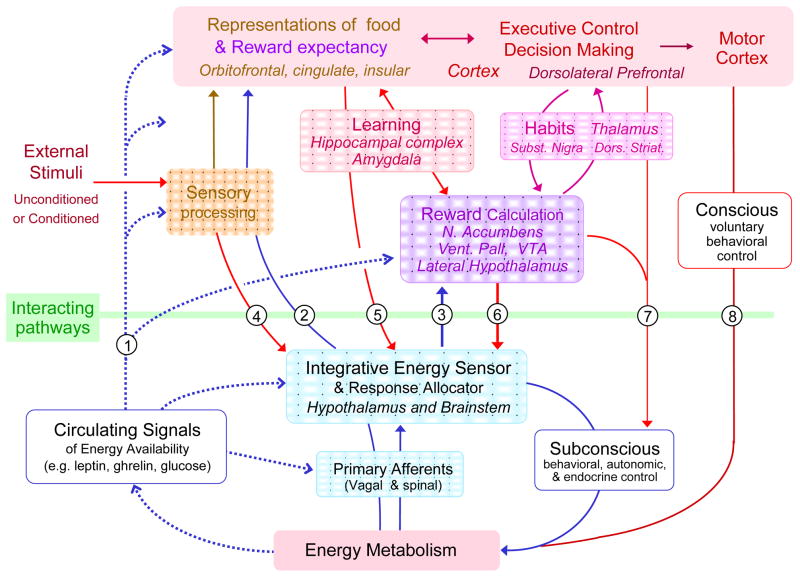
Modulation of gustatory and olfactory pathways by circulating hormones (Fig. 2, pathway 1)
Fig. 2.
Schematic diagram showing potential interactions between the so called “homeostatic” energy balance regulatory system (blue) and neural systems involved in external sensory information processing (yellowish-brown), reward processing (purple), and cognition and executive functions (red), collectively referred to as “hedonic systems”. Blue arrows indicate bottom-up modulation of hedonic systems by homeostatic signals. Broken blue lines represent circulating hormones, metabolites, and other factors; solid blue lines represent neural pathways. Red arrows indicate top-down modulation of homeostatic processes by hedonic drives. See text for discussion of specific interactive pathways.
One way in which a metabolic signal could increase the pleasure of eating is by enhancing the sensory properties of food. Perceived taste and odor are two such properties that can be potentially manipulated by alterations in sensory perception. The first indication that hormones reflecting metabolic state (caloric demand) may affect ingestive behavior (caloric intake) by modulating sensory perception was provided by the observations that leptin and insulin administration decreased gustatory and olfactory perception [5–7] [8]. Leptin may suppress sweet taste via leptin responsive lingual taste cells [5], and may modulate olfaction by changing mucous production in olfactory mucosal cells, which secrete odorant-binding proteins important in the detection of odorants by olfactory neurons [9].
Peripheral taste function appears to be modulated not just by leptin and insulin but also by other agents that reflect nutrient availability, including endocannabinoids, glucagon-like peptide-1 (GLP-1) and vasoactive intestinal peptide (VIP). Administration of the endocannabinoids AEA or 2-AG increases the electrical activity of taste receptor cells and gustatory nerve responses selectively to sweet but not other taste stimuli [10], suggesting that endocannabinoids may drive a metabolic need for food by perceptually enhancing the sweet properties of a given food. The receptor for GLP-1, a gut hormone that suppresses food intake, is also expressed in mammalian taste buds. Mice lacking the GLP-1 receptor (GLP-1R) exhibit a reduction in sensitivity to sweet taste but an enhancement of UMAMI taste [11], which suggests that by alterations in sensory perception, GLP-1 could enhance intake of sweet food, but reduce intake of foods with more savory properties. This apparent incongruence of effects on intake need further study in genetically “normal” animals. VIP may inhibit food intake by limiting sweet taste perception, as VIP knockout mice also exhibit enhanced taste preference for sweets. Because they also have elevated glucose, insulin, and leptin levels, it was suggested that the tongue could modulate energy intake to compensate for peripheral glycemic imbalances [12]. However, it is not yet clear whether the ligands for these taste bud receptors are locally produced and act in a paracrine fashion, or are gut derived.
Modulation of midbrain dopamine systems by leptin and insulin
There are two prevalent hypotheses suggested to explain the action of midbrain dopamine and excess food intake. The first is the “gluttony hypothesis”, which suggests that overindulgence in pleasurable stimuli is based on a positive correlation between the amount of dopamine signaling generated and pleasure derived from a sensory experience [23,24]. The second is the “reward-deficiency hypothesis”, which suggests that overindulgence is an attempt to self-medicate and bring deficient dopamine signaling to “pleasurable” levels [25]. There are data to support both hypotheses, as presented below.
Midbrain dopamine neurons with projections to the nucleus accumbens in the ventral striatum have been strongly implicated in food and drug reward with the potential to strongly affect the amount of energy consumed. The nucleus accumbens is particularly important for the control of reward functions, as opioid signaling within the shell portion of this nucleus plays a crucial role in hedonic value or “liking” and dopamine signaling plays a crucial role in translating motivation into action [13] by serving as an error signal for and attribution of incentive salience to reinforcing stimuli [3,14]. Earlier observations have demonstrated expression of insulin and leptin receptors, activation of intracellular signaling pathways, and modulation of electrical activity in mesolimbic dopamine neurons [15,16]. Furthermore, leptin receptor stimulation decreased dopamine release in the nucleus accumbens, while leptin receptor silencing increased dopamine release and increased sucrose preference and food intake [17,18]. In a recent series of papers, Myers and colleagues have started to further dissect this leptin-reward pathway and have produced several interesting observations [19–21]. Firstly, leptin modulation of midbrain dopamine neurons takes place not only by direct action on dopamine neurons, but also via orexin and neurotensin-expressing neurons located in the lateral hypothalamus [19]. Secondly, leptin responsive dopamine neurons project primarily to cocaine-and-amphetamine-regulated transcript- (CART)-expressing neurons in the extended central amygdala (but not the nucleus accumbens), which may be involved in processing of aversive stimuli and behaviors such as anxiety, depression, and stress responses [20]. Finally, systemic leptin administration in lean Sprague-Dawley rats stimulated dopamine transporter and tyrosine hydroxylase expression and enhanced amphetamine-stimulated dopamine efflux in the nucleus accumbens [21], suggesting up-regulation of dopamine signaling and leading to increased D2R occupation. Decreased D2R binding in leptin-treated mice [22], suggestive of higher occupation by endogenous dopamine corroborates these findings. However, the opposite was observed in leptin-deficient ob/ob mice: namely an observed increase in D2R availability after leptin-treatment, suggesting lower occupation by endogenous dopamine. Unfortunately, neither of these studies assessed the effects on food intake or preference, leaving open the ultimate question whether leptin decreases reward-driven calorie intake by inhibiting dopamine transmission or by rendering dopamine transmission more efficient. The former would be predicted by the “gluttony hypothesis”, as overindulgence in pleasurable stimuli would be positively correlated to the amount of dopamine signaling generated [23,24]. The latter would be predicted by the “reward-deficiency hypothesis”, in that the overindulgence could be interpreted as an attempt to normalize deficient dopamine signaling to “pleasurable” levels [25].
Other potential nutritional state modulators of midbrain dopamine function include ghrelin [26] and catecholaminergic afferents from hindbrain areas [27] known to process metabolic information. One of the few studies linking physical activity with the neural control of food preference suggests that the decreased preference for high fat diet in wheel-running rats may be mediated by enhanced leptin signaling in the ventral tegmental area [28]. These data suggest that the rewarding effects of wheel running, which may put animals in a state of metabolic need, substitutes for the consumption of a palatable high fat diet, thereby eliminating the preference for the HF food. This idea fits more along the lines of a “pleasure substitution” hypothesis; in that need for food reward is replaced by the perceived similar reward from physical activity, via enhanced dopamine signaling in VTA.
Given the complexity of midbrain dopamine projections to different portions of the striatum and other targets such as the amygdala, prefrontal cortex, hippocampus, and hypothalamus [29,30] it will be important in future experiments to delineate the specific subpopulations of these projecting dopamine neurons to better understand the different functions they subserve. Clearly, the above data show that metabolic inputs allows the brain to sense metabolic status (e.g. too few or too many calories) and put into play hedonic neurons that, via their actions on midbrain dopamine for instance, drive mechanisms that allow that metabolic need to be fulfilled. In this way, the metabolic and hedonic players interact to fulfill the energy need requirements.
Modulation of risk-based decisions by metabolic state
The decision to eat, and how much to eat is clearly a “free decision” [31], but one which should take into account the predicted reward values of different behavioral goals [32]. Yet animal and human studies demonstrate that a hungry state produces more risk-taking [33][34,35]. The act of impulsive eating is a risk-based decision that has been explained by over-activation of reward system components [37–41], hypo-functioning of an inhibitory network in obese and obesity-prone individuals [42,43], or defective signaling from the amygdala to the orbitofrontal cortex and nucleus accumbens [38] [44]. More work is needed to understand which of these predominate in impulsive eating, but what has become clear is that the neural processes underlying impulsive eating and other-risk based decisions are modified by metabolic state signals [33][34,35].
Two such metabolic state signals, ghrelin and leptin, have been shown to modulate activity of brain structures involved in memory and decision-making such as the hippocampus [45,46]. Recent studies in rats showed that hippocampal lesions impaired retention of discriminative responding to food cues in food-restricted rats, suggesting that the hippocampus may be involved in the inhibition of appetitive behavior by signals of energy availability [47]. In addition, spatial memory processing appears to be especially sensitive to disruption by high-fat diets [48,49], possibly mediated by changes in endocannabinoid signaling [50]. To further elucidate the effects of metabolic signals in humans, PET imaging studies will be necessary, as this is the only way to link neurochemical events to specific brain sites during the performance of particular behavioral tasks. In animals, the issue can be investigated through pharmacological or genetic manipulations of relevant brain structures or neuron populations.
Modulation of reward and cognitive functions by hypothalamic energy sensor circuitry (Fig. 2, pathway 2)
The first studies linking metabolic state signals with specific brain functions were carried out 50 years ago as part of the discovery of hypothalamic energy balance regulatory and self-stimulation circuits [51]. While it has long been known that rats electrically stimulate themselves more vigorously via lateral hypothalamic electrodes when food deprived or when glucose availability was decreased [52,53], the anatomical projections and chemistry were completely unknown. The mechanisms underlying this effect and the pathways involved have become much clearer during the last decade with the discovery of orexin and melanin concentrating hormone expressing neurons in the lateral hypothalamus, which act as metabolic sensors [54–56]. Through their widespread axonal projections they not only control effector pathways serving energy homeostasis [57] but also hedonic pleasure (liking) [58–60], reward seeking [61–66], and cognition [67]. These strategic anatomical projections put lateral hypothalamic orexin and melanin concentrating hormone neurons in an ideal position to link internal needs with choices available in the environment in order to make optimal adaptive choices. Specifically, it is tempting to speculate that the direct orexin actions on prefrontal cortex neurons [68] could be involved in reward and decision-making, but further work in this area is needed.
Viscero-and somatosensory afferent activity: generation of self-awareness (Fig. 2, pathway 3)
Finally, metabolic state information from the periphery can reach the brain via sensory neural pathways. Primary afferent neurons of both vagal and dorsal root/spinal origin can signal the state of the “rest of the body” to areas in the insular and orbitofrontal cortex as well as the amygdala and are thought to play an important role in the experience of feelings [69] and conscious awareness [70,71].
Top-down processes: reward and cognitive functions modulate metabolic state
Top-down processes include neural signals that influence either peripheral metabolism and/or the brain systems important in regulating energy state, and the recent obesity epidemic makes clear that “homeostatic” body weight regulatory processes can be overridden by other influences. This idea is supported by a recent study of overfed rats, which showed an orexigenic basomedial hypothalamic peptide expression profile that should signal for reduced energy intake. Yet despite increased expression of pre-pro-opiomelanocortin (POMC) and decreased expression of neuropeptide Y (NPY) in the hypothalamic arcuate nucleus, the animals overate and became obese on a high fat high sugar diet. These data suggest that while pattern of expression for hypothalamic homeostatic energy signals (NPY and POMC) reflect sensing of the nutrient surplus, the signals are unable to stop hyperphagia [72]. Although the role of voluntary and involuntary actions in this overriding process has been debated, we do not understand its basic neurological pathways and processes. Potential pathways of this “descending” or “top-down” interaction are schematically depicted in Fig. 2.
Modulation of the “homeostatic regulator” by reward and cognitive functions
Just as hypothalamic energy regulatory signals can modulate the activity of cortico-limbic structures (see above), signals generated by cortico-limbic structures processing sensory, cognitive, and reward information can likewise influence hypothalamic processes relevant for energy balance regulation. These potentially very important pathways have received little attention.
First, it has long been known that the lateral hypothalamus receives functional gustatory and olfactory input that is most likely independent of the cerebral cortex [73,74] (Fig. 2, pathway 4). As many of the neurons responding to gustatory stimuli are glucose sensitive and may express orexin, they may be involved in integrating external availability with internal needs as discussed above.
Second, the prominent projections from cortex, amygdala, and hippocampus to the hypothalamus ([75]and see [76] for a review) are likely to play an important role in cognitive suppression of metabolic satiation signals (Fig. 2, pathway 5). For example, intact projections from the amygdala and prefrontal cortex to the lateral hypothalamus are essential to elicit feeding in sated rats previously conditioned to associate a light or tone with food presentation while hungry [77,78].
Third, the hypothalamus receives direct input from the nucleus accumbens shell [79] (Fig. 2, pathway 6), shown to play a role in opioid-induced reward-driven food intake via activation of orexin neurons with projections to the ventral tegmental area [65]. This pathway may also be partly responsible for the development of diet-induced obesity in rats through chronic elevation of mu-opioid receptor signaling in the nucleus accumbens [80,81], as well as for the extinction of alcohol reward seeking [82].
Subconscious and conscious contributions of cortico-limbic structures to energy intake and expenditure (Fig 2, pathways 7 and 8)
It is generally assumed that hypothalamic energy balance signals drive behavioral, autonomic and endocrine effector pathways below the level of conscious awareness. While drug addiction clearly involves both conscious and unconscious components, the compulsive behavior of a drug addict is similar to the compulsive drive to eat in a child suffering from congenital leptin-deficiency or Prader-Willi syndrome. In the latter, this compulsion is clearly at the unconscious level. The effector pathways involved in subconscious drives are not well understood. Clearly, endocrine and autonomic nervous system outflow controlling many aspects of energy assimilation, metabolism, and energy expenditure operates outside awareness and these systems receive direct inputs from cortex and amygdala [83,84] (Fig. 2, pathway 7). Furthermore, an emotional (or limbic) motor system organized within longitudinal neuronal columns of the midbrain periaqueductal gray of rats [85] receives direct inputs from the hypothalamus [86], amygdala [87], and prefrontal cortex [88,89] and may be principally involved in human subconscious behavioral control. Activity along these “old” pathways has completely escaped attention by neuroimaging studies.
Motor control governed by the motor cortex is also not always under conscious voluntary control, in the sense that its actions can be determined by computations taking place outside awareness (Fig. 2, pathway 7). Specifically, activity in prefrontal and parietal cortex can encode the outcome of a decision before it enters awareness and precede activity in the motor cortex [31,90].
There also appear to be interactions between cognitive and emotional processes that could eventually lead to different responses to food cues and changes in food intake. Specifically affective responses in amygdala and orbitofrontal cortex elicited by emotionally charged pictures may be reduced within the context of increasing cognitive demands [91]. Similarly, the subjective taste of wine can be influenced by knowledge about its cost in the medial orbitofrontal cortex [92]. Also, when one interferes with the encoding of the memory of a meal, such as during television watching, there may be an increase in the subsequent consumption of snacks [93].
Finally, both food intake and physical activity level can be voluntarily controlled (Fig. 2, pathway 8). For example, humans routinely go on hunger strike based on religious or political ideas and can inhibit their drive to eat – even to the point of death [94]. The critical aspect of voluntary motor control appears to be inhibition of inappropriate behaviors, and inhibition/disinhibition are key descriptors in the control of food intake in humans.
In conclusion, it is clear that obesity is driven by many factors, the underlying bases of which are of brain origin. Eating can be triggered by metabolic need, hedonic drive, or an interaction between the two, and there are several neural circuits that represent this interface. Importantly, metabolic signals of energy status can modulate processing of cognitive and reward functions in cortico-limbic systems (bottom-up processing), which influence regulatory processes to restore energy status to the optimal level. Yet the cognitive and emotional brain can also override homeostatic regulation (top-down processing), to yield an energy imbalanced state. Clearly, the emerging obesity epidemic indicates that this top-down processing may be winning the battle of the bulge. Much more evidence on the mechanisms underlying this process is needed to effectively target this form of overindulgence and halt further increases in obesity.
Highlights.
Energy balance is normally tightly regulated by feedback signals from ingested and stored nutrients.
In our modern world, cognitive and emotional factors can overpower energy balance regulation.
Cognition and emotions can in turn be modulated by signals of nutrient availability.
Understanding the rules of interaction will help find new therapies to prevent or reverse obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoebel BG. Inhibition and disinhibition of self-stimulation and feeding: hypothalamic control and postingestional factors. J Comp Physiol Psychol. 1968;66:89–100. doi: 10.1037/h0025962. [DOI] [PubMed] [Google Scholar]
- *2.Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. This fMRI study shows that when metabolically hungry, pictures of high calorie foods were more appealing and produced higher neural activation in cortico-limbic structures than pictures of low calorie foods, and subjective rating and brain responses were positively correlated. [DOI] [PubMed] [Google Scholar]
- **3.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. This paper is a brief summary of the highly influential theoretical framework of distinctive behavioral functions and underlying neural circuitries contributing to food reward behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **4.Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. In a model of salt depletion in rats the authors show that mesocorticolimbic systems including the ventral pallidum can compute “wanting’ of a nutrient de novo by integrating novel physiological signals with a cue’s preexisting associations to an outcome that lacked hedonic value. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 6.Julliard AK, Chaput MA, Apelbaum A, Aime P, Mahfouz M, Duchamp-Viret P. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Getchell TV, Kwong K, Saunders CP, Stromberg AJ, Getchell ML. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol Behav. 2006;87:848–856. doi: 10.1016/j.physbeh.2005.11.016. [DOI] [PubMed] [Google Scholar]
- *8.Ketterer C, Heni M, Thamer C, Herzberg-Schafer SA, Haring HU, Fritsche A. Acute, short-term hyperinsulinemia increases olfactory threshold in healthy subjects. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.251. Using the euglycemic clamp technique the authors demonstrate that hyperinsulinemia leads to reduced smelling capacity, which could contribute to the process of satiation. [DOI] [PubMed] [Google Scholar]
- 9.Badonnel K, Durieux D, Monnerie R, Grebert D, Salesse R, Caillol M, Baly C. Leptin-sensitive OBP-expressing mucous cells in rat olfactory epithelium: a novel target for olfaction-nutrition crosstalk? Cell Tissue Res. 2009;338:53–66. doi: 10.1007/s00441-009-0846-2. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, et al. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A. 2010;107:935–939. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, et al. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 2010;59:1143–1152. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 14.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 15.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 16.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- **19.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. First systematic mapping of leptin receptor bearing neurons with direct and indirect projections to the ventral tegmental area further implicating lateral hypothalamic orexin/hypocretin neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. First systematic mapping of specific projection targets of leptin receptor bearing, mesolimbic dopamine neurons that unexpectedly identify CART neurons in the amygdala as major projection targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry ML, Leinninger GM, Chen R, Luderman KD, Yang H, Gnegy ME, Myers MG, Jr, Kennedy RT. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. J Neurochem. 2010;114:666–674. doi: 10.1111/j.1471-4159.2010.06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffly J, Michaelides M, Wang GJ, Pessin JE, Volkow ND, Thanos PK. Leptin increases striatal dopamine D2 receptor binding in leptin-deficient obese (ob/ob) mice. Synapse. 2010;64:503–510. doi: 10.1002/syn.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerink BH, Teisman A, de Vries JB. Increase in dopamine release from the nucleus accumbens in response to feeding: a model to study interactions between drugs and naturally activated dopaminergic neurons in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:230–235. doi: 10.1007/BF00169288. [DOI] [PubMed] [Google Scholar]
- 24.Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1846–1854. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. A series of studies with ghrelin agonists and antagonists administered both centrally and peripherally clearly demonstrate selective effects of ghrelin signaling in the ventral tegmental area on motivation to eat and consumption of palatable foods such as sucrose but not regular chow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarpace PJ, Matheny M, Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol Behav. 2010;100:173–179. doi: 10.1016/j.physbeh.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- **31.Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nat Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. Using fMRI and statistical pattern recognition techniques, it is shown that neural activity in the medial and lateral frontopolar cortex as well as the precuneus reflecting a future motor response (as for example eating) precedes the time of the decision entering consciousness by as much as 10 seconds. [DOI] [PubMed] [Google Scholar]
- 32.Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symmonds M, Emmanuel JJ, Drew ME, Batterham RL, Dolan RJ. Metabolic state alters economic decision making under risk in humans. PLoS One. 2010;5:e11090. doi: 10.1371/journal.pone.0011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brogan A, Hevey D, Pignatti R. Anorexia, bulimia, and obesity: shared decision making deficits on the Iowa Gambling Task (IGT) J Int Neuropsychol Soc. 2010;16:711–715. doi: 10.1017/S1355617710000354. [DOI] [PubMed] [Google Scholar]
- 35.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- **38.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. This fMRI study used hypothesis-based path analysis of food cue-induced neural activity in the orbitofrontal cortex, amygdala, and nucleus accumbens to demonstrate that obese individuals had a relative deficiency in the amygdala’s modulation of both OFC and NAc, but exaggerated modulation of OFC’s modulation of the amygdala. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 41.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. First study to demonstrate that weak inhibitory control over eating and enhanced impulsivity as manifested in reduced neural activity in the prefrontal cortex is associated with higher body mass index in adolescents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65. doi: 10.1038/oby.2008.469. First PET study showing a negative correlation between BMI and baseline metabolism (neural activity) in prefrontal/cingulate cortex, regions whose basal neural activity positively correlates with cognitive and executive functions. In contrast, changes in activity induced by cognitive stimulation were not associated with BMI or cognitive performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring) 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 45.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 46.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. Rats that were trained to solve a metabolic state discrimination problem with stimuli produced by 0-h or 24-h food deprivation serving as discriminative cues for the delivery of sucrose pellets showed reduced discrimination after hippocampal lesions and developed mild obesity, suggesting that the hippocampus is involved in the reduction of appetitive behavior by signals of increased energy availability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process. 2010;36:313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 49.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, Oliet SH, Geisslinger G, Lutz B. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30:6273–6281. doi: 10.1523/JNEUROSCI.2648-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 52.Blundell JE, Herberg LJ. Relative effects of nutritional deficit and deprivation period on rate of electrical self-stimulation of lateral hypothalamus. Nature. 1968;219:627–628. doi: 10.1038/219627a0. [DOI] [PubMed] [Google Scholar]
- 53.Berthoud HR, Baettig K. Effects of insulin and 2-deoxy-D-glucose on plasma glucose level and lateral hypothalamic eating threshold in the rat. Physiol Behav. 1974;12:547–556. doi: 10.1016/0031-9384(74)90202-9. [DOI] [PubMed] [Google Scholar]
- 54.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Silva JP, von Meyenn F, Howell J, Thorens B, Wolfrum C, Stoffel M. Regulation of adaptive behaviour during fasting by hypothalamic Foxa2. Nature. 2009;462:646–650. doi: 10.1038/nature08589. Lateral hypothalamic orexin/hypocretin neurons respond to nutrient availability via the insulin receptor and the transcription factor Foxa2 leading to adaptive changes in locomotor activity and reward seeking. [DOI] [PubMed] [Google Scholar]
- 56.Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150:1202–1216. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 59.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–8273. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 62.Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- *65.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. First study implicating an accumbens - lateral hypothalamus - ventral tegmental area loop in reward-driven eating, linking accumbens opioid and orexin signaling with the mesolimbic dopamine system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dranias MR, Grossberg S, Bullock D. Dopaminergic and non-dopaminergic value systems in conditioning and outcome-specific revaluation. Brain Res. 2008;1238:239–287. doi: 10.1016/j.brainres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 68.Tose R, Kushikata T, Yoshida H, Kudo M, Furukawa K, Ueno S, Hirota K. Interaction between orexinergic neurons and NMDA receptors in the control of locus coeruleus-cerebrocortical noradrenergic activity of the rat. Brain Res. 2009;1250:81–87. doi: 10.1016/j.brainres.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 69.Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 71.Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- *72.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. Only study to demonstrate that a paradoxical orexigenic basomedial hypothalamic peptide expression profile develops in rats becoming obese on a palatable choice diet. [DOI] [PubMed] [Google Scholar]
- 73.Norgren R. Gustatory responses in the hypothalamus. Brain Res. 1970;21:63–77. doi: 10.1016/0006-8993(70)90021-1. [DOI] [PubMed] [Google Scholar]
- 74.Karadi Z, Oomura Y, Nishino H, Scott TR, Lenard L, Aou S. Responses of lateral hypothalamic glucose-sensitive and glucose-insensitive neurons to chemical stimuli in behaving rhesus monkeys. J Neurophysiol. 1992;67:389–400. doi: 10.1152/jn.1992.67.2.389. [DOI] [PubMed] [Google Scholar]
- 75.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 76.Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neuroscience & Biobehavioral Reviews. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 77.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 78.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwindinger WF, Borrell BM, Waldman LC, Robishaw JD. Mice lacking the G protein gamma3-subunit show resistance to opioids and diet induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1494–1502. doi: 10.1152/ajpregu.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Lenard NR, Zheng H, Berthoud HR. Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond) 2010;34:1001–1010. doi: 10.1038/ijo.2009.297. Only study using chronic (5-week) suppression of mu-opioid signaling locally in the nucleus accumbens, resulting in suppression of food intake and adiposity selectively when on high-fat but not chow diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 84.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 85.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 86.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 87.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 88.An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 89.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 90.van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. Unconscious activation of the prefrontal no-go network. J Neurosci. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kellermann TS, Sternkopf MA, Schneider F, Habel U, Turetsky BI, Zilles K, Eickhoff SB. Modulating the processing of emotional stimuli by cognitive demand. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *92.Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci U S A. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. The same wine labeled as expensive is rated more pleasant than when labeled cheap, and the neural correlate consisted of a much stronger and longer neural activation in the medial orbitofrontal cortex, beautifully illustrating top-down modulation of taste perception by cognitive processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *93.Higgs S, Woodward M. Television watching during lunch increases afternoon snack intake of young women. Appetite. 2009;52:39–43. doi: 10.1016/j.appet.2008.07.007. The findings in this study support the idea formulated earlier by this investigator that information in memory about the most recently consumed meal is factored into decisions about future consumption. If the memory is weak because of distraction, it will have less power to curb future appetite. [DOI] [PubMed] [Google Scholar]
- 94.Montague PR. Free will. Curr Biol. 2008;18:R584–585. doi: 10.1016/j.cub.2008.04.053. [DOI] [PubMed] [Google Scholar]