Abstract
We used Near-Infrared Spectroscopy (NIRS) to simultaneously measure brain activity in two people while they played a computer-based cooperation game side by side. Inter-brain activity coherence was calculated between the two participants. We found that the coherence between signals generated by participants right superior frontal cortices increased during cooperation, but not during competition. Increased coherence was also associated with better cooperation performance. To our knowledge, this work represents the first use of a single NIRS instrument for simultaneous measurements of brain activity in two people. This study demonstrates the use of NIRS-based hyperscanning in studies of social interaction in a naturalistic environment.
Keywords: near-infrared spectroscopy, hyperscan, wavelet coherence, social cognition, cooperation, superior frontal cortex
Introduction
Human society has been shaped with complex and sophisticated social interactions across scales ranging from individual, family, community to nation. The complexity and scale of our social interactions arguably distinguish us from other species and explain the development of the relatively larger neocortex in the human brain (Adolphs, 2003; Dunbar, 2009). Understanding the dynamics of brain activity during social interaction is important for understanding our social nature and in turn for improving the life quality of people with social deficits, such as children with autism. Development of neuroimaging technologies, especially fMRI, has enabled noninvasive measurement of brain activity and has dramatically deepened our understanding of the neural basis of cognitive processes contributing to social behavior, including emotion (Phan et al., 2002; Hagan et al., 2008), theory of mind (Gallagher and Frith, 2003), moral judgment (Greene et al., 2001), trust (King-Casas et al., 2005), and agency (Tomlin et al., 2006). These technologies also enable us to better understand the structural and functional differences in the brains of people who have deficits in social cognition such as children with autism (Chiu et al., 2008; Piggot et al., 2004), fragile X syndrome (Watson et al., 2008), and Williams syndrome (Reiss et al., 2004).
However, two important limitations of the existing studies are evident. First, while by definition social interaction involves two or more individuals, most studies of social cognition have measured brain activity in only one person at a time (Montague et al., 2002). Single-person studies are useful in that they can localize and characterize brain activations related to certain social paradigms, but they cannot directly assess the dynamic interaction of two (or more) brains. Second, while social interaction in real life occurs in a naturalistic environment (e.g. face–to-face communication), most previous studies have employed highly artificial experimental settings in which participants are restricted from natural movement or direct communication with interacting partners.
Some previous experiments have sought to overcome one or both of these limitations. For example, measuring two brains simultaneously, termed hyperscanning, using fMRI has been successfully developed (Montague et al., 2002) and employed in the study of neuroeconomics (King-Casas et al., 2005; Tomlin et al., 2006; Chiu et al., 2008; Li et al., 2009). However, fMRI is unable to offer a real-world environment for social interaction: participants have to lie in a motionless position; verbal communication is discouraged; and the scanner is noisy and often emotionally daunting. On top of these limitations is the heavy cost of maintenance of MRI instrumentation. Another technology, EEG, has also been employed in hyperscanning (Babiloni et al., 2006). EEG-based hyperscanning has enabled the study of social interaction in a more naturalistic environment (Dumas et al., 2010; Astolfi et al., 2010; Lindenberger et al., 2009), for example in poker playing (Babiloni et al., 2006) or guitar playing (Lindenberger et al., 2009). However, EEG carries with it its own limitations, chiefly the inability to precisely localize the origin of the neuroelectrical signal (Michel et al., 2004).
On the other hand, NIRS offers a cost-effective, easy-to-use, non-invasive cortical imaging technology capable of measuring brain activity in a more naturalistic environment than fMRI experiments (Villringer and Chance, 1997; Rolfe, 2000). NIRS is a relatively flexible technology and to date has been successfully applied in several domains (Cui et al., 2011), including physiological mechanisms of the BOLD response (Toronov et al., 2003; Hoge et al., 2005; Kleinschmidt et al., 1996; Schroeter et al., 2006; Emir et al., 2008; Malonek et al., 1997; Huppert et al., 2006; 2009; 2007), non-invasive brain-computer interfaces (Power et al., 2010; Coyle et al., 2004; 2007; Sitaram et al., 2007; Utsugi et al., 2008), and brain activity in both active tasks and resting states (Boecker et al., 2007; Herrmann et al., 2005; White et al., 2009; Honda et al., 2010; Zhang et al., 2010; Lu et al., 2010). Particularly, NIRS has been used in real-world situations (Dresler et al., 2009) such as face-to-face communication (Suda et al., 2010), object manipulation (Okamoto et al., 2004), exercise (Ekkekakis, 2009), and driving (Harada et al., 2007; Tomioka et al., 2009).
While NIRS offers many opportunities for life-like experiments, its potential for hyperscanning has not been demonstrated. In this study, we developed an easy-to-use, cost-effective hyperscanning method with a single NIRS device, and used this method to study social interaction in a natural environment in which two participants sit side-by-side and perform a cooperation game. We demonstrate that the inter-brain coherence, a measure of the correlation between two signals of brain activity, increases during cooperation, but not during competition. To our knowledge, this work represents the first use of a single NIRS instrument for simultaneous measurements of brain activity in two-people1.
Materials and Methods
Participants
Twenty-two adults (11 pairs, mean age: 26 years with standard deviation 6, 12 females) participated in this study. Out of 11 pairs of participants, 8 pairs had been acquainted prior to the experiment. There were 2 female-female pairs, 1 male-male pair, and 8 male-female pairs. Written informed consent was obtained from all participants, and the study protocol was approved by the Stanford University Institutional Review Board. Participants were not paid for their performance.
Experimental Procedure
Each pair participated in four separate computer-based tasks: “cooperation,” “competition,” “single 1,” and “single 2,” as described below.
Cooperation
In the beginning of each trial, a hollow gray circle appeared and remained on the screen (Figure 1A). After a random delay of 0.6–1.5s (uniformly distributed), the gray circle filled with a green circle ( go signal). The participants were instructed to press their response keys only after the go signal. The participant on the left (denoted as participant #1) was instructed to use the z key, and the participant on the right (denoted as participant #2) was instructed to use the / key. We will denote the time between the go signal and the key press as the “response time.” If the difference between the response times of the two participants was smaller than a threshold (defined below), both participants earned one point; otherwise they both lost one point. They were instructed to maximize the number of points earned. Participants were also instructed to look at the screen during the experiment and, in most cases, their hands were covered with a cloth to reduce each participant s ability to view motor activity by the other member of the pair.
Figure 1.
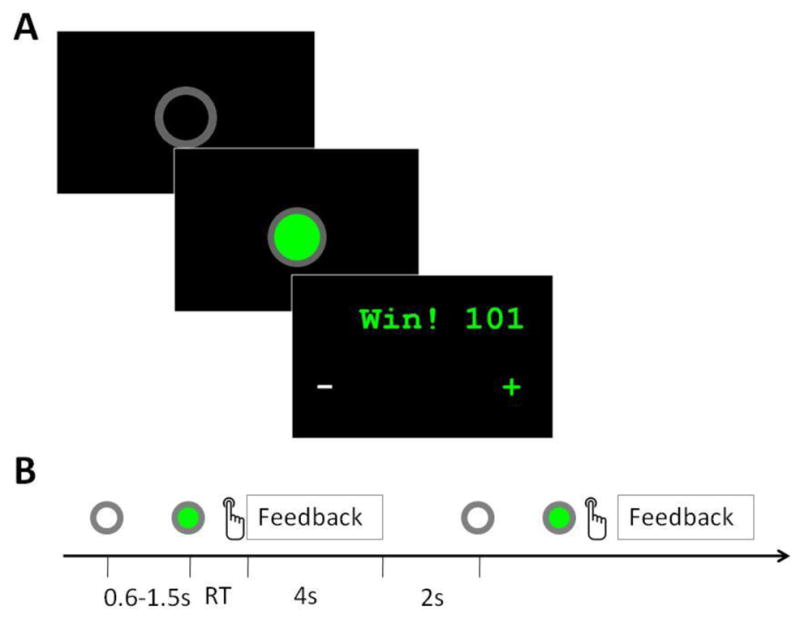
Task flow of the cooperation experiment. (A) Screenshots of the ready signal, “go” signal, and feedback window in a single trial. (B) Time flow of the experiment, showing two consecutive trials. The entire cooperation task consisted of two task blocks separated by a 30s rest period. Each task block consisted of 20 trials. RT: Response time.
The threshold was a function of the response time. Formally:
where T is the threshold, and R1 and R2 are the response times of the two participants, respectively. The parameter 1/8 was chosen so that the task achieved a reasonable level of difficulty. After both participants pressed their response buttons, a feedback screen was shown for 4s (figure 1A). This screen displayed whether they won or lost and their cumulative points. The screen also showed which participant was faster, indicated by a green plus sign on that participant s side, and which participant was slower, indicated by a white minus sign on that participant s side. The participants were able to use this information to adjust their response times. After a 2-second inter-trial interval, the next trial began. Figure 1B shows the time flow of this task. The symbols used in Figure 1B represent the screens depicted in Figure 1A and indicate when they are presented in a single trial. For example, in figure 1B, grey and green circles are used to indicate respectively the onset of the ready signal (the hollow grey circle, upper left of figure 1A) and the go signal (the filled green circle, center of figure 1A). The word “feedback” indicates when the feedback screen, depicted in the lower right of Figure 1A, is presented. The timeline in figure 1B depicts two trials, in order to show also the inter-trial interval.
Competition
This task was similar to the cooperation task, but the participants were given a different objective. Participants were instructed to respond faster than their partners. In each trial, the participant who responded faster won a point, and the other lost a point. Participants who responded before presentation of the go signal lost a point. In order to reduce the effectiveness of anticipatory responses, timing of go signal onset was randomized as in the cooperation task. The feedback screen, displayed for 1.5s, showed which player won, indicated by the word “win!” on that player s side, and which player lost, indicated by the word lose! on that player s side. The screen also showed plus and minus signs corresponding with which players were faster and slower, respectively, for consistency with the cooperation feedback screen, and the accumulative points for each participant.
Single 1
This task was similar to the competition task, but it involved only one participant responding while the other passively observed the screen. Before this task, participant 1 was instructed to respond as quickly as possible upon seeing the green circle, and participant 2 was instructed to passively view the screen. The feedback screen displayed participant 1 s points.
Single 2
This was the same as Single 1, but in this task, participant 2 responded while participant 1 observed.
Individual task organization and overall experimental organization
Each experiment was separated into five distinct sections ordered as follows: Rest, Task block, Rest, Task block, Rest. All rests lasted 30 seconds. Each task block consisted of 20 trials, lasting in total about 150s (in cooperation task), or 100s (in competition, single 1 and single 2). For example, in the competition task, participants rested for 30s, then competed for approximately 100s, then rested for 30s, then competed for approximately 100s, then rested for a final 30s. Each of the four task types was organized in this manner. Overall, the order in which participants performed the four tasks was randomized. For example, a pair of participants might complete the four tasks in the following order: Competition, Cooperation, Single 1, Single 2.
NIRS Data Acquisition
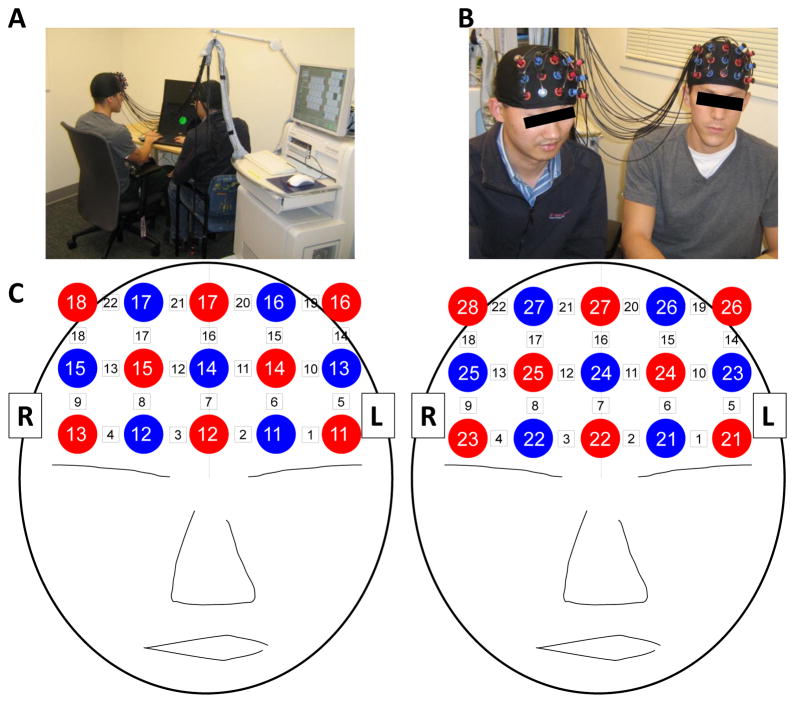
We used an ETG-4000 (Hitachi, Japan) Optical Topography system to measure the concentration changes in oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb). A single “3x5” measurement patch was attached to a regular swimming cap, which was positioned on each participant s head so that frontal cortex activity could be measured (Figure 2). Specifically, the patch was placed symmetrically over each participant s forehead, as shown in Figure 2A. Central emitters and detectors (eg. red 12, blue 14, and red 17 in left diagram of figure 2C) were aligned to the midline (ie, the arc running from the nasion through Cz to the inion). To align caps vertically, the caps were placed low over the forehead so that the bottom of the cap was touching the tops of the participant s eyebrows, which ensured adequate coverage of the forehead and resulted in a roughly uniform vertical position. Within participant pairs, caps were examined and adjusted to ensure similarity of position. In each patch, 8 emitters and 7 detectors were positioned alternatingly for a total of 15 probes, resulting in 22 measurement channels. The sampling frequency was 10Hz.
Figure 2.
Experimental Setup. (A & B) A pair of participants demonstrate the experimental setup. (C) Cap Configuration. Red circles indicate emitters; blue circles indicate detectors. White squares indicate measurement channels between emitters and detectors.
NIRS Data Analysis
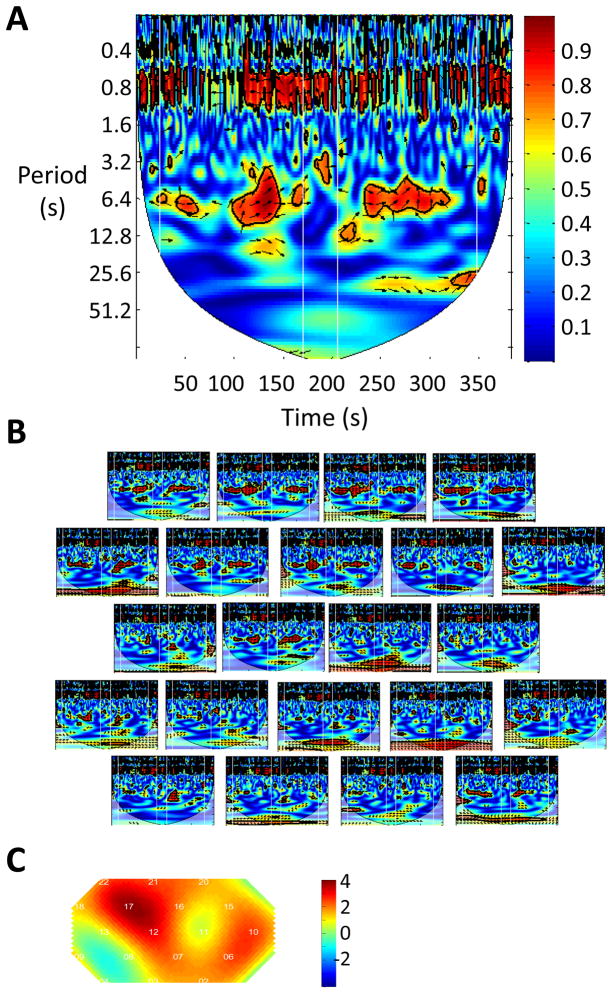
Wavelet coherence, also known as Wavelet Transform Coherence (WTC), was used to assess relationships between the NIRS signals generated by a pair of participants. WTC is a method of measuring the cross-correlation between two time series as a function of frequency and time (Torrence and Compo, 1998). As such, it is capable of uncovering locally phase-locked behavior that might not be discoverable with traditional time series analysis like Fourier analysis (Grinsted et al., 2004). WTC can be thought of as the local correlation between two time series (Grinsted et al., 2004). WTC has been used successfully in several studies in a wide variety of fields (Murphy et al., 2009), including fMRI signals in resting states (Chang and Glover, 2010). For more thorough explanations of continuous wavelet transform (CWT) and WTC as well as illustrative examples, please see Grinsted et al. (Grinsted et al., 2004) and Chang and Glover (Chang and Glover, 2010). We used the wavelet coherence MatLab package presented in Grinsted et al. (Grinsted et al., 2004) and provided on the authors website (http://www.pol.ac.uk/home/research/waveletcoherence/) to reveal the power of a time series as a function of time and frequency. Figures 6B and C show examples of CWT.
Figure 6.
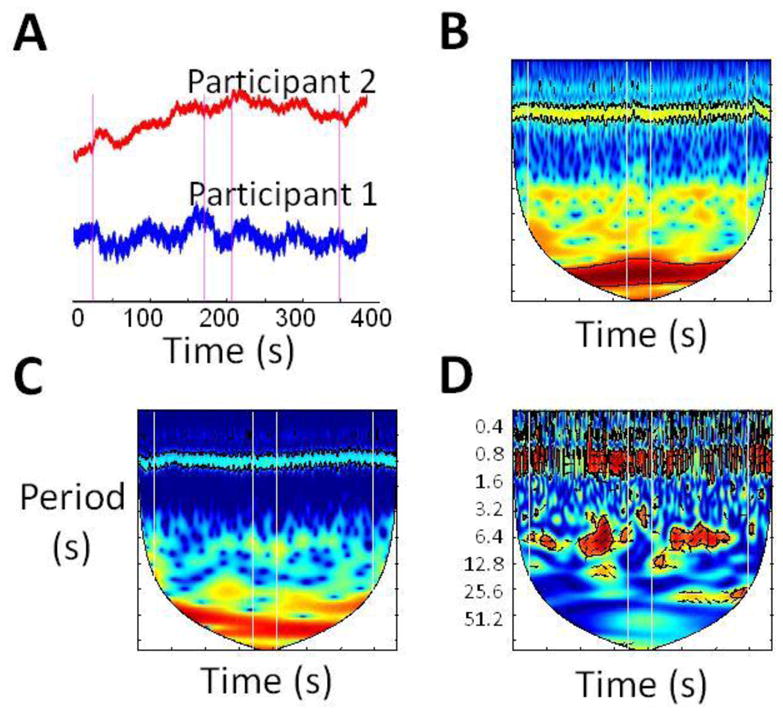
Hyperscanning reveals patterns not detectable by single-person brain scanning. (A) Raw oxy-Hb time courses from corresponding channels in each participant in a representative pair (i.e. channel 17 in each participant). Vertical lines indicate cooperation task onsets and offsets alternatingly. A 30s rest block separates the two task blocks. There is no obvious change in signal amplitude during the task block. (B & C) Continuous wavelet transform of time series from participant 1 and 2, respectively. There is no obvious change of power in the frequency domain during the task blocks. (D) Wavelet coherence between time courses from participants 1 and 2. The relationship between time courses is shown by high coherence at task-specific frequency.
For each channel from each pair of participants in the cooperation experiment, we had two oxy-Hb time series (e.g. oxy-Hb in channel 17 from participant 1, and oxy-Hb in channel 17 from participant 2). WTC analysis on these two time series generated a 2-D coherence map (e.g. Figure 3A). It should be noted that we did not perform any preprocessing on the oxy-Hb signals such as band pass filtering or detrending. We then identified a frequency band where the task occurred, which is between period 3.2s and 12.8s (corresponding to frequency 0.3Hz and 0.08Hz, respectively). We calculated the average coherence value in this band during the two task blocks and during the central rest period. “Coherence increase” is defined as the average coherence value in the two task blocks, minus the average coherence value in the central rest block. Then for each channel, we performed a one-sample t-test of “coherence increase” across all participant pairs and generated a t-map of coherence increase (e.g. Figure 3C). (Prior to the t-test, the coherence value was converted to Fisher z-statistics (Chang and Glover, 2010)). The t-map was smoothed using the spline method.
Figure 3.
The inter-brain coherence increases in the superior frontal cortex during cooperation. (A) Wavelet transform coherence (WTC) between the raw oxy-Hb signal from channel 17 from the 1st participant and the raw oxy-Hb signal from channel 17 from the 2nd participant in a representative participant pair. The vertical white lines indicate the onset and offset timing of the task block. The coherence, encoded by color, is higher during task than during rest in the task frequency band (3.2–12.8s). The high coherence in the ~0.8s frequency band is attributable to participant heartbeat. (B) Wavelet coherence in all channels in participant pair #1. The relative position of channels is identical to that shown in Figure 2, where the upper rows cover the superior frontal cortex while the lower rows cover the inferior frontal cortex. It is seen that the coherence increases during the task in the superior frontal cortex. (C) Group analysis of coherence increase in all 11 pairs of participants. Color indicates the t value. Coherence increase in channels 17 and 12, which are located in the right superior frontal cortex, is significant (p<0.05, FDR corrected).
Similar analysis procedures were performed for the competition, single 1, and single 2 experiments. All analyses utilized the oxy-Hb signals.
Results
Coherence increased in superior frontal cortex during cooperation
As demonstrated in one channel pair (channel 17, which corresponds to the superior frontal cortex, as seen in figure 2C) in an exemplary pair of participants in Figure 3A, higher coherence (shown in red color) is found during the task block in the frequency band between 3.2 and 12.8 seconds. This frequency band includes the period of the trials (~7s) in the cooperation game, indicating that coherence increase in this band is task-related. As shown in Figure 3B, this increase of coherence occurs in an array of channels, covering the bilateral superior frontal cortex in this pair of participants.
To quantify the coherence change in all 11 pairs of participants, we calculated the coherence increase in each channel in each subject pair, and then performed a t-test on the group level for each channel. The resulting t-test map is shown in Figure 3C. The coherence increase is significant in channel 17 that is most likely located in the right superior frontal cortex (SFC, p<0.05, FDR controlled).
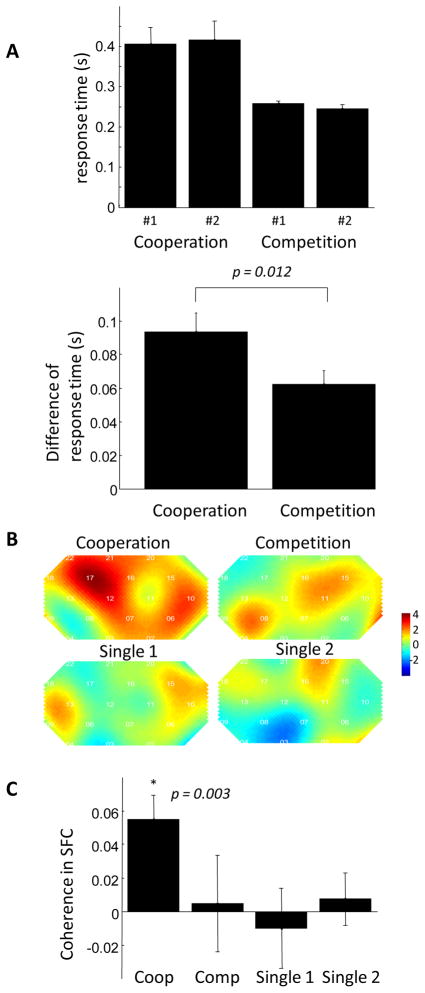
Closeness of action cannot explain the coherence increase
Can the significant increase of coherence in the cooperation game be explained by the closeness of actions (button responses) of the two participants? To this end, the same participants performed 3 additional experiments, competition (where they competed for a faster response time), single 1 (where participant #1 responded to the go cue and the other observed) and single 2 (where participant #2 responded and the other observed). We compared the average difference between response times in cooperation with the average difference between response times in competition. We found that the participant responses were actually closer during competition than they were during cooperation (p=0.012, Figure 4A), but at the same time the coherence increase is not significant during competition (Figure 4B and 4C). Indeed the coherence increase during competition is similar to that during a single player game where one participant responded and the other observed (Figure 4B and 4C). Thus the temporal proximity of participant response cannot fully account for coherence increase during cooperation.
Figure 4.
Coherence increase is not significant during the competition, single 1 and single 2 experiments. (A) The response time (top) and the difference of response time between the two participants (bottom) are plotted against the task type. The difference between response times of the two participants is smaller during the competition task than during the cooperation task. (B) The group t-test map of coherence increase in the four tasks. The coherence increase is significant only in the cooperation task. (C) The amplitude of coherence in superior frontal cortex (channel 12 and 17) in the four tasks.
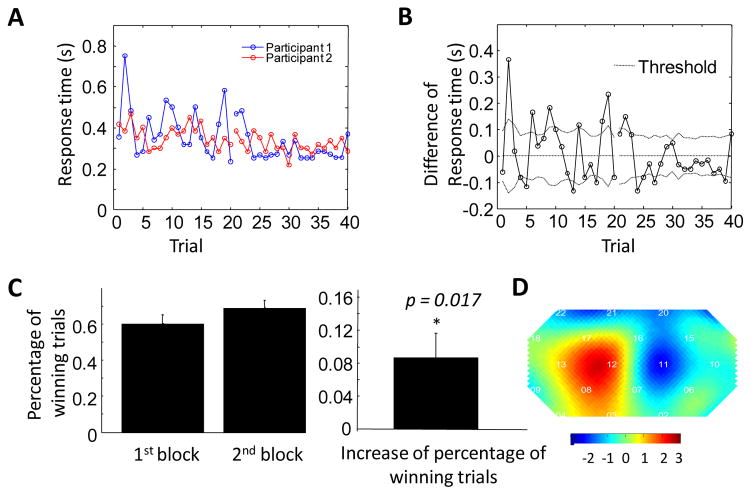
Learning improved cooperation and coherence
Participants completed two blocks in the cooperation experiment. Accordingly, we expected that they would show improved cooperation during the 2nd block relative to the 1st block. Figure 5A and 5B demonstrate that, in an exemplary pair of participants, the response time is closer during the 2nd block (trial 21–40) than during the 1st block (trial 1 to 20). On the group level, we find participants cooperated better in the 2nd block, demonstrated by the significant increase of the percentage of winning trials in the 2nd block than in the 1st block (p=0.017, Figure 5C).
Figure 5.
The inter-brain coherence is higher during the 2nd task block in the cooperation experiment. (A) Response times of the two participants in a representative pair as a function of trial number. It can be seen that the response time is closer in the 2nd block. (B) The difference between response times as a function of trial number. (C) The percentage of winning trials in the first and second cooperation block (left), and the increase in percentage of winning trials between the first and second cooperation task blocks, averaged across all participants. The percentage of winning trials in the second block is significantly higher than that in the first block, demonstrating a learning process. (D) t-test map of coherence change (coherence in the 2nd block minus that in the 1st block) shows that coherence in right frontal cortex is significantly higher in the second cooperation task block (p<0.02).
This increase of cooperation is reflected in the change of coherence in the right superior frontal cortex. Figure 5D shows the t-test map of the change of coherence from the 1st block to the 2nd block. Most significant increase of coherence is found in channel 12 which covers the right SFC (p<0.02).
Discussion
In this study, we used a single NIRS instrument to measure brain activation in two people simultaneously while they played a computer-based cooperation game side-by-side. We found that coherence between brain activation patterns in participants superior frontal cortices increased significantly during cooperation, but not during competition. Temporal proximity of response times could not account for this increased coherence, as participants responded closer together during competition than they did during cooperation. In addition, coherence increase accompanied increase in cooperation performance in the cooperation game.
This study has several implications. First, it establishes a novel experimental paradigm enabling multi-person studies of social cognition using a single NIRS instrument. The current NIRS community can readily take advantage of this method and use existing NIRS instruments to study two-person social interaction. Using a single NIRS instrument to measure two brains offers several advantages: (1) There is no need for additional NIRS instruments or network facilities, which can be costly; (2) There are no synchronization issues during data acquisition; (3) There is no need for complex coordinating and scheduling as often happens in cross-site hyperscanning; and (4) it is simple and easy to use. However, we believe two-brain simultaneous scanning is only the beginning of the study of social cognition. Many important social interactions occur among more than two individuals, such as those at auctions, in school classrooms, in political caucuses and in other group settings. For this purpose, one might further divide NIRS fibers and probes among multiple participants, which may inspire NIRS vendors to include additional fibers and probes in the original equipment. Alternatively, multiple labs could cooperate and connect NIRS instruments via internet, as has been done in fMRI hyperscanning, though this obviously eliminates some of the aforementioned advantages. In addition, NIRS offers certain advantages over fMRI, most notably the ability to measure hemodynamic activity in environments with greater ecological validity. This is especially important, as studies have shown that simulated tasks do not always generate the same brain activations as do their real-life counterparts (Okamoto et al., 2004). These advantages and those mentioned above potentially make single-instrument NIRS a powerful tool for studying social cognition.
Second, the results of the present study demonstrate the potential importance of simultaneously assessing brain activity in interacting participants for studies of social cognition. Measurement of inter-person brain activity allows assessment of the dynamic interaction between brains, which is distinct from what is measurable in traditional, one-person studies typically utilized in functional neuroimaging. In particular, in the experiment described here, a two-person, interactive paradigm allowed demonstration of increased inter-brain coherence during cooperation. To graphically illustrate this point, we plotted individual raw NIRS signal time courses in a representative channel from one of our participant pairs. The plot shows that there is no change in signal amplitude during the task blocks relative to the rest period in either of the individual participants (Figure 6A). To further illustrate the absence of individual changes in brain function for either participant, we also analyzed each of the raw time courses in the frequency domain using continuous wavelet transform (CWT) analysis. The power of the individual time series in the time-frequency domain (see Figures 6B and 6C) also failed to show changes during the task blocks relative to the rest blocks within the task frequency band (3.2s-12.8s) for either participant. In other words, individual time series analysis did not reveal task-specific patterns of brain activity. In contrast, inter-brain coherence analysis clearly revealed a task-specific pattern, namely, an increase in coherence during task blocks (in Figure 6D), suggesting that simultaneous collection and analysis of brain activity from multiple interacting humans can reveal an additional layer of information in the study of social cognition.
Third, the present study suggests a new class of neurobiological markers that could be used to diagnose social cognition-related disorders and quantify the effects of their treatment. Traditional neurobiological markers are usually based on the structure or activation of a single brain. In contrast, the class of biometrics described here is based on the correlation between neural signals from two interacting brains. This new layer of information could offer important insight into the study of social deficits (e.g. those in autism). Further investigation is needed to evaluate the feasibility and utility of such putative neurobiological markers and their potential use in diagnosis and treatment of disorders of social cognition.
Lastly, the increase of inter-brain coherence in SFC in cooperation may suggest a role for this region in modeling and predicting the behavior of others (i.e., theory of mind). This hypothesis is supported by the fact that the coherence increase was observed only during the cooperation block. Similar increases in coherence were not found during the rest period, nor any of the other tasks (competition, single 1 and single 2) where the participants did not have to model the behavior of their partners. A recent study (Dziobek et al., 2011) demonstrating the role of frontal cortex in empathy also supports the contention that the SFC is involved in theory of mind. The primary area of interest identified in this study is also within proximity of brain regions that contribute to response inhibition. Thus, response inhibition of self-performed action may also contribute to the cognitive basis of cooperation. Clearly, the elucidation of the precise role of the SFC in human cooperation is of great interest and requires additional research.
It should also be noted that this study has several limitations. First, the task timing was different in the cooperation and competition tasks, as noted in the methods section. Specifically, the feedback screen endured longer in the cooperation task. However, it is unlikely that this caused the observed coherence increase because coherence was calculated over the periods between 3.2 and 12.8 seconds, a range that includes both the period of the cooperation task and the period of the competition task, as well as that of Single 1 and Single 2. Second, the gender and familiarity of the subject-pair were not well controlled. Partners familiar with one another may demonstrate different levels of coherence. Males and females may also behavior differently in cooperation. Future studies should attempt to clarify the effect of sex and familiarity. Finally, given that participants sat side-by-side during the hyperscanning procedure, it is possible that observed coherence increases might have resulted, in part, from each participant's reaction to the other's motor activity.
In summary, NIRS-based hyperscanning combined with wavelet coherence analysis offers an easy-to-use, more ecologically valid, cost-effective approach to the study of social cognition.
Research highlights.
We used a single NIRS instrument to measure two brains simultaneously.
Participants played a computer-based cooperation game side by side.
The coherence of brain activities between two players increased during cooperation.
Acknowledgments
We thank Chris Gauthier for her assistance and Signe Bray, Catherine Chang and Eve-Marie Quintin for their helpful comments.
Footnotes
At the time of manuscript submission, April 6, 2011.
Competing Interest
The authors assert that they have no competing interests.
Financial Disclosure
This work is supported by S10 RR024657 (ALR PI) and the Stanford Institute for Neuro-Innovation and Translational Neurosciences (SINTN) fellowship (XC and ALR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Toppi J, De Vico Fallani F, Vecchiato G, Salinari S, Mattia D, Cincotti F, Babiloni F. Neuroelectrical hyperscanning measures simultaneous brain activity in humans. Brain Topogr. 2010;23:243–56. doi: 10.1007/s10548-010-0147-9. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Mattia D, Mattiocco M, De Vico Fallani F, Tocci A, Bianchi L, Marciani MG, Astolfi L. Hypermethods for EEG hyperscanning. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3666–9. doi: 10.1109/IEMBS.2006.260754. [DOI] [PubMed] [Google Scholar]
- Boecker M, Buecheler MM, Schroeter ML, Gauggel S. Prefrontal brain activation during stop-signal response inhibition: an event-related functional near-infrared spectroscopy study. Behavioural brain research. 2007;176:259–66. doi: 10.1016/j.bbr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–73. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle S, Ward T, Markham C, McDarby G. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces. Physiological Measurement. 2004;25:815–822. doi: 10.1088/0967-3334/25/4/003. [DOI] [PubMed] [Google Scholar]
- Coyle SM, Ward TE, Markham CM. Brain-computer interface using a simplified functional near-infrared spectroscopy system. Journal of neural engineering. 2007;4:219–26. doi: 10.1088/1741-2560/4/3/007. [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–21. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler T, Obersteiner A, Schecklmann M, Vogel AC, Ehlis AC, Richter MM, Plichta MM, Reiss K, Pekrun R, Fallgatter AJ. Arithmetic tasks in different formats and their influence on behavior and brain oxygenation as assessed with near-infrared spectroscopy (NIRS): a study involving primary and secondary school children. Journal of neural transmission. 2009 doi: 10.1007/s00702-009-0307-9. [DOI] [PubMed] [Google Scholar]
- Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. Inter-brain synchronization during social interaction. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36:562–72. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Preiβler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. NeuroImage. 2011;57:539–548. doi: 10.1016/j.neuroimage.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P. Illuminating the black box: investigating prefrontal cortical hemodynamics during exercise with near-infrared spectroscopy. Journal of sport & exercise psychology. 2009;31:505–53. doi: 10.1123/jsep.31.4.505. [DOI] [PubMed] [Google Scholar]
- Emir UE, Ozturk C, Akin A. Multimodal investigation of fMRI and fNIRS derived breath hold BOLD signals with an expanded balloon model. Physiological Measurement. 2008;29:49–63. doi: 10.1088/0967-3334/29/1/004. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of 'theory of mind'. Trends Cogn Sci (Regul Ed) 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes In Geophysics. 2004;11:561–566. [Google Scholar]
- Hagan CC, Hoeft F, Mackey A, Mobbs D, Reiss AL. Aberrant neural function during emotion attribution in female subjects with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 2008;47:1443–354. doi: 10.1097/CHI.0b013e3181886e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Nashihara H, Morozumi K, Ota H, Hatakeyama E. A comparison of cerebral activity in the prefrontal region between young adults and the elderly while driving. J Physiol Anthropol. 2007;26:409–14. doi: 10.2114/jpa2.26.409. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Ehlis AC, Wagener A, Jacob CP, Fallgatter AJ. Near-infrared optical topography to assess activation of the parietal cortex during a visuo-spatial task. Neuropsychologia. 2005;43:1713–20. doi: 10.1016/j.neuropsychologia.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Franceschini MA, Covolan RJM, Huppert T, Mandeville JB, Boas DA. Simultaneous recording of task-induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin-labeling MRI. Neuroimage. 2005;25:701–707. doi: 10.1016/j.neuroimage.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Honda Y, Nakato E, Otsuka Y, Kanazawa S, Kojima S, Yamaguchi MK, Kakigi R. How do infants perceive scrambled face?: A near-infrared spectroscopic study. Brain Res. 2010;1308:137–46. doi: 10.1016/j.brainres.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Allen MS, Benav H, Jones PB, Boas DA. A multicompartment vascular model for inferring baseline and functional changes in cerebral oxygen metabolism and arterial dilation. J Cereb Blood Flow Metab. 2007;27:1262–79. doi: 10.1038/sj.jcbfm.9600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Allen MS, Diamond SG, Boas DA. Estimating cerebral oxygen metabolism from fMRI with a dynamic multicompartment Windkessel model. Hum Brain Mapp. 2009;30:1548–67. doi: 10.1002/hbm.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage. 2006;29:368–82. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. Journal Of Cerebral Blood Flow And Metabolism. 1996;16:817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Li J, Xiao E, Houser D, Montague PR. Neural responses to sanction threats in two-party economic exchange. Proc Natl Acad Sci USA. 2009;106:16835–40. doi: 10.1073/pnas.0908855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Li SC, Gruber W, Müller V. Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 2009;10:22. doi: 10.1186/1471-2202-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CM, Zhang YJ, Biswal BB, Zang YF, Peng DL, Zhu CZ. Use of fNIRS to assess resting state functional connectivity. J Neurosci Methods. 2010;186:242–9. doi: 10.1016/j.jneumeth.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94:14826–31. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115:2195–222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS, Cohen JD, McClure SM, Pagnoni G, Dhamala M, Wiest MC, Karpov I, King RD, Apple N, et al. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 2002;16:1159–64. doi: 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti–correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Shimizu K, Takeo K, Amita T, Oda I, Konishi I, Sakamoto K, Isobe S, Suzuki T, et al. Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. Neuroimage. 2004;21:1275–88. doi: 10.1016/j.neuroimage.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, Bookheimer S, Reiss AL. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:473–80. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Power SD, Falk TH, Chau T. Classification of prefrontal activity due to mental arithmetic and music imagery using hidden Markov models and frequency domain near-infrared spectroscopy. Journal Of Neural Engineering. 2010;7:026002. doi: 10.1088/1741-2560/7/2/026002. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–15. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe P. In vivo near-infrared spectroscopy. Annu Rev Biomed Eng. 2000;2:715–54. doi: 10.1146/annurev.bioeng.2.1.715. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Kupka T, Mildner T, Uludag K, von Cramon DY. Investigating the post-stimulus undershoot of the BOLD signal--a simultaneous fMRI and fNIRS study. NeuroImage. 2006;30:349–58. doi: 10.1016/j.neuroimage.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Zhang HH, Guan CT, Thulasidas M, Hoshi Y, Ishikawa A, Shimizu K, Birbaumer N. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain-computer interface. Neuroimage. 2007;34:1416–1427. doi: 10.1016/j.neuroimage.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Suda M, Takei Y, Aoyama Y, Narita K, Sato T, Fukuda M, Mikuni M. Frontopolar activation during face-to-face conversation: An in situ study using near-infrared spectroscopy. Neuropsychologia. 2010;48:441–447. doi: 10.1016/j.neuropsychologia.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Yamagata B, Takahashi T, Yano M, Isomura AJ, Kobayashi H, Mimura M. Detection of hypofrontality in drivers with Alzheimer's disease by near-infrared spectroscopy. Neurosci Lett. 2009;451:252–6. doi: 10.1016/j.neulet.2008.12.059. [DOI] [PubMed] [Google Scholar]
- Tomlin D, Kayali MA, King-Casas B, Anen C, Camerer CF, Quartz SR, Montague PR. Agent-specific responses in the cingulate cortex during economic exchanges. Science. 2006;312:1047–50. doi: 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- Toronov V, Walker S, Gupta R, Choi JH, Gratton E, Hueber D, Webb A. The roles of changes in deoxyhemoglobin concentration and regional cerebral blood volume in the fMRI BOLD signal. Neuroimage. 2003;19:1521–1531. doi: 10.1016/s1053-8119(03)00152-6. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. Bulletin Of The American Meteorological Society. 1998;79:61–78. [Google Scholar]
- Utsugi K, Obata A, Sato H, Aoki R, Maki A, Koizumi H, Sagara K, Kawamichi H, Atsumori H, Katura T. GO-STOP Control Using Optical Brain-computerInterface during Calculation Task. IEICE Transactions on Communications. 2008;E91-B:2133–2141. [Google Scholar]
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends in neurosciences. 1997;20:435–42. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry. 2008;65:1315–23. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BR, Snyder AZ, Cohen AL, Petersen SE, Raichle ME, Schlaggar BL, Culver JP. Resting-state functional connectivity in the human brain revealed with diffuse optical tomography. NeuroImage. 2009;47:148–56. doi: 10.1016/j.neuroimage.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang YJ, Lu CM, Ma SY, Zang YF, Zhu CZ. Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements. Neuroimage. 2010;51:1150–61. doi: 10.1016/j.neuroimage.2010.02.080. [DOI] [PubMed] [Google Scholar]