Abstract
Background
The transcription factor FoxO3a is highly expressed in brain, but little is known about the response of FoxO3a to behavioral stress and its impact in the associated behavioral changes.
Methods
We tested the response of brain FoxO3a in the learned helplessness (LH) paradigm and tested signaling pathways that mediate the response of FoxO3a.
Results
A single session of inescapable shocks (IES) in mice reduced FoxO3a phosphorylation at the Akt-regulating serine/threonine residues and induced prolonged nuclear accumulation of FoxO3a in the cerebral cortex, both indicate activation of FoxO3a in brain. The response of FoxO3a is accompanied by a transient inactivation of Akt and a prolonged activation of glycogen synthase kinase-3beta (GSK3β). Noticeably, FoxO3a formed a protein complex with GSK3β in the cerebral cortex, and the interaction between the two proteins was stronger in IES-treated mice. Inhibition of GSK3 was able to abolish IES-induced LH behavior, disrupt IES-induced GSK3β-FoxO3a interaction, and reduce nuclear FoxO3a accumulation. In vitro approaches further revealed that the interaction between GSK3β and FoxO3a was strongest when both were active, FoxO3a was phosphorylated by recombinant GSK3β, and GSK3 inhibitors effectively reduced FoxO3a transcriptional activity. Importantly, IES-induced LH behavior was markedly diminished in FoxO3a-deficient mice that have minimal FoxO3a expression and reduced levels of FoxO3a-inducible genes.
Conclusions
FoxO3a is activated in response to IES by interacting with GSK3β, and inhibition of GSK3β or reducing FoxO3a expression promotes resistance to stress-induced behavioral disturbance by disrupting this signaling mechanism.
Keywords: FoxO3a, GSK3beta, Akt, stress, learned helplessness, signal transduction
INTRODUCTION
FoxO3a is a subtype of mammalian FoxO transcription factors (1,2) that are identified as orthologs of the C. elegans DAF16 (3). FoxO3a is active when it is localized in the nucleus (4-6), a process tightly regulated by posttranslational modification. Activation of Akt by trophic signals phosphorylates FoxO3a at Thr32, Ser253, and Ser315 residues, which promotes sequestration of FoxO3a from the nucleus to the cytosol by the chaperone protein 14-3-3, therefore inactivates FoxO3a (7-11). Besides responding to trophic signals, environmental and physiological stresses, such as oxidative stress, UV irradiation, and food restriction, can activate FoxO3a, a response regulated by Akt-independent mechanisms, such as Jun N-terminal Kinase (JNK), acetyltransferase CBP and p300, and sirturin deacetylases (12-18).
FoxO3a is highly expressed and widely distributed in adult brain (2,4,19,20). Destructive brain insults, such as ischemia and epileptic seizures, have been shown to increase the level of active FoxO3a that acts to eliminate damaged neurons by apoptosis (21-23). However, it is less known if brain FoxO3a is only active during the extreme apoptotic insults or FoxO3a has other functions in response to abnormal brain activity, such as behavioral stress.
Behavioral stress often induces mood-related behavioral disturbance in vulnerable individuals, such as depression (24,25), as a result of disturbed neurotransmission, brain gene expression, and neuroplasticity (26,27). We and others previously reported that neurotrophins phosphorylate and inactivate FoxO3a in neuronal cells (28,29). Enhancing serotonin neurotransmission in animal brain also strongly phosphorylates and inactivates brain FoxO3a (30), a result in agreement with findings in C. elegans that activation of serotonin receptors led to inhibition of DAF-16 transcriptional activity (31). Furthermore, both the monoamine reuptake inhibitor antidepressant imipramine and the mood stabilizer lithium suppress FoxO3a activity in mouse brain via different mechanisms of action (30,32). In accordance with these findings, mice with FoxO3a-deficiency have higher resistance to stress-induced despair behavior in the forced swim and tail suspension tests (30).
We therefore hypothesize that brain FoxO3a may be overactive in response to behavioral stress. In this study, we investigated the response of mouse brain FoxO3a to inescapable foot shocks (IES) in the learned helplessness (LH) paradigm, and examined the underlying mechanisms mediating the response of FoxO3a and the behavioral impact of FoxO3a.
MATERIALS AND METHODS
In addition to the brief descriptions of methods below, detailed Materials and Methods can be found online in Supplemental Information.
Animals
The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved the experimental protocol using mice. Adult (10-12 wk old) male mice were used for all experiments. GSK3 inhibitor BIP-135 (33,34) or saline was infused into the right cerebral ventricle of mice via a cannula once daily. Behavioral stress was induced by repeated inescapable foot shocks (IES) (35,36). Escape latency and failure were recorded as described (37), and social interaction was tested with a modified protocol (38).
Cells
Human SH-SY5Y neuroblastoma cells and embryo kidney (HEK)-293 cells were used for adenovirus infection and DNA plasmid transfection of FoxO3a and GSK3β DNA constructs.
Bioassays
For brain protein assays, mice were sacrificed on day-1, day-3 and day-8 after IES (Fig. S1A in the Supplement). Proteins from homogenate and nuclear/cytosolic extracts of mouse cerebral cortex were prepared as described (30,39). Proteins were immunobloted with antibodies to phosphorylated or total FoxO3a, Akt, GSK3β, GSK3α, and JNK-1. Immunoprecipitation was performed using anti-FoxO3a and anti-GSK3β antibodies (40).
Bioluminescence resonance energy transfer (BRET) assay (41) was performed in HEK cells transiently co-transfected with RLuc-FoxO3a and YFP-GSK3β or their mutants as described (40).
FoxO3a phosphorylation by GSK3β was performed in vitro using immunoprecipitated HA-tagged wild type FoxO3a and recombinant human GSK3β in the presence of 32P-ATP.
FoxO3a transcriptional activity was measured by luciferase assay in HEK cells co-transfected with FoxO3a and 6xDBE-firefly/renilla dual-luciferase plasmids (32).
Gene expression of FoxO3a, Bim, p27, and GADD-45 in the cerebral cortex of mouse brain were measured by quantitative Real-time PCR (qRT-PCR) using the TaqMan Universal PCR system (Applied Biosystems).
Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were conducted using SigmaStat 10.0. All data were checked for assumptions of normal distribution and homogeneity of variances in study samples, and any unexplained outlier value greater than ±2 standard deviations from the mean of the group was excluded. For a two-group comparison, statistical analysis was performed using unpaired Student’s t-test. For comparison of more than three experimental groups, one-way or two-way analysis of variance (ANOVA) was used to test for significant differences (p≤0.05) between groups. Any significant difference detected by ANOVA was followed by post hoc or inter-group comparisons using Tukey’s, Holm-Sidak, or Tamhane’s test.
RESULTS
To induce behavioral stress, adult (10-12 wk old) male wild type C57BL/6 mice were subjected to a single session of IES that includes 180 repeated inescapable foot shocks delivered at unpredictable shock durations and intervals. Control mice were exposed to the foot shock apparatus for the same length of time without receiving IES. When behaviors were tested on post-IES day-1, IES-treated mice exhibited significantly longer escape latency than control mice and higher numbers of escape failure (77.6±7.1% of IES-treated mice and 5.4±3.6% of control mice failed more than 15 of the 30 escape trials) (Fig. S1B in the Supplement). In addition, IES-treated mice exhibited poorer social interaction when compared to control mice (Fig. S1C in the Supplement). When the duration of LH behavior after a single session of IES was tested along a 15-day period of time, the elevated escape latency peaked on post-IES day-1, gradually reduced to approximately 50% of maximal but significantly higher than control level between day-3 and day-8, and remained at a residual longer escape latency even during day-11 and day-15 (Fig. S1D in the Supplement).
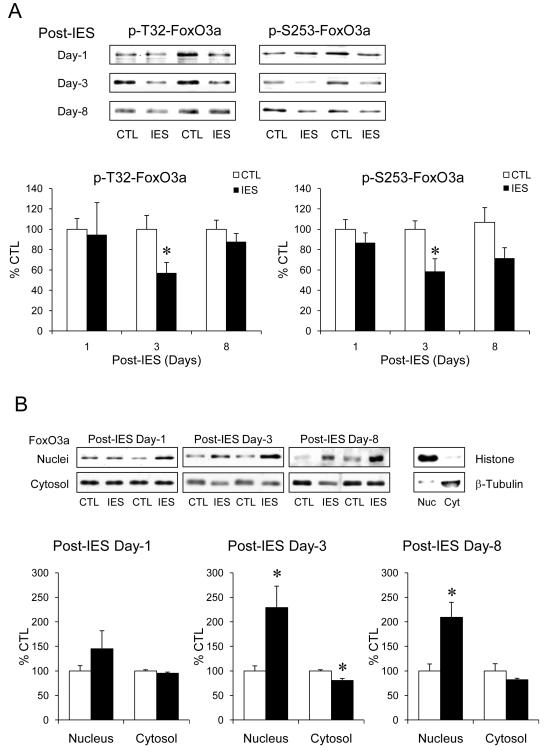
The response of FoxO3a to IES was measured in the cerebral cortex that is a major stress-responding brain area (42-44). Although the levels of phospho-T32-FoxO3a and phospho-S253-FoxO3a (representing the Akt-regulated inactive state of FoxO3a (8)) were not different between IES-treated and control mice on post-IES day-1 (Fig. 1A), both were significantly lower in IES-treated mice than control mice on post-IES day-3. On post-IES day-8, there was no significant difference on phospho-T32-FoxO3a between IES-treated and control mice, but phospho-S253-FoxO3a showed a trend of reduction (p=0.057) in IES-treated mice.
Figure 1.
IES-induced activation of FoxO3a in the cerebral cortex. The cerebral cortex from control (CTL) and IES-treated mice were dissected on post-IES day-1 (n=6/group), day-3 (n=14/group), and day-8 (n=8/group). (A) Protein homogenates were immunobloted for phospho-T32-FoxO3a, phospho-S253-FoxO3a, and total FoxO3a. (B) Nuclear and cytosolic proteins were immunobloted separately for total FoxO3a. Histone and β-tubulin were immunobloted as quality control of nuclear/cytosolic extraction. Data is expressed as % control (no IES). Mean ± SEM, *p<0.05 in Student’s t-test when IES-treated mice were compared to control mice.
Since active FoxO3a locates in the nucleus, the levels of nuclear and cytosolic FoxO3a in the cerebral cortex were measured. On post-IES day-1, IES did not cause significant change of nuclear or cytosolic FoxO3a, whereas on post-IES day-3, nuclear FoxO3a in IES-treated mice elevated to a significantly higher level than in control mice, and this was accompanied by a small but significant reduction of cytosolic FoxO3a (Fig. 1B). Surprisingly, the nuclear FoxO3a remained at a significantly higher level in IES-treated mice than control mice on post-IES day-8, suggesting that IES causes a prolonged activation of FoxO3a in the cerebral cortex.
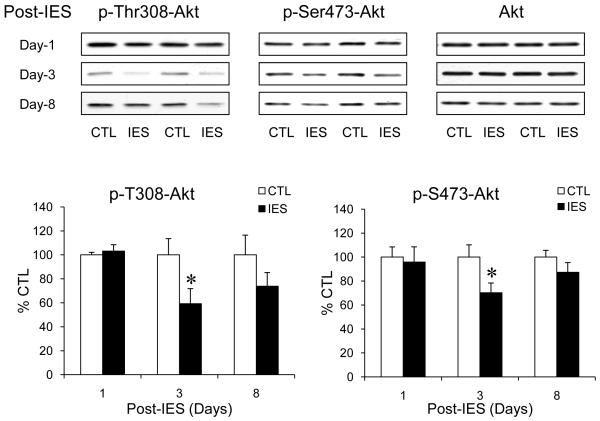
Since Akt-dependent FoxO3a phosphorylation was decreased following IES treatment, we measured the levels of phospho-T308-Akt and phospho-S473-Akt that represent active Akt to determine if Akt activity is decreased by IES. No significant difference of phospho-T308-Akt or phospho-S473-Akt between IES-treated and control mice was found on post-IES day-1, but on post-IES day-3, the levels of both phospho-T308-Akt and phospho-S473-Akt reduced significantly in IES-treated mice without a change of total Akt (Fig. 2). The reduction, however, was no longer significant on post-IES day-8. Therefore, IES caused a transient inactivation of Akt, which is in accordance with the time of reduction in Akt-dependent FoxO3a phosphorylation. However, the transient reduction of Akt activity does not concur with the prolonged robust nuclear accumulation of FoxO3a, suggesting that other regulatory mechanisms may be involved.
Figure 2.
IES inactivates Akt in the cerebral cortex. Protein homogenates from the cerebral cortex of control (CTL) and IES-treated mice were immunobloted for phospho-T308-Akt, phospho-S473-Akt, and total Akt. Data is expressed as % control (no IES). Mean ± SEM, n=6-14/group, *p<0.05 in Student’s t-test when IES-treated mice were compared to control mice.
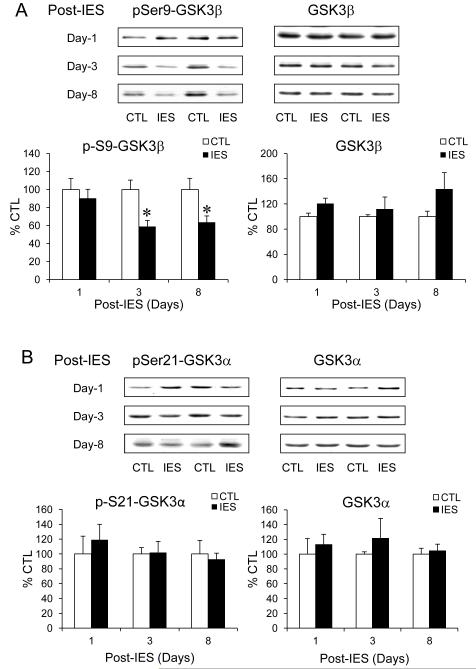
We previously reported (37) that the sensitivity of developing IES-induced LH behavior is markedly enhanced in GSK3 knock-in mice (45) that express constitutively active GSK3, a protein kinase that is also negatively regulated by Akt (46). We therefore measured the inhibitory serine phosphorylation and the total level of GSK3β and GSK3α, the two isoforms of GSK3. On post-IES day-1, there was no significant change of phospho-S9-GSK3β in the cerebral cortex when IES-treated mice were compared to control mice (Fig. 3A). However, the level of phospho-S9-GSK3β was significantly lower in IES-treated mice than in control mice on post-IES day-3, but the level of total GSK3β did not change. Different from Akt phosphorylation, phospho-S9-GSK3β remained significantly lower in IES-treated than in control mice on post-IES day-8, and there was a trend of increase in total GSK3β (p=0.064), both suggest a prolonged active state of GSK3β, which has a similar time course as the prolonged elevation of nuclear FoxO3a. In contrast, there was no significant change of phospho-Ser21-GSK3α or total GSK3α at the tested post-IES days (Fig. 3B).
Figure 3.
IES decreases the inhibitory serine phosphorylation of GSK3β in the cerebral cortex. Protein homogenates from the cerebral cortex of control (CTL) and IES-treated mice were immunobloted for (A) phospho-S9-GSK3β and total GSK3β, (B) phospho-S21-GSK3α and total GSK3α. Data is expressed as % control (no IES). Mean ± SEM, n=6-14/group, *p<0.05 in Student’s t-test when IES-treated mice were compared to control mice.
JNK-1 was reported to activate FoxO3a during oxidative and physical stresses (12-14), but no significant changes of phosphorylated or total JNK-1 was found in the cerebral cortex after IES (Table S1 in the Supplement).
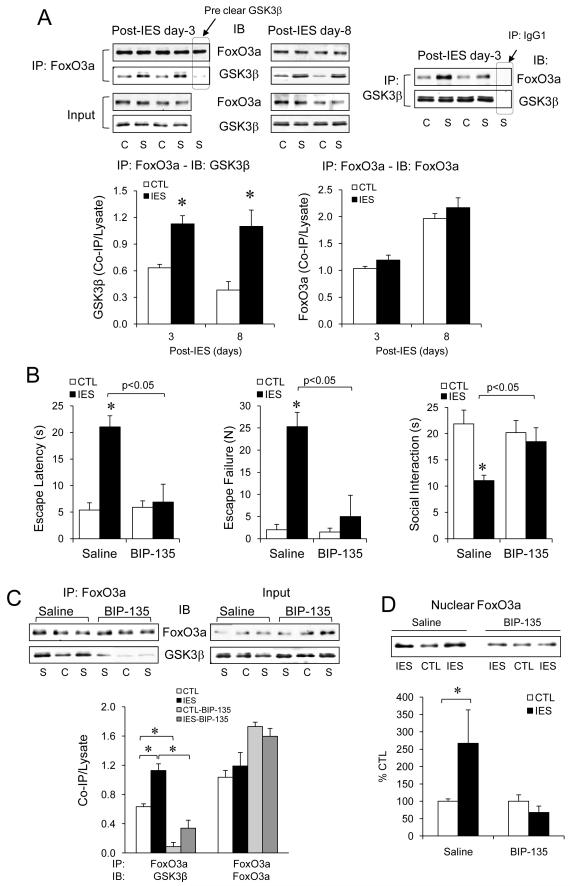
With the prominent effect of IES inducing a concomitant activation of GSK3β and FoxO3a, we next tested if there is an interaction between GSK3β and FoxO3a in mouse brain when they respond to IES. Interestingly, not only that GSK3β and FoxO3a from the cerebral cortex co-immunoprecipitated with each other, but the amount of co-immunoprecipitated proteins was significantly higher in IES-treated mice than in control mice on both post-IES day-3 and day-8 (Fig. 4A). To further test the GSK3β-FoxO3a interaction during IES, control and IES-treated mice were infused with the GSK3 inhibitor BIP-135 (0.08 nmol, i.c.v.). A single BIP-135 infusion 2 hr prior to behavior tests on post-IES day-1 significantly reduced LH behavior and normalized social interaction (Fig. 4B). Remarkably, once daily BIP-135 infusion on post-IES day-1, day-2, and day-3 largely reduced the interaction between GSK3β and FoxO3a (Fig. 4C) and completely occluded IES-induced increase in nuclear FoxO3a (Fig. 4D) on post-IES day-3.
Figure 4.
GSK3β interacts and regulates FoxO3a in mouse cerebral cortex. (A) The cerebral cortex from control (C) and IES (S)-treated mice were dissected on post-IES day-3 and day-8. Protein homogenates were immunoprecipitated with anti-FoxO3a or anti-GSK3β, and immunocomplex and homogenates (input) were immunobloted for FoxO3a and GSK3β. Data is expressed as the ratio of the protein in the immunocomplex (Co-IP) to total lysate. Mean ± SEM, n=4-6/group, *p<0.05 in Student’s t-test when IES-treated mice were compared to control mice. (B-D) Control and IES-treated mice received daily i.c.v. infusion of saline or the GSK3 inhibitor BIP-135 (0.08 nmol). (B) LH behavior and social interaction were tested on post-IES day-1, 2 hr after GSK3 inhibitor treatment. Mean ± SEM, n=6/group, *p<0.05 in Student’s t-test. (C) After 3 doses of BIP-135 on post-IES day-1, 2, and 3, protein homogenates from the cerebral cortex were immunprecipitated for FoxO3a and the immunocomplexes were immunobloted for FoxO3a and GSK3β. Data is expressed as the ratio of the protein in the immunocomplex (Co-IP) to total lysate. Mean ± SEM, n=6/group, *p<0.05 in two-way ANOVA with post-hoc analysis. (D) After BIP-135 on post-IES day-1, 2, and 3, nuclear proteins from the cerebral cortex were immunobloted for FoxO3a. Data is expressed as % control (no IES). Mean ± SEM, n=6/group, *p<0.05 in Student’s t-test.
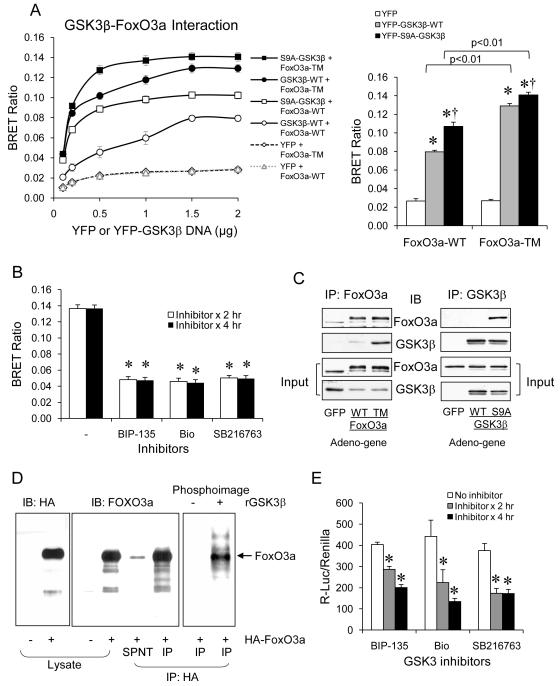
To support a GSK3β-dependent regulation of FoxO3a, we performed additional experiments in vitro. Using the distance-based BRET assay that detects protein interaction (41), we tested if the activation state of GSK3β and FoxO3a affects their interaction. In this assay, a fixed concentration (0.5 μg DNA) of RLuc-FoxO3a-WT or RLuc-FoxO3a-TM (the triple mutant that is resistant to Akt-regulated phosphorylation (8)) was co-transfected with different concentrations of YFP, YFP-GSK3β-WT, or YFP-S9A-GSK3β (Mutant GSK3β that is unable to be phosphorylated at the inhibitory Ser-9 residue (47,48)) in HEK cells. YFP alone did not interact with FoxO3a, but YFP-GSK3β-WT and FoxO3a-WT had a low level of interaction in a DNA concentration-dependent manner, reaching maximal interaction at 1.5 μg YFP-GSK3β-WT (Fig. 5A). Interestingly, the interaction was stronger when GSK3β-WT was co-transfected with the active FoxO3a-TM or when the active S9A-GSK3β was co-transfected with FoxO3a-WT, and the interaction was strongest when the active S9A-GSK3β was co-transfected with the active FoxO3a-TM. This interaction pattern was indicated by a left shift of maximal effective concentration of YFP-GSK3β and a significant difference in the maximal interaction at 1.5 μg YFP-GSK3β. Furthermore, the interaction between S9A-GSK3β and FoxO3a-TM was significantly reduced by three GSK3 inhibitors, BIP-135, Bio, and SB216763 (Fig. 5B). To test if activation states of GSK3β and FoxO3a affect their protein complex formation in cells, SH-SY5Y cells were infected with the adenovirus-carried HA-tagged FoxO3a-WT or FoxO3a-TM. When FoxO3a was immunoprecipited, endogenous GSK3β was found to co-immunoprecipitate strongly with FoxO3a-TM, but less with FoxO3a-WT (Fig. 5C). Vice versa, when SH-SY5Y cells were infected with the adenovirus-carried HA-tagged GSK3β-WT or S9A-GSK3β, only S9A-GSK3β, but not GSK3β-WT, co-immunoprecipitated with endogenous FoxO3a.
Figure 5.
Interaction and regulation of FoxO3a in vitro by GSK3β. (A) BRET assay in HEK cells transfected with a fixed concentration (0.5 μg DNA) of RLuc-FoxO3a-WT or RLuc-FoxO3a-TM and different concentrations of YFP, YFP-GSK3β-WT, or YFP-S9A-GSK3β. Right panel shows statistical analysis from 1.5 μg of YFP or GSK3β transfection. Data is expressed as BRET ratio. Mean ± SEM, n=3/group, *p<0.05 in two-way ANOVA. (B) HEK cells expressing S9A-GSK3β and FoxO3a-TM were treated with GSK3 inhibitors BIP-135, Bio, and SB216763 (10 μM) for 2 or 4 hr before BRET assay. Mean ± SEM, n=6/group, *p<0.05 in Student’s t-test when GSK3 inhibitor-treated cells were compared to no inhibitor treatment. (C) SH-SY5Y cells infected with the adenovirus-carried HA-tagged FoxO3a-WT or FoxO3a-TM were immunoprecipitated with anti-FoxO3a, and immunocomplexes were immunobloted for endogenous GSK3β. SH-SY5Y cells infected with the adenovirus-carried HA-tagged GSK3β-WT or the S9A-GSK3β were immunoprecipitated with anti-GSK3β, and immunocomplexes were immunobloted for endogenous FoxO3a. (D) Protein lysate from SH-SY5Y cells infected with adeno-HA-FoxO3a were immunoprecipitated with anti-HA-FoxO3a. The immunocomplex was then incubated in vitro with recombinant GSK3β in the presence of 32P-ATP. HA and FoxO3a in the cell lysates and immunocomplex were detected by immunoblots, and phosphorylated FoxO3a were detected by a phosphoimager. (E) HEK cells were transfected with FoxO3a-WT and 6xDBE-luciferase, and treated with the GSK3 inhibitors BIP-135, Bio, or SB216763 (10 μM) for 2 or 4 hr, followed by measuring 6xDBE-luciferase activity. Data is expressed as relative luciferase activity. Mean ± SEM, n=4/group, *p<0.05 in one-way ANOVA.
To test if GSK3β phosphorylates FoxO3a, GSK3β kinase assay was performed using immunoprecipitated HA-FoxO3a as the candidate substrate, which was indeed phosphorylated by recombinant GSK3β in vitro (Fig. 5D). To also test if GSK3β affects FoxO3a transcriptional activity, HEK cells transfected with FoxO3a-WT and the canonical 6xDBE-Luc were treated with GSK3 inhibitors BIP-135, Bio, or SB216763 under a serum-free condition (to reduce FoxO3a phosphorylation and increase its activity) followed by measuring luciferase activity (32). All three GSK3 inhibitors significantly reduced FoxO3a-induced luciferase activity (Fig. 5E).
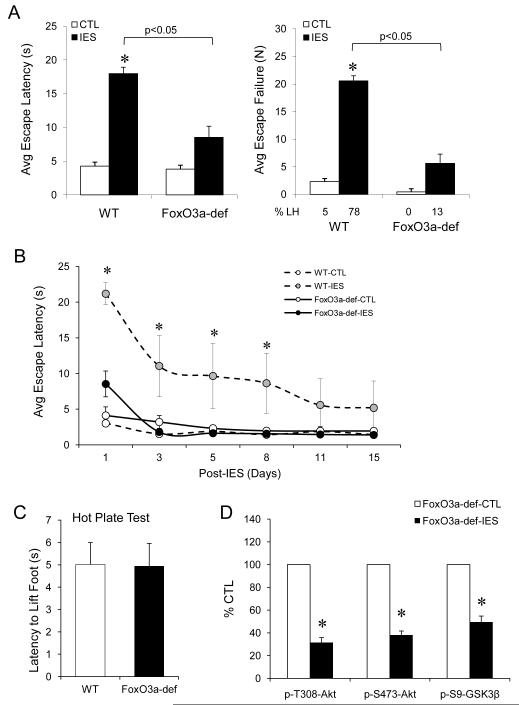
To further understand the role of FoxO3a in IES-induced behavior, we tested LH behavior in FoxO3a-deficient mice that do not express functional FoxO3a mRNA and protein (30,49). When LH behavior was tested on post-IES day-1, control FoxO3a-deficient mice displayed similar escape latency as control wild type mice. However, IES-induced escape latency in FoxO3a-deficient mice was significantly lower than in wild type mice (Fig. 6A), and the escape failure only occurred in 12.5±9.2% of IES-treated FoxO3a-deficient mice, which was significantly lower than IES-treated wild type mice (77.6±7.1%). Also, when the escape latency was tested along the 15 days of post-IES period, in remarkable contrast to wild type mice, the mildly elevated escape latency in IES-treated FoxO3a-deficient mice rapidly returned to basal level on post-IES day-3, and there was no further observable LH behavior throughout the 15-day test (Fig. 6B). The IES-resistant behavior in FoxO3a-deficient mice was unlikely due to insensitivity to pain because these mice had similar sensitivity as wild type mice in the hot plate test.
Figure 6.
Effects of FoxO3a-deficiency in LH behavior. (A) Control (CTL) and IES-treated wild type and FoxO3a-deficient (F3a-def) mice were tested for LH behavior on post-IES day-1. Mean ± SEM, n=8-10/group. *p<0.05 in two-way ANOVA. (B) LH behavior in wild type and FoxO3a-deficient mice were tested on post-IES day-1, 3, 5, 8, 11, and 15. Mean ± SEM, n=6/group, *p<0.05 among groups in repeated ANOVA. (C) Wild type and FoxO3a-deficient mice were tested for sensitivity to hot plate. Mean ± SEM, n=5/group. (D) The cerebral cortex of control (CTL) and IES-treated FoxO3a-deficient mice were dissected on post-IES day-3, and protein homogenates were immunobloted for phospho-T308-Akt, phospho-S473-Akt, and phospho-S9-GSK3β. Data is expressed as % control (no IES). Mean ± SEM, n=4/group, *p<0.05 in Student’s t-test.
Since FoxO3a-deficient mice are deprived of FoxO3a prior to IES, the observed behavioral effect could be the result of either resistance to stress or to developing LH behavior. We therefore measured phosphorylation of Akt and GSK3β that were reduced in response to IES in wild type mice on post-IES day-3. In FoxO3a-deficient mice, IES treatment was able to significantly reduce phospho-T308-Akt, phospho-S473-Akt, and phospho-Ser9-GSK3β (Fig. 6C), which is a similar effect as found in wild type mice (Fig. 2 and Fig. 3), supporting a role of FoxO3a in developing LH behavior.
To test if altered level of FoxO3a in the cerebral cortex is associated with change of gene expression, the levels of several FoxO-regulated genes, Bim, p27, and GADD-45 (29,50-53) in the cerebral cortex were measured by qRT-PCR. In wild type mice, neither of these genes was changed significantly on the post-IES day-3, but IES caused a small albeit significant increase of P27 on post-IES day-8 (Fig. S2A in the Supplement). In FoxO3a-deficient mice that essentially had no FoxO3a expression, both Bim and p27 were significantly reduced by 37%, and GADD-45 was reduced by 27% (Fig. S2B in the Supplement).
DISCUSSION
Findings of this study strongly support the hypothesis that FoxO3a is activated by behavioral stress, indicated by IES-induced reduction of the inhibitory phosphorylation of FoxO3a and elevation of nuclear FoxO3a in the cerebral cortex. Since the cerebral cortex is a major brain area within the stress-regulating neurocircuit (42,43,54), and FoxO3a is highly expressed in the cerebral cortex (Allen Brain Atlas) (55), the study mainly tested the response of FoxO3a in this brain area. The significant activation of FoxO3a observed from the entire cerebral cortex suggests that the effect of IES to FoxO3a is robust, although it is possible that the increased activity of FoxO3a preferentially occurs in a sub-region of the cerebral cortex, such as the prefrontal cortex, which remains to be determined in detail. Among the FoxO subtypes expressed in brain, FoxO3a has the widest brain expression, whereas FoxO1 is mostly expressed in the hippocampus and striatum, and FoxO6 is mostly expressed during developmental stage with a nucleus-predominant distribution (2,4,19,20). In our previous studies (29,30,32,56), we demonstrated prominent regulation of FoxO3a by neurotrophins, serotonin, and psychotropics, and an antidepressant-like phenotype of FoxO3a-deficiency mice. Therefore, this study focused on studying FoxO3a, whereas it may also be important in the future to determine the similarity and difference of FoxO subtypes in response to stress.
Although our initial measurement of FoxO3a activity on post-IES day-1 did not reveal a significant change, it does not exclude an early response of FoxO3a to IES. IES can cause acute biochemical changes of the brain, such as increasing serotonin (57). Serotonin has been shown to increase FoxO3a phosphorylation by activating brain Akt (30), which could mask IES-responsive signals that lead to activation of FoxO3a at an early post-IES period. Despite being an acute stress, IES-induced biological changes in brain may last for several days (58,59), such as differentially changing the levels of neurotrophins (58), down-regulating signal transducers activators of transcription 3 (Stat3)-interacting protein (StIP1), up-regulating the suppressor of cytokine signaling 3 (Socs3) (60), and elevating the immediate early gene nerve growth factor-inducible gene A (NGFI-A) (59). The robust increase in FoxO3a activity on post-IES day-3 and day-8 highly suggests that there are FoxO3a-regulating machineries responsible for the prolonged activation of FoxO3a, and this study focused on identifying the underlying mechanism.
Since Akt is a major regulator of FoxO3a (7-9,11,61,62), and Akt-dependent FoxO3a phosphorylation decreased following IES treatment, we speculated that IES may inactivate Akt in the cerebral cortex, which was indicated by the reduction of Akt phosphorylation in IES-treated mice on post-IES day-3. Since it occurred at the same time point as the change of FoxO3a, it could be responsible for the initial reduction of FoxO3a phosphorylation and nuclear localization observed on post-IES day-3. However, the lack of significant reduction of Akt activity on post-IES day-8 suggests that additional stress-responsive mechanisms other than Akt may be involved in maintaining the prolonged nuclear FoxO3a accumulation.
JNK was reported to activate FoxO3a during oxidative and physical stresses (12-14), but significant changes of total and phosphorylated active JNK-1 were not found in the cerebral cortex after IES. The negative finding suggests that activation of FoxO3a by behavioral stress may represent a very different pathological process from the better understood activation of FoxO3a by oxidative stress (15).
We measured phosphorylation of GSK3 in this study because we previously found (37) that the sensitivity of developing IES-induced LH behavior is markedly enhanced in mice that express constitutively active GSK3 that is resistant to phosphorylation at the N-terminal serine (45). Although the study found a robust dephosphorylation of GSK3β by IES, but less effect on GSK3α in the cerebral cortex, we do not exclude a response of GSK3α to IES since GSK3 phosphorylation is measured in only one brain area for the purpose of this study, and phosphorylation at the N-terminal serine is not the only mechanism of regulating GSK3 activity. As the phosphorylation effect of IES appeared most significant on GSK3β in the cerebral cortex, we chose to focus this current study on this GSK3 isoform, whereas the role of GSK3α on FoxO3a activity remains to be studied in future.
GSK3β is negatively regulated by Akt at serine-9 residue (46). The reduced phospho-S9-GSK3β on post-IES day-3 has the same time point of change as seen with Akt, suggesting that activation of GSK3β is at least partially the result of IES-induced inactivation of Akt. However, the reduced phospho-S9-GSK3β and the trend of increase in total GSK3β were prolonged to post-IES day-8 when Akt activity has recovered. The uncoupling between Akt and GSK3β may be due to a selective dephosphorylation mechanism that affects GSK3β, but spares dephosphorylation of Akt. For example, protein phosphatase-1 inhibitor-2 was reported to differentially regulate the KCl-mediated dephosphorylation of GSK3β and Akt (63). Although the actual mechanism remains to be determined, the similar time course of the prolonged activation state of GSK3β and FoxO3a let us hypothesize that GSK3β could be an upstream regulator of FoxO3a in response to IES (Fig. S3 in the Supplement).
GSK3β is a protein kinase that regulates many transcription factors either by phosphorylation or direct interaction (64,65), but regulation of FoxO3a by GSK3β in mammalian brain has never been reported. We previously reported that chronic lithium treatment robustly inactivates FoxO3a in mouse brain (32). Since lithium is a GSK3 inhibitor both in vitro and in vivo (66-68), it raises the possibility that the effect of lithium on FoxO3a is via its inhibition of GSK3. A recent study with RNAi screening for kinase and phosphatase regulators of dFoxO in Drosophila S2 cells identified GSK3β as one of the regulators of dFoxO (69). Our sequence search also revealed enriched GSK3 consensus phosphorylation sites (S/TXXXS/T) throughout FoxO3a protein sequence, suggesting that FoxO3a is a candidate phosphorylation substrate of GSK3. Results of this study provided convincing evidence that GSK3β is a direct regulator of FoxO3a in response to IES by direct interaction and phosphorylation. Our data appears to suggest that the non-Akt-phosphorylated FoxO3a is a better substrate for GSK3β, whereas dephosphorylated active GSK3β has better access to FoxO3a, both processes can be facilitated by inactivating Akt (Fig. S3 in the Supplement). However, once GSK3β and FoxO3a form a protein complex, GSK3β becomes the driving force to maintain nuclear FoxO3a activity independently from Akt regulation.
While there is prolonged activation of FoxO3a after IES, It is also noticeable that both FoxO3a deficiency and GSK3 inhibitor reduced LH behavior on post-IES day-1. This effect may partly due to disrupting the baseline GSK3β-FoxO3a complex, which is present at a lower level in control mice. The behavioral effect in FoxO3a-deficient mice was not due to a pre-existing stress-resistant phenotype because IES is able to induce dephosphorylation of Akt and GSK3β in FoxO3a-deficient mice despite of no observable LH behavior. In addition, GSK3 inhibitor administered after IES delivery was able to reduce LH behavior. Therefore, our data suggests a behavioral-biochemical model in that GSK3β-FoxO3a complex is required for stress to induce behavioral disturbance. Meanwhile, stress triggers signal transduction mechanisms to build up a stronger GSK3β-FoxO3a complex that may contribute to prolonged behavioral disturbance as well as induce gene effect. The significance of interrupting the GSK3β-FoxO3a signaling pathway is therefore not to prevent stress, but to occlude stress-induced abnormal behaviors, which may serve as an effective treatment in stress-induced behavioral disturbance, such as depression.
As a transcription factor, activation of FoxO3a by IES is expected to induce gene expression. This study, however, only detected a small increase of p27 at post-IES day-8. The small change of p27 may be the initial gene response to IES-activated FoxO3a, but this result does not exclude the possibility that FoxO3a may regulate other neuronal genes that remain to be identified. On the other hand, prominent reduction of Bim and p27 were detected in FoxO3a-deficient mice, which is likely the result of lifetime FoxO3a deficiency. Bim is a proapoptotic gene, and reduced expression may have subtle neuroprotective effect (29,70,71). p27 is a cell cycle regulator (72,73), and in the cerebral cortex, p27 was found to promote neuronal differentiation and migration (74,75). Although not the focus of this study, it could be interesting to further determine if deletion of Bim or p27 or other FoxO3a-targeting genes has a direct effect in reducing IES-induced behavioral disturbance.
Supplementary Material
ACKNOWLEDGMENT
The authors thank R.S. Jope (University of Alabama at Birmingham, AL) for scientific advices and providing YFP-tagged GSK3β, A.P. Kozikowski (University of Illinois at Chicago, IL) for generous supply of benzofuranyl-3-yl-(indol-3-yl)maleimides, S. Peng (Virginia Mason Medical Center, WA) for providing breeders of FoxO3a-trap mice, M.E. Greenburg (Harvard Medical School, Boston, MA) and B. Burgering (University Medical Center, Utrecht, Netherlands) for FoxO3a cDNAs, and A.M. Polter for technical training. This research was supported by NIH grants MH73723 and MH86622 (XL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 4.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katoh M. Human FOX gene family. Int J Oncol. 2004;25:1495–1500. [PubMed] [Google Scholar]
- 6.Brunet A. The multiple roles of FOXO transcription factors. Med Sci (Paris) 2004;20:856–859. doi: 10.1051/medsci/20042010856. [DOI] [PubMed] [Google Scholar]
- 7.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 10.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 12.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 16.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 20.van der Heide LP, Jacobs FM, Burbach JP, Hoekman MF, Smidt MP. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J. 2005;391:623–629. doi: 10.1042/BJ20050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukunaga K, Ishigami T, Kawano T. Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons. J Pharmacol Sci. 2005;98:205–211. doi: 10.1254/jphs.fmj05001x3. [DOI] [PubMed] [Google Scholar]
- 22.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 23.Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, et al. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–1068. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 27.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126:45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, et al. Forkhead box, class o transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry. 2009;65:150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang B, Moussaif M, Kuan CJ, Gargus JJ, Sze JY. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 2006;4:429–440. doi: 10.1016/j.cmet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Mao Z, Liu L, Zhang R, Li X. Lithium reduces FoxO3a transcriptional activity by decreasing its intracellular content. Biol Psychiatry. 2007;62:1423–1430. doi: 10.1016/j.biopsych.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Gaisina IN, Gallier F, Ougolkov AV, Kim KH, Kurome T, Guo S, et al. From a natural product lead to the identification of potent and selective benzofuran-3-yl-(indol-3-yl)maleimides as glycogen synthase kinase 3beta inhibitors that suppress proliferation and survival of pancreatic cancer cells. J Med Chem. 2009;52:1853–1863. doi: 10.1021/jm801317h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Zhou W, Chen P, Gaisina I, Yang S, Li X. Glycogen Synthase Kinase-3beta is a Functional Modulator of Serotonin 1B Receptors. Mol Pharmacol. 2011;79:974–986. doi: 10.1124/mol.111.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anisman H, Merali Z. Rodent models of depression: learned helplessness induced in mice. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0810cs14. Chapter 8:Unit 8 10C. [DOI] [PubMed] [Google Scholar]
- 36.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Polter AM, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Salinas GD, Li X. Regulation of serotonin 1B receptor by glycogen synthase kinase-3. Mol Pharmacol. 2009;76:1150–1161. doi: 10.1124/mol.109.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, et al. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci U S A. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 47.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 51.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 52.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 53.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr., DiStefano PS, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 54.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Mai L, Jope RS, Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem. 2002;82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 57.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- 58.Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144:1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baranova KA, Rybnikova EA, Mironova VI, Samoilov MO. Effects of hypoxic preconditioning on expression of transcription factor NGFI-A in the rat brain after unavoidable stress in the “learned helplessness” model. Neurosci Behav Physiol. 2010;40:693–700. doi: 10.1007/s11055-010-9313-5. [DOI] [PubMed] [Google Scholar]
- 60.Mingmalairak S, Tohda M, Murakami Y, Matsumoto K. Possible involvement of signal transducers and activators of transcription 3 system on depression in the model mice brain. Biol Pharm Bull. 2010;33:636–640. doi: 10.1248/bpb.33.636. [DOI] [PubMed] [Google Scholar]
- 61.Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci U S A. 1999;96:11836–11841. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Peso L, Gonzalez VM, Hernandez R, Barr FG, Nunez G. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene. 1999;18:7328–7333. doi: 10.1038/sj.onc.1203159. [DOI] [PubMed] [Google Scholar]
- 63.King TD, Gandy JC, Bijur GN. The protein phosphatase-1/inhibitor-2 complex differentially regulates GSK3 dephosphorylation and increases sarcoplasmic/endoplasmic reticulum calcium ATPase 2 levels. Exp Cell Res. 2006;312:3693–3700. doi: 10.1016/j.yexcr.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 65.Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trehds in Biochemical Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 69.Mattila J, Kallijarvi J, Puig O. RNAi screening for kinases and phosphatases identifies FoxO regulators. Proc Natl Acad Sci U S A. 2008;105:14873–14878. doi: 10.1073/pnas.0803022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 71.Linseman DA, Phelps RA, Bouchard RJ, Le SS, Laessig TA, McClure ML, et al. Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci. 2002;22:9287–9297. doi: 10.1523/JNEUROSCI.22-21-09287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blain SW, Scher HI, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 73.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 74.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.