Abstract
Mechanisms of digitoxin-inhibited cell growth and induced apoptosis in human non-small cell lung cancer (NCI-H460) cells remain unclear. Understanding how digitoxin or derivate analogs induce their cytotoxic effect below therapeutically relevant concentrations will help in designing and developing novel, safer and more effective anti-cancer drugs. In this study, NCI-H460 cells were treated with digitoxin and a synthetic analog D6-MA to determine their anti-cancer activity. Different concentrations of digitoxin and D6-MA were used and the subsequent changes in cell morphology, viability, cell cycle, and protein expressions were determined. Digitoxin and D6-MA induced dose-dependent apoptotic morphologic changes in NCI-H460 cells via caspase-9 cleavage, with D6-MA possessing 5-fold greater potentcy than digitoxin. In comparison, non-tumorigenic immortalized bronchial and small airway epithelial cells displayed significantly less apoptotic sensitivity compared to NCI-H460 cells suggesting that both digitoxin and D6-MA were selective for NSCLC. Furthermore, NCI-H460 cells arrested in G(2)/M phase following digitoxin and D6-MA treatment. Post-treatment evaluation of key G2/M checkpoint regulatory proteins identified down-regulation of cyclin B1/cdc2 complex and survivin. Additionally, Chk1/2 and p53 related proteins experienced down-regulation suggesting a p53-independent cell cycle arrest mechanism. In summary, digitoxin and D6-MA exert anti-cancer effects on NCI-H460 cells through apoptosis or cell cycle arrest, with D6-MA showing at least 5-fold greater potency relative to digitoxin.
INTRODUCTION
Appropriate cell cycle progression is crucial for cell viability (Lapenna and Giordano, 2009; Schwartz and Shah, 2005; Lapenna and Giordano, 2009). Cardiac glycosides (CGs) are a class of natural products known for their cardiotonic and anti-neoplastic effects (Newman et al., 2008). In vitro studies on CG pharmacodynamics showed apoptosis, autophagy, and cell cycle arrest; however, such effects were selective against tumor cells when compared to normal cells (Daniel et al., 2003; Lawrence, 1988; López-Lázaro, 2007; Newman et al., 2008).
Digitoxin, a clinically approved CG for heart failure has shown anti-cancer effect in several types of cancer (López-Lázaro, 2007). For instance, Stenkvist et al. (1982) found that breast cancer patients on digitoxin regiment (20 – 33 nM) displayed improved outcomes than untreated patients (Lopez-Lazaro et al., 2005). Digitoxin induces apoptosis and inhibits cancer cell growth by binding to the Na+/K+ATPase pump (López-Lázaro, 2007; Newman et al., 2008). Digitoxin-bound Na+/K+ ATPase activates tyrosine kinase Src, PI3K, phospholipase C and Ras/MAPK pathways which leads to anti-proliferative downstream effects related to cell growth and apoptosis (Xie and Cai, 2003; Newman et al., 2008). Given that the integrated sugars give the unique biological function of CGs (Iyer et al., 2010; Langenhan et al., 2008; Zhou and O’Doherty, 2008), digitoxin provides an excellent model to efficiently modify its carbohydrate moiety and assess the biological impact of novel synthetic derivatives using cellular-based experiments. Therefore, synthesis of CG derivatives has become a potential approach for developing safer and more effective anti-neoplastic drugs.
Previous studies showed that modifying the glycosidic linkage or the oligosaccharide moiety of digitoxin could significantly enhance its anti-neoplastic activity (Iyer et al., 2010; Langenhan et al., 2008; Zhou and O’Doherty, 2008;). In other studies, Wang et al. (2010) synthesized and compared stereochemistry structure/activity relationships of the carbohydrate moiety of several digitoxin monosaccharide analogs based on a 60 cancer cell line NCI screening for lethal and growth inhibitory effects. This screening showed that non-small cell lung carcinoma (NSCLC) cells were more sensitive to digitoxin and its analogs than many other cancer cell lines being tested (Iyer et al., 2010; Wang et al., 2010). Additionally, three monosaccharide analogues, namely β-D-digitoxose, α-L-rhamnose and α-L-amicetose showed at least a 5-fold increase in potency to induce apoptosis and growth inhibition in NSCLC (Wang et al., 2010; Wang et al., 2011a; Wang et al., 2011b). However, these studies failed to identify the mechanisms mediating the anti-neoplastic effects of digitoxin or its synthetic analogs in cancer cells at/or below therapeutically relevant concentrations. Understanding the cytotoxic mechanism of digitoxin and its analogs in NSCLC will help in designing and developing safer and more effective CG-based anti-cancer therapies.
Based on the sensitivity to digitoxin and its analogs (Wang et al., 2010) and increased resistance to chemotherapy (Mijatovic et al., 2006), NCI-H460 cells were chosen as a NSCLC model in this study. We examined the cytotoxic mechanism of digitoxin and α-L-rhamnose digitoxin analog (namely D6-MA) below therapeutically relevant concentrations. We hypothesized that exposure of NCI-H460 cells to therapeutically relevant doses of digitoxin and D6-MA would decrease cell viability due to G2/M arrest and induce apoptosis, with greater potency for D6-MA. By comparing cytotoxic mechanisms of digitoxin to D6-MA, we aim to demonstrate that improved anti-cancer activity can be obtained by sugar-based modifications of natural products.
MATERIALS AND METHODS
Reagents and chemicals
Digitoxin was purchased from Sigma Chemicals (St. Louis, MO). Hoechst 33342, RNase, and propidium iodide (PI) were purchased from Molecular Probes (Eugene, OR). MTT cell viability assay kit, Triton X-100, sodium dodecyl sulfate (SDS), Tris-HCl, EDTA, NaCl, and Complete Mini cocktail protease inhibitors were purchased from Roche Applied Science (Indianapolis, IN). BCA assay kit was purchased from Thermo Scientific (Rockford, IL). Cdc2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other primary and horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling (Boston, MA). Acrylamide/Bis solution was purchased from Bio-Rad Laboratories (Hercules, CA). The iBlot® dry blotting and transferring system, PVDF transfer membranes, Countess automated cell counter, Countess cell counting chamber slides, and trypan blue stain 0.4% were purchased from Invitrogen (Carlsbad, CA). High Throughput Colorimetric ATPase Assay kit was purchased from Innova Biosciences Ltd. (Babraham, Cambridge, UK). Small airway cell basal medium (SABM), SAGM SingleQuots supplementation, and Cabrex medium were purchased from Lonza (Walkersville, MD). Purified Na+/K+ adenosine 5′-triphosphatase (ATPase) isolated from porcine cerebral cortex, and all other chemicals and reagents (including ethanol, isopropanol, Tween 20, Tris-HCl, DMEM medium, and RPMI 1640 medium) were purchased from Sigma Chemicals (St. Louis, MO).
Synthesis of D6-MA
D6-MA, an α-L-rhamnose monosaccharide analog of digitoxin, was synthesized from digitoxin based on a previously described method (Wang et al., 2011a; Zhou and O’Doherty, 2008). Briefly, digitoxin was first subjected to acid hydrolysis to cleave off the trisaccharide moiety and generate free aglycone moiety, digitoxigenin (Fig. 1A). The monosaccharide moiety was then synthesized from α-L-pyranose generated from acetyl furan by asymmetric reduction and oxidative rearrangement followed by tert-butoxycarbonyl (BOC) protection of the amino group. Subsequently, α-L-pyranose was conjugated with the digitoxigenin moiety via palladium-catalyzed glycosylation. The resulting digitoxin-α-L-pyranose was subjected to Luche reduction to generate digitoxin-α-L-rhamnoside.
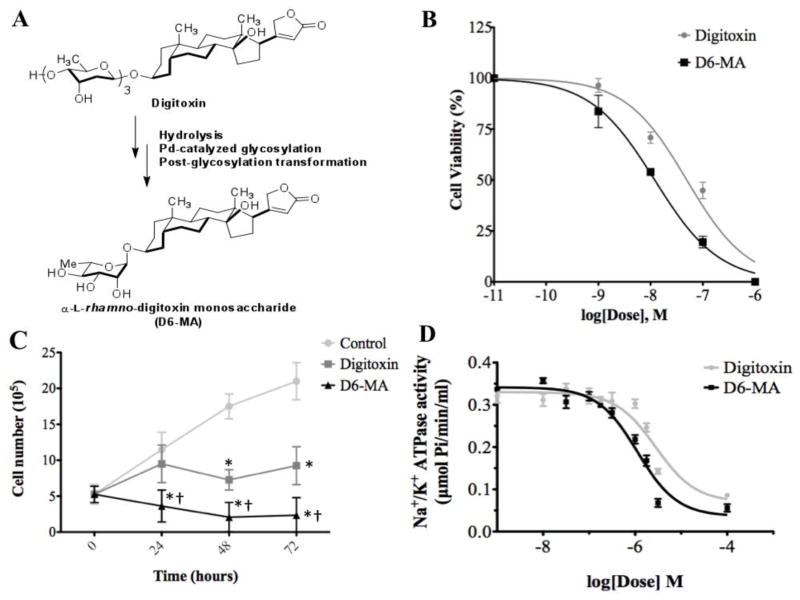
Fig. 1.
Digitoxin and D6-MA inhibit NCI-H460 cell viability in a dose dependent manner. (A) α-L-rhamnose monosaccharide analog (D6-MA) was synthesized from digitoxin. (B) Dose-response curve showing a 4-fold difference in potency between digitoxin and D6-MA for inhibition of cell viability generated by colorimetric MTT cell viability assay. (C) Time-response inhibition of cell proliferation after treatment with 10 nM of either digitoxin or D6-MA; (*) indicates significantly different from control, (†) indicates significantly different from digitoxin. (D) Dose-response inhibition of Na+/K+ATPase activity.
Cell culture
Human NSCLC cells (NCI-H460) and non-tumorigenic human bronchial epithelial cells (BEAS-2B) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Dr. Tom Hei generously provided immortalized small airway epithelial cells (hTERT SAEC) cells. The hTERT SAEC cells were developed and authenticated by the ectopic expression of human telomerase reverse transcriptase (hTERT) in normal human small airway epithelial cells according to the procedure previously described (Piao et al., 2005). Primary human respiratory epithelial cells (pSAEC), isolated from the small airway of a normal human donor (Lonza, Walkersville, MD), were also examined. pSAEC were cultured following manufacturer’s directions. Electron microscope and cytokeratin 8 and 18 immunostaining were used to verify the phenotype of the cells. pSAEC were identified as type I and type II lung epithelial cells with a normal diploid human male karyotype. Cells of at least 90% purity and 80% viability from a single lot were used for all experiments. All cell lines were tested for Mycoplasma contamination using Hoechst fluorescence staining. Briefly, cells were seeded overnight in a 12 well plate at 1×105 cells/ml, stained with 10 μg/ml of Hoechst 33342 for 30 min and analyzed for the presence of foreign or Mycoplasma nuclear material. No Mycoplasma was detected in any of the cell lines tested.
NCI-H460 cells were cultured in RPMI 1640 medium supplemented with 10% bovine fetal serum (FBS), 2 mM L-glutamine and 100-units/ml penicillin/streptomycin. BEAS-2B cells were cultured in DMEM medium supplemented with 5% bovine fetal serum (FBS), 2 mM L-glutamine and 100-units/ml penicillin/streptomycin. hTERT SAEC cells were cultured in SABM medium supplemented with 1% bovine serum albumin and SAGM SingleQuots growth factors. Cells were maintained in culture in a humid atmosphere containing 5% CO2 at 37°C. All experiments with NCI-H460 and BEAS-2B cells were performed in medium enriched with 1% FBS serum, 2 mM L-glutamine and 100-units/ml penicillin/streptomycin. 1% FBS was used due to existing concerns about digitoxin binding to serum proteins (Baggot and Davis, 1973; Lohman and Merkus, 1987).
Cell viability tests with MTT assay
Cells were seeded overnight in 96 well plates at a concentration of 1×104 cells/well, and then treated for 48 h with a log10 scale dilution series of either digitoxin or D6-MA dissolved in sterile filtered DMSO. Subsequently, 10 μl of 5 mg/ml MTT reagent was added to each well and then incubated for 4 h at 37 °C. Isopropanol acidified with 0.04 N HCl was used to dissolve converted dye. Absorbance at 570 nm was measured using an Automated Microplate Reader ELx800 (BioTek, Winooski, VT). Each experiment was conducted 4 times with 4 replicate wells per concentration.
Trypan blue exclusion assay
NCI-H460 cells were seeded overnight in 60 mm2 dishes at 5×105 cell/dish, and subsequently treated with 10 nM of either digitoxin or D6-MA for 24 h, 48 h and 72 h. After treatment, cells were collected, stained with 0.4% trypan blue, and counted using a Countess automated cell counter.
Na+/K+ ATPase activity assay
Na+/K+ ATPase activity assay for release of inorganic phosphate was performed on Na+/K+ ATPase isolated from porcine cerebral cortex following exposure to each compound according to the manufacturer’s protocol. Briefly, serial dilutions of each compound were prepared in a buffer containing 50 mM Tris, 25 mM MgCl2, 0.5 mM ATP, 130 mM NaCl, and 20 mM KCl at pH 7.5, then plated in a 96 well plate in triplicate. Subsequently, diluted Na+/K+ ATPase was added to each well and the reaction allowed to proceed for 15 minutes. The reaction was stopped with Pi ColorLock Gold for 30 minutes, and then the absorbance of each well was determined at 595 nm.
Apoptosis assay
Cells were seeded overnight in 12 or 24 well plates at a concentration of 1×105 cell/ml and subsequently treated with different concentrations of either digitoxin or D6-MA for 24 h. After treatment, cells were incubated with 10 μg/ml of Hoechst 33342 for 30 min and analyzed for apoptosis by scoring the percentage of cells having intensely condensed chromatin and/or fragmented nuclei using fluorescence microscopy (Leica Microsystems, Bannockburn, IL). Approximately 1,000 nuclei from ten random fields were analyzed for each sample. The apoptotic index was calculated as the percentage of cells with apoptotic nuclei over total number of cells.
Cell cycle analysis
NCI-H460 cells were seeded in 60 mm2 cell culture dishes at a concentration of 5×105 cells/dish, starved overnight in serum-free media, and then treated with 1, 5, 10, and 20 nM of either digitoxin or D6-MA for 48 h. Treated cells were then trypsinized, collected, washed with PBS and fixed in 70% ethanol at 4°C overnight. Subsequently, cells were washed with PBS and stained with propidium iodide containing 0.05% RNase. For cell cycle analysis, the DNA content was determined using a FACScan laser flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA). Data were analyzed using MODFIT software (Verity Software House, Topsham, ME). Experiments were repeated 4 times to conduct statistical analysis.
Western blot analysis
Cells were seeded in 60 mm2 cell culture dishes at a concentration of 1×106 cell/plate, starved overnight, and then treated with 5 to 50 nM of either digitoxin or D6-MA for 24 h. After treatment, cells were collected and lysed for 30 min on ice in lysis buffer containing 2% Triton X-100, 1% sodium dodecyl sulfate (SDS), 100 mM NaCl, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, and Complete Mini cocktail protease inhibitors. Insoluble debris was pelleted by centrifugation at 4 °C and 6800 g for 15 min. Subsequently, the supernatant was collected and used to determine protein content using BCA assay. Briefly, diluted supernatant samples and bovine serum albumin standards were plated in duplicate to a 96 well plate. Working reagent (1000 μL) was prepared by mixing 50 parts of reagent A (1000 μL) with 1 part of reagent B (20 μL), added to each well (200 μL each), and incubated at 37°C for 30 min. Absorbance of each well was measured at 562 nm with a Varioskan spectrophotometer (Thermo, Waltham, MA). BSA protein standard curves were plotted to determine sample protein content.
Samples were next separated on 12% SDS-PAGE and transferred to PVDF membranes using the iBlot® Dry Blotting System. Membranes were blocked in 5% skim milk in TBST (25 mM Tris–HCl, pH 7.4, 125 mM NaCl, 0.1% Tween 20) for 1 h, and subsequently incubated with appropriate primary antibodies at 4°C overnight. Membranes were washed three times for 10 min each with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. The immune complexes formed were detected by chemiluminescence (Supersignal West Pico; Pierce, Rockford, IL). Band quantification via densitometry was performed using ImageJ software version 10.2.
Statistical analysis
All results are presented as mean ± standard deviation. For cell viability, ATPase activity and apoptosis assays, dose-response curves and concentrations that caused 50% effect (i.e. IC50) were calculated for both digitoxin and D6-MA in all the cell lines tested using non-linear regression analysis in GraphPad Prism 5.0 (San Diego, CA). Two-way analysis of variance (ANOVA) and unpaired two-tailed Student’s t-test with α = 0.05 were performed to compare the effect of compounds and administered dose on cell viability, apoptosis and quantified protein expression data. Post-hoc Tukey-Kramer HSD tests were conducted on significant ANOVA results. Results were considered significant when p ≤ 0.05.
RESULTS
D6-MA causes inhibition of NCI-H460 cell viability and Na+/K+ATPase enzyme activity
D6-MA, a digitoxin analog, was prepared by subjecting digitoxin-α-L-pyranoside to post-glycosylation modification (Fig. 1A) as previously described (Wang et al., 2010; Wang et al., 2011a). To evaluate whether D6-MA exhibits greater potency than digitoxin to inhibit NCI-H460 cell viability, we performed colorimetric MTT and trypan blue exclusion assays. Non-linear regression analysis (Fig. 1B) showed that D6-MA exhibited about 4-fold greater potency (IC50 = 12.0 nM) than digitoxin (IC50 = 49.4 nM) while Student’s t-test detected digitoxin’s and D6-MA’s inhibition lowest observed effective concentration (LOEC) at 10 nM and 1nM (p<0.001) respectively. Additionally, two-way ANOVA test showed that while both compounds significantly inhibited NCI-H460 cell viability in a time dependent manner, D6-MA was significantly more potent than digitoxin (Fig. 1C; F = 18.11, p <0.0001). Next, we tested whether the compounds inhibit Na+/K+ ATPase enzyme activity. Non-linear regression analysis showed that D6-MA is more potent (IC50 = 1.1 μM) than digitoxin (IC50 = 2.8 μM) at inhibiting activity of isolated Na+/K+ ATPase (Fig. 1D).
D6-MA induces apoptosis in NCI-H460 cells
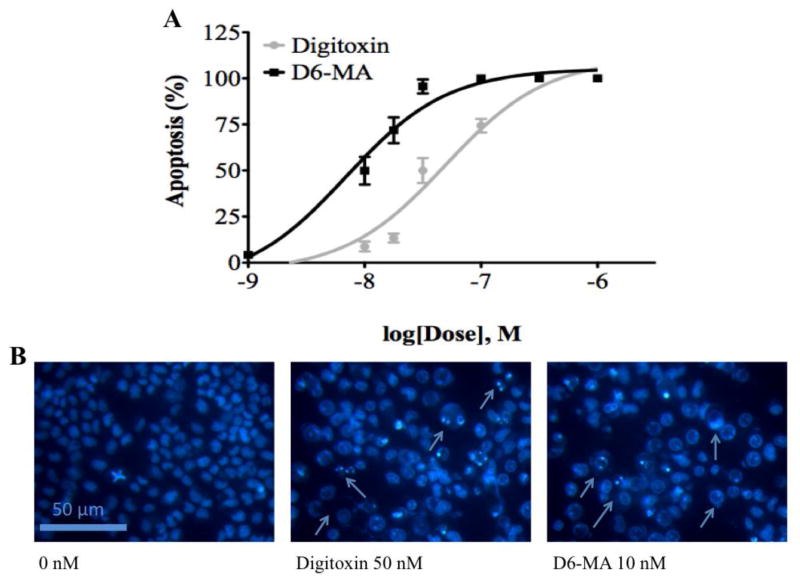
Previous studies attributed the anti-neoplastic effects of CG to their capacity to induce apoptosis (Newman et al., 2008). To investigate whether D6-MA is more potent than digitoxin in inducing apoptosis, we exposed NCI-H460 cells to each of the compounds and used Hoechst 33342 DNA fragmentation assay to evaluate the apoptotic effects. Non-linear regression analysis showed that the percentage of NCI-H460 cells displaying apoptotic nuclei was both dose and compound dependent (Fig. 2A and 2B), with D6-MA displaying about 5-fold greater potency (IC50 = 10 nM) when compared to digitoxin (IC50 = 48 nM).
Fig. 2.
Digitoxin and D6-MA induce NCI-H460 cell apoptosis in a dose dependent manner. (A) Dose-response induced apoptosis. (B) Hoechst staining and fluorescence microscopy images of NCI-H460 cells. Arrows point to apoptotic cells.
D6-MA exhibits selective cytotoxicity to NSCLC cells
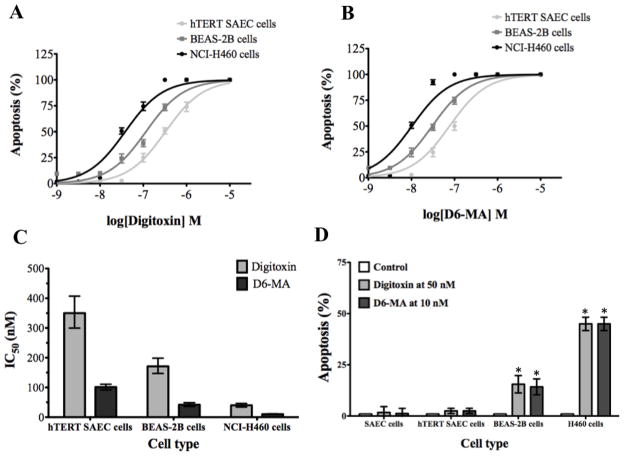
Anti-cancer drugs ought to be selectively toxic toward cancer cells and not normal cells. To test whether digitoxin and D6-MA are selectively toxic to NSCLC cells, we exposed primary small airway epithelial cells (pSAEC), immortalized non-tumorigenic human small airway epithelial cells (hTERT SAEC), and immortalized non-tumorigenic human bronchial epithelial cells (BEAS-2B) to digitoxin and D6-MA, and used Hoechst 33342 DNA fragmentation assay to evaluate apoptosis in these cell lines. Interestingly, non-linear regression analysis showed that treated hTERT SAEC and BEAS-2B cells had significantly less sensitivity to apoptosis for either of the compounds when compared to NCI-H460 cells (Fig. 3A and B). To validate this finding, an apoptosis assay with set doses of digitoxin and D6-MA on all four cell lines was conducted. Two-way ANOVA showed a significant difference between cell line sensitivity (Fig. 3C; F=91.84, p<0.0001) while no difference existed between the compounds with dosing placed at a 5-fold interval. Specifically, NCI—H460 and BEAS-2B cells showed greater sensitivity to each compound while both SAEC cell lines displayed minimal sensitivity. In addition, D6-MA exhibited a 4-fold greater potency than digitoxin to induce apoptosis regardless of cell type (Fig. 3D).
Fig. 3.
The apoptotic effect of digitoxin and D6-MA is selective to non-small cell lung cancer cells. (A) Digitoxin dose-response in hTERT SAEC, BEAS-2B, and NCI-H460 cells. (B) D6-MA dose-response in hTERT SAEC, BEAS-2B, and NCI-H460 cells. (C) Non-tumorigenic hTERT SAEC cells and BEAS-2B cells have higher IC50 values than NCI-H460 cells. Error bars represent IC50 values at 95% confidence intervals. (D) Primary SAEC cells, non-tumorigenic hTERT SAEC cells, and BEAS-2B cells are less sensitive to apoptosis for either digitoxin (50 nM) or D6-MA (10 nM). Error bars represent standard deviation; (*) indicates significantly different from untreated cells.
D6-MA induces expression of cytochrome c and extensive caspase-9 cleavage
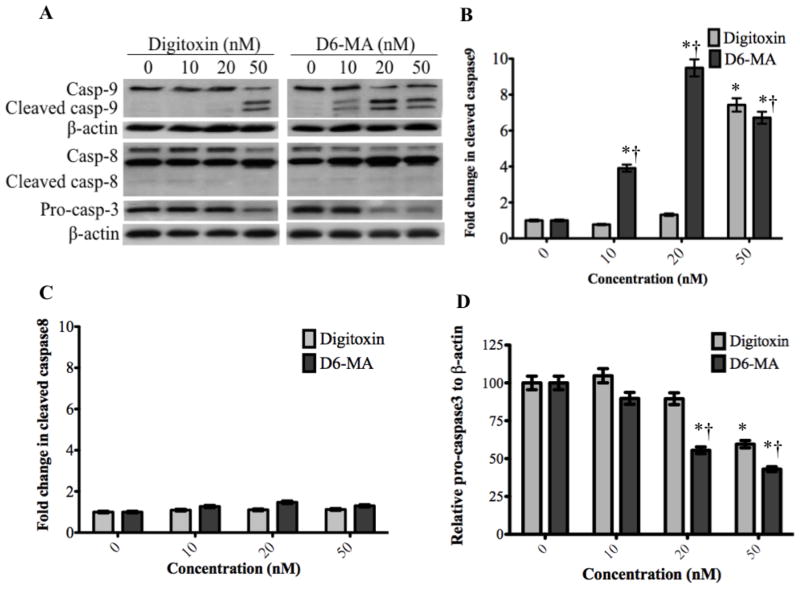
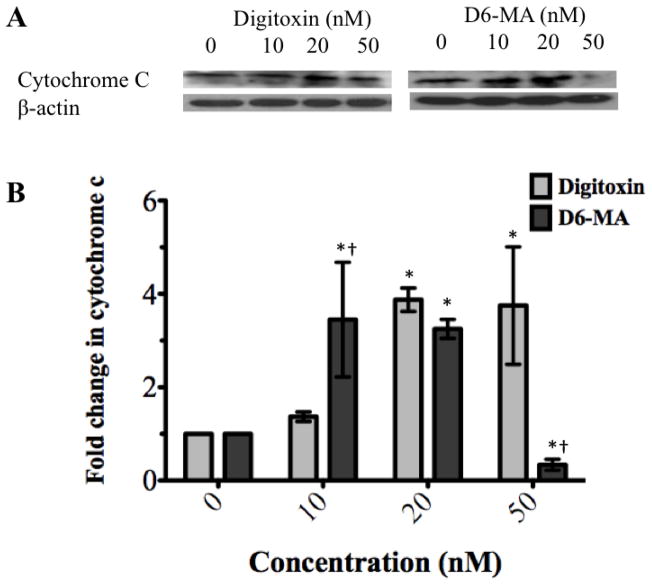
Previous studies have shown that sequential activation of caspases and increased cytochrome c expression play a central role in the execution phase of apoptosis (Ashkenazi, 2008). Specifically, caspase-8 and caspase-9 mediate the extrinsic (death ligand) and intrinsic (mitochondrial) apoptotic pathways, respectively. Studies have also shown that caspase-3 interacts with both caspase-8 and -9 and experiences activation in both apoptotic signaling pathways (Ashkenazi, 2008). With persistent apoptotic stimulus, increased cytochrome c is released from mitochondria into the cytoplasm, recruits pro-caspase-9 and APAF-1 and forms the apoptosome that causes caspase-9 activation (Ashkenazi, 2008). To examine which apoptotic pathway predominates in NCI-H460 cells exposed to digitoxin or D6-MA, we performed Western blot analysis for cytochrome c and caspase cleavage respectively. Based on β-actin expression (used as control), two-way ANOVA with subsequent post-hoc tests showed that D6-MA exhibited greater potency than digitoxin in inducing substantial caspase-9 cleavage (Fig. 4A, and 4B; F=28.78, p<0.0001). Pro-caspase-3 experienced a significant drop in expression (Fig. 4A, and 4C; F=7.19, p<0.0001) while caspase-8 displayed slight cleavage following digitoxin and D6-MA treatment (Fig. 4A, and 4D; F=16.06, p=0.066). Additionally, two-way ANOVA and post-hoc testing showed that D6-MA was more potent than digitoxin in inducing cytochrome c expression (Fig. 5A) at 10 nM while at 50 nM, D6-MA significantly reduced cytochrome c expression (Fig. 5B; F=25.52, p<0.001),.
Fig. 4.
Western blot analysis show changes in caspase 9, 8, and 3. (A) Digitoxin and D6-MA induced differential cleavage of caspase 9. Digitoxin caused pro-caspase 3 to drop at 50 nM, while D6-MA caused pro-caspase 3 to drop at 20 nM. (B) Quantification of cleaved caspase 9 blots shows fold change in cleavage following treatment. (C) Quantification of cleaved caspase 8 blots shows fold change in cleavage following treatment. (D) Quantification of pro-caspase 3 blots shows decrease in quantity following treatment; (*) indicates significantly different from control, (†) indicates significantly different from digitoxin.
Fig. 5.
Western blot analysis shows changes in cytochrome c expression. (A) Digitoxin and D6-MA induce cytochrome c expression in a dose dependent manner. (B) D6-MA is more potent than digitoxin in inducing cytochrome c expression (F=25.51, p<0.001). Quantification of cytochrome c blots shows increased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to digitoxin treatment).
Digitoxin and D6-MA induce G2/M phase arrest
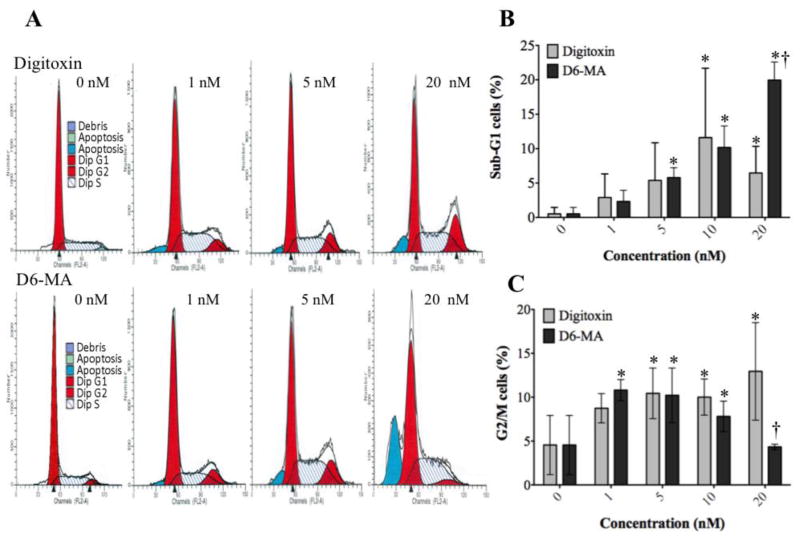
Previous research suggested that cell cycle arrest causes apoptosis or loss of cell viability (Dickson and Schwartz, 2009; Schwartz and Shah, 2005). To investigate whether digitoxin or D6-MA induces cell cycle arrest, we exposed NCI-H460 cells to different doses of either digitoxin or D6-MA for 48 h. Using two-way ANOVA our results showed that digitoxin and D6-MA significantly increased the percentage of cells in sub-G1 phase in a dose dependent manner (Fig. 6A and Fig. 6B; F = 16.1, p < 0.0001). Additionally, D6-MA was significantly more potent than digitoxin at 20 nM (Fig. 6B; p=0.0071). Both compounds induced G2/M arrest and was dose-dependent (Fig. 6A and Fig. 6C; p = 0.0099). D6-MA’s LOEC for inducing G2/M phase arrest was at 1 nM, while digitoxin showed comparable effect at 5 nM. Moreover, digitoxin increased G2/M phase arrest in a dose dependent manner, while D6-MA G2/M phase arrest peaked between 1 and 5 nM and then decreased to control levels at 20 nM. Our results suggest that the drop in the percentage of D6-MA-treated cells in G2/M phase coincide with a significant increase of cells in sub-G1 phase.
Fig. 6.
Digitoxin and D6-MA induce G2/M arrest in NCI-H460 cells. (A) Digitoxin and D6-MA induced an increase in the G2/M and sub-G1 cell populations. (B) Digitoxin and D6-MA pushed cells into sub-G1 phase. (C) Digitoxin and D6-MA induced significant G2/M phase arrest compared to controls; (*) indicates significantly different from control, (†) indicates significantly different from digitoxin, p<0.05).
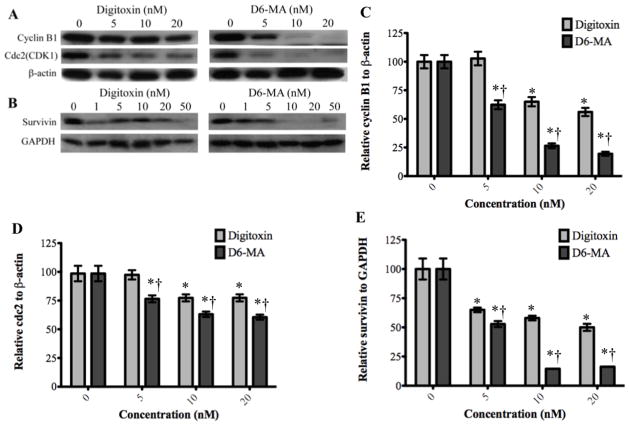
Digitoxin and D6-MA induce down-regulation of cyclin B, cdc2, and survivin
Inducing G2/M phase arrest usually occurs following an alteration in the signaling pathways that control cell progression through G2/M phase (Stark and Taylor, 2006). Since our results showed that the percentage of cells in G2/M phase increased upon exposure to digitoxin and D6-MA, we further investigated how digitoxin and D6-MA induce G2/M phase arrest. Specifically, we examined the expression of key molecular drivers of G2/M phase, namely cyclin B1, cdc2, and survivin, using Western blot analysis. Cdc2 requires both cdc2 and cyclin B1 in the complex form for adequate activity. Cyclin B1/cdc2 complex is known to catalyze the chromatin condensation as well as nuclear envelope breakdown during mitosis, thus performing a key and rate limiting function in the cell transition from G2 to M phase (Stark and Taylor, 2006). Survivin promotes mitosis by activating the chromosomal passenger complex (Mita et al., 2008). Moreover, the phosphorylation of survivin by cyclin B1/cdc2 complex facilitates the association of survivin/caspase-9, which inhibits caspase-9 activity (O’Connor et al., 2000). Two-way ANOVA testing revealed that D6-MA was significantly more potent than digitoxin in inducing dose-dependent down-regulation of cyclin B1, cdc2, and survivin (Fig. 7A–E; F= 29.06, 6.8, and 25.43; p< 0.0001, 0.0036, and 0.0001 respectively) based on β-actin and GAPDH expression.). D6-MA’s LOEC for reducing expression of both cyclin B and cdc2 was 5 nM while digitoxin showed similar effect at 10 nM (Fig. 7C and D). Both compounds exhibited reduced survivin expression LOEC at 5 nM (Fig. 7E). Interestingly, the doses that resulted in reduced expression of each protein correlated with the observed arrest in G2/M phase; moreover, this was dependent on compound treatment, thus implicating these proteins in cell cycle arrest signaling.
Fig. 7.
Western blot analysis show decreased expression of cyclinB1, cdc2, and survivin. (A) Digitoxin and D6-MA inhibited cyclinB1 and cdc2 expression in a dose dependent manner. (B) Digitoxin and D6-MA inhibited survivin expression in a dose dependent manner. (C) D6-MA is more potent than digitoxin in inhibiting cyclinB1 expression (F=29.06, p<0.001). Quantification of cyclinB1 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment). (D) D6-MA is more potent than digitoxin in inhibiting cdc2 expression (F=6.805, p=0.0036). Quantification of cdc2 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment). (E) D6-MA is more potent than digitoxin in inhibiting survivin expression (F=25.43, p<0.001). Quantification of survivin blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment).
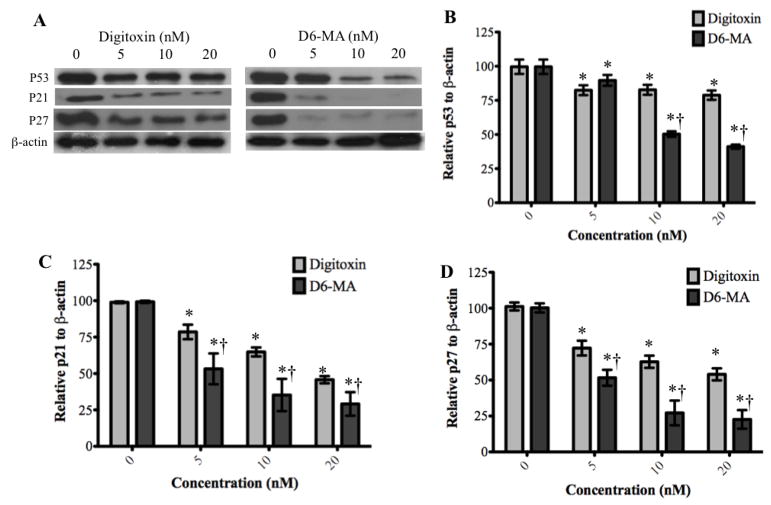
Digitoxin and D6-MA-mediated G2/M phase arrest does not correlate with up-regulation of p53-related signaling protein or Chk1/2
We further aimed to identify the signaling pathways that mediate G2/M phase arrest following exposure to digitoxin or D6-MA. For this we examined the key upstream regulatory pathways of the cyclin B1/cdc2 complex. Since p53 functions as a key coordinator of cell cycle check point activity and as an effective promoter of apoptosis, (Stark and Taylor, 2006; Vermeulen et al., 2003), we also examined p53-related signaling including p53, p21, and p27 (Vermeulen et al., 2003). Our results showed that while digitoxin significantly inhibited the expression of p53, p21, and p27 at concentrations between 5 to 20 nM, D6-MA was significantly more potent in inducing down-regulation of p53, p21, and p27 (Fig. 8A). Specifically, two-way ANOVA and post-hoc testing confirmed that p53, p21 and p27 expression was compound and dose dependent (Fig. 8B–D; F = 52.89, 2.06, and 4.23; p < 0.001, 0.0008, and 0.0155 respectively).
Fig. 8.
Western blot analysis show decreased expression of p53, p21, and p27. (A) Digitoxin and D6-MA down-regulated p53, p21, and p27 in a dose dependent manner. (B) D6-MA is more potent than digitoxin in inhibiting p53 expression (F=52.89, p<0.001). Quantification of p53 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment). (C) D6-MA is more potent than digitoxin in inhibiting p21 expression (F=4.23, p<0.001). Quantification of p21 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment). (D) D6-MA is more potent than digitoxin in inhibiting p27 expression (F=2.06, p=0.008). Quantification of p27 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment).
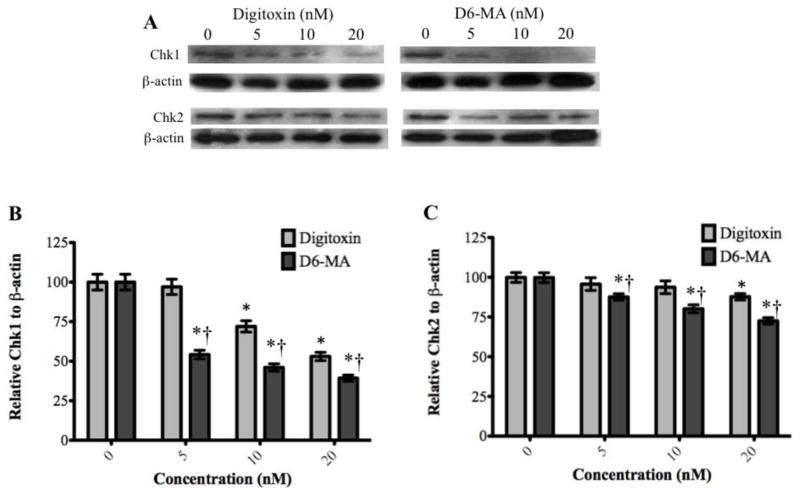
We also examined whether checkpoint kinase 1 and 2 (Chk1/2) up-regulation contributed to G2/M phase arrest signaling. Chk1/2 is involved in DNA damage response and in normal cell cycle progression by controlling cell cycle checkpoints (Bartek and Lukas, 2003; Wang et al., 2009a; Zhou and Bartek, 2004). Our results and two-way ANOVA analysis showed that digitoxin and D6-MA induced inhibition of Chk1 and Chk 2 expression and was dose-dependent (Fig. 9A–C; F = 36.11 and 7.982; p<0.001 and 0.002 respectively). Notably, Chk1 experienced a 2-fold decrease in expression while Chk2 exhibited a less substantial decrease. Reduced expression of both proteins correlated with similar trends observed with cyclin B and cdc2.
Fig. 9.
Western blot analysis show decreased expression of Chk1 and Chk2. (A) Digitoxin and D6-MA down-regulated Chk1 and Chk2 in a dose dependent manner. (B) D6-MA is more potent than digitoxin in inhibiting Chk1 expression (F=10.17, p<0.001). Quantification of Chk1 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment). (C) D6-MA is more potent than digitoxin in inhibiting Chk2 expression (F=7.982, p<0.001). Quantification of Chk2 blots shows decreased expression following treatment (*p<0.05 compared 0 nM, †p<0.05 compared to respective digitoxin treatment).
DISCUSSION
Our study aimed to evaluate and compare the cytotoxic potency of digitoxin and D6-MA in NCI-H460 cells in order to derive the mechanisms and signaling pathways responsible for cell cycle arrest. Such studies could lead to feasible, reliable and more potent drug therapies, which exhibit potent anti-cancer effects at safe and therapeutically relevant doses. Our results showed that digitoxin and D6-MA modulates one or several signaling pathways controlling cell cycle progression, cell proliferation, and apoptosis.
Cardiac glycosides (CGs) are known to mediate their cellular effects by inhibiting Na+/K+ ATPase activity, by affecting ion homeostasis at high doses and activating the ATPase ‘signalasome’ at low doses (Newman et al., 2008; Smith, 1989). Our studies showed that D6-MA is more potent than digitoxin at inhibiting Na+/K+ ATPase activity. Interestingly, concentrations of digitoxin and D6-MA that inhibited Na+/K+ ATPase activity were more than 10-fold greater than their cytotoxic concentrations. This result suggests that Na+/K+ ATPase inhibition by either digitoxin or D6-MA does not account for drug or analog cytotoxic effects, thus leaving the possibility for ‘signalasome’ activation.
We hypothesized that the apoptotic effect of digitoxin and D6-MA are selective to NSCLC cells. To test this hypothesis, we performed Hoechst apoptosis assay on primary human lung epithelial cells and non-tumorigenic human lung epithelial cells treated with each compound. Our results showed that D6-MA caused increased apoptosis when compared to digitoxin in all the cell lines being tested. Moreover, digitoxin and D6-MA showed higher IC50 values in non-tumorigenic lung epithelial cells than in NSCLC cells. Thus, this confirmed that primary human lung epithelial cells and non-tumorigenic human lung epithelial cells are significantly less sensitive to digitoxin and D6-MA when compared to NCI-H460 cells.
We further hypothesized that for both digitoxin and D6-MA, apoptosis is mediated through the mitochondrial pathway. Mitochondrial pathway involvement leads to cytochrome c release and association with pro-caspase-9 and APAF1 respectively (Ashkenazi, 2008). Such association leads to caspase-9 and caspase-3 activation and induced cell apoptosis (Ashkenazi, 2008). To test this hypothesis, we performed Western blot analysis of NCI-H460 cells exposed to digitoxin and D6-MA, respectively. Our results showed that D6-MA was more potent than digitoxin in inducing cytochrome c expression and cleavage of caspase-9, while for pro-caspase-3 there was a drop in expression and only slight differential cleavage for caspase-8. In addition, increased cytochrome c expression suggests that H460 cells retained their ability to synthesize protein, which contrasts previous claims that CG induced cytotoxicity is due to general inhibition of protein synthesis (Perne et al. 2009).
We further investigated cyclin B1 and cdc2 regulation; cyclin B1 and cdc2 form cyclin B1/cdc2 complex that is crucial for progression of cells through G2/M phase, protects mitotic cells from apoptosis, and maintains cancer cell viability (Allan and Clarke, 2007; Stark and Taylor, 2006; Yuan et al., 2004). We showed down-regulation of cyclin B1 and cdc2 by both digitoxin and D6-MA, with D6-MA exhibiting greater potency in reducing cell viability.
We also tested whether survivin, a protein that promotes mitosis by activating the chromosomal passenger complex (Mita et al., 2008) is involved in the reduced NCI-H460 cell viability. Our results showed that digitoxin and D6-MA induced survivin down-regulation at sub-therapeutic concentrations, with D6-MA being significantly more potent than digitoxin. Such down-regulation further explains the observed G2/M phase arrest and points to survivin as being a viable target for digitoxin or D6-MA mediated anti-neoplastic activity in NCI-H460 cells.
Our results provide further support that cytotoxic effects of CGs are independent of p53 status. Digitoxin’s ability to down-regulate p53, even though is first demonstrated in this study, is supported by previous reports employing other CGs (such as oubain and digoxin) in both breast and lung cancer cells (Kometiani et al., 2005; Wang et al., 2009b). For instance, p53 null and p53 wild type lung cancer cells were previously shown to exhibit equal cell death when exposed to CGs (Wang et al. 2009b). In addition, we also report for the first time on the down-regulation of p21 and p27 by digitoxin and D6-MA. Several studies suggested that p21 possesses oncogenic properties in promoting mitosis and cell migration (Abbas and Dutta, 2009; Kumar et al., 2006; Roninson, 2002). In contrast to our findings, p21 up-regulation in breast cancer cells following oubain exposure was reported (Kometiani et al., 2005). p21 down-regulation possibly explains the reduced NCI-H460 cell viability following digitoxin and D6-MA exposure.
We also studied Chk1/2 expression to further explain the reduced cell viability associated with G2/M arrest,. Chk1/2 is known to mediate cell cycle arrest following DNA damage or stress response (Bartek and Lukas, 2003; Wang et al., 2009a; Zhou and Bartek, 2004). Abrogating cell cycle checkpoints by chemotherapeutic agents that specifically target Chk1/2 was shown to be an effective chemotherapeutic alternative for several types of cancer (Bartek and Lukas, 2003; Zhou and Bartek, 2004). We are first to show that digitoxin and D6-MA inhibit Chk1/2 expression at sub-therapeutic concentrations in NCI-H460 cells, with the D6-MA being more potent than digitoxin. These results indicate that following digitoxin or D6-MA treatment neither G2/M phase arrest, nor down-regulation of cyclin B1 and cdc2 is mediated by the up-regulation of Chk1/2. However, down-regulation of Chk1/2 following treatment with digitoxin or D6-MA at sub-therapeutic concentrations can explain the reduced cell viability found in our studies.
The results presented herein further advance our understanding of the selective anti-neoplastic mechanism of sub-therapeutic digitoxin concentrations towards NSCLC cancer cells. In addition, enhanced and selective anti-neoplastic activity of D6-MA for NSCLC opens new perspectives for more effective chemotherapeutic strategies based on artificially synthesized compounds.
CONCLUSIONS
Our study is the first to focus on determining anti-neoplastic effects of realistic, sub-therapeutic doses of digitoxin and D6-MA in NCI-H460 cancer cells. Moreover, we show for the first time that sub-therapeutic concentrations of digitoxin and D6-MA induce G2/M phase arrest and cyclinB1 and cdc2 down-regulation, with D6-MA exhibiting greater potency than digitoxin. Our results also suggest that G2/M phase arrest and down regulation of cyclinB1 and cdc2 by digitoxin and D6-MA are not directly controlled by up-regulation of p53 signaling or checkpoint kinase signaling.
Highlights.
Digitoxin and synthetic analog D6-MA induced apoptotic morphologic changes in NCI-H460 cells in a dose-dependent manner
Apoptotic cell death induced by analog was 5-fold more potent when compared to digitoxin
NCI-H460 cells arrested in G(2)/M phase following digitoxin (≥5 nM) and analog (≥1 nM) treatment
Digitoxin inhibited the expression of cyclin B1/cdc2 complex and survivin at sub-therapeutic concentrations
D6-MA was 4-fold more potent than digitoxin
Acknowledgments
The authors thank Y. Lu, S. Talbot, D. Medan, M. Chen, V. Pongrakhananon, and S. Luanpitpong for their technical assistance. We also thank Dr. Kathleen Brundage for her help with the flow cytometry experiments, performed in West Virginia University Flow Cytometry Core Facility, under COBRE NCRR P20 RR016440. This work is supported by the NIH grants R01-HL076340, GM090259, GM088839 and the NSF grant EPS-1003907. Disclaimer: Research findings and conclusions are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LA, Clarke PR. Phosphorylation of Caspase-9 by CDK1/Cyclin B1 Protects Mitotic Cells against Apoptosis. Molecular Cell. 2007;26:301–310. doi: 10.1016/j.molcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- Baggot JD, Davis LE. Plasma protein binding of digitoxin and digoxin in several mammalian species. Res Vet Sci. 1973;15:81–87. [PubMed] [Google Scholar]
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bøhmer T, Røseth A. Prolonged digitoxin half-life in very elderly patients. Age Ageing. 1998;27:222–224. doi: 10.1093/ageing/27.2.222. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Daniel D, Süsal C, Kopp B, Opelz G, Terness P. Apoptosis-mediated selective killing of malignant cells by cardiac steroids: maintenance of cytotoxicity and loss of cardiac activity of chemically modified derivatives. Int Immunopharmacol. 2003;3:1791–1801. doi: 10.1016/j.intimp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16:36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AKV, Zhou M, Azad N, Elbaz H, Wang L, Rogalsky DK, Rojanasakul Y, O’Doherty GA, Langenhan JM. A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependent Induction of Caspase-9-Mediated Apoptosis. ACS Medicinal Chemistry Letters. 2010;1:326–330. doi: 10.1021/ml1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometiani P, Liu L, Askari A. Digitalis-Induced Signaling by Na+/K+-ATPase in Human Breast Cancer Cells. Mol Pharmacol. 2005;67:929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Langenhan JM, Engle JM, Slevin LK, Fay LR, Lucker RW, Smith KR, Endo MM. Modifying the glycosidic linkage in digitoxin analogs provides selective cytotoxins. Bioorganic & Medicinal Chemistry Letters. 2008;18:670–673. doi: 10.1016/j.bmcl.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. Int J Radiat Oncol Biol Phys. 1988;15:953–958. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- Lohman JJHM, Merkus FWHM. Plasma protein binding of digitoxin and some other drugs in renal disease. Pharmacy World & Science. 1987;9:75–78. doi: 10.1007/BF01960739. [DOI] [PubMed] [Google Scholar]
- López-Lázaro M. Digitoxin as an anticancer agent with selectivity for cancer cells: possible mechanisms involved. Expert Opin Ther Targets. 2007;11:1043–1053. doi: 10.1517/14728222.11.8.1043. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortes F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J Nat Prod. 2005;68:1642–1645. doi: 10.1021/np050226l. [DOI] [PubMed] [Google Scholar]
- Mijatovic T, Op De Beeck A, Van Quaquebeke E, Dewelle J, Darro F, de Launoit Y, Kiss R. The cardenolide UNBS1450 is able to deactivate nuclear factor κB–mediated cytoprotective effects in human non–small cell lung cancer cells. Molecular Cancer Therapeutics. 2006;5:391–399. doi: 10.1158/1535-7163.MCT-05-0367. [DOI] [PubMed] [Google Scholar]
- Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: Key Regulator of Mitosis and Apoptosis and Novel Target for Cancer Therapeutics. Clinical Cancer Research. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- Newman RA, Yang P, Pawlus AD, Block KI. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Molecular Interventions. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- O’Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proceedings of the National Academy of Sciences. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, Sloane M, Uras IZ, Hoermann G, Nijman SM, Mayerhofer M. Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One. 2009;4:e8292. doi: 10.1371/journal.pone.0008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CQ, Liu L, Zhao YL, Balajee AS, Suzuki M, Hei TK. Immortalization of human small airway epithelial cells by ectopic expression of telomerase. Carcinogenesis. 2005;26:725–731. doi: 10.1093/carcin/bgi016. [DOI] [PubMed] [Google Scholar]
- Roninson IB. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Letters. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Shah MA. Targeting the Cell Cycle: A New Approach to Cancer Therapy. Journal of Clinical Oncology. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- Smith TW. The fundamental mechanism of inotropic action of digitalis. Therapie. 1989;44:431–435. [PubMed] [Google Scholar]
- Stark G, Taylor W. Control of the G2/M transition. Molecular Biotechnology. 2006;32:227–248. doi: 10.1385/MB:32:3:227. [DOI] [PubMed] [Google Scholar]
- Stenkvist B, Pengtsson E, Dahlqvist B, Eriksson O, Jarkrans T, Nordin B. Cardiac glycosides and breast cancer, revisited. N Engl J Med. 1982;306:484. [PubMed] [Google Scholar]
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Proliferation. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HYL, Rojanasakul Y, O’Doherty GA. Synthesis and Evaluation of the α-d-/α-l-Rhamnosyl and Amicetosyl Digitoxigenin Oligomers as Antitumor Agents. ACS Medicinal Chemistry Letters. 2011a;2:264–269. doi: 10.1021/ml100290d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HYL, Wu B, Zhang Q, Kang SW, Rojanasakul Y, O’Doherty GA. C5′-Alkyl Substitution Effects on Digitoxigenin α-l-Glycoside Cancer Cytotoxicity. ACS Medicinal Chemistry Letters. 2011b;2:259–263. doi: 10.1021/ml100291n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HYL, Xin W, Zhou M, Stueckle TA, Rojanasakul Y, O’Doherty GA. Stereochemical Survey of Digitoxin Monosaccharides. ACS Medicinal Chemistry Letters. 2010;2:73–78. doi: 10.1021/ml100219d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ji P, Liu J, Broaddus RR, Xue F, Zhang W. Centrosome-associated regulators of the G2/M checkpoint as targets for cancer therapy. Mol Cancer. 2009a;8:8. doi: 10.1186/1476-4598-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, Zheng W, Xie Z, Sun Y. Cardiac Glycosides Inhibit p53 Synthesis by a Mechanism Relieved by Src or MAPK Inhibition. Cancer Research. 2009b;69:6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+/K+ATPase-Mediated Signal Transduction: From Protein Interaction to Cellular Function. Molecular Interventions. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, Strebhardt K. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene. 2004;23:5843–5852. doi: 10.1038/sj.onc.1207757. [DOI] [PubMed] [Google Scholar]
- Zhou BBS, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- Zhou M, O’Doherty G. The De Novo Synthesis of Oligosaccharides: Application to the Medicinal Chemistry SAR-Study of Digitoxin. Current Topics in Medicinal Chemistry. 2008;8:114–125. doi: 10.2174/156802608783378828. [DOI] [PubMed] [Google Scholar]