Abstract
N-3 polyunsaturated fatty acids(PUFA) from fish oil exert their functional effects by targeting multiple mechanisms. One mechanism to emerge in the past decade is the ability of n-3 PUFA acyl chains to perturb the molecular organization of plasma membrane sphingolipid/cholesterol enriched lipid raft domains. These domains are nanometer scale assemblies that coalesce to compartmentalize select proteins for optimal function. Here we review recent evidence on how n-3 PUFAs modify lipid rafts from biophysical and biochemical experiments from several different model systems. A central theme emerges from these studies. N-3 PUFA acyl chains display tremendous conformational flexibility and a low affinity for cholesterol and saturated acyl chains. This unique flexibility of n-3 PUFA acyl chains impacts the organization of inner and outer leaflet lipid rafts by disrupting acyl chain packing and molecular order within rafts. Ultimately, the disruption in raft organization has consequences for protein clustering and thereby signaling. Overall, elucidating the complex mechanisms by which n-3 PUFA acyl chains reorganize membrane architecture will enhance the translation of these fatty acids into the clinic for treating several diseases.
Consumption of Fish Oil Has Health Benefits
Dietary consumption of fish oil is increasingly recognized to have beneficial health effects, especially for the prevention or treatment of specific diseases. As examples, fish oil intake is associated with decreased risk for coronary heart disease and prescription fish oil supplements lower serum triglycerides(1, 2). There is also emerging evidence that fish oil has immunosuppressive properties, which may have clinical applications for the treatment of symptoms associated with autoimmune and inflammatory diseases(3). Finally, there is some suggestion that fish oil may lower the risk of cognitive disorders, prevent the progression of specific cancers, and improve insulin sensitivity(4, 5).
One major obstacle in effectively translating fish oil into the clinic for the treatment of some of the aforementioned afflictions is a limited understanding of its molecular mechanisms. The bioactive components of fish oil are the n-3 polyunsaturated fatty acids (PUFA) eicosapentaenoic (EPA, 20:5) and docosahexaenoic (DHA, 22:6) acids. Mechanistically, these fatty acids modify gene expression, give rise to bioactive oxygenated metabolites known as resolvins and protectins, disrupt cellular signaling, protein trafficking and modify plasma membrane domains(6, 7). This review focuses on recent advances in n-3 PUFA and plasma membrane lipid raft domains.
Lipid Rafts Are a Molecular Target of N-3 PUFAs
Lipid rafts are operationally defined as sphingolipid/cholesterol enriched domains that under specific circumstances serve to compartmentalize signaling(8). Since the inception of the lipid raft model, there has been considerable debate on their existence, composition, size, and lifetime. Much of the controversy arose from the use of indirect methods to study rafts; predominately, the use of biochemical detergent extraction, which has utility as a predictive tool but can also introduce artifacts(9). Recent advances in lipidomics and high-resolution imaging, which resolves domain sizes below the diffraction limit of a microscope, have provided stronger evidence for the existence of lipid rafts(10).
The current model proposes lipid rafts are fluctuating assemblies that exist as nanometer sized domains(10). In response to stimuli such as a ligand binding to its receptor, the nanometer scale domains coalesce. The coalesced domains are larger in size, up to the micrometer size scale, and display high molecular order relative to the surrounding non-rafts(10). The formation of these ordered domains is driven by favorable molecular interactions between sphingolipids and cholesterol and is regulated by the underlying actin cytoskeleton. Lipid raft domains are not limited to the plasma membrane; for instance, they exist in intracellular organelles such as the Golgi where they regulate protein trafficking(10). Their existence is questionable in other endomembranes; for instance, mitochondrial membranes, which have low levels of cholesterol, are unlikely to form rafts(11).
A key component of the lipid raft model is that coalescence of nanometer scale assemblies, driven by both lipid-lipid and lipid-protein interactions, serve to enhance or optimize the function of specific plasma membrane proteins(10). Compartmentalization of signaling is central to the lipid raft concept, and is a target of n-3 PUFAs(12).
Biophysical Studies On An Atomic Scale Show N-3 PUFAs Adopt Unique Molecular Orientations That Do Not Pack Efficiently With Raft Molecules
Some of our understanding of how n-3 PUFAs from fish oil impact the physical organization of membranes comes from biophysical experiments using model membranes (e.g. lipid vesicles, supported bilayers, monolayers) and molecular dynamic (MD) simulations(13). The advantage of model membrane and MD simulation studies is they provide a highly controlled environment for investigating the dynamic nature of n-3 PUFA acyl chains in the presence of sphingolipids and/or cholesterol. Of course, this approach has a major limitation, that is, they are not an accurate depiction of the plasma membrane that contains hundreds of different lipid species and proteins. Nevertheless, these studies provide mechanistic details on an atomic scale that are not readily discerned from cellular studies.
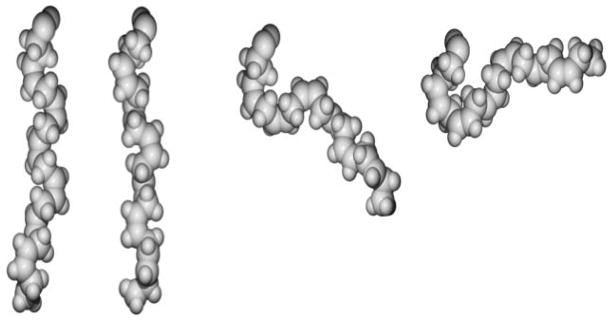
The majority of work on n-3 PUFAs in model membranes has focused on DHA(14). The molecular structure of DHA is unique. It is somewhat polar, due to the presence of six double bonds, and is highly flexible with rapid reorientations through multiple conformational states(15). A combination of NMR, neutron diffraction and MD simulations show the DHA acyl chain can be displaced toward the headgroup region, with the terminal methyl at times approaching the water interface (Figure 1)(16–19). Addition of cholesterol can partially redistribute the DHA acyl chain toward the bilayer center but the ordering effect of cholesterol is still more pronounced when cholesterol interacts with saturated acyl chains(20).
Figure 1. DHA acyl chains adopt unique conformations.
The different conformational states of DHA were obtained with molecular dynamic simulations. The images show the DHA acyl chain is highly flexible and the terminal methyl can even approach the headgroup region. This degree of flexibility likely contributes to its ability to disrupt lipid raft molecular organization. The figure was obtained with permission from the Feller lab.
The flexible and therefore disordered structure of DHA renders it incompatible with surrounding ordered saturated acyl chains and cholesterol. Rosetti and Pastorino very recently reported with MD simulations that DHA acyl chains do not pack efficiently with saturated acyl chains(21). Similarly, we reported, in collaboration with others, that DHA-containing phospholipids did not mix ideally with sphingolipids with predominately saturated acyl chains in the absence of cholesterol(22).
The Stillwell and Wassall labs over the past decade have demonstrated a packing inability between saturated acyl chains and DHA, which was further enhanced in the presence of cholesterol(14). Spectroscopic and calorimetric studies from their labs established DHA-containing phosphatidylcholines and phosphatidylethanolamines have a low affinity for cholesterol in the presence of sphingomyelin(23, 24). Their NMR data suggest DHA-containing phospholipids are forming their own organizationally distinct domains (<20 nm in size) in the presence of sphingolipids and cholesterol as a mechanism to avoid saturated acyl chains and cholesterol(23). Lately, novel data from the Katsaras and Wassall labs imply the presence of select PUFAs in the membrane can force cholesterol to adopt unusual orientations in the membrane; for instance, forcing the polar hydroxyl group of cholesterol to be buried into the interior of the bilayer(25, 26).
Overall, model membrane and MD simulation data suggest that when disordered DHA acyl chains incorporate into the plasma membrane of cells, they do not pack efficiently with saturated acyl chains or cholesterol, both of which are essential for the formation of lipid rafts.
Biochemical Studies In Vitro and Ex Vivo Demonstrate N-3 PUFAs Disrupt Detergent Resistant “Rafts” and Associated Protein Distribution
The initial observation of how n-3 PUFAs disrupted lipid domain organization in cells came from studies using biochemical detergent extraction(27–29). For instance, the Stulnig lab showed treatment of T cells with n-3 PUFAs modified detergent resistant membranes (DRM), which are a crude cellular fraction that supposedly represent cellular “lipid rafts” (we use quotes to remind the reader that DRMs are not the same as lipid rafts)(27). A significant proportion of n-3 PUFAs were localized into DRMs with the remaining portion in “non-raft” detergent soluble membranes (DSMs). The intriguing aspect of this work was proteins localized in DRMs were displaced in response to n-3 PUFA treatment.
More recent in vitro and ex vivo measurements in several cell types have verified the basic hypothesis that n-3 PUFAs incorporate directly into DRMs to displace proteins either into or out of these “raft”-like fractions(30–36). In some cell types, n-3 PUFAs lower the levels of cholesterol and sphingolipids in the DRMs(31). Grimm et al., recently demonstrated that treatment of SH-SY5Y cells with DHA displaced cholesterol from DRMs to DSMs(37). We and others have also observed a trend for cholesterol to move from DRMs to DSMs with DHA treatment (29, 38, 39). Thus, DHA and perhaps EPA, may be promoting the displacement of cholesterol from DRMs to DSMs, which is consistent with NMR and X-ray diffraction data to show DHA has a low affinity for cholesterol(14, 40). Once the cholesterol is displaced toward non-rafts, it is likely to associated with saturated acyl chains.
Several questions arise from the biochemical detergent extraction experiments: 1) How does one effectively connect findings from different model systems: i.e. model membranes, in vitro, ex vivo data? 2) Are n-3 PUFAs exerting their effects by directly incorporating into lipid rafts? Alternatively, are n-3 PUFAs exerting their effects indirectly on rafts (e.g. by targeting the cytoskeleton, non-raft domains, etc.)?3) What is the molecular nature of lipid rafts upon incorporation of n-3 PUFAs into the raft? In other words, are these domains still ordered rafts or are they simply disrupted rafts? 4) Where are n-3 PUFA acyl chains localizing to (e.g. what lipid species, which membrane leaflet, etc?). To address these questions, sophisticated imaging and lipidomic methods are starting to provide some insights.
Quantitative Imaging and Lipidomics Reveal N-3 PUFAs Disrupt Raft Composition and Molecular Order to Alter Protein Distribution
Recently, several labs, including our own, have corroborated biochemical methods with quantitative imaging methods to address some of the aforementioned questions on how n-3 PUFAs disrupt lipid rafts. We very recently reported using a combination of confocal and FRET imaging to show that in vitro and ex vivo, n-3 PUFAs selectively disrupted the clustering of lipid rafts of EL4 and B cells(38, 41). However, n-3 PUFAs had no impact on non-raft organization under physiologically relevant conditions(41). Our model system addressed raft formation in the outer leaflet of the plasma membrane since we measured cholera toxin binding to GM1 molecules. Our data were in agreement with an electron microscopy study in vitro with HeLa cells, which showed DHA treatment selectively disrupted lipid raft, but not non-raft, domains of the inner leaflet(42). Thus, recent data suggest that n-3 PUFAs are selectively disrupting lipid rafts of both the outer and inner leaflets with little impact on non-raft organization.
If n-3 PUFAs directly incorporate into lipid rafts, then what is the nature of the lipid raft domains? Given the steric incompatibility between DHA acyl chains and lipid raft molecules, one would predict that incorporation of n-3 PUFA acyl chains in to rafts would disrupt their molecular order. Two different labs have attempted to address this issue, which has yielded opposite results.
The Chapkin lab showed incorporation of n-3 PUFAs into the immunological synapse of CD4+ T cells from the fat-1 transgenic mouse increased the molecular order of lipid rafts, as measured with fluorescence polarization microscopy(43). In contrast, the Harder lab demonstrated, also with fluorescence polarization imaging, that EPA treatment of Jurkat T cells in vitro decreased the molecular order of lipid rafts in the synapse(44). Lipidomics in the same study revealed significant incorporation of EPA into differing phospholipids with an increase in a select sphingolipid species(44). One reason for the discrepancy was differential uptake of fatty acids between in vitro and ex vivo studies(45). Furthermore, in vitro studies only provided a single fatty acid whereas both EPA and DHA accumulated in T cells from the fat-1 transgenic mouse. Clearly, more studies are needed to resolve how n-3 PUFAs are modifying the lipidome and molecular order of rafts.
The consequences of disrupting lipid rafts on protein lateral organization are also emerging. Recent imaging studies are overall confirming the biochemical observations that n-3 PUFAs displace proteins into or out of rafts. For instance, we discovered that DHA, upon disrupting lipid raft clustering, forced the non-raft immunological protein, MHC class I, into lipid rafts(38). We also recently observed with FRET imaging that DHA and EPA could modify MHC I spatial organization on a nanometer scale, which was driven by changes in plasma membrane size(41). Similarly, several other labs have shown with imaging or biochemical approaches that protein-protein interactions, which are central to generate intracellular signaling, are modified with dietary n-3 PUFAs. For instance, proteins essential for the activation of immune cells or growth of differing cell types are modified by n-3 PUFAs, which leads to changes in downstream signaling, gene expression, and ultimately function(31, 33–36, 43, 46).
Integrated Model
In Figure 2, we present a model on how n-3 PUFAs could disrupt lipid raft organization and thereby protein lateral distribution. The model is based on data from our lab and others from several model systems. The model shows, as an example, MHC class I proteins localized to non-rafts. When n-3 PUFA acyl chains incorporate into the raft (possibly as nanodomains as suggested by recent NMR data in model membranes), the raft becomes de-clustered. The poor affinity between n-3 PUFAs and cholesterol forces some of the cholesterol molecules to move out of the raft into non-rafts, which contributes to the de-clustering. The de-clustering event allows a protein such as MHC class I to now move into the disrupted raft. The model is also applicable to proteins localized within the lipid rafts. In this case, incorporation of n-3 PUFA acyl chains into lipid rafts and subsequent de-clustering will force raft-localized proteins from rafts into non-rafts.
Figure 2. Proposed model on how n-3 PUFA acyl chains disrupt the molecular organization of membrane lipid rafts and proteins.
The model shows n-3 PUFAs de-clustering rafts by directly incorporating into the raft and then redistributing cholesterol toward non-rafts. As a consequence, a non-raft protein such as MHC class I is forced into the lipid raft. Similarly, raft proteins are likely de-clustered and forced into non-rafts. The order of events is shown for simplicity and may occur simultaneously.
Future Directions
Determining the mechanism by which n-3 PUFA acyl chains disrupt lipid raft spatial and temporal organization is in its infancy. As listed above, there are several questions that arise from the biochemical studies that are currently being addressed. Furthermore, there are many other questions that require attention that are central to developing a complete mechanism by which n-3 PUFAs disrupt raft molecular organization. For instance, do EPA and DHA exert differential effects on membrane raft organization? Our quantitative studies in vitro with EL4 cells showed that DHA, but not EPA, disrupted the clustering of lipid rafts(38). However, are data were not in agreement with another lab that showed both EPA and DHA disrupted lipid raft spatial distribution(30). More studies are needed to understand what are the molecular differences, especially at an atomic scale, between EPA and DHA. As another example, how do EPA and DHA modify the plasma membrane lipidome? Recent advances have been with cell culture experiments(44), but studies at the animal and human level are needed. Finally, it is essential to determine how changes in lipid raft organization with n-3 PUFAs impact not just downstream signaling and gene expression but the impact on function at the tissue and whole animal level.
Conclusion
The goal of this review was to highlight recent mechanistic advances in biophysical and biochemical studies with n-3 PUFAs and lipid rafts. As the field is rapidly evolving, some central themes are emerging. DHA, and perhaps EPA, acyl chains are highly disordered which renders them incompatible with packed lipid raft molecules. As a consequence, when lipid rafts are exposed to n-3 PUFAs, their molecular composition and order is changed, which has consequences for protein clustering and ultimately cellular function.
Acknowledgments
This work was supported by a grant from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (R15AT006122) (to S.R.S).
We thank Dr. Scott Feller of Wabash University for providing the MD simulation results shown in Figure 1 and Benjamin Drew Rockett for his assistance in designing the molecular model shown in Figure 2.
Footnotes
There are no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth EM, Harris WS. Fish oil for primary and secondary prevention of coronary heart disease. Curr Atheroscler Rep. 2010;12:66–72. doi: 10.1007/s11883-009-0079-6. [DOI] [PubMed] [Google Scholar]
- 2.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, Ballantyne CM, Ginsberg HN. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hyper triglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–67. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann Nutr Metab. 2009;55:123–39. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 4.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–19. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opion Clin Nutr Metab Care. 2009;12:138–46. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 6.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leuk Essent Fatty Acid. 2009;81:187–91. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am J Clin Nutr. 2006;84:1277–89. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- 8.Pike LJ. Rafts defined: A report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protocols. 2007;2:2159–65. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 10.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 11.Zheng YZ, Berg KB, Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J Lipid Res. 2009;50:988–98. doi: 10.1194/jlr.M800658-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaqoob P, Shaikh SR. The nutritional and clinical significance of lipid rafts. Curr Opin Clin Nutr Metab Care. 2010;13:156–66. doi: 10.1097/MCO.0b013e328335725b. [DOI] [PubMed] [Google Scholar]
- 13.Stillwell W, Wassall SR. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 14.Wassall SR, Stillwell W. Polyunsaturated fatty acid–cholesterol interactions: Domain formation in membranes. Biochim Biophys Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Wassall SR, Stillwell W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Mihailescu M, Gawrisch K. The structure of polyunsaturated lipid bilayers important for rhodopsin function: A neutron diffraction study. Biophys J. 2006;90:L04–6. doi: 10.1529/biophysj.105.071712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feller SE, Gawrisch K. Properties of docosahexaenoic-acid-containing lipids and their influence on the function of rhodopsin. Curr Opin Struct Biol. 2005;15:416–22. doi: 10.1016/j.sbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J Am Chem Soc. 2003;125:6409–21. doi: 10.1021/ja029029o. [DOI] [PubMed] [Google Scholar]
- 19.Feller SE, Gawrisch K, MacKerell AD. Polyunsaturated fatty acids in lipid bilayers: Intrinsic and environmental contributions to their unique physical properties. J Am Chem Soc. 2002;124:318–26. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- 20.Mihailescu M, Soubias O, Worcester D, White SH, Gawrisch K. Structure and dynamics of cholesterol-containing polyunsaturated lipid membranes studied by neutron diffraction and NMR. J Membr Biol. 2011;239:63–71. doi: 10.1007/s00232-010-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosetti C, Pastorino C. Polyunsaturated and saturated phospholipids in mixed bilayers: A study from the molecular scale to the lateral lipid organization. J Phys Chem B. 2011;115:1002–13. doi: 10.1021/jp1082888. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh SR, LoCascio DS, Soni SP, Wassall SR, Stillwell W. Oleic- and docosahexaenoic acid-containing phosphatidylethanolamines differentially phase separate from sphingomyelin. Biochim Biophys Acta. 2009;1788:2421–6. doi: 10.1016/j.bbamem.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soni SP, LoCascio DS, Liu Y, Williams JA, Bittman R, Stillwell W, Wassall SR. Docosahexaenoic acid enhances segregation of lipids between raft and nonraft domains: 2H-NMR study. Biophys J. 2008;95:203–14. doi: 10.1529/biophysj.107.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh SR, Dumaual AC, Castillo A, LoCascio D, Siddiqui RA, Stillwell W, Wassall SR. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: A comparative NMR, DSC, AFM, and detergent extraction study. Biophys J. 2004;87:1752–66. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucerka N, Marquardt D, Harroun TA, Nieh MP, Wassall SR, de Jong DH, Schafer LV, Marrink SJ, Katsaras J. Cholesterol in bilayers with PUFA chains: Doping with DMPC or POPC results in sterol reorientation and membrane-domain formation. Biochemistry. 2010;49:7485–93. doi: 10.1021/bi100891z. [DOI] [PubMed] [Google Scholar]
- 26.Harroun TA, Katsaras J, Wassall SR. Cholesterol is found to reside in the center of a polyunsaturated lipid membrane. Biochemistry. 2008;47:7090–6. doi: 10.1021/bi800123b. [DOI] [PubMed] [Google Scholar]
- 27.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–40. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 28.Ma DWL, Seo J, Davidson LA, Callaway ES, Fan Y, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–2. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133(6):1913–20. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 30.Altenburg JD, Siddiqui RA. Omega-3 polyunsaturated fatty acids down-modulate CXCR4 expression and function in MDA-MB-231 breast cancer cells. Mol Cancer Res. 2009;7:1013–20. doi: 10.1158/1541-7786.MCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 31.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–53. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 32.Duraisamy Y, Lambert D, O’Neill CA, Padfield PJ. Differential incorporation of docosahexaenoic acid into distinct cholesterol-rich membrane raft domains. Biochem Biophys Res Comm. 2007;360:885–90. doi: 10.1016/j.bbrc.2007.06.152. [DOI] [PubMed] [Google Scholar]
- 33.Langelier B, Linard A, Bordat C, Lavialle M, Heberden C. Long chain-polyunsaturated fatty acids modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures. J Cell Biochem. 2010;110:1356–64. doi: 10.1002/jcb.22652. [DOI] [PubMed] [Google Scholar]
- 34.Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, Pardini RS. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–30. doi: 10.1093/carcin/bgq111. [DOI] [PubMed] [Google Scholar]
- 35.Wong SW, Kwon M, Choi AMK, Kim H, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284(40):27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye S, Tan L, Ma J, Shi Q, Li J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins Leukot Essent Fatty Acids. 2010;83:37–43. doi: 10.1016/j.plefa.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Grimm MOW, Kuchenbecker J, Groesgen S, Burg VK, Hundsdoerfer B, Rothhaar TL, Friess P, de Wilde MC, Broersen LM, et al. Docosahexaenoic acid reduces amyloid β production via multiple, pleiotropic mechanism. J Biol Chem. 2011;286:14028–39. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 39.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–60. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 40.Shaikh SR, Cherezov V, Caffrey M, Soni SP, LoCascio D, Stillwell W, Wassall SR. Molecular organization of cholesterol in unsaturated phosphatidylethanolamines: X-ray diffraction and solid state 2H NMR reveal differences with phosphatidylcholines. J Am Chem Soc. 2006;128:5375–83. doi: 10.1021/ja057949b. [DOI] [PubMed] [Google Scholar]
- 41.Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonrafts of EL4 and B cells. J Nutr. 2011;141:1041–8. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapkin RS, Wang N, Fan Y, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–71. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–76. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010;49:250–61. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonilla DL, Ly LH, Fan YY, Chapkin RS, McMurray DN. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to mycobacterium tuberculosis. PLoS One. 2010;5:e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]