Abstract
Our ability to reason by analogy facilitates problem solving and allows us to communicate ideas efficiently. In this study, we examined the neural correlates of analogical reasoning and, more specifically, the contribution of rostrolateral prefrontal cortex (RLPFC) to reasoning. This area of the brain has been hypothesized to integrate relational information, as in analogy, or the outcomes of subgoals, as in multi-tasking and complex problem solving. Using fMRI, we compared visuospatial analogical reasoning to a control task that was as complex and difficult as the analogies and required the coordination of subgoals but not the integration of relations. We found that analogical reasoning more strongly activated bilateral RLPFC, suggesting that anterior prefrontal cortex is preferentially recruited by the integration of relational knowledge. Consistent with the need for inhibition during analogy, bilateral, and particularly right, inferior frontal gyri were also more active during analogy. Finally, greater activity in bilateral inferior parietal cortex during the analogy task is consistent with recent evidence for the neural basis of spatial relation knowledge. Together, these findings indicate that a network of frontoparietal areas underlies analogical reasoning; we also suggest that hemispheric differences may emerge depending on the visuospatial or verbal/semantic nature of the analogies.
Keywords: analogical reasoning, relational integration, rostrolateral prefrontal cortex, inferior frontal gyrus, spatial relations
1. Introduction
The ability to reason by analogy has been claimed to be at the “core of cognition” (Hofstadter 2000). Two objects, concepts, or even real-world situations are analogous if they are dissimilar on the surface but share some higher-order, structural similarities. For instance, consider this analogy often attributed to Einstein: “You see, wire telegraph is a kind of a very, very long cat. You pull his tail in New York, and his head is meowing in Los Angeles.” Superficially, a telegraph and a cat have little in common, and yet this analogy conveys an important point about the way in which a local action can have long-distance consequences. Similarly, analogies are used frequently and naturally to communicate ideas in a wide-variety of domains (Holyoak and Thagard 1995), including science (Dunbar 1995) and politics (Blanchette and Dunbar 2001), and their use promotes learning in education (Loewenstein et al. 1999) and rehabilitation (Wai-Kwong Man et al. 2006).
Perhaps because of analogy’s critical role in human cognition (and some primates, Gillan et al. 1981; Flemming et al. 2008), analogical reasoning has been the focus of many behavioral and computational investigations in the past three decades. However, not much is known about analogy’s neural basis or the way in which cognitive accounts of analogy map onto neural dynamics. Of the functional magnetic resonance imaging (fMRI) studies that have examined analogical reasoning, most point to the rostrolateral prefrontal cortex (RLPFC) as playing a pivotal role in mediating analogy. For instance, activity in RLPFC is sensitive to the number of relations, or the relationships between components of an analogy, that need to be considered (Christoff et al. 2001; Kroger et al. 2002; Cho et al. 2010; Krawczyk et al. 2011). The degree to which an analogy’s components are dissimilar on the surface also modulates RLPFC activation (Green et al. 2010; Hampshire et al. 2011). For instance, cross-domain analogies (e.g., nose:scent::antenna:signal) elicit greater frontopolar activity than within-domain analogies (e.g., nose:scent::tongue:taste) (Green et al. 2010). The development of analogical reasoning ability in children is also accompanied by changes in the way in which RLPFC is engaged (Wright et al. 2008; Crone et al. 2009; see also Dumontheil et al. 2008), and damage to prefrontal cortex (PFC) is associated with impaired performance on analogies (Morrison et al. 2004; Krawczyk et al. 2008).
The recruitment of RLPFC during analogical (and other types of relational) reasoning has been hypothesized to reflect the need to simultaneously compare and integrate multiple relations between things – “relational integration” (Christoff et al. 2001; Kroger et al. 2002; Bunge et al. 2005; Wendelken et al. 2008; Bunge et al. 2009). For example, determining whether or not two pairs of words are analogous to each other (e.g., bouquet:flower::chain:link?), a task that requires relational integration, more strongly recruits left RLPFC than determining whether or not the words in each pair are semantically related, a task that does not require relational integration (Bunge et al. 2009). Successful analogy-making requires comparing and evaluating relational similarities between concepts (Gentner 1983, 2003; Holyoak and Thagard 1997; Holyoak 2005), and the need for relational integration distinguishes analogical reasoning from other complex cognitive skills.
However, anterior PFC activity is also observed during a wide range of non-analogical cognitive tasks, including episodic memory retrieval (Ranganath et al. 2000, 2003), prospective memory (Burgess et al. 2001; Simons et al. 2006), and multi-tasking (Koechlin et al. 1999; Braver and Bongiolatti, 2002; Dreher et al. 2008). This domain-general involvement of anterior PFC has led to comprehensive theories of its function. On several accounts, this area of the brain coordinates the outcomes of subgoals in the service of fulfilling a main goal (Koechlin et al. 1999; Braver and Bongiolatti 2002; Ramnani and Owen 2004; Reynolds et al. 2006; De Pisapia and Braver 2008). For instance, greater frontopolar activity was observed when participants detected that any concrete word followed any abstract word in a stream of words relative to detecting that a specific concrete word (“lime”) followed a specific abstract word (“fate”) (Braver and Bongiolatti 2002). In this way, the task that elicited greater frontopolar activity required participants to semantically classify the probe words (subgoal tasks) and integrate their outcomes with the context (concrete follows abstract) to make a response. On this view, integrating the relations between entities is just a special case of coordinating subgoals, and even non-analogical reasoning tasks may require “the integration of the results of two or more separate cognitive operations” (Ramnani and Owen 2004, p. 190). Thus, a comparison of analogy to another complex task that requires the coordination of subgoals but not the integration of relations would test the specificity of RLPFC for relational integration.
A challenge for any neuroimaging study of analogy is the difficulty of analogical reasoning relative to most other tasks – and the observation that the tasks that engage RLPFC are frequently the most difficult to solve (Velanova et al. 2003; Braver et al. 2003). Among neuroimaging studies of analogical reasoning, analogies are often solved less accurately and more slowly than the non-analogical task to which they are compared (Prabhakaran et al. 1997; Wharton et al. 2000; Luo et al. 2003; Bunge et al. 2005; Bunge et al. 2009; Volle et al. 2010; but see Krawczyk et al. 2010, 2011). A non-analogical task as difficult as analogy natively is needed to more precisely investigate the neural basis of analogical reasoning.
In the current study, we investigated the neural correlates of analogical reasoning relative to a task that, while necessitating the coordination of subgoals, did not require integration of relations. This comparison task was both similarly difficult and perceptually-matched to the analogy task. To create our stimuli, we drew inspiration from developmental studies of analogical reasoning (e.g., Kotovsky and Gentner 1996) and used sets of colored shapes as the basis for our reasoning tasks (Figure 1). In the “analogy” condition, the correct answer is arrived at by considering the spatial relations between the colored shapes in the source set and selecting the response with the same set of relations. In the “item” condition, however, the relationships between the colored shapes are not relevant: the correct answer is arrived at by selecting the choice that contains the same three colored shapes as the source, in any order. In this way, both reasoning tasks required evaluation of multiple subgoals to select an answer. Only the analogy condition, however, requires an integration of relations, in particular (here, relations in space).
Figure 1.
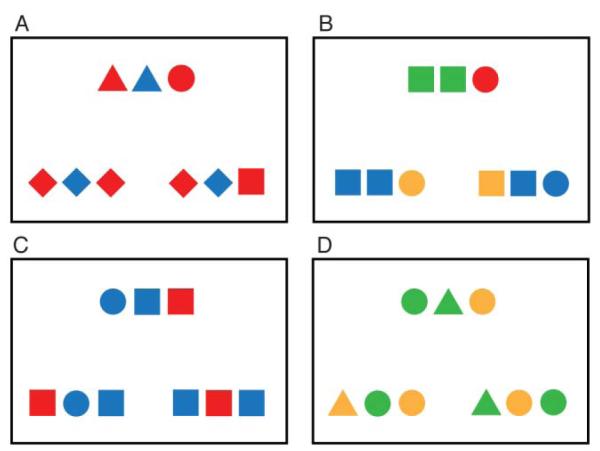
Stimuli from the analogy and item tasks. A) An analogy trial on which the critical relations between source and correct answer are between shapes. B) An analogy trial on which the critical relations between source and correct answer are between colors. C) An item trial on which the correct answer and foil differ only in shape. D) An item trial on which the correct answer and foil differ only in color.
If RLPFC is more strongly driven by relational integration, we expect greater activity in this region during the analogy condition. However, if this area responds to the coordination of subgoals, more generally, we expect no differences in this area between conditions. In fact, because the item condition was slightly more difficult than the analogy condition for participants in the scanner (see Results), a degree of difficulty account would predict greater activity in RLPFC during the item condition.
Of course, relational integration is only one part of the analogical reasoning process; many accounts of analogy also include inhibition as a crucial component (Robin and Holyoak 1995; Morrison et al. 2004; Viskontas et al. 2004; Krawczyk et al. 2008). Inhibitory control is necessary to minimize interference from responses that are based on superficial (e.g., semantic or perceptual) similarity rather than deeper, relational similarity. As a result, patients with impaired inhibitory processes as a result of frontotemporal lobar degeneration show deficits on relational reasoning tasks (Morrison et al. 2004; Krawczyk et al. 2008). Similarly, several fMRI studies have found greater activity within inferior frontal gyrus (IFG) during analogy (Christoff et al. 2001; Luo et al. 2003; Green et al. 2006; Cho et al. 2010; Green et al. 2010; Krawczyk et al. 2011; Volle et al. 2010), an area of the brain that, in the right hemisphere, also participates in response inhibition (e.g., Aron et al. 2003; for a review, see Aron et al. 2004) and, in the left hemisphere, in selecting among competing semantic representations in memory (e.g., Thompson-Schill et al. 1997; Novick et al. 2005; Moss et al. 2005). In the current task, responses in the analogy condition were based on relational rather than perceptual similarities. On the assumption that participants’ dominant, prepotent responses are driven by perceptual cues, we predicted greater activity in IFG, particularly in the right hemisphere, during the analogy task compared to the “item” task.
In addition to this network of frontal regions, posterior areas of the brain that mediate the visuospatial (or semantic) information upon which analogies are based are also recruited during analogical reasoning (Krawczyk 2010). In the present task, analogies are solved based on the spatial relationships between colored shapes. A recent fMRI study from our lab of the neural basis of categorical spatial relations like “in” and “above” found that attending to spatial relations produced greater activity in bilateral superior and inferior parietal cortices and middle frontal gyrus relative to attending to the identity of objects (Amorapanth et al. 2010). Several neuroimaging studies of visuospatial analogies have also implicated parietal cortex (Wharton et al. 2000; Geake and Hansen 2005; Crone et al. 2009; Wartenburger et al. 2009; Cho et al. 2010; Preusse et al. 2010; Volle et al. 2010). Therefore, we predicted greater activation in this area during the analogy task relative to the item task, in which spatial relations were uninformative. By contrast, we predicted greater activation by the item task in areas involved in processing shape and color – surface, not relational, aspects of the stimuli. Amorapanth & colleagues (2010) found greater activity in left fusiform gyrus when participants attended to object identity relative to spatial relations, and fusiform gyri have also been implicated in visual semantic processing (Martin et al. 1995; Martin and Chao 2001; Simons et al. 2003). Although visual information like color and shape is relevant to both tasks at some point in the reasoning process, we speculated that heightened attention to structural features in the analogy condition might lessen sustained activity in areas responsible for processing visual information relative to the item condition.
2. Materials and Methods
2.1. Stimuli
Stimulus materials consisted of a visuospatial analogical reasoning task (“analogy” task), a visuospatial, non-analogical reasoning task (“item” task), and an identity matching task used as a baseline (“matching” task). In all three conditions, each trial was made up of a display of three sets of colored shapes. The source set, presented at the top of the screen, was designed to match one of the response sets at the bottom. The nature of this match was determined by the task at hand (Figure 1).
As described above, a correct response in the analogy condition was the set of colored shapes that contained the same pattern of spatial relations as the source. On any given trial, only one type of information, color (blue, green, red, yellow) or shape (circle, diamond, square, triangle), was relevant for selecting a response. When the basis of an analogy was the system of relations between shapes, all three sets contained the identical pattern of colors (Figure 1A). Conversely, when the basis of an analogy was relations between colors, all sets contained the identical pattern of shapes (Figure 1B). In the item condition, relations between colored shapes were not relevant for selecting a response. Instead, a correct response was the set of colored shapes that contained the same items as the source, in any order. Distinguishing correct and incorrect responses could be a difference in shape (Figure 1C) or a difference in color (Figure 1D). Finally, a correct response in the matching condition (not pictured) was the single colored shape that was perceptually identical to the single colored shape presented as the source. In all three tasks, the location of the correct answer (left or right) was randomly determined on each trial.
The item task was matched in difficulty and complexity to the analogy task, requiring subgoal coordination without the need to integrate spatial relations between items. We verified that this manipulation produced tasks of similar difficulty by collecting pilot reaction time and accuracy data from 22 participants at the University of Pennsylvania. Participants performed 100 analogy trials and 100 item trials, with no time limit required for a response. Trials of each type were presented in alternating blocks of 10 trials each. One subject was excluded from our analyses for having an average reaction time on all trials of greater than 2.5 standard deviations from the group mean. On the basis of this pilot data, we determined the average accuracy and reaction time for each stimulus item. We then used the SOS stimulus matching software (B Armstrong, CE Watson, DC Plaut, in preparation) to select 72 analogy trials and 72 item trials that differed non-significantly on accuracy [Manalogy = 97.88%, SD = 3.19, Mitem = 97.55%, SD 3.57, t(142) = .59, p = .56] and reaction time [Manalogy = 2517.64, SD = 454.80, Mitem = 2509.98, SD = 499.00, t(142) = .10, p = .92].
2.2. Participants
We recruited 23 participants from the University of Pennsylvania (Mage = 22.80 years, range: 18 – 27). Fourteen participants were female, and all were right-handed, native speakers of English with corrected-to-normal vision. All participants gave informed consent in accordance with the procedures of the University of Pennsylvania and were paid $20 for their participation. Prior to entering the scanner, each participant completed approximately 5 minutes of practice trials of each type (analogy, item, and matching).
2.3. Behavioral Task
In the scanner, participants completed 216 individual trials: 72 analogy trials, 72 item trials, and 72 matching trials. Responses and reaction times were recorded via a button box held with both hands. On each trial, the correct answer was selected by pressing a button on the left or right side with the left or right thumb, respectively. All stimuli were presented on a black background.
Trials were presented in four separate scanning runs. During a given run, trials were blocked by task type. Each block consisted of 6 trials, and participants had 5s to respond to each trial. If no response was recorded after 5 seconds, the experiment advanced to the next trial. Trials were separated by a 500ms fixation cross. Additionally, each block began with a 5s instruction screen alerting participants to the nature of the upcoming trials; trials in the analogy condition, for instance, were preceded by a screen with the words “Analogy Trials”. Prior to entering the scanner, participants were familiarized with the names of each condition.
In total, each block of trials lasted 38s. Each of four scanning runs contained 3 blocks of each condition (9 blocks total). The order in which blocks were presented cycled between conditions so that all three conditions were seen every within every 3 blocks (e.g., analogy-item-matching-analogy-item-matching...). This constraint allowed for 6 possible block sequences. We counterbalanced the occurrence of each sequence across participants, but the location of an individual trial was randomly determined for every participant. Including time spent collecting both structural and functional MRI data (and resting state activity not reported here), the entire scanning session lasted approximately 40 minutes.
2.4. MRI Data Acquisition
Structural and functional data were collected on a 3.0 Tesla Siemens Trio scanner with an eight-channel head coil. High-resolution T1-weighted structural images were collected for each participant using a 3-D MPRAGE pulse sequence (TR = 1620 ms, TE = 3.87 ms, TI = 950 ms, flip angle = 15°). Each volume consisted of 160 axial slices and near isotropic voxels (0.9766mm × 0.9766mm × 1.0000mm). Functional volumes were acquired using a BOLD EPI sequence (TR = 3000ms, TE = 30ms, flip angle = 90°). Each volume consisted of 50 interleaved axial slices approximately 3mm thick. During each of four scanning runs, 118 functional volumes were acquired, for a total of 472 volumes. The first three volumes of each run consisted of fixation screens and were discarded to allow for steady state magnetization.
2.5. Image Processing
Data analysis was performed using VoxBo (www.voxbo.org) and SPM2 (www.fil.ion.ucl.ac.uk/spm). Data preprocessing procedures included slice timing correction by sinc interpolation, and standard alignment, smoothing, and normalization procedures. Each participant’s structural data were normalized to the standard MNI template using the SPM2 normalization routine. Functional BOLD data were then realigned with the participant’s first functional volume using rigid body alignment. Normalization of the functional data was accomplished by using the parameters generated by SPM2 during normalization of the participant’s structural data. Functional data were smoothed with a 3 × 3 × 3mm full width at half maximum (FWHM) Gaussian smoothing kernel.
2.6. Data Analysis
2.6.1. First-level analyses
We used the modified general linear model (Worsley and Friston 1995) to analyze BOLD data from each subject as a function of condition. Task covariates were boxcar waveforms convolved with an estimate of the BOLD hemodynamic response function empirically derived from motor cortex in a large group of subjects (Aguirre et al. 1998). Nuisance covariates for effects of scan and six types of rigid-body motion were also included. Because reaction time differences between analogy and item conditions emerged in the scanner (see Results), reaction time was included as a covariate of non-interest and convolved with an estimate of the BOLD hemodynamic response function (Aguirre et al. 1998). Time series data were subjected to a high-pass filter.
2.6.2. Second-level analyses
We evaluated the effect of condition on BOLD signal differences with second-level, random effects analyses performed on beta values obtained from the first-level analyses. To do so, we used a multiple regression analysis with subject as a random effect. To derive group activation maps for whole-brain analyses, the false positive rate was controlled by performing 2000 Monte-Carlo permutation tests on the data (Nichols and Holmes 2002). The result was a t-statistic threshold and minimum cluster size of 20 voxels that corrected for multiple comparisons at p < .05. For region-of-interest (ROI) analyses, we calculated beta values for each ROI from a single, spatially averaged time series separately for each participant. Paired or one-sample t-tests were then used to determine if a given contrast was consistently different between hemispheres or consistently greater than zero, respectively, across all participants.
2.6.3. Regions-of-interest
We defined ROIs in two different ways. First, left and right RLPFC ROIs were determined by averaging the peak voxels that had been reported in these regions in previous fMRI studies of analogical reasoning. For the average in each hemisphere, we only considered peaks that emerged from corrected, whole-brain analyses. Two peaks were determined to be located within medial anterior PFC (Green et al. 2006; Green et al. 2010) and were excluded from the average given recent evidence that lateral and medial rostral PFC are cytoarchitectonically and functionally distinct (Koechlin et al. 2000; Burgess et al. 2003; Gilbert et al. 2006). Average coordinates were calculated separately for each hemisphere, with six studies reporting peaks in left RLPFC (Christoff et al. 2001; Bunge et al. 2005; Wendelken et al. 2008; Bunge et al. 2009; Volle et al. 2010; Hampshire et al. 2011) and two in right RLPFC (Wendelken et al. 2008; Bunge et al. 2009). In the left, the average was determined to be x = 42, y = 50, z = 6 (MNI coordinates). In the right, the average was determined to be x = −42, y = 44, z = 0 (MNI coordinates). ROIs were created by placing 10mm spheres around each point.
All other ROIs were defined anatomically using the Automated Anatomical Labeling (AAL) atlas of the MNI single-subject brain (Tzourio-Mazoyer et al. 2002). For each structure, separate ROIs were created for left and right hemispheres. ROIs consisted of: left and right inferior frontal gyri, inferior parietal cortex, superior parietal cortex, and fusiform gyri.
3. Results
3.1. Behavioral Results
By design, the matching task was much easier than the analogy and item task. Therefore, statistics were computed only on data from analogy and item tasks. Analogy (M = 95.69%, SD = .03) and item conditions (M = 94.79%, SD = .03) did not differ in accuracy [t(19) = 1.14, p = .27] (Figure 2). However, despite having been matched for accuracy and reaction time using pilot data, analogy trials (M = 2043.01ms, SD = 356.62) were completed more quickly than item trials (M = 2183.61, SD = 287.42) in the scanner [t(19) = 2.96, p < .01] (Figure 2). Because reaction time differences can produce activation differences between conditions, we included in our statistical model of BOLD data regressors to covary out the effects of reaction time (Kable et al. 2004).
Figure 2.
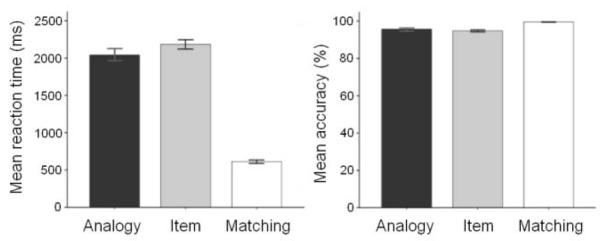
Mean reaction time (correct trials only) and accuracy by condition. Analogy trials are significantly faster than item trials [t(19) = 2.96, p < .01], but analogy and item trials do not differ on accuracy [t(19) = 1.14, p = .27].
3.2. Imaging Results
3.2.1. Whole-brain analyses (Table 1, Figure 3)
Table 1.
| Results for the whole-brain analysis for Analogy > Item contrast | ||||||
|---|---|---|---|---|---|---|
| Brain region | Hemisphere | Peak voxel t-value | Cluster size (voxels) | X | Y | Z |
| Inferior frontal gyrus (pars triangularis) |
R | 5.67* | 58 | 52 | 40 | 10 |
| Middle temporal gyrus | R | 4.33 | 34 | 64 | −47 | −5 |
| Inferior frontal gyrus (pars opercularis) |
R | 4.38 | 20 | 52 | 7 | 19 |
Note: peaks of activation exceeded p < .001 (uncorrected) threshold, with at least 20 contiguous voxels. Coordinates are reported in standardized MNI space. Peaks that survived correction for multiple comparisons (p < .05) are in boldface.
Significant at p < .05 level, corrected for multiple comparisons.
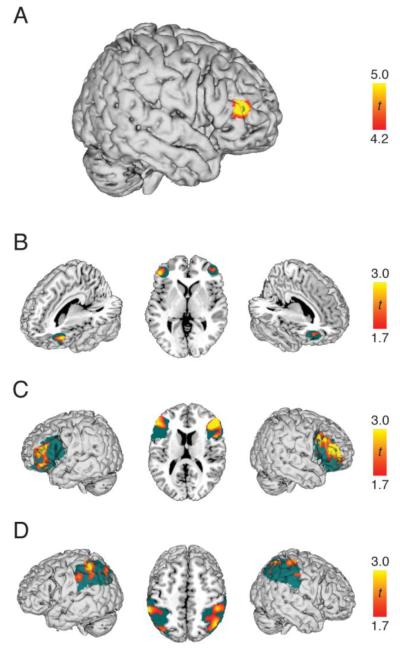
Figure 3.
A) Whole-brain activation map for regions that responded more strongly in the analogy task compared to the item task. Map has been thresholded at α = .05 (t = 4.22) corrected for multiple comparisons with at least 20 contiguous voxels. B) Right and left empirically-defined RLPFC ROIs for analogy > item contrast, both ps < .05. Although ROI statistics were computed on average activity, voxel-wise activation is depicted here at a level of p < .05 (uncorrected). The extent of the ROI is shown in green. C) Right and left IFG ROIs for analogy > item contrast, both ps < .05. D) Right and left inferior parietal ROIs for analogy > item contrast, both ps < .05.
First, we determined brain areas that were more active during the analogy condition than the item condition. This analysis resulted in a single cluster of 26 voxels within right inferior frontal gyrus (Figure 3) (BA 6/45, x = 52, y = 40, z = 10, MNI coordinates). For descriptive purposes, we also report all peaks of activation that exceeded p < .001, uncorrected, with a minimum cluster size of 20 (Table 1).
No brain areas were more active during the item condition relative to the analogy condition. Because participants responded significantly more slowly in the item condition than the analogy condition, we included reaction time as a covariate in our analyses to ensure that activation differences were not due to longer processing times. To determine if including reaction time as a covariate had, however, eliminated regions that responded more strongly to the item condition, we performed a subsequent analysis in which reaction time was not included as a covariate. Even so, the same pattern of results emerged for both analogy > item and item > analogy contrasts.
3.2.1. Anatomical regions-of-interest (Table 2, Figure 3)
Table 2.
| Results for the region-of-interest analysis for Analogy > Item contrast | |||
|---|---|---|---|
| Region of interest | Hemisphere | t-values | p-values (one-tailed) |
| RLPFC | L | 2.32 | .016 |
| R | 1.97 | .032 | |
| Inferior frontal gyrus | L | 2.03 | .029 |
| R | 2.66 | .008 | |
| Inferior parietal cortex | L | 1.81 | .043 |
| R | 1.81 | .043 | |
| Superior parietal cortex | L | .65 | .261 |
| R | .46 | .325 | |
| Results for the region-of-interest analysis for Item > Analogy contrast | |||
|---|---|---|---|
| Region of interest | Hemisphere | t-values | p-values (one-tailed) |
| Fusiform gyrus | L | −1.35 | .904 |
| R | −2.13 | .977 | |
Note: contrasts that are significantly different from zero (p < .05) are in boldface.
Within each ROI, we determined if a contrast was significantly greater than zero across participants using a one-sample t-test. In some cases, we also determined if a contrast was significantly greater in one hemisphere or the other using a paired-samples t-test. ROIs predicted to exhibit greater activation during the analogy condition relative to the item condition included left and right RLPFC, inferior frontal gyri, and inferior and superior parietal cortex. Within our empirically-defined RLPFC ROIs, both left [t(19) = 2.31, p < .05, one-tailed] and right [t(19) = 1.97, p < .05, one-tailed] ROIs exhibited greater activity during the analogy condition relative to the item condition (Figure 3B). The strength of RLPFC recruitment during analogy did not differ by hemisphere [t(19) = .30, p = .77].
Analogical reasoning also produced greater activation in right [t(19) = 2.66, p < .01, one-tailed] and left [t(19) = 2.03, p < .05, one-tailed] IFG (Figure 3C) and right [t(19) = 1.81, p < .05, one-tailed] and left [t(19) = 1.81, p < .05, one-tailed] inferior parietal cortex (Figure 3D). Greater activity in the analogy relative to the item condition was not observed in left [t(19) = .65, p = .26, one-tailed] or right [t(19) = .46, p = .33, one-tailed] superior parietal cortex. Additionally, none of the significant ROIs showed hemispheric differences: right versus left IFG [t(19) = .39, p = .70, one-tailed], right versus left inferior parietal cortex [t(19) = .18, p = .86, one-tailed].
Activity in fusiform gyrus, predicted to be greater during the item condition relative to the analogy condition, did not exhibit a stronger response to the item condition in either the left [t(19) = 1.34, p = .90, one-tailed] or right [t(19) = 2.13, p = .97, one-tailed] hemispheres.
4. Discussion
In the present study, we investigated the neural basis of analogical reasoning. To determine whether or not the integration of relations or the coordination of multiple subgoals more strongly engages RLPFC, we compared an analogy task to another reasoning task that did not require extracting relational information. We also predicted greater activity in inferior frontal gyrus during the analogy task given the need for inhibition during analogical reasoning (Robin and Holyoak 1995; Morrison et al. 2004). Additionally, the relevance of spatial relations in the analogy task led to a proposed role for parietal cortex in analogical relative to non-analogical reasoning; conversely, the need to attend to object identity led to a prediction for greater activity in fusiform gyrus in the non-analogical, item condition. The major strengths of our design were the difficulty of the non-analogical item task and the fact that both analogy and item tasks required the use of subgoals operating on similar visual stimuli.
To test our primary hypothesis of interest – the contribution of RLPFC to reasoning – we examined activity within ROIs based on peak voxels reported in previous fMRI studies of analogy. Both left and right RLPFC were more active during the analogy condition relative to the item condition. Because the analogy task alone required integration of relations, these results suggest that RLPFC activity is more strongly driven by the need to integrate relational information rather than coordinate subgoal outcomes, more generally. The task to which analogical reasoning was compared was, in fact, more difficult than the analogy condition. Contrasting analogy with a task that is at least as difficult is rare. We know of only two other studies to have done so (Krawczyk et al. 2010, 2011), and, in both of these studies, the greater speed with which participants solved analogies relative to control problems may have been artificially affected by the smaller number of possible correct responses in the analogy condition. In the current study, stimuli in both conditions were nearly perceptually identical with an equal number of possible responses (2) to each item. Additionally, both tasks required the coordination of multiple subgoals. Thus, increased reaction times during the item task could have been interpreted as reflecting increased demands on subgoal coordination. If RLPFC participates in the coordination of multiple cognitive operations, then we should have observed greater activity within this region for the item condition. However, ROI analyses revealed the opposite result: despite being solved more quickly, analogies elicited greater average activity within left and right RLPFC. This pattern of results suggests both that RLPFC activity is not necessarily modulated by task difficulty (see also Wendelken et al. 2008) and that it is more strongly engaged during tasks that require simultaneous integration of multiple relations rather than coordinating subgoals.
In some ways, our item task differed from tasks previously used to investigate the coordination of multiple subgoals. For instance, Koechlin & colleagues (Koechlin et al. 1999; Dreher et al. 2008) hypothesize that frontopolar cortex performs cognitive “branching” – maintaining one goal in working memory while processing a secondary goal at the same time. These goals are typically unrelated. Our item task, however, required participants to maintain multiple, related goals in working memory (verifying that each item in the target occurred in an answer) and to integrate these goals in order to fulfill a main goal (selecting the appropriate response). Despite these differences, both the current conception of subgoal coordination and previous ones demand more than merely the use of working memory (maintaining a single goal over time) or dual task performance (allocating attention between alternative goals), neither of which activate frontopolar cortex alone (Koechlin et al. 1999).
We observed greater RLPFC activity during the analogy task relative to the item task. Thus, taken in isolation, our results could suggest that RLPFC activation is unique to relational integration. However, the current study occurs within the context of other studies that have reported RLPFC involvement in non-relational, non-analogical task domains (e.g., multi-tasking, Koechlin et al. 1999; Braver and Bongiolatti, 2002; Dreher et al. 2008). What account of this area’s function, then, can integrate both patterns of results? One possible resolution is the view that increasingly abstract information preferentially recruits RLPFC, even when task or stimulus complexity are held constant (Christoff and Keramatian 2007; Christoff et al. 2009). For instance, solving anagrams whose solutions were abstract words (e.g., “myth”, “appeal”) relative to those whose solutions were concrete (“desk”, “motor”) was associated with stronger recruitment of RLPFC and other aspects of PFC (Christoff et al. 2009); no differences between abstract and concrete conditions emerged in accuracy or reaction time, so this pattern could not be attributed to differences in difficulty. In the current task, analogy and item conditions were also comparably complex. However, only successful analogy-making required extracting an abstract pattern of relations – a pattern did not depend on the specific perceptual features of the stimuli. Thus, greater RLPFC activity observed during the analogy condition could be due to the more abstract nature of the information relevant to the task.
Unlike some studies of analogy’s neural correlates (e.g., Goel et al. 1997; Wharton et al. 2000; Christoff et al. 2001; Kroger et al. 2002; Bunge et al. 2005; Bunge et al. 2009; Volle et al. 2010; Hampshire et al. 2011), we did not find evidence for a differential recruitment of left relative to right RLPFC. However, a few studies have reported activity in right RLPFC during analogy (Wendelken et al. 2008; Bunge et al. 2009), leading Bunge & colleagues (2009) to speculate that there may be hemispheric differences in the recruitment of RLPFC based on the nature of the stimuli: right RLPFC may be specialized for processing visuospatial relations and left RLPFC for verbal or semantic relations. During either kind of task, contralateral RLPFC may be recruited to “*assist+ with relational integration as needed” (p. 341). In our own experiment, we did not observe differential recruitment of right or left RLPFC during analogical reasoning. This result could indicate that, as Bunge & colleagues (2009) suggest, left RLPFC is aiding in difficult relational integration while right RLPFC processes visuospatial relations. Alternatively, because (to our knowledge) right RLPFC activity has never been observed in isolation during analogical reasoning, left RLPFC may be specialized for relational integration, with right RLPFC assisting under difficult conditions.
A secondary question of interest concerned the possibility of overlap between the neural bases of spatial relation knowledge and visuospatial analogical reasoning. Because prior research in our lab has suggested that inferior and superior parietal cortex are involved in the representation of categorical spatial relations (Amorapanth et al. 2010), we predicted that the need to extract spatial relations during analogical reasoning would produce greater activity in these areas relative to a task in which spatial relations were irrelevant. Both right and left inferior parietal cortex were indeed more strongly activated during the analogy condition relative to the item condition. Other studies of analogical or relational reasoning have also reported greater activity within inferior parietal cortex (Wharton et al. 2000; Geake and Hansen 2005; Crone et al. 2009; Golde et al. 2010; Preusse et al. 2010; Volle et al. 2010) or decreased activity here with repeated exposure to analogy problems (Wartenburger et al. 2009). Interestingly, all of these studies used visuospatial or non-semantic (e.g., letter strings, Geake and Hansen 2005) analogies as stimuli. Bilateral parietal damage is often associated with simultanagnosia, a disorder in which individuals find it difficult to process multiple objects simultaneously as is required when judging spatial relations (Coslett and Chatterjee 2003). The present and previous findings suggest that inferior parietal cortex is more active when a task (e.g., analogy) requires extraction of the relative spatial locations of multiple objects.
A final question we addressed with the current study concerned the role of inhibition in analogical reasoning. In the right hemisphere, both whole-brain and ROI analyses were in agreement: analogical reasoning produced greater activity within right IFG than the item task. In ROI analyses only, this effect was also observed in left IFG. Bilateral IFG have been implicated in inhibitory control: in the right hemisphere, by the inhibition of a dominant response (e.g., Aron et al., 2003), and in the left, by the need to resolve conflict among competing semantic representations (e.g., Thompson-Schill et al. 1997). Similarly, given the need to inhibit incorrect responses based on surface similarities, inhibition has been proposed as a necessary component of the analogical reasoning process (Robin and Holyoak 1995; Morrison et al. 2004; Viskontas et al. 2004; Krawczyk et al. 2008). Several other neuroimaging studies using either semantic or visuospatial analogies have also found greater activity in IFG during analogy (Christoff et al. 2001; Luo et al. 2003; Green et al. 2006; Cho et al. 2010; Green et al. 2010; Krawczyk et al. 2011; Volle et al. 2010). Cho & colleagues (2010), who also used a visuospatial analogy task, manipulated both relational complexity and the need for interference resolution. Similar to our own whole-brain analysis, a cluster of activation was identified in right lateral PFC (right middle frontal gyrus and IFG) that responded exclusively to demands on interference resolution. Thus, IFG may be engaged during analogy when it is necessary to ignore irrelevant aspects of the stimuli in order to identify relational similarity or to inhibit prepotent responses based on superficial, not relational, dimensions.
Given that activity in right IFG during analogy emerged in whole-brain and ROI analyses, our results (and those of Cho et al., 2010) suggest, if anything, a more important role for right IFG in analogical reasoning. However, greater activation of IFG by analogy has more frequently been reported in the left hemisphere (Christoff et al. 2001; Luo et al. 2003; Bunge et al. 2005; Green et al. 2006; Wendelken et al. 2008; Green et al. 2010; Volle et al. 2010; Krawczyk et al. 2010). This pattern of results leads to the question: what factors influence the degree to which IFG recruitment is lateralized? One possibility is that at least some aspects of inferior PFC are sensitive to the domains from which stimuli are drawn (Wagner et al. 1998; Braver et al. 2003; Hazeltine et al. 2003; Bunge et al. 2004). For example, among studies employing verbal analogies, IFG activity during analogy tends to be left-lateralized (Luo et al. 2003; Bunge et al. 2005; Green et al. 2006; Wendelken et al. 2008; Green et al. 2010). When visuospatial analogies without any linguistic information are used, activity in IFG tends to be bilateral (Christoff et al. 2001; Volle et al. 2010) or right-lateralized (the current study and Cho et al. 2010). Thus, hemispheric differences in IFG recruitment during analogy may depend on the linguistic or visuospatial nature of the stimuli.
According to one influential set of accounts of the general function of prefrontal cortex, the PFC is responsible for top-down biasing of processing in posterior areas when information in those areas is relevant to the task at hand (Cohen and O’Reilly 1996; O’Reilly et al. 2002). Hemispheric differences in PFC activity may be observed, then, depending on posterior regions receiving a top-down bias, with right IFG being in a privileged position to bias processing of knowledge more strongly represented in the right hemisphere – like visual and spatial knowledge. Left IFG, on the other hand, may be better suited to biasing processing of conceptual or linguistically-based knowledge in left temporal cortex.
A more specific possibility is that the semantic or visuospatial nature of the stimuli determines the kind of inhibitory processes that are required during analogical reasoning. For instance, left IFG has been hypothesized to resolve conflict among competing semantic representations (e.g., Thompson-Schill et al. 1997). Thus, left IFG may be more active when analogies are formed over semantic knowledge, and compelling semantic, but non-relational, associations need to be suppressed. On the other hand, right IFG has been implicated in the inhibition of prepotent responses, for example, during tasks in which participants make speeded responses on “go” trials but withhold those responses on infrequent “no-go” trials (e.g., Menon et al. 2001; Rubia et al. 2003). Thus, right IFG may be more active when participants solve visuospatial analogies and must overcome a dominant response to respond perceptually. In the current study, this inclination is helpful during the item task. In the analogy task, however, responses must be based on relational similarity, and response inhibition may be required to suppress the urge to respond to a perceptually similar answer. (In fact, both answers are equally perceptually related to the target, so participants may have been driven to respond to the option to which they first attended.) Thus, semantic and visuospatial analogies may put differential demands on control processes dependent on right or left IFG, respectively.
However, we did not explicitly manipulate demands for response inhibition in the current experiment, so we cannot rule out other explanations for greater IFG activity during the analogy task. In fact, activation in this area has been observed during a wide-variety of cognitive tasks (Duncan and Owen 2000) – even during tasks that do not require inhibition of motor responses (Hampshire et al. 2009) or any motor responses at all (Hampshire et al. 2010). Hampshire & colleagues (2010) have recently attempted to unify these findings with an alternative account of this region’s function: the RIFG is recruited whenever important cues are detected that “trigger effortful and task relevant behaviours” (p. 1318). In the current experiment, these cues could have been the perceptual mis-match between target and answers during the analogy task, indicating to participants that attention to relational, rather than perceptual, information was required.
5. Conclusions
In sum, we found evidence that bilateral RLPFC is more strongly engaged during the integration of relations in analogy relative to the coordination of subgoal outcomes, more generally, even when the non-analogical task was more difficult for participants to solve. Additionally, the neural basis of analogical reasoning lies not only in the anterior aspects of prefrontal cortex, but also in areas of the brain involved in inhibition or cognitive control and, for visuospatial analogies, areas that represent spatial relation knowledge. Together, these areas work in concert during analogical reasoning to extract and integrate relational information while suppressing the tendency to think superficially rather than structurally.
Highlights.
We use fMRI to investigate the neural correlates of visuospatial analogies.
We compare relational to subgoal integration in rostrolateral prefrontal cortex.
Bilateral RLPFC was more active during relational integration (analogies).
Analogies recruit bilateral inferior frontal gyri and inferior parietal cortices.
These areas overlap with inhibitory processes and knowledge of spatial relations.
Acknowledgements
We thank Matt Lehet and Bianca Bromberger for their assistance with behavioral data collection and figure generation, respectively, and Dan Kimberg and Olufunsho Faseyitan for guidance on fMRI data analysis. We also thank Eileen Cardillo and Alexander Kranjec for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Amorapanth PX, Widick P, Chatterjee A. The neural basis for spatial relations. J Cogn Neurosci. 2010;22:1739–1753. doi: 10.1162/jocn.2009.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Blanchette I, Dunbar K. Analogy use in naturalistic settings: The influence of audience, emotion, and goals. Mem Cogn. 2001;29:730–735. doi: 10.3758/bf03200475. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56:141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. NeuroImage. 2009;46:338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, Knowlton BJ, Holyoak KJ. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 2010;20:524–533. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Christoff K, Keramatian K. Abstraction of mental representations: theoretical considerations and neuroscientific evidence. In: Bunge SA, Wallis JD, editors. Perspectives on rule-guided behavior. Oxford University Press; New York (NY): 2007. pp. 107–126. [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JDE. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cohen JD, O’Reilly RC. A preliminary theory of the interactions between prefrontal cortex and hippocampus that contribute to planning and prospective memory. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: theory and applications. Lawrence Erlbaum Associates; Hillsdale (NJ): 1996. pp. 267–295. [Google Scholar]
- Coslett HB, Chatterjee A. Balint’s Syndrome and related disorders. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. McGraw-Hill; New York (NY): 2003. pp. 325–336. [Google Scholar]
- Crone EA, Wendelken C, Van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Dev Sci. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N, Braver TS. Preparation for integration: the role of anterior prefrontal cortex in working memory. Neuroreport. 2008;19:15–19. doi: 10.1097/WNR.0b013e3282f31530. [DOI] [PubMed] [Google Scholar]
- Dreher J, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS ONE. 2008;3:e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore S. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol. 2008;50:168–181. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar K. How scientists really reason: Scientific reasoning in real-world laboratories. In: Sternberg RJ, Davidson JE, editors. The nature of insight. MIT Press; Cambridge (MA): 1995. pp. 365–395. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Flemming TM, Beran MJ, Thompson RKR, Kleider HM, Washburn DA. What meaning means for same and different: Analogical reasoning in humans (Homo sapiens), chimpanzees (Pan troglodytes), and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2008;122:176–185. doi: 10.1037/0735-7036.122.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. NeuroImage. 2005;26:555–564. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Gentner D. Structure-mapping: A theoretical framework for analogy. Cogn Sci. 1983;7:155–170. [Google Scholar]
- Gentner D. Why we’re so smart. In: Gentner D, Goldin-Meadow S, editors. Language in mind: Advances in the study of language and thought. MIT Press; Cambridge (MA): 2003. pp. 195–235. [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain-behavior associations. Cereb Cortex. 2006;16:1783–1789. doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- Gillan DJ, Premack D, Woodruff G. Reasoning in the chimpanzee: I. Analogical reasoning. J Exp Psychol: Anim Behav Process. 1981;7:1–17. [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. Neuroreport. 1997;8:1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Golde M, von Cramon DY, Schubotz RI. Differential role of anterior prefrontal and premotor cortex in the processing of relational information. NeuroImage. 2010;49:2890–2900. doi: 10.1016/j.neuroimage.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJM, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Res. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, Dunbar KN. Connecting long distance: semantic distance in analogical reasoning modulates frontopolar cortex activity. Cereb Cortex. 2010;20:70–76. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Neurosci. 2009;9:103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Lateral prefrontal cortex subregions make dissociable contributions during fluid reasoning. Cereb Cortex. 2011;21:1–10. doi: 10.1093/cercor/bhq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JDE. Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Hofstadter DR. Analogy as the core of cognition. In: Gentner D, Holyoak KJ, Kokinov BJ, editors. Analogy: Perspectives from cognitive science. MIT Press; Cambridge (MA): 2000. pp. 499–538. [Google Scholar]
- Holyoak KJ. Analogy. In: Holyoak KJ, Morrison R, editors. The Cambridge handbook of thinking and reasoning. Cambridge University Press; Cambridge (UK): 2005. pp. 117–142. [Google Scholar]
- Holyoak KJ, Thagard P. Mental leaps: Analogy in creative thought. MIT Press; Cambridge (MA): 1995. [Google Scholar]
- Holyoak KJ, Thagard P. The analogical mind. Am Psychol. 1997;52:35–44. [PubMed] [Google Scholar]
- Kable JW, Kimberg DY, Chatterjee A. Dealing with potential reaction time confounds in the interpretation of fMRI results; Poster presented at 11th Annual Meeting of the Cognitive Neuroscience Society; San Francisco (CA). 2004. [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. PNAS. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotovsky L, Gentner D. Comparison and categorization in the development of relational similarity. Child Dev. 1996;67:2797–2822. [Google Scholar]
- Krawczyk DC. The cognition and neuroscience of relational reasoning. Brain Res. 2010 doi: 10.1016/j.brainres.2010.11.080. in press. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, McClelland MM, Donovan CM. A hierarchy for relational reasoning in the prefrontal cortex. Cortex. 2011;47:588–597. doi: 10.1016/j.cortex.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, McClelland MM, Donovan CM, Tillman GD, Maguire MJ. An fMRI investigation of cognitive stages in reasoning by analogy. Brain Res. 2010;1342:63–73. doi: 10.1016/j.brainres.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Morrison RG, Viskontas I, Holyoak KJ, Chow TW, Mendez MF, Miller BL, Knowlton BJ. Distraction during relational reasoning: the role of prefrontal cortex in interference control. Neuropsychologia. 2008;46:2020–2032. doi: 10.1016/j.neuropsychologia.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Loewenstein J, Thompson L, Gentner D. Analogical encoding facilitates knowledge transfer in negotiation. Psychon Bull Rev. 1999;6:586–597. doi: 10.3758/bf03212967. [DOI] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: an fMRI study. Cogn Brain Res. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RG, Krawczyk DC, Holyoak KJ, Hummel JE, Chow TW, Miller BL, Knowlton BJ. A neurocomputational model of analogical reasoning and its breakdown in frontotemporal lobar degeneration. J Cogn Neurosci. 2004;16:260–271. doi: 10.1162/089892904322984553. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler LK. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex. 2002;12:246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE. Neural substrates of fluid reasoning: An fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Preusse F, Meer E, Ullwer D, Brucks M, Krueger F, Wartenburger I. Long-term characteristics of analogical processing in high-school students with high fluid intelligence: an fMRI study. ZDM. 2010;42:635–647. [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Robin N, Holyoak KJ. Relational complexity and the functions of the prefrontal cortex. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT Press; Cambridge (MA): 1995. pp. 987–997. [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Simons JS, lvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory?: Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. PNAS. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Morrison RG, Holyoak KJ, Hummel JE, Knowlton BJ. Relational integration, inhibition, and analogical reasoning in older adults. Psychol Aging. 2004;19:581–591. doi: 10.1037/0882-7974.19.4.581. [DOI] [PubMed] [Google Scholar]
- Volle E, Gilbert SJ, Benoit RG, Burgess PW. Specialization of the rostral prefrontal cortex for distinct analogy processes. Cereb Cortex. 2010;20:2647–2659. doi: 10.1093/cercor/bhq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wai-Kwong Man D, Soong WYL, Tam S, Hui-Chan CWY. Development and evaluation of a pictorial-based analogical problem-solving programme for people with traumatic brain injury. Brain Inj. 2006;20:981–990. doi: 10.1080/13561820600909852. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Preusse F, Kramer J, Van Der Meer E. Cerebral correlates of analogical processing and their modulation by training. NeuroImage. 2009;48:291–302. doi: 10.1016/j.neuroimage.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to??”: Investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20:682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wharton CM, Grafman J, Flitman SS, Hansen EK, Brauner J, Marks A, Honda M. Toward neuroanatomical models of analogy: A positron emission tomography study of analogical mapping. Cogn Psychol. 2000;40:173–197. doi: 10.1006/cogp.1999.0726. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Wright SB, Matlen BJ, Baym CL, Ferrer E, Bunge SA. Neural correlates of fluid reasoning in children and adults. Front Hum Neurosci. 2008;1:8. doi: 10.3389/neuro.09.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]