Abstract
4-vinylcyclohexene diepoxide (VCD) is a metabolite of 4-vinylcyclohexene (VCH) which has the potential to be formed in the ovary through CYP2E1 activity. VCD specifically destroys primordial and small primary follicles in the rodent ovary. Mouse ovaries exposed to VCD demonstrate increased mRNA and protein expression of microsomal epoxide hydrolase (mEH), and an inactive tetrol metabolite (4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane) can be formed in mouse ovarian follicles, potentially through detoxification action of mEH. In contrast, mEH can bioactivate another ovotoxic chemical, 7,12-dimethylbenz[a]anthracene (DMBA) to a more toxic compound, DMBA-3,4-diol-1,2-epoxide. Thus, the present study evaluated a functional role for mEH during detoxification of VCD. Additionally, because inhibition of the phosphatidyinositol-3 kinase (PI3K) signaling pathway in a previous study protected primordial follicles from VCD-induced destruction, but accelerated DMBA-induced ovotoxicity, a role for PI3K in ovarian mEH regulation was evaluated. Using a post-natal day (PND) 4 Fischer 344 rat whole ovary culture system inhibition of mEH using cyclohexene oxide during VCD exposure resulted in a greater (P < 0.05) loss of primordial and small primary follicles relative to VCD-treated ovaries. Also, relative to controls, meh mRNA was increased (P < 0.05) on day 4 of VCD (30 μM) exposure, followed by increased (P < 0.05) mEH protein after 6 days. Furthermore, inhibition of PI3K signaling increased mEH mRNA and protein expression. Thus, these results support a functional role for mEH in the rat ovary, and demonstrate the involvement of PI3K signaling in regulation of ovarian xenobiotic metabolism by mEH.
Keywords: microsomal epoxide hydrolase, 4-vinylcyclohexene, phosphatidylinositol-3 kinase, ovary, primordial follicle
Introduction
The ovary is the primary reproductive organ in females, and contains follicles at various stages of development. The primordial follicle is the most immature form, and consists of a meiotically-arrested oocyte surrounded by a single layer of squamous granulosa cells. Females are born with a finite number of primordial follicles, which once depleted, cannot be replaced (Hirshfield, 1991). The phosphatidylinositol-3 kinase (PI3K) signaling pathway is critically involved in maintaining primordial follicle viability (Parrott and Skinner, 1999; Yoshida et al., 1997) and regulates the rate at which primordial follicles are recruited into the growing follicular pool (Yoshida et al., 1997; Kissel et al., 2000; Castrillon et al., 2003; Reddy et al., 2005; John et al., 2008; Reddy et al., 2008). This survival pathway is activated when granulosa cell-expressed Kit Ligand (KITL) binds to the oocyte expressed receptor, c-KIT (Ismail et al., 1996). In response, c-KIT undergoes autophosphorylation, which leads to activation of PI3K (Castrillon et al., 2003; Reddy et al., 2005; John et al., 2008; Liu et al., 2006; Reddy et al., 2008), eventually resulting in phosphorylation of a key downstream molecule, AKT (Nicholson and Anderson, 2002; Datta et al., 1999).
Exposure to a chemical that accelerates depletion of the finite primordial follicle pool can lead to premature ovarian failure. 4-vinylcyclohexene (VCH) is a byproduct of the pesticide, rubber, plastic and flame retardant industries (Rappaport and Fraser, 1977). Bioactivation of VCH to the ovotoxic form, 4-vinylcyclohexene diepoxide (VCD) occurs via the cytochrome P450 family of enzymes, including ovarian expressed CYP 2E1 (Cannady et al., 2002; 2003; Rajapaksa et al., 2007a). Human exposure to VCH and VCD are limited, however, VCD is a useful model ovotoxicant due to its capacity to selectively destroy primordial and small primary follicles in ovaries of mice and rats (Smith et al., 1990; Doerr et al., 1985).
The enzyme microsomal epoxide hydrolase (mEH) is expressed in multiple tissues, including the ovary, and has wide substrate specificity (Dannan and Guengerich, 1982; Mukhtar et al., 1978). mEH mRNA and enzyme activity expression were shown to increase in small pre-antral follicles of mouse ovaries following repeated daily dosing (15d) with VCD (0.57 mmol/kg/day) (Cannady et al., 2002). Furthermore, mEH mRNA and protein were up-regulated in cultured postnatal day 4 (PND4) B6C3F1 mouse ovaries in response to VCD exposure (Keating et al., 2008a), and formation of the inactive tetrol metabolite [4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane] in ovarian follicles of VCD dosed mice has been demonstrated (Flaws et al., 1994). Based on these collective data, a functional role for mEH in VCD detoxification is hypothesized but has not yet been established.
In contrast to VCD, the ovotoxic polycyclic aromatic hydrocarbon, 7,12-dimethylbenz[a]anthracene (DMBA), liberated during combustion of organic matter (Gelboin, 1980), destroys follicles at all stages of development (Mattison and Schulman, 1980). A study in which an ovotoxic index (concentration required for 50% primordial follicle loss) was calculated found that DMBA is approximately 20 times more ovotoxic than VCD (Borman et al., 2000). It has been demonstrated that DMBA is bioactivated to a more potent ovotoxicant DMBA-3,4-diol, 1,2-epoxide via the mEH enzyme (Rajapaksa et al., 2007; Igawa et al., 2009). Thus, mEH is involved in bioactivation of DMBA and the action of mEH enhances the ovotoxic effects of DMBA.
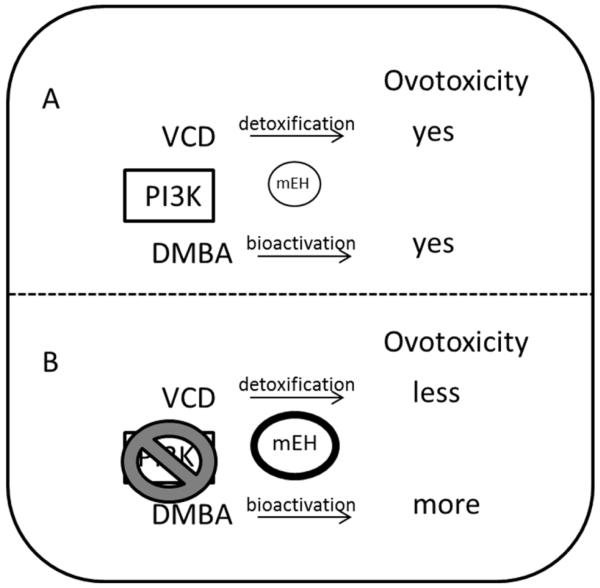
Inhibition of PI3K protects primordial but not small primary follicles from VCD-induced destruction (Keating et al., 2009). In contrast, ovarian DMBA exposure during inhibition of PI3K accelerates the amount of ovotoxicity observed (Keating et al., 2009). Due to the divergent role of mEH in bioactivation of DMBA and potential detoxification of VCD, it is proposed that changes in mEH action may be involved in the different levels of ovotoxicity observed with these two chemicals during PI3K inhibition (Figure 1). The purpose of this study therefore was to 1) establish that mEH has a functional role in VCD detoxification and, 2) to investigate if the previously observed divergent levels of ovotoxicity induced by VCD and DMBA that occur during PI3K inhibition can be attributed to changes in ovarian mEH expression.
Figure 1. Proposed effect of PI3K inhibition on VCD- and DMBA-induced ovotoxicity.
PI3K signaling is proposed to regulate mEH expression. (A) VCD and DMBA both cause depletion of primordial follicles, however, (B) inhibition of PI3K signaling repressed VCD-induced but accelerated DMBA-induced ovotoxicity (Keating et al., 2009). These events are proposed to be due to increased mEH expression (B) since mEH is proposed to detoxify VCD, and is known to bioactivate DMBA (Igawa et al., 2009). Thus, the divergent levels of ovotoxicity observed due to inhibited PI3K signaling during VCD and DMBA exposure are hypothesized to be due to increased detoxification of VCD, but increased bioactivation of DMBA.
Materials and Methods
Reagents
4-vinylcyclohexene diepoxide (VCD), bovine serum albumin (BSA), ascorbic acid, transferrin, cyclohexene oxide (CHO), 2-β-mercaptoethanol, 30% acrylamide/0.8% bisacrylamide, ammonium persulphate, glycerol, N’N’N’N’-Tetramethylethylenediamine (TEMED), Tris base, Tris HCL, sodium chloride, Tween-20 were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1x (DMEM/Ham’s F12), Albumax, penicillin (5000U/ml), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2 or MgSO4) from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts and 48 well cell culture plates were obtained from Millipore (Bedford, MA) and Corning Inc. (Corning, NY) respectively. RNeasy Mini kit, QIA shredder kit, RNeasy Min Elute kit, and Quantitect™ SYBR Green PCR kit were purchased from Qiagen Inc (Valencia, CA). RNAlater was obtained from Ambion Inc. (Austin, TX). With the exception of 18S rRNA primers, all primers were obtained from IDT (Coralville, IA). The 18S rRNA primer was obtained from Applied Biosystems (Carlsbad, CA). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002; CAS#154447-36-6) was purchased from A.G. Scientific, Inc. (San Diego, CA). The mEH antibody was purchased from Detroit R and D (Detroit, MI). Donkey anti-goat secondary antibody was purchased from Vector (Burlingame, CA). The polyclonal β-actin and goat anti-rabbit secondary were obtained from Santa Cruz Biotechnology, (Santa Cruz, CA) and Pierce Biotechnology (Rockford, IL) respectively. Ponceau S was from Fisher Scientific. ECL plus chemical luminescence detection kit was obtained from GE Healthcare, Amersham (Buckinghamshire, UK).
Animals
All animals were housed one per cage in plastic cages and maintained in a controlled environment (22 ± 2°C; 12h light/12h dark cycles). The animals were provided a standard diet with ad libidum access to food and water, and allowed to give birth. All animal experimental procedures were approved by the University of Arizona and Iowa State University Institutional Animal Care and Use Committees.
In vitro ovarian cultures
Ovaries were collected from PND4 female F344 rats and cultured as described by Devine et al., 2002. Briefly, PND4 female F344 rat pups were euthanized by CO2 inhalation followed by decapitation. Ovaries were removed, trimmed out of oviduct and other excess tissues and placed onto Millicell-CM membrane floating on 250 μl of DMEM/Ham’s F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 μg/ml ascorbic acid, 5 U/ml penicillin and 27.5 μg/ml transferrin per well in a 48 well plate previously equilibrated to 37°C. A drop of medium was placed on top of each ovary to prevent it from drying. Ovaries were treated with vehicle control medium (1% DMSO), VCD (30 μM), the PI3K inhibitor LY294002 (20 μM), and/or the mEH inhibitor CHO (2 mM) and maintained at 37°C and 5% CO2 for 2-8 days. The concentrations of LY294002 and CHO were previously determined to be effective in the PND4 rat ovary culture system (Keating et al., 2009; Igawa et al., 2009, respectively). For treatments lasting for more than two days, media was changed every alternate day. The concentration (30 μM) and times of VCD exposure (day 6) were previously determined to cause 50% primordial and small primary follicle loss (Devine et al., 2002; Keating et al., 2009).
Histological evaluation of follicle numbers
Following treatment, ovaries were placed in Bouin’s fixative for 1.5 hr, paraffin embedded and serially sectioned (5 μM). Every 6th section was mounted and stained with hematoxylin and eosin. Healthy oocyte-containing follicles were identified and counted in every 12th section. Unhealthy follicles were distinguished by their granulosa cell content of pyknotic bodies and intense eosinophilic staining of oocytes (Devine et al., 2002). Follicle population classification was performed as previously described (Keating et al., 2009).
RNA isolation and polymerase chain reaction (PCR)
Following 2, 4, 6 or 8 days of in vitro culture, ovaries were stored in RNA later at −80°C. Total RNA was isolated from ovaries (n=3; 10 ovaries per pool) using an RNeasy Mini kit according to the manufacturer’s instructions. RNA was eluted in 14 μl of RNase-free water and concentration quantified using a NanoDrop (λ=260/280 nm; ND 1000; Nanodrop Technologies Inc, Wilmington, DE). Total RNA (500 ng) was reverse transcribed to cDNA using Superscript III One- Step RT-PCR System (Invitrogen). Genes of interest were amplified using an Eppendorf mastercycler (Hauppauge, NY) using a Quantitect™ SYBR Green PCR kit (Qiagen Inc.Valencia, CA). The primers used were: meh forward primer: 5′GGC ATC GTC CAT AAA CA; meh reverse primer: 5′ TCT TCA AAG GCA GCA AAG TG, (NCBI GenBank accession number M26125), β-actin forward primer: 5′ TCT ATC CTG GCC TCA CTG TC; β-actin reverse primer: 5′ACG CAG CTC AGT AAC AGT CC, (NCBI GenBank accession number NM_007393) and commercially available primers to detect 18S rRNA. A melting curve analysis was used to ensure that a single product was amplified for each primer set. For the VCD experiments, there was no effect of VCD on β-actin mRNA expression, thus, meh mRNA was normalized to β-actin. Inhibition of PI3K did result in decreased β-actin mRNA expression, thus, 18S rRNA was used as a housekeeping gene for the PI3K inhibition experiments, since there was no impact of PI3K inhibition on 18S rRNA expression. Quantification of fold-change in gene expression was performed using the 2−ΔΔCt method (Pfaffl, 2001; Livak and Schmittgen, 2001). The PCR conditions used were: 15 min hold at 95°C and 40 cycles of: denaturing at 95°C for 15 s, annealing at 58°C for 15 s, and extension at 72°C for 15 s.
Protein isolation and Western blot analysis
Following 4, 6 or 8 days of culture, protein was isolated from ovaries (n=3; 10 ovaries/pool) as previously described (Thompson et al., 2005). Protein concentration was measured using a standard BCA protocol. Emission absorbance values were detected with a λ=540 nm excitation on a Synergy™ HT Multi-Detection Microplate Reader using KC4™ software (BioTekR Instruments Inc. Winooski, VT).
SDS-PAGE (10%) was used to separate proteins (10 μg; n=3) in the homogenates followed by transfer onto nitrocellulose membranes as previously described (Thompson et al., 2005). Membranes were blocked for 1 h with shaking at 4°C in 5% milk in Tris-buffered saline with Tween-20 (TTBS). Membranes were incubated with primary antibody in 5% milk in TTBS overnight at 4°C. The mEH antibody dilution used was 1:2000. Following three washes (10 min) in TTBS, membranes were incubated in HRP-conjugated secondary antibody (1:2000) for 1hr at room temperature. Membranes were washed three times (10 min) in TTBS followed by a single wash for 10 min in Tris Buffered Saline (TBS). Membranes were incubated in chemiluminescence detection substrate (ECL plus) for 5 min and exposed to X-ray film. Densitometry of the appropriate bands was performed using ImageJ software (NCBI). Equal protein loading was confirmed by Ponceau S staining of membranes and mEH protein was normalized to Ponceau S densitometry values.
Statistical analysis
Comparisons were made between treatments for follicle count experiments using Statview software analysis of variance (ANOVA). Quantitative RT-PCR and Western blotting data were analyzed by paired t-tests comparing treatment with control raw data at each individual time-point using Prism 5.04 software (GraphPad Software). Statistical significance was defined as P < 0.05. For graphical purposes, protein expression is presented as a percentage of the respective controls, and only one control value of 100% is presented.
Results
Effect of mEH inhibition on VCD-induced ovotoxicity in PND4 rat ovaries
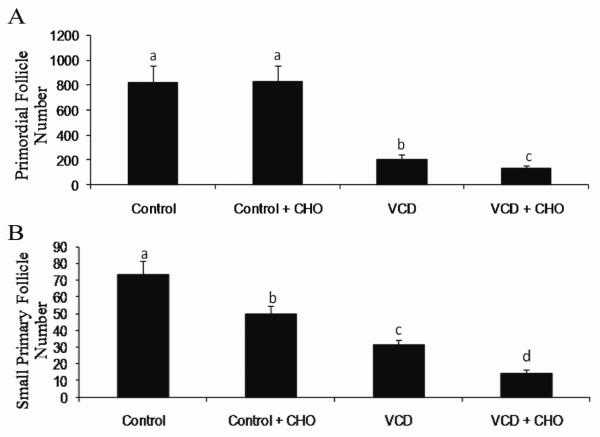
Cyclohexene oxide (CHO) is a competitive inhibitor of mEH. In order to evaluate a functional role for mEH in VCD-induced ovotoxicity, F344 rat ovaries were cultured in vehicle control or VCD (30 μM) ± CHO (2 mM) for 8 days, followed by histological evaluation of follicles. This time-point was chosen since primordial and small follicle destruction is established at this time (Keating et al., 2009). There was no effect of CHO on primordial follicle number. Relative to control, VCD depleted (P < 0.05) primordial follicles by 74.5%. When ovaries were exposed to VCD in the presence of mEH inhibition (CHO), there was greater (P < 0.05) primordial follicle loss (36% fewer follicles) relative to VCD alone (Figure 2). Surprisingly, there was an effect of CHO on small primary follicle number (32.5% fewer follicles than control treatment; P < 0.05). As expected, relative to control, VCD depleted (P < 0.05) small primary follicles (57.5% fewer follicles). Inhibition of mEH in the presence of VCD resulted in additional loss (P < 0.05) of small primary follicles compared to VCD alone (55% fewer small primary follicles; Figure 2).
Figure 2. Effect of mEH inhibition on VCD-induced follicle loss.
PND4 F344 rat ovaries were cultured in media containing vehicle control or VCD (30 μM), ± CHO (2 mM) for 8 days. Ovaries were processed for histological evaluation and healthy follicles were classified and counted as described in methods. Values are expressed as mean ± SE total follicles counted/ ovary, n=5. Different letters indicate significant difference; P < 0.05.
Effect of VCD exposure on meh mRNA in PND4 rat ovaries
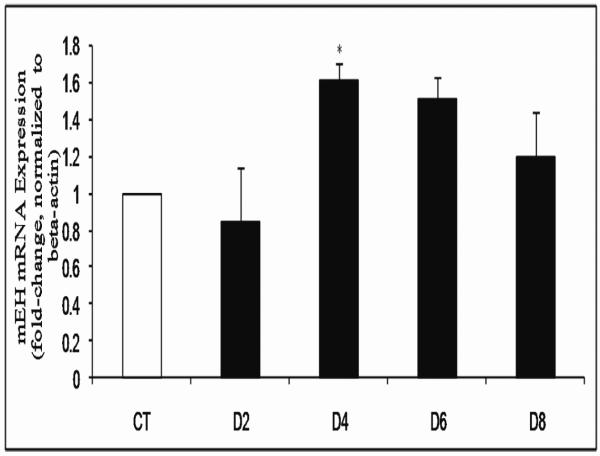
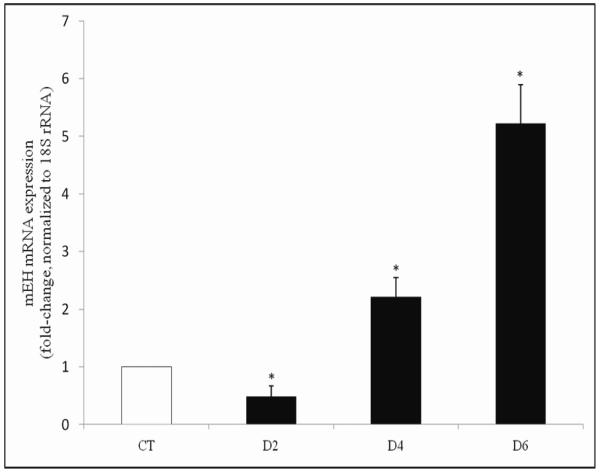
It has previously been determined that VCD exposure (30 μM) causes significant loss of both primordial and small primary follicles from 6 days of exposure onwards in cultured PND4 F344 rat ovaries (Keating et al., 2009). To determine the temporal pattern of meh mRNA expression in response to VCD exposure, PND4 F344 ovaries were cultured in VCD (30 μM) for 2-8 days (Figure 3). Relative to control treated ovaries, there was no change in meh mRNA level following 2 days of VCD exposure. On day 4 of VCD exposure, there was an increase (P < 0.05) in meh mRNA expression (0.61-fold increase) and a trend for increased meh mRNA after 6 days (0.51-fold, P = 0.08; Figure 3).
Figure 3. Temporal effect of VCD on meh mRNA expression.
PND4 F344 rat ovaries were cultured in media containing vehicle control (CT) or VCD (30 μM) for 2-8 days. Following incubation, total RNA was isolated and meh and β-actin mRNA levels were quantified by RT-PCR as described in methods. Values are expressed as mean fold change ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control.
Effect of VCD exposure on mEH protein in PND4 rat ovaries
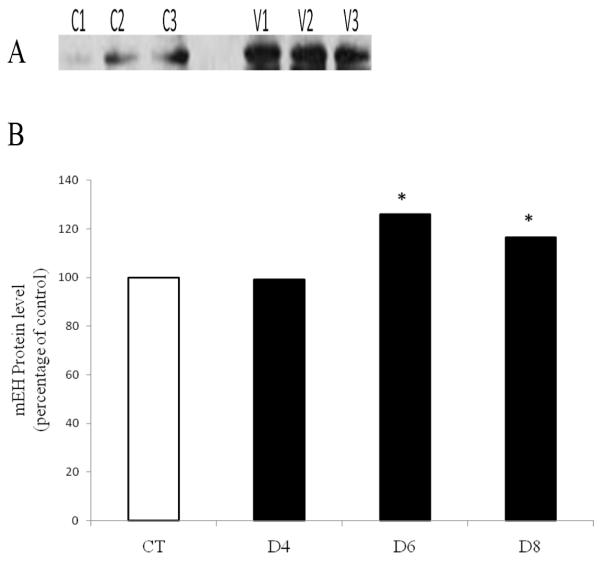
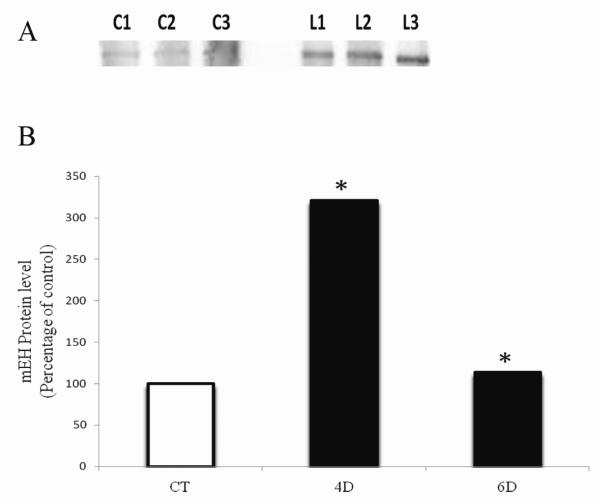
Since mEH mRNA level did not change until 4 days after VCD exposure, changes in mEH protein levels were investigated on days 4, 6 and 8 (Figure 4). There was no effect of VCD on mEH protein level at the 4 day time-point, but mEH protein was increased (P < 0.05) after 6 and 8 days of VCD exposure, by 26 and 17%, respectively, relative to control.
Figure 4. Temporal effect of VCD on mEH protein expression.
PND4 F344 rat ovaries were cultured in media containing vehicle control (CT) or VCD (30 μM) for 4-8 days. Total protein was isolated and Western blotting was performed for mEH protein as described in methods. (A) Representative Western blot day 6; Control = C; VCD = V. (B) Values are expressed as a percentage of control mean ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control.
Effect of PI3K inhibition on ovarian expression of mRNA encoding meh
To investigate a potential role of PI3K signaling in regulation of meh, PND4 F344 ovaries were cultured in vehicle control ± LY294002 (20 μM; PI3K inhibitor) for 2 or 4 days followed by quantitative RT-PCR to determine changes in mRNA encoding meh. The housekeeping gene 18S rRNA was used as an internal control because PI3K inhibition affected the mRNA level for β-actin (data not shown). There was a decrease (P < 0.05) in meh mRNA expression following 2 days of PI3K inhibition (0.51-fold decrease) compared to vehicle control. In contrast, meh mRNA expression was increased (P < 0.05) by PI3K inhibition after 4 days (1.21-fold increase; Figure 5).
Figure 5. Effect of PI3K inhibition on meh mRNA expression.
PND4 F344 rat ovaries were cultured in media containing vehicle control (CT), ± 20 μM LY294002 for 2 or 4 days. Total RNA was isolated and meh and 18S rRNA levels were quantified by RT-PCR as described in methods. Values are expressed as mean fold change ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control.
Effect of PI3K inhibition on ovarian expression of mEH protein
To evaluate the effect of PI3K inhibition on mEH protein, PND4 F344 rat ovaries were cultured in vehicle control ± LY294002 for 4 and 6 days (time-points lagging the mRNA increase) followed by Western blotting to detect mEH protein levels. Due to the observed effects of PI3K on the mRNA encoding the cytoskeleton protein, β-actin, Ponceau S staining was used to confirm equal protein loading across samples and densitometry values for mEH were normalized to those of Ponceau S. mEH protein was significantly increased (P < 0.05) by PI3K inhibition by 221% on day 4 and 14% on day 6 relative to control-treated ovaries (Figure 6).
Figure 6. Temporal effect of PI3K inhibition on mEH protein.
PND4 F344 rat ovaries were cultured in media containing vehicle control (CT), ± 20 μM LY294002 for 4 or 6 days. Total protein was isolated and Western blotting was performed to detect mEH protein. (A) Representative Western blot is shown on day 4; Control = C; LY294002 = L. (B) Values are expressed as a percentage of control mean ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control.
Discussion
Previously, it has been shown that VCD exposure to cultured PND4 B6C3F1 mice resulted in increased mEH mRNA and protein levels (Keating et al., 2008a). It has also been demonstrated that follicles isolated from ovaries of VCD-dosed mice produce an inactive tetrol metabolite, [4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane] (Flaws et al., 1994), potentially through the action of mEH. Demonstration of a functional role for mEH during VCD detoxification, however, has been lacking, therefore this study investigated a functional effect of mEH during VCD exposure, using the competitive inhibitor of mEH, cyclohexene oxide (CHO), followed by assessment of follicle number. CHO has previously been determined to be effective in the ovary culture system at the concentration used (Igawa et al., 2009). As expected, VCD depleted primordial and small primary follicle number. While there was no impact on primordial follicle number, surprisingly, small primary follicle number was reduced by mEH inhibition (in the absence of VCD). mEH protein is distributed throughout the rat ovary, including the cytoplasm of oocytes, and in granulosa and interstitial cells (Igawa et al., 2009). In humans, mEH protein has been detected in granulosa and theca interna cells, where inhibition of mEH in cultured granulosa cells was found to result in decreased Estradiol (E2) production from testosterone (Hattori et al., 2000), independent of an effect on aromatase activity. Female aromatase knockout mice (ArKO) are deficient in E2 and have higher numbers of primary follicles relative to age matched control mice indicating increased activation from the primordial follicle pool (Britt et al., 2002). Thus, E2 may inhibit primordial follicle activation supporting that the decreased number of small primary follicles due to mEH inhibition in the current study may reflect some increased recruitment from the small primary pool as a consequence of decreased estradiol production. When mEH was inhibited in the presence of VCD, relative to VCD alone, there was greater loss of both primordial follicles and small primary follicles, and loss of small primary follicles was greater than that caused by CHO alone. Since inhibition of mEH would result in exposure to greater persistent concentrations of VCD, these data provide confirmation that ovarian mEH is involved in detoxification of VCD.
meh mRNA was increased in cultured rat ovaries in response to VCD, following 4 days of exposure. Additionally, an increase in mEH protein was observed to follow the increase in mRNA (d4 mRNA; d6 protein). Interestingly, the rise in meh mRNA expression was not sustained, despite continuous exposure to VCD. However, mEH protein levels remained elevated, indicating either an increase in mEH protein expression or an extension of mEH protein half-life. Thus, these data confirm that mEH expression is activated in PND4 cultured rat ovaries in a temporal response to VCD exposure. It is likely that despite the increase in ovarian mEH level, that continuous exposure (every two days) to VCD overwhelms the capacity of mEH to detoxify VCD, leading to loss of primordial and small primary follicles,
In a previous study, inhibition of PI3K signaling resulted in decreased ovotoxicity induced by VCD, but accelerated DMBA-induced ovotoxicity (Keating et al., 2009). These results suggested that an alteration in metabolism of VCD and DMBA might be occurring. Further, a role for mEH in mediating the divergent ovotoxic effects of VCD and DMBA in CYP 2E1 null mice has been reported (Keating et al., 2008b). Thus, mEH is an ovarian expressed enzyme whose action may determine the extent of ovotoxicity caused by exposure to different xenobiotic chemicals.
To further investigate regulation of mEH expression in ovarian tissue, a specific inhibitor of PI3K signaling, LY294002, was employed. After two days in culture, meh mRNA was decreased, potentially due to a lag in transcriptional activation. Conversely, PI3K inhibition resulted in increased meh mRNA on days 4. mEH protein was increased by PI3K inhibition on days 4 and 6. These results indicate that mEH is downstream of PI3K signaling. Whether PI3K signaling is altered during mEH inhibition by CHO is unclear at this point, but is possible considering the effect of mEH deficiency on small primary follicle number. Increased mEH mRNA and protein supports that increased VCD detoxification and DMBA bioactivation are likely to be involved in the previously observed reduction in VCD-induced follicle loss and the increase in DMBA-induced follicle loss observed with PI3K inhibition (Keating et al., 2009).
The PI3K pathway has been identified as a major initial target of VCD (Keating et al., 2011). VCD exposure to cultured PND4 rat ovaries reduces c-KIT phosphorylation (Mark-Kappeler et al., 2011), resulting in downstream post-translational reductions in oocyte phosphorylated AKT (pAKT) and FOXO3 proteins (Keating et al., 2011). Depressed PI3K signaling by VCD exposure is consistent with the increase in mEH protein caused by both VCD and PI3K inhibition reported in the current study. Insulin has been reported to positively regulate hepatic mEH expression (Thomas et al., 1989 Kim et al., 2003; Kim and Novak, 2007) while glucagon inhibits mEH expression (Kim et al., 2003). Induction of Type I diabetes, characterized by insulin deficiency, in rats resulted in approximately 71% less mEH activity compared to the littermate controls. Treatment with insulin restored the activity of mEH. In addition, starvation, a physiological state known to reduce insulin levels, reduced mEH activity by approximately 33% of the control values in rats, while re-feeding restored mEH to control levels (Thomas et al., 1989). Thus, insulin plays a role in induction of mEH activity. It has also been shown that treatment of cultured primary rat hepatocytes with insulin increased mEH mRNA and protein in a time- and concentration-dependent manner (Kim et al., 2003), while use of the PI3K inhibitors, Wortmannin and LY294002, reduced pAKT level and prevented the insulin-induced increase in mEH (Kim et al., 2003), supporting that PI3K signaling is involved in insulin-induced hepatic regulation of mEH. Use of mTOR inhibitor, rapamycin, also prevented the insulin-induced induction of mEH protein (Kim et al., 2003). Recently, mTOR phosphorylated at position Ser2448 (activated form) was demonstrated to be increased by DMBA exposure in neonatal mouse ovaries (Sobinoff et al., 2011). Another study has demonstrated the involvement of PI3K signaling in induction of mEH through the transcription factors C/EBPα and C/EBPβ (Ki and Kim, 2008). Taken together, these data support a role for PI3K signaling in regulation of ovarian metabolism by mEH, potentially through the action of mTOR or C/EBP transcription factors, and also support that mEH is a downstream target of PI3K. Whether over-stimulation of PI3K signaling would further alter ovarian metabolism of VCD and DMBA is unclear at this point, however there are indications that altered insulin signaling (through PI3K) as seen during diabetes, can impact mEH expression in extra-ovarian tissues (Thomas et al., 1989; Kim and Novak, 2007).
In summary, a functional role for ovarian expressed mEH in VCD detoxification has been demonstrated, along with the temporal response of mEH mRNA and protein to VCD exposure. Results from the current study also support that PI3K signaling impacts mEH expression. These results further underline the potential impact of ovarian xenobiotic metabolism on the extent of follicular damage during chemical-induced ovotoxicity.
Highlights of this research.
Ovarian mEH functions to metabolize VCD to a less toxic compound
mEH expression is increased in a temporal pattern in response to VCD exposure
PI3K signaling is involved in regulation of ovarian mEH expression
Acknowledgements
The project described was supported by the National Institutes of Environmental Health Sciences [R00ES016818 to A.F.K.; R01 ES09246 to P.B.H. and the Southwest Environmental Health Sciences Center grant, ES0669344]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Conflicts of Interest Statement: There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones MEE, Simpson ER, Findlay JK. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J. Steroid Biochem. Mol. Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol. Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochrome P450 2E1, 2A, and 2B in the mouse ovary: The effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol. Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Dannan GA, Guengerich FP. Immunochemical comparison and quantitation of microsomal flavin-containing monooxygenases in various hog, mouse, rat, rabbit, dog, and human tissues. Mol. Pharmacol. 1982;22:787–794. [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol. Appl. Pharmacol. 2002;184:107–115. [PubMed] [Google Scholar]
- Doerr JK, Hooser SB, Smith BJ, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene and related olefins in B6C3F1 mice:role of diepoxides. Chem. Res. Toxicol. 1995;8:963–969. doi: 10.1021/tx00049a010. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB. Involvement of the KIT/KITL signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol. Reprod. 2008;79:318–327. doi: 10.1095/biolreprod.108.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Hattori N, Fujiwara H, Maeda M, Fujii S, Ueda M. Epoxide hydrolase affects estrogen production in the human ovary. Endocrinol. 2000;141:3353–3365. doi: 10.1210/endo.141.9.7682. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol. Appl. Pharmacol. 2009;234:361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail RS, Okawara Y, Fryer JN, Vanderhyden BC. Hormonal regulation of the ligand for c-kit in the rat ovary and its effects on spontaneous oocyte meiotic maturation. Mol. Reprod. Dev. 1996;43:458–469. doi: 10.1002/(SICI)1098-2795(199604)43:4<458::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sipes IG, Hoyer PB. Expression of ovarian microsomal epoxide hydrolase and glutathione S-transferase during onset of VCD-induced ovotoxicity in B6C3F(1) mice. Toxicol. Appl. Pharmacol. 2008a;230:109–116. doi: 10.1016/j.taap.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Effect of CYP2E1 gene deletion in mice on expression of microsomal epoxide hydrolase in response to VCD exposure. Toxicol. Sci. 2008b;105:351–359. doi: 10.1093/toxsci/kfn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 2009;241:127–134. doi: 10.1016/j.taap.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Fernandez SM, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol. Reprod. 2011;84:743–751. doi: 10.1095/biolreprod.110.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Kim SG. Phase II enzyme induction by α-lipoic acid through phosphatidylinositol 3-kinase-dependent C/EBPs activation. Xenobiotica. 2008;38:587–604. doi: 10.1080/00498250802126920. [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol. Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Kim SG, Novak RF. Insulin and glucagon signaling in regulation of microsomal epoxide hydrolase expression in primary cultured rat hepatocytes. Drug Metab. Dispos. 2003;31:1260–1268. doi: 10.1124/dmd.31.10.1260. [DOI] [PubMed] [Google Scholar]
- Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow MR, Manova K, Besmer P. Point mutation in Kit receptor tyrosine kinase reveals essential roles for Kit signaling in spermatogenesis and oogenesis without affecting other Kit responses. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev. Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Mark-Kappeler CJ, Sen N, Lukefahr A, McKee L, Sipes IG, Konhilas J, Hoyer PB. Inhibition of ovarian KIT phosphorylation by the ovotoxicant 4-vinylcyclohexene diepoxide in Rats. Biol. Reprod. 2011 doi: 10.1095/biolreprod.111.092742. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar H, Philpot RM, Bend JR. The postnatal development of microsomal epoxide hydrolase cytosolic gliutathione S-transferase and mitochondrial and microsomal cytochrome P-450 in adrenals and ovaries of female rats. Drug. Metab. Dispos. 1978;6:577–583. [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinol. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Rajapaska KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol. Appl. Pharmacol. 2007a;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaska KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase in ovotoxicity caused by 7,12-dimethylbenz(a)anthracene. Toxicol. Sci. 2007b;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Fraser DA. Air sampling and analysis in a rubber vulcanization area. Am. Ind. Hyd. Assoc. J. 1977;38:205–210. doi: 10.1080/0002889778507601. [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol. Appl. Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the villain: DMBA induced pre-antral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol. Sci. 2011;123:563–75. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol. Appl. Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Thomas H, Schladt L, Knehr M, Oesch F. Effect of diabetes and starvation on the activity of rat liver epoxide hydrolases, glutathione Stransferases and peroxisomal beta-oxidation. Biochem. Pharmacol. 1989;38:4291–4297. doi: 10.1016/0006-2952(89)90528-5. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev. Biol. 1997;184:122–137. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]