Abstract
Inflammatory microglia modulate a host of cellular processes in the central nervous system that include neuronal survival, metabolic fluxes, foreign body exclusion, and cellular regeneration. Elucidation of the pathways that oversee microglial survival and integrity may offer new avenues for the treatment of neurodegenerative disorders. Here we demonstrate that erythropoietin (EPO), an emerging strategy for immune system modulation, prevents microglial early and late apoptotic injury during oxidant stress through Wnt1, a cysteine-rich glycosylated protein that modulates cellular development and survival. Loss of Wnt1 through blockade of Wnt1 signaling or through the gene silencing of Wnt1 eliminates the protective capacity of EPO. Furthermore, endogenous Wnt1 in microglia is vital to preserve microglial survival since loss of Wnt1 alone increases microglial injury during oxidative stress. Cellular protection by EPO and Wnt1 intersect at the level of protein kinase B (Akt1), the mammalian target of rapamycin (mTOR), and p70S6K, which are necessary to foster cytoprotection for microglia. Downstream from these pathways, EPO and Wnt1 control “anti-apoptotic” pathways of microglia through the modulation of mitochondrial membrane permeability, the release of cytochrome c, and the expression of apoptotic protease activating factor-1 (Apaf-1) and X-linked inhibitor of apoptosis protein (XIAP). These studies offer new insights for the development of innovative therapeutic strategies for neurodegenerative disorders that focus upon inflammatory microglia and novel signal transduction pathways.
Keywords: Akt, Apaf-1, apoptosis, cytochrome c, erythropoietin, inflammation, microglia, mitochondria, mTOR, oxidative stress, phosphatidylserine, XIAP, wingless, Wnt
INTRODUCTION
Inflammatory microglia oversee a broad spectrum of disorders that can affect the central nervous system. These disease processes can range from acute processes such as halting the invasion of foreign organisms [1] to limiting the progression of chronic neurodegenerative disorders such as in Alzheimer’s disease [2–5]. New studies suggest that microglia may have a role during dietary oxidative stress [6], may mediate the release of neurotrophic factors during excitotoxicity [7], and also allow for regeneration and brain plasticity [8]. In a number of disorders, microglia serve an essential function to quarantine and remove non-functional neuronal and vascular cells [9–11]. Yet, potentially excessive activation of microglia also may contribute to the progression of neurodegeneration [3] and the loss of functional neuronal cells [12]. Inflammatory cells including microglia can play a critical role during the signal transduction of oxidant stress pathways [13–15] and can potentiate free radical release [16]. Given the dual modulatory role of microglia to not only confer cellular protection but also promote cellular demise in the nervous system, elucidating critical cellular mechanisms that oversee microglial survival may form a vital basis for the development of novel treatment strategies for multiple disorders of the nervous system.
In this regard, erythropoietin (EPO) has emerged as an exciting potential strategy for immune system modulation [17–24]. EPO limits leukocyte inflammation [25], prevents transplant cell loss and fosters angiogenesis [26], blocks the progression of ulcers in scleroderma [27], reduces renal inflammation during injury [28], is effective against experimental models of arthritis [29], limits immune mediated fibrosis [30], protects against pancreatic inflammation [31], and may be effective against cell injury during demyelinating disease [32]. At the cellular level, EPO is protective against tumor necrosis factor apoptosis [33], blocks cytokine gene expression [34], controls pro-inflammatory mediators [35], governs microglial activation and proliferation [10, 36, 37], and prevents the disposal of functional cells targeted by phosphatidylserine exposure [38–41]. Recently, Wnt1, a cysteine-rich glycosylated protein associated with stem cell growth, tumorigenesis and cellular senenscence [42–45] has been shown to be a vital component for the control of microglial integrity and proliferation [15, 46]. Furthermore, in neuronal cell populations, Wnt1 has been shown to prevent apoptotic neuronal injury in models of Alzheimer’s disease that is medicated by EPO [47].
Here we show that during oxidant stress through oxygen-glucose deprivation (OGD) exposure, EPO governs both early apoptotic membrane PS exposure and later genomic DNA degradation in microglia through Wnt1, since blockade of Wnt1 signaling or the gene silencing of Wnt1 eliminates the protective capacity of EPO. Loss of Wnt1 alone increases microglial injury during oxidative stress, illustrating that endogenous Wnt1 in microglia is a vital component to maintain microglial integrity. Furthermore, EPO is necessary to maintain the endogenous expression of Wnt1 in microglia that is otherwise lost during oxidant stress in the absence of EPO. Downstream from EPO and Wnt1, novel signaling through Akt1, mammalian target of rapamycin (mTOR), and p70S6K are necessary to implement protection for microglia against oxidative stress. Ultimately, EPO and Wnt1 oversee mitochondrial membrane permeability, cytochrome c release, and the expression of apoptotic protease activating factor-1 (Apaf-1) and X-linked inhibitor of apoptosis protein (XIAP) to foster microglial survival. Our work highlights the critical link between EPO and Wnt1 for the maintenance of microglia and elucidates several novel downstream therapeutic targets for inflammatory microglia that may be vital for the nervous system.
MATERIALS AND METHODS
Microglia Cell Cultures
Per our prior protocols, the microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA.) [11, 14, 15]. Cells were maintained in Dulbecco’s modified Eagle medium (ATTC, Manassas, VA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 µg/ml penicillin and streptomycin and 20% media from the LADMAC cell line (ATCC, Manassas, VA) which contains colony stimulating factor-1 (CSF-1) secreted by LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5 × 106 cells per well or 4 × 106 cells per dish.
Experimental Treatments
Per our prior work, oxygen-glucose deprivation (OGD) in microglia was performed by replacing the media with glucose-free HBSS containing 116 mmol/l NaCl, 5.4 mmol/l KCl, 0.8 mmol/l MgSO4, 1 mmol/l NaH2PO4, 0.9 mmol/l CaCl2, and 10 mg/l phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 °C per the experimental paradigm [11, 48, 49]. For treatments applied prior to OGD, human recombinant erythropoietin (EPO) (Sigma, St. Louis, MO), EPO blocking antibody (EPO Ab, 2 µg/ml), human recombinant Wnt1 protein (R&D Systems, Minneapolis, MN), mouse monoclonal antibody against Wnt1 (Wnt1 Ab) (1 µg/ml, R&D Systems, Minneapolis, MN), the recombinant Wnt antagonist dickkopf related protein 1 (DKK-1, 500 ng/ml, R&D Systems, Minneapolis, MN), rapamycin (RAPA, 20 nM, R&D Systems, Minneapolis, MN), or Ku 0063794 (KU, 100 nM, R&D Systems, Minneapolis, MN) were continuous.
Assessment of Cell Survival
Microglial injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with OGD per our previous protocols [50, 51]. The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10–20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures.
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay [52, 53]. Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3’-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Assessment of Membrane Phosphatidylserine (PS) Residue Externalization
Externalization of membrane PS residues was determined by using Annexin V labeling per our prior studies [50, 51, 54, 55]. A 30 µg/ml stock solution of Annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2). Plates were incubated with 500 µl of diluted Annexin V for 10 minutes. Images were acquired with "blinded" assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm [38].
Expression of Phosphorylated Akt1, Total Akt1, Phosphorylated mTOR, Total mTOR, Phosphorylated p70S6K, Total p70S6K, Apaf-1, Wnt1, GSK-3α/β, XIAP, and Cytochrome c
Cells were homogenized and each sample (50 µg/lane) was subjected to SDS-polyacrylamide gel electrophoresis [7.5% for Akt, mTOR, p70S6K, Apaf-1; 12.5% for Wnt1, GSK-3α/β, XIAP, and cytochrome c). After transfer, the membranes were incubated with a rabbit polyclonal antibody against Wnt1 (1:1000, R&D Systems, Minneapolis, MN), a rabbit monoclonal antibody against phospho-Akt1 (Ser473, 1:1000 and total Akt1 (1:1000) (Cell Signaling, Beverly, MA), a rabbit antibody against total Akt1, a rabbit monoclonal antibody against phospho-mTOR (Ser2448, 1:1000) and total mTOR (Cell Signaling, Beverly, MA), a rabbit antibody against phospho-p70S6K (Thr389, 1:1000) and total p70S6K (1:1000) (Cell signaling Technology, Beverly, MA), a primary rabbit antibody against Apaf-1, a primary rabbit antibody against XIAP (1:1000), or a primary rabbit antibody against cytochrome c (1:1000) (Cell Signaling Technology, Beverly, MA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (1:5000, Zymed Laboratories, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Akt Kinase Activity Assessment
Per our prior work [52, 56], Akt1 activity was determined by using a commercially available nonradioactive Akt1 kinase assay kit with a GSK-3β fusion protein. Cells were lysed in ice with 150 µl of lysis buffer containing 1% Triton X-100, 10% glycerol, 137 mM NaCl, 20 mM Tris-HCl (pH 7.5), 2 µg/ml aprotinin, 2 µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM Na2PPi, and 1 mM Na3VO4. Equal amounts of lysates (200 µg) were pre-cleared by centrifugation and pre-absorbed with protein A-protein G (1:1) agarose slurry. Immunoprecipitation was carried out over night using the immobilized anti-Akt1G1 monoclonal antibody (Cell Signaling Technology, Beverly, MA) cross-linked to agarose. Immunoprecipitates were washed three times with lysis buffer and twice with Akt kinase buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2). Kinase assays were performed for 30 min at 30°C under continuous agitation in kinase buffer containing 200 µM ATP and 1 µg of GSK-3 fusion protein according to the manufacturer's instructions (Cell Signaling Technology, Beverly, MA). Samples were analyzed by Western blot analysis using 12.5% SDS-polyacrylamide gel and rabbit antibody against p-GSK-3α/β (Cell Signaling Technology, Beverly, MA). Data for the kinase activity are expressed as percentage of control activity.
Assessment of Mitochondrial Membrane Potential
The fluorescent probe JC-1 (Molecular Probes, Eugene, OR), a cationic membrane potential indicator, was used to assess the mitochondrial membrane potential [57, 58]. Microglia in 35 mm dishes were incubated with 2 µg/ml JC-1 in growth medium at 37 °C for 30 min. The cultures were washed three times using fresh growth medium. Mitochondria were then analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, USA) with a dual emission fluorescence filter with 515–545 nm for green fluorescence and emission at 585–615 nm for red fluorescence [50].
Preparation of Mitochondria for the Analysis of Cytochrome c Release
After washing once with ice-cold PBS, cells were harvested at 10,000g for 15 min at 4°C and the resulting pellet was re-suspended in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenylmethylsulfonylfluoride) containing 250 mM sucrose and used as the mitochondrial fraction. The supernatant was subjected to ultracentrifugation at 50,000 g for 1 hour at 4 °C with the resultant supernatant used as the cytosolic fraction [59].
Statistical Analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett's test. Statistical significance was considered at P<0.05.
RESULTS
EPO Prevents Injury in Microglia During Oxygen-Glucose Deprivation (OGD)
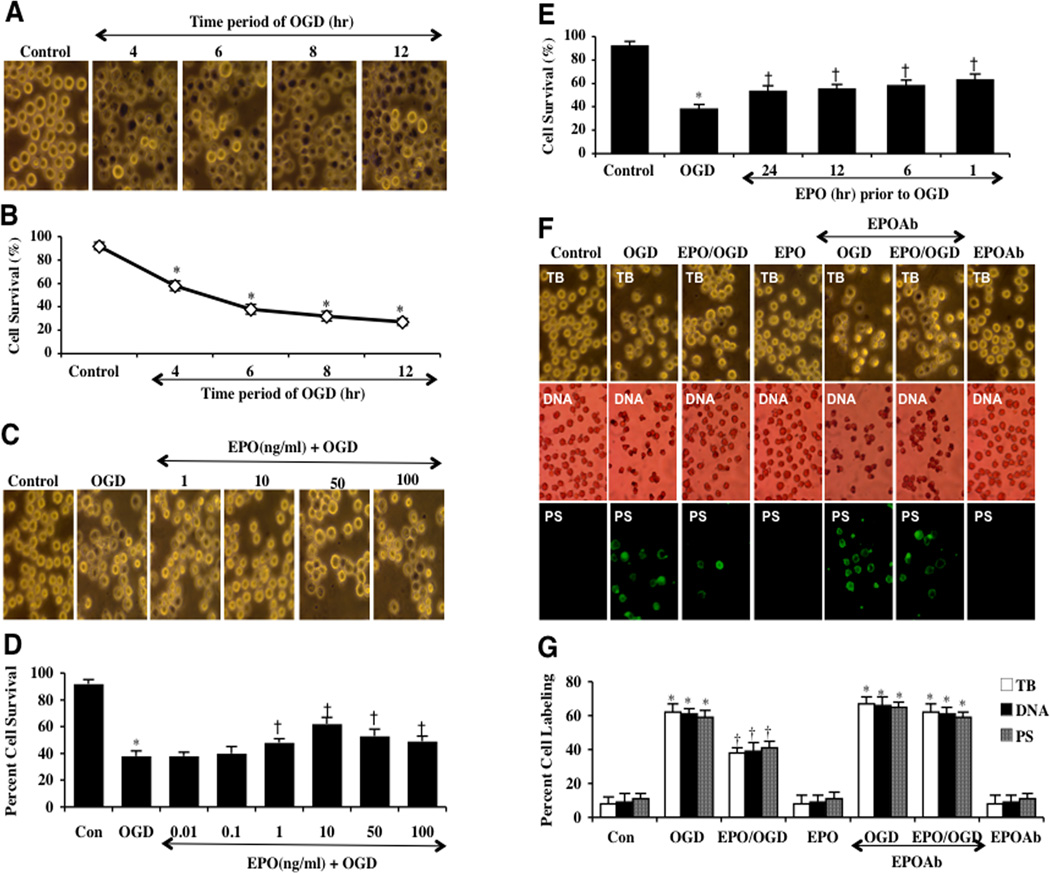
We examined microglial survival after exposure to OGD for the periods of 4 hours, 6 hours, 8 hours, and 12 hours. Cell survival was assessed with trypan blue exclusion method 24 hours after OGD exposure. As shown in Fig. (1A), representative images illustrate that OGD leads to trypan blue staining in microglia, but no significant staining is present in control cells not exposed to OGD. In Fig. (1B), quantitative data demonstrate that microglial survival was progressively reduced over periods following OGD application to 58 ± 4% (4 hours), 38 ± 4% (6 hours), 32 ± 4% (8 hours), and 27 ± 3% (12 hours) when compared to untreated control cultures (92 ± 3%, p<0.01). Since OGD exposure for a period of 6 hours resulted in survival rate of approximately 40% (a 60% microglial cell loss), this duration of OGD was used for the reminder of the experimental paradigms.
Fig. (1). EPO is necessary to foster protection in microglia during OGD exposure.
(A) Microglia was exposed to OGD for 4, 6, 8 and 12 hours and cell survival was determined 24 hours after OGD by using trypan blue exclusion method. Representative images illustrate that OGD leads to progressively increased microglial injury over time. Control = untreated microglia. (B) Quantitative analysis shows that microglial survival was significantly decreased following OGD exposure when compared with untreated control cultures (*P <0.01 vs. Control). Each data point represents the mean and SEM from 6 experiments. (C and D) EPO was applied to microglial cultures at the concentrations of 0.01, 0.1, 1,10, 50, and 100 ng/ml 1 hour prior to OGD and cell survival was determined 24 hours after OGD with the trypan blue dye exclusion method. Representative images show that EPO (1–100 ng/ml) significantly reduced trypan blue staining and increased cell survival following OGD (*P<0.01 vs. untreated control; †P <0.05 vs. OGD). Each data point represents the mean and SEM from 6 experiments. (E) EPO was applied to microglial cultures 24, 12, 6 or 1 hour prior to a 6 hour period of OGD and cell survival was determined 24 hours after OGD with the trypan blue dye exclusion method. The quantitative results show that 1 hour is the most effective pretreatment time with maximal survival achieved after EPO application (*P<0.01 vs. untreated control; †P <0.05 vs. OGD). Each data point represents the mean and SEM from 6 experiments. (F) Representative images demonstrate that OGD led to a significant increase in trypan blue staining, DNA fragmentation, and membrane PS exposure in microglia at 24 hours after OGD compared to untreated control cultures, which was prevented by EPO (10 ng/ml) application. Yet, inhibition of EPO with an EPO blocking antibody (EPO Ab, 2 µg/ml) abrogates the efficacy of EPO on cell survival and apoptotic injury. (G) Quantification of the results in F illustrate that EPO (10 ng/ml) application significant decreased percent trypan blue uptake, DNA fragmentation, and membrane PS exposure 24 hours after OGD when compared to OGD treated alone. Inhibition of EPO with EPO Ab (2 µg/ml) diminishes the efficacy of EPO with a decrease in cell survival and an increase in percent apoptotic DNA fragmentation and PS exposure (*P < 0.01 vs. untreated control; †P <0.05 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
We next investigated the ability of EPO to prevent microglial cell injury following OGD exposure. EPO (0.1, 1, 10, 50 and 100 ng/ml) was given 1 hour prior to OGD and cell survival was determined 24 hours after OGD by the trypan blue dye exclusion method. As shown in Figures 1C and 1D, EPO at the concentrations of 1, 10, 50, and 100 ng/ml significantly reduced trypan blue uptake in microglia and the concentration of 10 ng/ml provided the highest cell survival for microglia, which was used for the later experiments. Concentrations lower than 0.1 ng/ml did not improve cell survival during OGD (Fig. 1D).
Interestingly, pre-treatment of EPO appears to provide the highest level of cell protection when applied 1 hour prior to OGD exposure with cell survival increasing from 38 ± 4% in cells exposed to OGD alone to 63 ± 5% with EPO (Fig. 1E). However, other pre-treatment regimens at 6, 12, and 24 hours prior to OGD also increased microglial cell survival from 38 ± 4% in microglia exposed to OGD alone to 58 ± 5%, 55 ± 4%, and 53 ± 5% respectively, but administration of EPO closest to the point of OGD exposure yielded the greatest degree of cytoprotection for microglia (Fig. 1E). We therefore utilized a 1 hour application of EPO prior to OGD exposure for subsequent studies.
EPO is Necessary to Offer Protection Against Apoptotic Injury in Microglia During OGD Exposure
We next investigated whether specific antagonism against exogenous EPO application with an antibody to EPO (EPO Ab) could neutralize the protective capacity of EPO. EPO Ab (2 µg/ml) in conjunction with EPO (10 ng/ml) was applied to microglial cultures 1 hour prior to a 6 hour period of OGD. Prior studies have shown that concentrations of EPO Ab of 0.50 and greater can significantly neutralize the protective capacity of EPO in other cell systems [40]. Microglial cells were exposed to OGD and either cellular genomic DNA fragmentation was assessed with TUNEL or cellular membrane PS exposure was determined by annexin V labeling method 24 hours later. In Figs. (1F and 1G), representative images demonstrate that OGD leads to DNA fragmentation and membrane PS externalization in microglia, but that pretreatment with EPO (10 ng/ml) 1 hour prior to OGD significantly prevent DNA nuclear condensation and membrane PS exposure. Yet, inhibition of EPO with EPO blocking antibody (EPO Ab, 2 µg/ml) abrogated EPO protection for apoptotic microglial injury. Application of EPO Ab alone did not significantly alter microglial survival when compared to untreated control cultures (data not shown). Quantitative results illustrate that EPO (10 ng/ml) application significant decreased trypan blue dye uptake, DNA fragmentation, and membrane PS exposure 24 hours after OGD. Combined application of EPO with EPO Ab (2 µg/ml) significantly blocks the protective capacity of EPO resulting in a decrease in cell survival and an increase in apoptotic DNA fragmentation and PS exposure (Figs. 1F and 1G).
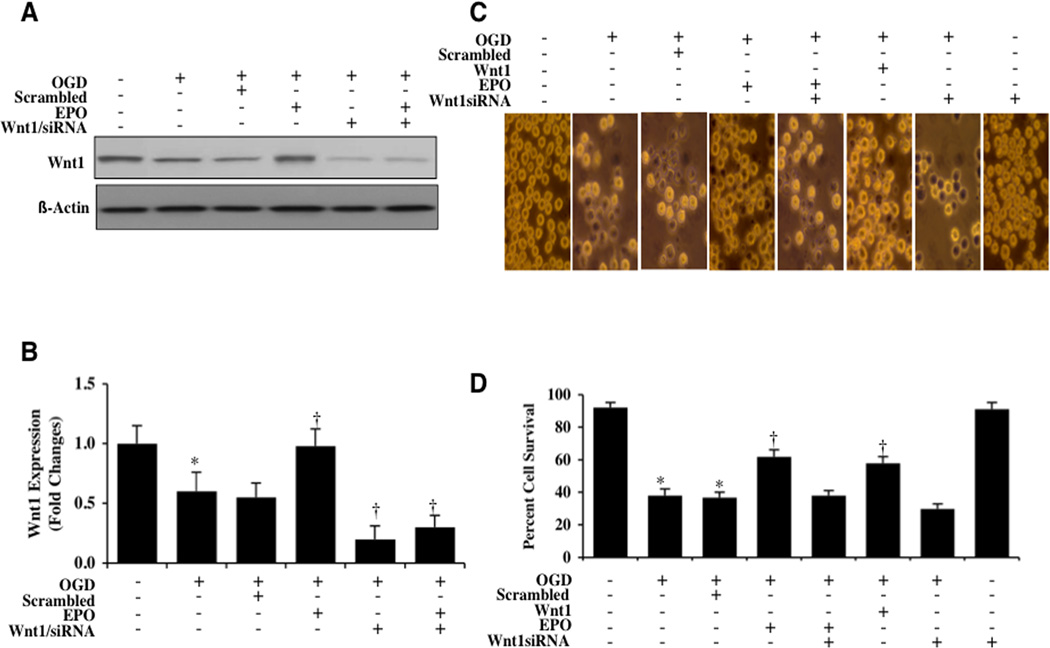
EPO Maintains Expression of Wnt1 During OGD
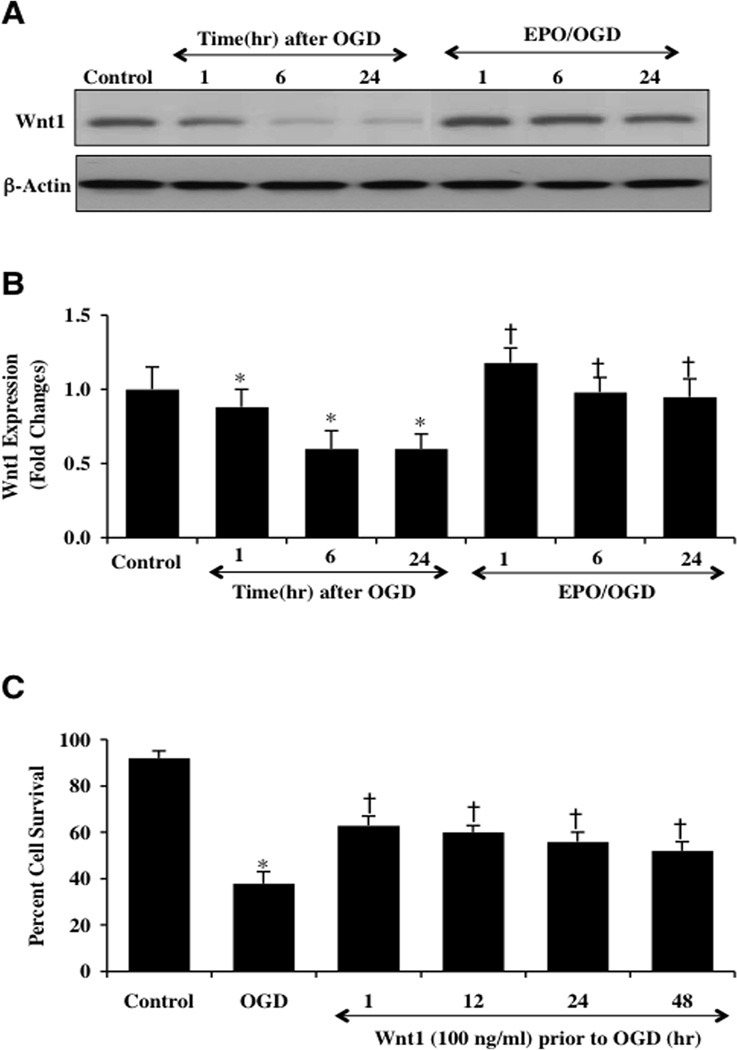
Western blot assay was performed for the endogenous cellular expression of Wnt1 at 1, 6, and 24 hours following a 6 hour period of OGD. A representative Western blot in Fig. (2A) demonstrates that Wnt1 expression was present within 1 hour after OGD, but the expression of Wnt1 was diminished within 6 and 24 hours following OGD exposure (Figs. 2A and 2B). Application of EPO (10 ng/ml) in microglia significantly maintained the expression of Wnt1 at 1, 6, and 24 hours after OGD exposure (Figs. 2A and 2B), suggesting that EPO can prevent the degradation of Wnt1 during OGD.
Fig. (2). EPO maintains the expression of Wnt1 and the highest microglial survival occurs with early Wnt1 application.
(A and B) Microglial protein extracts (50 µg/lane) were immunoblotted with anti-Wnt1 at 1, 6 and 24 hours after OGD. Wnt1 expression was progressively reduced over 24 hours after OGD exposure (*P<0.01 vs. control). In contrast, EPO (10 ng/ml) given 1 hour prior to OGD significantly increased Wnt1 expression at 1, 6 and 24 hours respectively compared with OGD alone (†P <0.01 vs. OGD). (C) Wnt1 was applied to microglial cultures 1, 12, 24, or 48 hours prior to a 6 hour period of OGD and cell survival was determined 24 hours after OGD with the trypan blue dye exclusion method. Wnt1 application significantly increased cell survival 24 hours following OGD with maximal efficacy with a 1 hour pretreatment (*P<0.01 vs. untreated control; †P <0.01 vs. OGD). Each data point represents the mean and SEM from 3 experiments.
Wnt1 Prevents Microglial Injury Following OGD Exposure
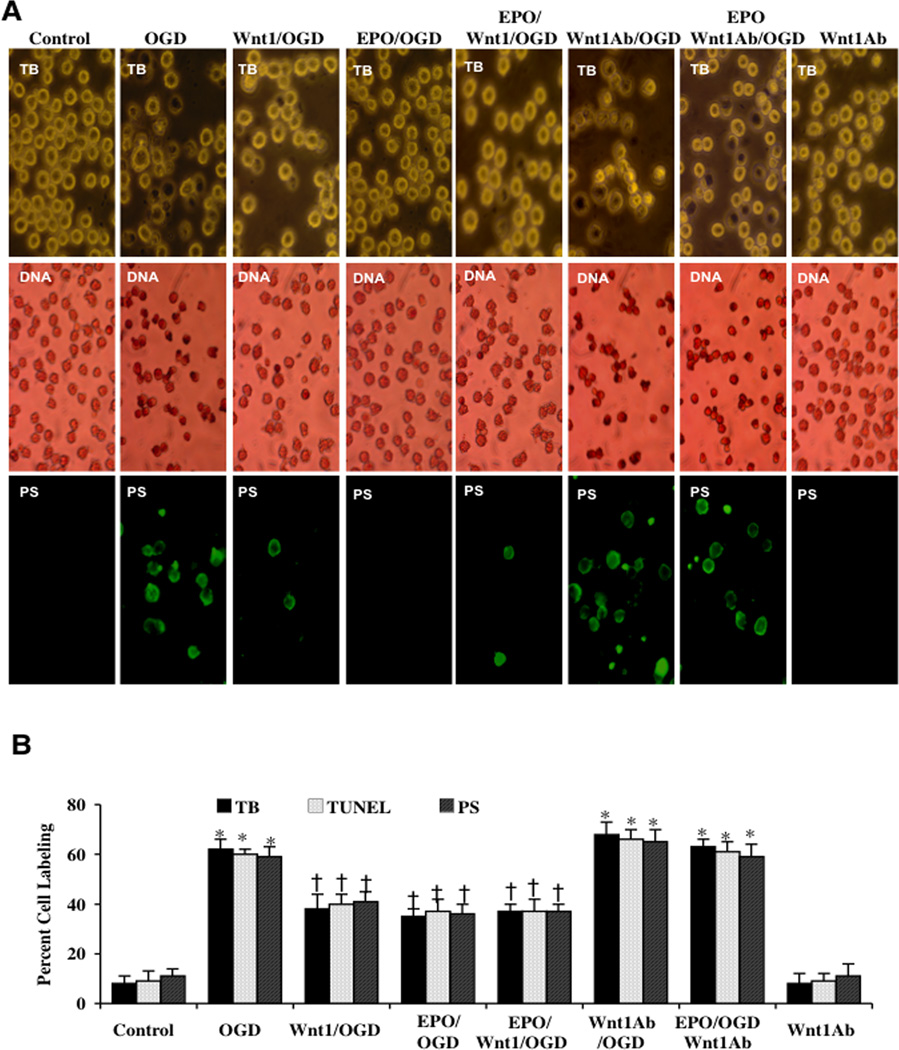
Application of recombinant human Wnt1 protein (100 ng/ml) in microglia at 1, 12, 24, or 48 hours prior to OGD significantly increased cell survival as assessed by trypan blue dye uptake. The greatest degree of protection was achieved with a 1 hour pre-treatment period of Wnt1 (Fig. 2C). In Figs. (3A and 3B), microglial cell survival and apoptosis were assessed with trypan blue staining, apoptotic genomic DNA fragmentation (TUNEL), and membrane PS exposure (Annexin V staining) 24 hours following OGD exposure. As shown in Fig. (3A), representative images demonstrate that untreated control microglia have minimal trypan blue dye uptake, TUNEL staining, or Annexin V staining. In contrast, exposure to OGD leads to a significant increase in trypan blue staining, DNA fragmentation and membrane PS exposure in microglia 24 hours after OGD. Application of EPO (10 ng/ml) applied 1 hour prior to OGD significantly reduced trypan blue staining, DNA fragmentation, and membrane PS exposure. Furthermore, Wnt1 administration (100 ng/ml) alone or in combination with EPO provided a similar degree of protection as EPO alone, suggesting that EPO and Wnt1 may be dependent upon similar protective pathways (Figs. 3A and 3B).
Fig. (3). Wnt1 antibody administration decreases the ability of EPO and Wnt1 to protect microglia against OGD.
(A) Microglial cells were exposed to OGD for 6 hours and cell survival, DNA fragmentation, and PS exposure were determined 24 hours after OGD with the trypan blue dye exclusion method, TUNEL, and annexin V labeling method respectively. Representative images illustrate that Wnt1 (100 ng/ml) and EPO (10 ng/ml) administration during OGD significantly reduced trypan blue staining, genomic DNA degradation, and membrane PS externalization (green fluorescence). In contrast, blockade of Wnt1 with Wnt1 Ab (1 µg/ml) resulted in increased trypan blue staining, DNA fragmentation, and membrane PS exposure and also attenuated the protective ability of EPO. Combined Wnt1 and EPO application yielded a similar protection to EPO applied only during OGD. (B) Quantification of data illustrates that percent trypan blue staining, DNA fragmentation, and membrane PS externalization were significantly increased following a 6 hour period of OGD when compared to untreated microglial control cultures, but Wnt1 (100 ng/ml), EPO (10 ng/ml), or EPO/Wnt1 combined therapy increased cell survival and prevented DNA fragmentation and membrane PS exposure during OGD. Inhibition of Wnt1 with Wnt1 Ab (1µg/ml) abrogates the efficacy of EPO during OGD (*P < 0.01 vs. untreated control; †P < 0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
Blockade of Wnt1 Inhibits EPO Cytoprotection in Microglia During OGD
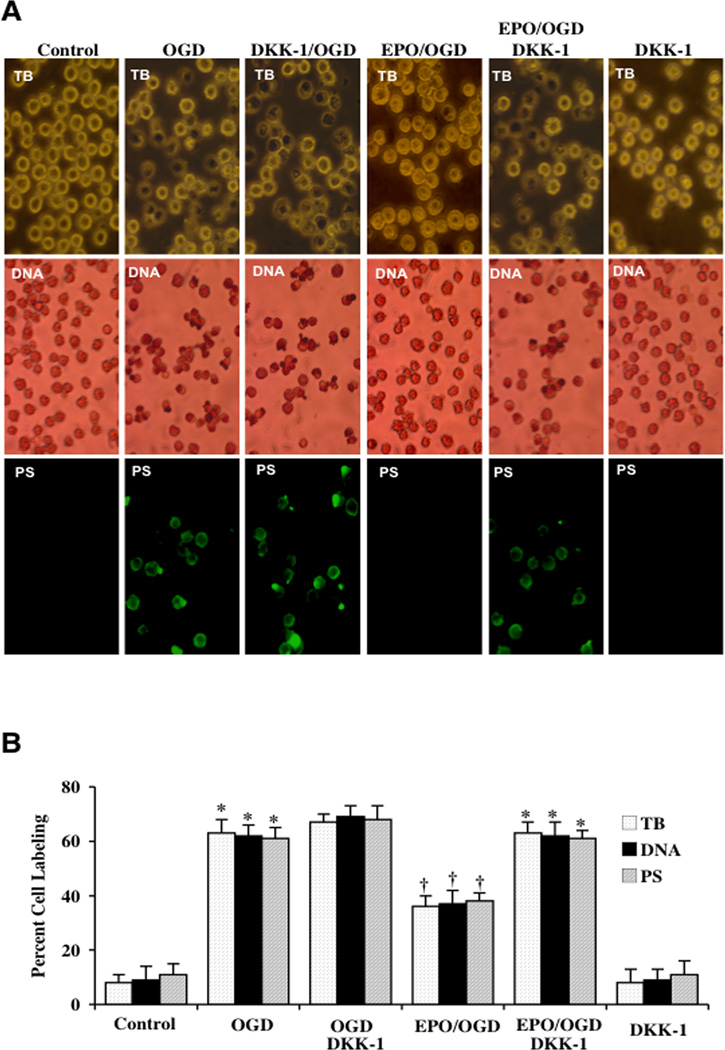
Application of Wnt1 Ab (1 µg/ml) (Fig. 3A) or the Wnt antagonist DKK-1 (500 ng/ml) (Fig. 4A) with EPO increased cell injury, DNA fragmentation, and PS membrane exposure following OGD when compared with OGD alone, suggesting that endogenous Wnt1 activity may offer a level of protection against OGD exposure. Wnt1 Ab or DKK-1 applied to untreated control cultures were not toxic. Protection by EPO was significantly reduced during blockade of Wnt1 with Wnt1 Ab or during DKK-1 application. Quantification of results illustrate that OGD led to a significant increase in percent trypan blue staining (60 ± 4%), DNA fragmentation (59 ± 3%) and membrane PS exposure (58 ± 5%) in microglia 24 hours after OGD when compared to untreated control cultures for trypan blue staining, for DNA staining, and for PS staining respectively (Figs. 3B and 4B). Application of EPO (10 ng/ml) or Wnt1 (100 ng/ml) significantly decreased percent trypan blue staining, DNA fragmentation, and membrane PS exposure. Yet, protection with EPO was markedly reduced during Wnt1 Ab treatment or DKK-1 application (Figs. 3B and 4B).
Fig. (4). Wnt antagonist DKK-1 blocks protection by EPO during OGD.
(A) Microglial cells were exposed to OGD for 6 hours and cell survival, DNA fragmentation, and PS exposure were determined 24 hours after OGD with the trypan blue dye exclusion method, TUNEL, and annexin V labeling respectively. Representative images illustrate that EPO (10 ng/ml) administration during OGD significantly reduced trypan blue staining, genomic DNA degradation, and membrane PS externalization (green fluorescence). In contrast, blockade of Wnt cell signaling with DKK-1 (500 ng/ml) resulted in increased trypan blue staining, DNA fragmentation, and membrane PS exposure and also blocked protection by EPO during OGD exposure. DKK-1 (500 ng/ml) application alone to microglial cultures increased cell injury during OGD. (B) Quantification of data illustrates that percent trypan blue staining, DNA fragmentation, and membrane PS externalization were significantly increased following a 6 hour period of OGD when compared to untreated microglial control cultures, but EPO (10 ng/ml) increased cell survival and prevented apoptotic DNA fragmentation and membrane PS exposure during OGD. DKK-1 application abrogates the protective capacity of EPO during OGD (*P < 0.01 vs. untreated control; †P < 0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments (*P < 0.01 vs. untreated control; †P < 0.01 vs. OGD).
Gene Silencing of Wnt1 Abrogates the Protective Capacity of EPO Against OGD
As shown in Figs. (5A and 5B), expression of Wnt1 was significantly decreased at 6 hours following OGD exposure. Application of EPO (10 ng/ml) prior to OGD maintained the expression of Wnt1. Yet, transfection with Wnt1 siRNA in microglia resulted in significant reduction of the expression of Wnt1 protein as revealed with Western blot analysis at 6 hours after OGD (Figs. 5A and 5B). As a control, non-specific scrambled siRNA did not alter Wnt1 protein expression in untreated control microglia or microglia exposed to OGD, demonstrating the specificity of Wnt1 siRNA to block protein expression of Wnt1.
Fig. (5). Gene silencing of Wnt1 abrogates the protective capacity of EPO against OGD.
(A and B) Gene silencing of Wnt1 was performed with transfection of Wnt1 siRNA prior to OGD in microglia. Wnt1 expression was determined at 6 hours following a 6 hour period of OGD. Transfection with Wnt1 siRNA significantly reduced expression of Wnt1 following a 6 hour period of OGD or during EPO (10 ng/ml) application with OGD, but non-specific scrambled siRNA did not alter Wnt1 expression (*P < 0.01 vs. OGD). In B, western band intensity was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image). (C) Gene silencing of Wnt1 was performed with transfection of Wnt1 siRNA prior to OGD in microglia and cell survival was determined by using trypan blue dye exclusion method 24 hours following a 6 hour period of OGD. Transfection with Wnt1 siRNA significantly increased cell staining during OGD and prevented protection by EPO (10 ng/ml) during OGD exposure resulting in increased trypan blue staining. Non-specific scrambled siRNA did not alter trypan blue staining during OGD. (D) Transfection with Wnt1 siRNA in microglia prior to OGD significantly reduced cell survival and blocked the ability of EPO (10 ng/ml) to protect microglia during OGD (*P < 0.01 vs. untreated control; †P<0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
We next assessed the ability of EPO to offer protection during gene silencing of Wnt1. As shown in Fig. (5C), representative images demonstrate that OGD leads to a significant increase in trypan blue staining in microglia 24 hours following OGD exposure. EPO (10 ng/ml) or Wnt1 (100 ng/ml) prevented cell injury during OGD. However, gene knockdown of Wnt1 with siRNA significantly increased cell injury when compared with OGD alone, suggesting that endogenous Wnt1 protein is necessary for microglial protection (Fig. 5D). In addition, gene knockdown of Wnt1 with siRNA during EPO application significantly reduced the protective capacity of EPO, further supporting that Wnt1 is necessary for EPO cytoprotection in microglia (Fig. 5D).
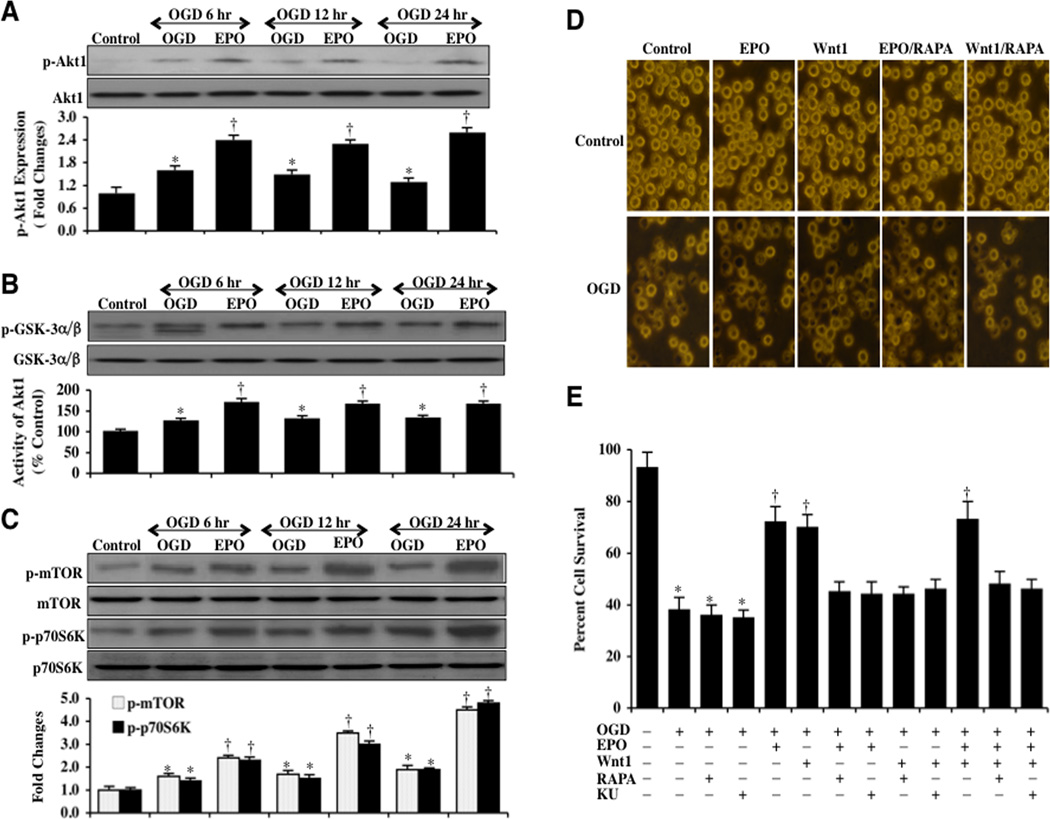
EPO Activates Akt1 and Requires mTOR to Protect Microglia Against OGD
Western blot assay for the cellular expression of p-Akt1 (active) was performed following OGD exposure. As shown in Fig. (6A), the expression of p-Akt1 was slightly increased at 6 hours following OGD exposure, but was progressively lost over a 24 hour course. Application of EPO (10 ng/ml) 1 hour prior to OGD significantly increased and maintained the expression of p-Akt1 over a 24 hour course following OGD (Fig. 6A). Similarly, EPO administration 1 hour prior to OGD also significantly increased and maintained the activity of Akt1 determined by the expression of p-GSK-α/β when compared to microglia exposed to OGD only (Fig. 6B).
Fig. (6). EPO activates Akt1 and employs mTOR to protect microglia against OGD.
(A) Microglial protein extracts (50 µg/lane) were immunoblotted with p-Akt1 (active form) at 6, 12, and 24 hours following a 6 hour period of OGD. OGD resulted in a slight increase in the expression of p-Akt1 but was progressively lost over a 24 hour period. EPO (10 ng/ml) with a 1 hour pretreatment significantly increased the expression of p-Akt1 (*P <0.01 vs. Control; †P<0.01 vs. OGD of corresponding time point). In all cases, each data point represents the mean and SEM from 6 experiments. (B) The activity of Akt1 was determined by assessing the expression of p-GSK-3α/β after incubation of the substrate GSK-3 infusion protein with protein extracts from microglia following OGD. EPO (10 ng/ml) with 1 hour pretreatment significantly increased the activity of Akt1 over 24 hours following a 6 hour period of OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD of corresponding time point). In all cases, each data point represents the mean and SEM from 6 experiments. (C) Microglial protein extracts (50 µg/lane) were immunoblotted with phosphorylated (p)-mTOR (Ser2448) and p-p70S6K (Th389) antibodies at 6, 12, and 24 hours following a 6 hour period of OGD. OGD resulted in a slight increase in the expression of p-mTOR and p-p70S6K, but a 1 hour pretreatment with EPO (10 ng/ml) significantly increased and maintained the expression of p-mTOR and p-p70S6K over a 24 hour period following OGD exposure (*P <0.01 vs. Control; †P<0.01 vs. OGD of corresponding time point). In all cases, each data point represents the mean and SEM from 3 experiments. (D) EPO (10 ng/ml) or Wnt1 (100 ng/ml) was applied to microglial cultures 1 hour prior to a 6 hour period of OGD and cell survival was determined 24 hours following OGD by using trypan blue dye exclusion method. Representative pictures demonstrate that EPO or Wnt1 application significantly reduced trypan blue staining following OGD. Application of the mTOR specific inhibitor rapamycin (RAPA, 20 nM) combined with EPO (10 ng/ml) administration blocked protection by EPO resulting in an increased staining of trypan blue in microglia. (E) Quantification of data illustrates that microglial cell survival was significantly decreased 24 hours following a 6 hour period of OGD when compared to untreated microglial control cultures. In contrast, EPO (10 ng/ml), Wnt1 (100 ng/ml) or EPO combined with Wnt1 administration significantly increased cell survival to a similar level. In contrast, the mTOR specific inhibitors rapamycin (RAPA, 20 ng/ml) or Ku 0063794 (KU, 100 ng/ml) blocked protection by EPO, Wnt1 or EPO combined with Wnt1 during OGD resulting in a decrease in microglial cell survival (*P < 0.01 vs. untreated control; †P < 0.01 vs. OGD). Each data point represents the mean and SEM from 6 experiments.
Downstream from Akt1, mTOR and p70S6K are phosphorylated and activated [48, 60]. Therefore, we next investigated the ability of EPO (10 ng/ml) to alter the mTOR signaling pathway. Western blot assay for the expression of p-mTOR (active) and p-p70S6K (active) were performed following OGD. As shown in Fig. (6C), the expression of p-mTOR and p-p70S6K were slightly increased 6, 12, and 24 hours following OGD exposure. In contrast, treatment with EPO (10 ng/ml) prior to OGD significantly increased and maintained the expression of p-mTOR and p-p70S6K over a 24 hour period following OGD exposure (Fig. 6C).
Given that EPO activates the Akt1 pathways of mTOR and p70S6K, we assessed whether either EPO or Wnt1 require mTOR to foster protection of microglia during OGD. Microglial cell survival was assessed with the trypan blue dye exclusion method 24 hours following OGD exposure. Representative images demonstrate that untreated control microglia were with minimal trypan blue staining (Fig. 6D), but OGD leads to a significant increase in trypan blue staining in microglia 24 hours after OGD. Both EPO (10 ng/ml) and Wnt1 (100 ng/ml) prevent trypan blue uptake in microglia during OGD. However, inhibition of mTOR activity with the specific inhibitors rapamycin (RAPA, 20 nM) and Ku 0063794 (KU, 100 nM) significantly blocks protection by EPO and Wnt1 (Figs. 6D and 6E). Furthermore, combined application of EPO (10 ng/ml) and Wnt1 (100 ng/ml) failed to protect microglia in the presence of either rapamycin or Ku 0063794, supporting the premise that EPO as well as Wnt1 rely upon mTOR pathways to offer cytoprotection to microglia (Fig. 6E).
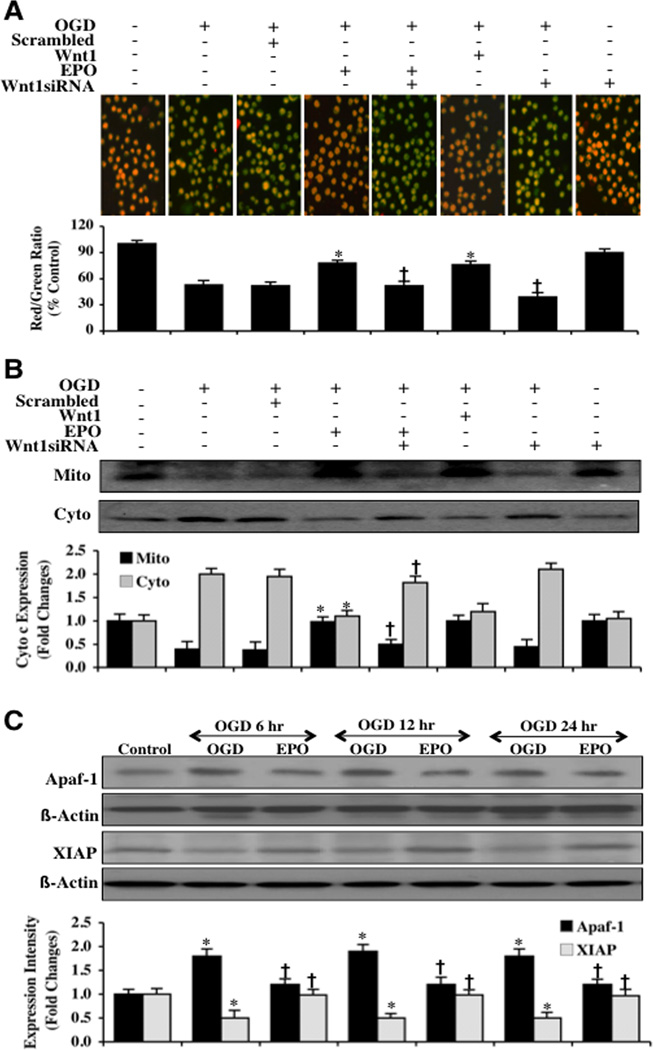
EPO Utilizes Wnt1 to Modulate Mitochondrial Depolarization, Cytochrome c Release, and Apaf-1 and XIAP Expression in Microglia During OGD
Using the cationic membrane potential indicator JC-1, we determined mitochondrial depolarization in microglia during OGD exposure. In Fig. (7A), OGD exposure produces a significant decrease in the microglia mitochondrial red/green fluorescence intensity ratio at 6 hours after OGD (53 ± 5%) when compared to untreated control mitochondria (100 ± 4%). Gene knockdown of Wnt1 with siRNA (Fig. 7A) during OGD further decreased mitochondrial membrane red/green fluorescence ratio to 39 ± 5%, suggesting that endogenous Wnt1 also provides protection against mitochondrial depolarization. EPO (10 ng/ml) or Wnt1 (100 ng/ml) administration 1 hour prior to OGD significantly increased the red/green fluorescence intensity of the mitochondria to 78 ± 34% and 76 ± 4% respectively, illustrating that EPO and Wnt1 can significantly improve mitochondrial permeability transition pore membrane potential. The ability of EPO to control mitochondrial depolarization was dependent upon the presence of Wnt1 since gene knockdown of Wnt1 with siRNA blocked the ability of EPO to prevent mitochondrial depolarization to 52 ±5% (Fig. 7A). Non-specific scrambled siRNA did not alter mitochondrial depolarization during OGD when compared to OGD alone (Fig. 7A).
Fig. (7). EPO through Wnt1 inhibits mitochondrial depolarization and subsequent cytochrome c release and modulates the expression of Apaf-1 and XIAP.
(A) OGD produced a significant decrease in the red/green fluorescence intensity ratio of mitochondria using a cationic membrane potential indicator JC-1 within 6 hours when compared with untreated control cultures demonstrating that OGD results in mitochondrial membrane depolarization. EPO (10 ng/ml) or Wnt1 (100 ng/ml) application during OGD prevented mitochondrial depolarization and significantly increased the red/green fluorescence intensity of mitochondria in microglia. In contrast, inhibition of Wnt1 with transfection of Wnt1 siRNA (siRNA) increased mitochondrial membrane depolarization to a greater degree than OGD alone and blocked the ability of EPO to prevent mitochondrial depolarization during OGD. The relative ratio of red/green fluorescent intensity of mitochondrial staining was measured in 6 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (untreated microglia = Control, *P<0.01 vs. OGD; †P <0.01 vs. EPO/OGD). (B) Equal amounts of mitochondrial (Mito) or cytosol (Cyto) protein extracts (50 µg/lane) were immunoblotted demonstrating that EPO or Wnt1 administration significantly prevented cytochrome c release from mitochondria during OGD (*P<0.01 vs. OGD; †P <0.01 vs. EPO/OGD). Transfection with Wnt1 siRNA blocked the ability of EPO to prevent mitochondrial release of cytochrome c. Non-specific scrambled siRNA did not affect mitochondrial depolarization. Each data point represents the mean and SEM from 6 experiments. (C) Microglial protein extracts (50 µg/lane) were immunoblotted with Apaf-1 and XIAP antibodies 6, 12, and 24 hours following OGD. OGD significantly increased Apaf-1 and significantly decreased XIAP expression. In contrast, EPO (10 ng/ml) application decreased Apaf-1 and increased XIAP expression following OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM from 3 experiments.
In Fig. (7B), subsequent cytochrome c release from mitochondria was determined by Western blot for cytochrome c expression in both mitochondrial and cytosol extractions. Following OGD exposure, a significant release of cytochrome c from the mitochondria occurred indicated by increased expression of cytochrome c in cytosol. Gene knockdown of Wnt1 further increased the release of cytochrome c into the cytosol, further supporting an endogenous protective role for Wnt1 (Fig. 7B). Yet, EPO (10 ng/ml) or Wnt1 (100 ng/ml) administration prevented cytochrome c release from the mitochondria. Furthermore, Wnt1 was necessary for EPO to prevent cytochrome c release from the mitochondria since transfection of Wnt1 siRNA blocked the ability of EPO to prevent cytochrome c release from the mitochondria (Fig. 7B).
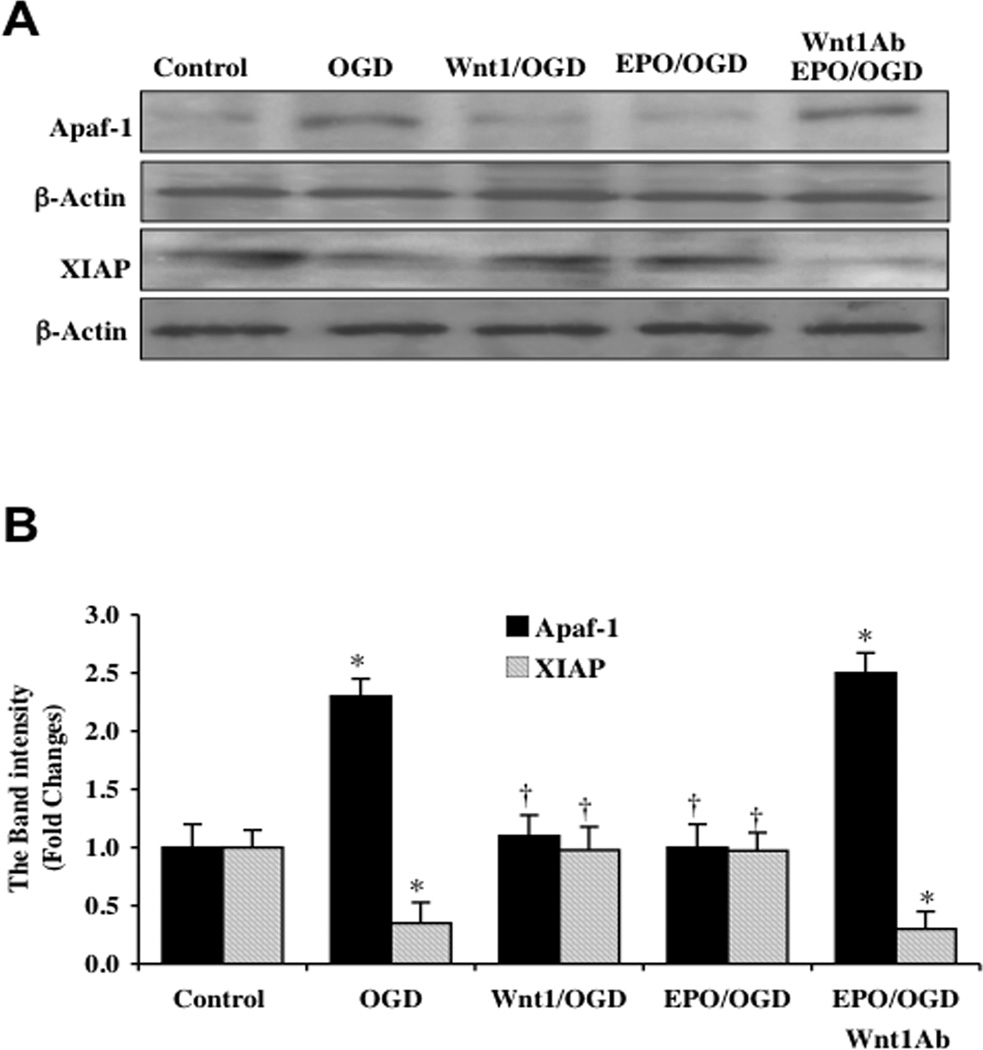
Since mitochondrial release of cytochrome c is an important mechanism in the apoptotic cascade that can lead to caspase activation [61–66], we next examined the role of two critical components in this pathway for EPO and Wnt1, namely apoptotic protease activating factor-1 (Apaf-1) and X-linked inhibitor of apoptosis protein (XIAP). Cytochrome c in the cytoplasm binds to Apaf-1 leading to the oligomerization of Apaf-1 under the assistance of dATP/ATP and the subsequent activation of the apoptotic cascade [20]. In addition, EPO has previously been shown to prevent apoptosis through parallel pathways that prevent the induction of Apaf-1 and caspase 9 [67]. In regards to XIAP, XIAP binds to caspases such as caspase 9 to block caspase activity [2, 68]. Western blot assay was performed for Apaf-1 and XIAP (Figs. 7C, 8A, and 8B). At 6, 12, and 24 hours following OGD, Apaf-1 expression was significantly increased and XIAP expression was significantly decreased (Fig. 7C). However, EPO (10 ng/ml) (Figs. 7C, 8A, and 8B) or Wnt1 (100 ng/ml) (Figs. 8A and 8B) administered prior to OGD significantly reduced the expression of Apaf-1 and increased the expression of XIAP, suggesting that both EPO and Wnt1 block apoptotic downstream pathways from mitochondrial depolarization through inhibition of Apaf-1 expression and enhancement of XIAP expression. Furthermore, Wnt1 is necessary for EPO to modulate Apaf-1 and XIAP expression since application of Wnt1 Ab (1 µg/ml) with EPO blocks the ability of EPO during OGD exposure to decrease Apaf-1 expression (Figures 8A and 8B) and increase XIAP expression (Figs. 8A and 8B).
Fig. (8). EPO requires Wnt1 to govern expression of Apaf-1 and XIAP in microglia during OGD.
Microglial protein extracts (50 µg/lane) were immunoblotted with Apaf-1 and XIAP antibodies 6 hours following a 6 hour period of OGD exposure. (A and B) A representative Western blot demonstrates that OGD exposure significantly increased Apaf-1 and significantly decreased XIAP expression. Application of EPO (10 ng/ml) 1 hour prior to OGD significantly decreased Apaf-1 and significantly increased XIAP expression following OGD. In addition, Wnt1 is necessary for EPO to control Apaf-1 and XIAP expression since application of Wnt1 Ab (1 µg/ml) with EPO blocks the ability of EPO during OGD exposure to decrease Apaf-1 expression (Figs. 8A and 8B) and increase XIAP expression. In B, quantification of western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (*P <0.01 vs. Control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM from 3 experiments.
DISCUSSION
EPO has broad protective abilities in a number of cell types related to the nervous system that include neurons [10, 36, 40, 69–74], vascular cells, [39, 41, 47, 49, 67, 75–78], and non-neuronal cells [10, 37, 74, 79]. Separately, the Wnt signaling pathway [44, 80] has been shown to protect against dopamine loss in models of Parkinson’s disease [81, 82], reduce cardiomyocyte injury [83], promote hematoendothelial cell development [84], stimulate neurite extension [85], improve neurological function after injury [46], protect against amyloid neurodegeneration [56], and promote microglial integrity [15]. Interestingly, prior studies have illustrated that EPO relies upon Wnt1 to block endothelial injury in models of diabetes [38, 47].
Our present work demonstrates that Wnt1 with EPO has much broader implications in other cell types as well as models of oxidative stress with OGD. EPO maintains the expression of Wnt1 in microglial cells during oxidant stress. In addition, endogenous Wnt1 in microglia is critical to preserve microglial survival, since loss of Wnt1 alone increases microglial injury during oxidative stress. EPO requires the presence of Wnt1 in microglia to block early apoptotic PS exposure and subsequent nuclear DNA fragmentation, since blockade of Wnt1 signaling or gene silencing of Wnt1 eliminates the protective capacity of EPO. Prevention of early apoptotic PS exposure in cells can be critical for their function and survival. If left unchecked, cells tagged by PS can be removed and destroyed by inflammatory cells during acute or chronic insults and lead to disability via loss of functional cells [11, 14, 53, 86–89]. In this regard, benefits of inflammatory cell activation during oxidative stress could be lost in the absence of the necessary signaling pathways of EPO and Wnt1.
The protective pathways of EPO and Wnt1 intersect at the level of Akt1. Akt1 controls multiple cellular mechanisms that involve processes such metabolism, vascular disease, neurodegeneration, and inflammation [1, 64, 68, 86, 90]. Under most circumstances, Akt increases cell survival, may promote tumorigenesis, but is protective against toxic insults [52, 91–96]. EPO relies upon the expression and activation of Akt1 for cytoprotection [38, 39, 97–101]. Interestingly, Wnt also has recently been shown to utilize Akt1 which previously was not considered in the Wnt signaling canonical and non-canonical pathways [38, 42, 46, 56, 83, 102–104]. Here we show that EPO and Wnt1 phosphorylate and activate Akt1 that is necessary for microglial cytoprotection during oxidative stress.
Furthermore, we demonstrate that the downstream activetion of mTOR and p70S6K by both EPO and Wnt1 are critical components for maintaining microglial integrity during oxidative stress. Prior work has shown that mTOR signaling protects neurons during oxidative stress [48, 60, 105–107], limits cardiomyocyte injury [108], may control synaptic plasticity [109], and can foster microglial activation and survival [16, 48, 60]. In addition, Wnt1 may employ mTOR to control hair follicle proliferation [110] while EPO may rely upon mTOR for bone formation [111] and to affect renal cell survival at lower concentrations [112]. Our demonstration that mTOR and p70S6K are governed by EPO and Wnt1 for microglial survival opens new possibilities for modulation of immune cell response during neurodegenerative insults.
EPO also relies upon Wnt1 to modulate mitochondrial permeability as well as the expression of Apaf-1 and XIAP in microglial cells. Loss of mitochondrial permeability leads to cytochrome c release and the induction of apoptotic and autophagic cascades [2, 61, 62, 66, 113–118]. We illustrate that both EPO and Wnt1 can block mitochondrial membrane depolarization and the release of cytochrome c, similar to prior studies with EPO [38, 41, 67, 101, 119] or Wnt1 [15, 38, 46]. However, we also demonstrate that Wnt1 is necessary for EPO to control these pathways in microglia during oxidative stress, since loss of Wnt1 during gene silencing abrogates the ability of EPO to control mitochondrial permeability and cytochrome release.
Given that EPO through Wnt1 can modulate microglial cell death though mitochondrial pathways, we also examined the role of Apaf-1 and XIAP. In vascular cells, EPO blocks cell death by preventing Apaf-1 and caspase 9 activation [67]. EPO may employ XIAP in this process since XIAP can block caspase 9 activity [2, 68] and prior work has shown an up-regulation of XIAP by EPO in renal cells [120]. We now show that EPO as well as Wnt1 in microglial cells significantly reduce Apaf-1 expression and increase XIAP expression during oxidative stress, suggesting that EPO and Wnt1 prevent the induction of microglial apoptosis not only through the control of mitochondrial membrane permeability, but also through downstream apoptotic pathways that involve Apaf-1 and XIAP. In addition, Wnt1 is required for EPO to modulate Apaf-1 and XIAP expression, since blockade of Wnt1 signaling eliminates the ability of EPO to control Apaf-1 or XIAP expression during oxidative stress.
ACKNOWLEDGEMENTS
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association [National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS [P30 ES06639), NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010 Mar;45(3):217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005 Feb;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrmann M, Bittner T, Jung CK, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010 Mar;21 doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiese K, Chong ZZ, Hou J, et al. New strategies for Alzheimer's disease and cognitive impairment. Oxid Med Cell Longev. 2009 Nov–Dec;2(5):279–289. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer's disease. J Mol Med. 2009 Jul;87(7):697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 6.Nerurkar PV, Johns LM, Buesa LM, et al. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang J, Takeuchi H, Jin S, et al. Glutamate induces neurotrophic factor production from microglia via protein kinase C pathway. Brain Res. 2010 Mar 31;1322:8–23. doi: 10.1016/j.brainres.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 8.Madinier A, Bertrand N, Mossiat C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS ONE. 2009;4(12):e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong ZZ, Kang J, Li F, et al. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005 Jul;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003 Mar;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang YC, Chong ZZ, Hou J, et al. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009 Nov;6(4):223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey TJ, Fossum SL, Fimbel SM, et al. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010 Nov;91(5):601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Kim HY, Kim H, et al. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free Radic Biol Med. 2009 Jun 15;46(12):1694–1702. doi: 10.1016/j.freeradbiomed.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Shang YC, Chong ZZ, Hou J, et al. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang YC, Chong ZZ, Hou J, et al. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010 Sep;22(9):1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dello RC, Lisi L, Tringali G, et al. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. 2009 Nov 1;78(9):1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 17.Kumral A, Tuzun F, Oner MG, et al. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev. 2011 Sep;33(8):632–643. doi: 10.1016/j.braindev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Lanfranconi S, Locatelli F, Corti S, et al. Growth factors in ischemic stroke. J Cell Mol Med. 2011 Aug;15(8):1645–1687. doi: 10.1111/j.1582-4934.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78(1):41–53. doi: 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Li F, et al. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008 Jun;85(2):194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008 Apr;19(2):145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiese K, Hou J, Chong ZZ, et al. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. Scientific World Journal. 2009;9:1072–1104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005 Jan 5;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walden AP, Young JD, Sharples E. Bench to bedside: A role for erythropoietin in sepsis. Crit Care. 2010;14(4):227. doi: 10.1186/cc9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contaldo C, Elsherbiny A, Lindenblatt N, et al. Erythropoietin enhances oxygenation in critically perfused tissue through modulation of nitric oxide synthase. Shock. 2009 Jun;31(6):599–606. doi: 10.1097/SHK.0b013e31818b9cc4. [DOI] [PubMed] [Google Scholar]
- 26.Hamed S, Egozi D, Kruchevsky D, et al. Erythropoietin improves the survival of fat tissue after its transplantation in nude mice. PLoS ONE. 2010;5(11):e13986. doi: 10.1371/journal.pone.0013986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferri C, Giuggioli D, Sebastiani M, et al. Treatment of severe scleroderma skin ulcers with recombinant human erythropoietin. Clin Exp Dermatol. 2007 May;32(3):287–290. doi: 10.1111/j.1365-2230.2007.02363.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang YK, Choi DE, Na KR, et al. Erythropoietin attenuates renal injury in an experimental model of rat unilateral ureteral obstruction via anti-inflammatory and anti-apoptotic effects. J Urol. 2009 Mar;181(3):1434–1443. doi: 10.1016/j.juro.2008.10.105. [DOI] [PubMed] [Google Scholar]
- 29.Cuzzocrea S, Mazzon E, di Paola R, et al. Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum. 2005 Mar;52(3):940–950. doi: 10.1002/art.20875. [DOI] [PubMed] [Google Scholar]
- 30.Sigounas G, Salleng KJ, Mehlhop PD, et al. Erythropoietin ameliorates chemotherapy-induced fibrosis of the lungs in a preclinical murine model. Int J Cancer. 2008 Jun 15;122(12):2851–2857. doi: 10.1002/ijc.23426. [DOI] [PubMed] [Google Scholar]
- 31.Ucan BH, Irkorucu O, Cakmak GK, et al. Erythropoietin: a possible cytoprotective cytokine in acute necrotizing pancreatitis. J Hepatobiliary Pancreat Surg. 2009;16(4):530–537. doi: 10.1007/s00534-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 32.Thorne M, Moore CS, Robertson GS. Lack of TIMP-1 increases severity of experimental autoimmune encephalomyelitis: Effects of darbepoetin alfa on TIMP-1 null and wild-type mice. J Neuroimmunol. 2009 Jun 25;211(1–2):92–100. doi: 10.1016/j.jneuroim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Pregi N, Wenker S, Vittori D, et al. TNF-alpha-induced apoptosis is prevented by erythropoietin treatment on SH-SY5Y cells. Exp Cell Res. 2009 Feb 1;315(3):419–431. doi: 10.1016/j.yexcr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25(2):183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- 35.Mihaila RG, Rezi EC, Boitan M, et al. Erythropoietin and the pro-inflammatory cytokines in chronic C hepatitis. Hepatogastroenterology. 2009 May–Jun;56(91–92):751–755. [PubMed] [Google Scholar]
- 36.Chong ZZ, Lin SH, Kang JQ, et al. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003 Mar 1;71(5):659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006 Aug;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong ZZ, Hou J, Shang YC, et al. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005 Dec;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou J, Wang S, Shang YC, et al. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011 Aug 1;8(3):220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binet R, Ythier D, Robles AI, et al. WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2009 Dec 15;69(24):9183–9991. doi: 10.1158/0008-5472.CAN-09-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008 April – May;62(4):218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiese K, Li F, Chong ZZ, et al. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008 Apr;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang LH, Xu HT, Han Y, et al. Axin downregulates TCF-4 transcription via beta-catenin, but not p53, and inhibits the proliferation and invasion of lung cancer cells. Mol Cancer. 2010;9:25. doi: 10.1186/1476-4598-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong ZZ, Shang YC, Hou J, et al. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010 Mar–Apr;3(2):153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007 Aug;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007 Feb;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- 49.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007 Apr;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003 Sep;64(3):557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 51.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003 Oct 1;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 52.Chong ZZ, Kang JQ, Maiese K. AKT1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp Cell Res. 2004 Jun 10;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010 May;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 Oct 10;283(41):27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008 Aug;5(3):159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007 Jun;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong ZZ, Lin SH, Li F, et al. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated "anti-apoptotic" pathways. Curr Neurovasc Res. 2005 Oct;2(4):271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39(2):131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- 59.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004 Jul;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 60.Chong ZZ, Shang YC, Zhang L, et al. Mammalian target of rapamycin: hitting the bull' s-eye for neurological disorders. Oxid Med Cell Longev. 2010 Nov–Dec;3(6):374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deruy E, Gosselin K, Vercamer C, et al. MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS One. 2010;5(9):e12712. doi: 10.1371/journal.pone.0012712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011 Aug;45(8):888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 63.Maiese K, Chong ZZ, Shang YC, et al. Therapeutic promise and principles: Metabotropic glutamate receptors. Oxid Med Cell Longev. 2008 Jul 1;1(1):1–14. doi: 10.4161/oxim.1.1.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007 Feb;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maiese K, Shang YC, Chong ZZ, et al. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010 Feb 1;7(1):59–64. doi: 10.2174/156720210790820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J, Song R, Song W, et al. Chlorpromazine protects against apoptosis induced by exogenous stimuli in the developing rat brain. PLoS ONE. 2011;6(7):e21966. doi: 10.1371/journal.pone.0021966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003 Mar;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 68.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005 Jul;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keswani SC, Bosch-Marce M, Reed N, et al. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):4986–4990. doi: 10.1073/pnas.1019591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kollensperger M, Krismer F, Pallua A, et al. Erythropoietin is neuroprotective in a transgenic mouse model of multiple system atrophy. Mov Disord. 2011 Feb 15;26(3):507–515. doi: 10.1002/mds.23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kook YH, Ka M, Um M. Neuroprotective cytokines repress PUMA induction in the 1-methyl-4-phenylpyridinium (MPP(+)) model of Parkinson's disease. Biochem Biophys Res Commun. 2011 Jul 29;411(2):370–374. doi: 10.1016/j.bbrc.2011.06.151. [DOI] [PubMed] [Google Scholar]
- 72.Loeliger MM, Mackintosh A, De Matteo R, et al. Erythropoietin protects the developing retina in an ovine model of endotoxin-induced retinal injury. Invest Ophthalmol Vis Sci. 2011 May;52(5):2656–2661. doi: 10.1167/iovs.10-6455. [DOI] [PubMed] [Google Scholar]
- 73.Simon F, Scheuerle A, Groger M, et al. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med. 2011 Sep;37(9):1525–1533. doi: 10.1007/s00134-011-2303-4. [DOI] [PubMed] [Google Scholar]
- 74.Yamada M, Burke C, Colditz P, et al. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. J Pathol. 2011 May;224(1):101–109. doi: 10.1002/path.2862. [DOI] [PubMed] [Google Scholar]
- 75.Ogino A, Takemura G, Kawasaki M, et al. Erythropoietin receptor signaling mitigates renal dysfunction-associated heart failure by mechanisms unrelated to relief of anemia. J Am Coll Cardiol. 2010 Nov 30;56(23):1949–1958. doi: 10.1016/j.jacc.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 76.Taniguchi N, Nakamura T, Sawada T, et al. Erythropoietin prevention trial of coronary restenosis and cardiac remodeling after ST-elevated acute myocardial infarction (EPOC-AMI): a pilot, randomized, placebo-controlled study. Circ J. 2010 Oct 25;74(11):2365–2371. doi: 10.1253/circj.cj-10-0267. [DOI] [PubMed] [Google Scholar]
- 77.Warren JS, Zhao Y, Yung R, et al. Recombinant human erythropoietin suppresses endothelial cell apoptosis and reduces the ratio of bax to bcl-2 proteins in the aortas of apolipoprotein e-deficient mice. J Cardiovasc Pharmacol. 2011 Apr;57(4):424–433. doi: 10.1097/FJC.0b013e31820d92fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Tian Y, Wei HJ, et al. Erythropoietin increases circulating endothelial progenitor cells and reduces the formation and progression of cerebral aneurysm in rats. Neuroscience. 2011 May 5;181:292–299. doi: 10.1016/j.neuroscience.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 79.Kato S, Aoyama M, Kakita H, et al. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011 Oct 10;89:1566–1574. doi: 10.1002/jnr.22702. [DOI] [PubMed] [Google Scholar]
- 80.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006 Jan;21(1):103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.L'Episcopo F, Serapide MF, Tirolo C, et al. A Wnt1 regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: Therapeutical relevance for neuron survival and neuroprotection. Molecular neurodegeneration. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.L'Episcopo F, Tirolo C, Testa N, et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011 Feb;41(2):508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venkatesan B, Prabhu SD, Venkatachalam K, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008 Jan 1;111(1):122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Endo Y, Beauchamp E, Woods D, et al. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3-and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008 Apr;28(7):2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maiese K, Chong ZZ, Hou J, et al. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 88.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010 Aug 12;116(6):993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010 Sep;15(9):1072–1082. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007 Nov;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011 Feb 15;14(4):649–661. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cui W, Matsuno K, Iwata K, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011 May;26 doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 93.Hou J, Chong ZZ, Shang YC, et al. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010 Mar 4;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koh PO. Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett. 2011 Jul 8;498(2):105–109. doi: 10.1016/j.neulet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 95.Le XF, Mao W, Lu Z, et al. Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010 Jul;13 doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson MS, Brosens JJ, Schwenen HD, et al. FOXO and FOXM1 in Cancer: The FOXO-FOXM1 Axis Shapes the Outcome of Cancer Chemotherapy. Curr Drug Targets. 2011 Aug 1;12(9):1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]
- 97.Chattopadhyay M, Walter C, Mata M, et al. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009 Apr;132(Pt 4):879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008 Jan;324(1):160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 99.Koh SH, Noh MY, Cho GW, et al. Erythropoietin increases the motility of human bone marrow-multipotent stromal cells (hBM-MSCs) and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev. 2009 Apr;18(3):411–421. doi: 10.1089/scd.2008.0040. [DOI] [PubMed] [Google Scholar]
- 100.Toba H, Sawai N, Morishita M, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009 Jun 10;612(1–3):106–114. doi: 10.1016/j.ejphar.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 101.Wang ZY, Shen LJ, Tu L, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009 Apr 15;46(8):1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 102.Almeida M, Han L, Bellido T, et al. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005 Dec 16;280(50):41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 103.Mercado-Gomez O, Hernandez-Fonseca K, Villavicencio-Queijeiro A, et al. Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: role of tau phosphorylation. Neurochem Res. 2008 Aug;33(8):1599–1609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]
- 104.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen L, Xu B, Liu L, et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest. 2010 May;90(5):762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010 Jun;90(6):718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu LY, Sun ZG, Wen YM, et al. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010 Sep 1;169(3):1046–1062. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 108.Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010 Aug 2;120(8):2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma T, Hoeffer CA, Capetillo-Zarate E, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Castilho RM, Squarize CH, Chodosh LA, et al. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009 Sep 4;5(3):279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2011 Sep;6 doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andreucci M, Fuiano G, Presta P, et al. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009 Aug;42(4):554–561. doi: 10.1111/j.1365-2184.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arboleda G, Morales LC, Benitez B, et al. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009 Mar;59(2):333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Astiz M, de Alaniz MJ, Marra CA. Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol Environ Saf. 2009 Oct;72(7):2025–2032. doi: 10.1016/j.ecoenv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 115.Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004 Apr;19(2):495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paiva MA, Rutter-Locher Z, Goncalves LM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011 Jun;300(6):H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Silva DF, Esteves AR, Oliveira CR, et al. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer's disease. Curr Alzheimer Res. 2011 Aug;8(5):563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 118.Wilk A, Urbanska K, Yang S, et al. Insulin-like growth factor-I-forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-alpha-mediated neuronal damage: implications for human immunodeficiency virus encephalitis. J Neurosci Res. 2011 Feb;89(2):183–198. doi: 10.1002/jnr.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miki T, Miura T, Yano T, et al. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006 Apr;317(1):68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- 120.Sharples EJ, Patel N, Brown P, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004 Aug;15(8):2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]