Abstract
In this study, expressions of aquaporin (AQP) 1, AQP4, endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor in blood-cerebrospinal fluid (CSF) barrier and blood-brain barrier (BBB) are examined in rat choroid plexus and peri-infarcted hippocampal formation (HF) following systemic hyponatremia (SH) and permanent middle cerebral artery occlusion (pMCAO). These events are thought to cause the development of hydrocephalic and vasogenic edemas. The importance of CSF overproduction and intact blood-CSF barrier during hydrocephalic edema formation is demonstrated by the high expression of AQP1 (329.86±10.2%, n=4 , P<0.01) and trapped plasma immunoglobulin G (IgG) in choroid plexus epithelium after 24 hours of SH. However, the increased eNOS expression in peri-infarcted HF (130±3%, n=4, P<0.01) and extravasation of plasma IgG into the extravascular compartment after 24 hours of pMCAO suggest that increased microvascular permeability, probably due to elevated levels of nitric oxide, leads to development of vasogenic brain edema via BBB breakdown. Based on these findings, the authors suggest that modulation of different protein expression, dependent on the type of brain edema, is required for primary (pMCAO) and secondary (SH) brain injuries to attenuate brain edema and neuronal degeneration.
Keywords: Aquaporin 1, Blood-CSF barrier, eNOS, Blood-brain barrier, Brain edema
Introduction
The term brain edema refers to swelling of the brain caused by the accumulation of excess water [1]. Brain edema is associated with several brain pathologies, such as hydrocephalus, traumatic brain injury, stroke, and brain tumors. It is also linked to extracranial pathologies that affect the brain secondarily, such as systemic hyponatremia (SH), organ failure (liver, kidney), and sepsis [2, 3]. There are two major types of cerebral edema: cytotoxic and vasogenic edema. However, a third type, termed hydrocephalic edema, has also been recently described [4]. Cytotoxic edema, as seen in SH and early cerebral ischemia, is caused by the intracellular accumulation of water due to cellular energy failure and the inability of cells to regulate their volumes. In contrast, vasogenic edema, of which brain ischemia and brain abscess edema are prime examples, involves disruption of the blood-brain barrier (BBB) [5]. Hydrocephalic edema refers to the movement of cerebrospinal fluid (CSF) from the ventricles across the ependyma into the interstitial space of the brain in hydrocephalus.
The largest extracellular fluid (ECF) compartment in the brain is made up of CSF, which is mainly produced by the choroid plexus of ventricles in the brain and constitutes the major blood-CSF barrier [6]. It has been suggested that aquaporin1 (AQP1) plays an important role in CSF formation, because it is specifically expressed in the apical mem brane of choroid plexus epithelium [7]. Recent studies have shown that AQP1 deletion in mice reduces the osmotic water permeability of the choroid plexus and the production of CSF [8, 9]. It was also seen that AQP1 deletion decreases intracranial pressure (ICP) [9]. These results strongly suggest that AQP1 expression may regulate ICP by modulating the rate of CSF production.
In the brain, the BBB is composed of the capillary endothelium, basement membrane, and numerous astrocytic foot processes. The BBB effectively separates plasma from the extracellular space. The barrier properties of the BBB result from the absence of fenestrations, the low number of pinocytotic vesicles, and the presence of tight intercellular junctions between endothelial cells [10]. Aquaporin4 (AQP4) is expressed in the astrocytic foot processes that form the BBB and the glia limitans, a structure that lines the pial and ependymal surfaces in contact with the CSF in the subarachnoid space and ventricular system [11]. This localization pattern suggests that AQP4 plays a critical role in both edema formation and resolution [12].
Endothelial nitric oxide synthase (eNOS) is a constitutive enzyme of endothelial cells found in the choroid plexus and brain parenchyma. Elevated levels of nitric oxide (NO) increase the vascular permeability of endothelial cells [13, 14]. Recent studies have demonstrated that NO can influence vascular permeability by regulating endothelial cell shape and intercellular junction formation [15, 16]. Vascular endothelial growth factor (VEGF), also referred to as the vascular permeability factor, is an endothelial cell-specific mitogen in the brain. VEGF can induce endothelial cell associated changes, such as the separation of intercellular tight junctions, increased vesicle transport, and the formation of vesicovacuolar organelles. These changes can lead to increased macromolecular transport through the endothelial barrier [17]. It has also been shown that the intraventricular infusion of VEGF and the exposure of a normal rat brain to VEGF result in BBB breakdown and brain edema, respectively [18].
The present study examines various types of brain edema in vivo using an immunohistochemical method. This paper also explores the possible roles of the altered expressions of AQP1, AQP4, eNOS, and VEGF in the blood-CSF barrier and the BBB during brain edema formation using SH and permanent middle cerebral artery occlusion (pMCAO) rat models. The hippocampal formation (HF) has been chosen in investigations of SH and pMCAO-induced brain damage because of its close proximity to the ventricular CSF and cortical infarction area. Quantitative immunoblot analysis is used to measure the expression levels of AQP1, AQP4, eNOS, and VEGF in choroid plexus and in peri-infarcted HF.
Materials and Methods
Experimental animals
The present study is performed using 24 adult Sprague-Dawley rats with initial weights of 270±8 g. Animals are main tained on a standard rodent diet with free access to water. Experimental procedures used have been reviewed and approved by the Animal Care and Use Committee of Dongguk University. Animal care and use are in accordance with the guidelines of the National Institute of Health (Bethesda, MD, USA).
Induction of SH
Rats are anesthetized with isoflurane and then implanted with an osmotic minipump subcutaneously in the neck region. The osmotic minipumps (model 2001, Alzet, Palo, CA, USA) are prepared and equilibrated in saline for at least 4 hours at 37℃ prior to implantation. SH is induced by simultaneous water loading (140 mmol/L dextrose solution) and implanting an osmotic minipump containing 8-deaminoarginin vasopressin (dDAVP, V-1005, Sigma Aldrich Corp., St. Louis, MO, USA) in saline and DMSO (30% v/v) for 24 hours (30 ml [-12% body weight] dextrose solution i.p. and 50 ng/µl/h dDAVP s.c., followed by repeated doses of 20 ml [-8% body weight] of dextrose i.p. every 4 hours after the injection). Animals are sacrificed 24 hour post-injection. After completing the hydration protocols, rats are anesthetized with isoflurane, venous blood is sampled to determine serum osmolarity and serum sodium concentration, and brains are removed.
Induction of focal cerebral ischemia
As described previously, pMCAO is induced by occluding the left middle cerebral artery [19]. Anesthesia is briefly induced with 3% isoflurane in a mixture of oxygen/nitrous oxide (30 : 70) and animals are maintained on 1% isoflurane in the same oxygen/NO gas mixture. A catheter is then inserted and positioned in the femoral artery and arterial blood pressure is continuously recorded throughout the procedure. Similarly, body temperature is monitored using a rectal thermometer probe. Temperature control is accomplished using a heating pad maintained at 37℃.
Under the dissecting microscope, the left middle cerebral artery is occluded for 24 hours using a 4-0 mono filament (3 cm long) coated with silicone resin. Sham-operated control subjects are subjected to middle cerebral artery surgery without occlusion. After 24 hours of occlusion, animals are reanesthetized with isoflurane and brain tissues are removed for 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma Aldrich Corp.) staining.
TTC staining of infarction and penumbra zones
Once the subjects are sacrificed the brains are quickly re moved and sectioned into 2-mm thick vertical sections, starting from the frontal pole using a Brain Matrix Slicer (Vibratome Co., St. Louis, MO, USA) (n=5). Slices are then immersed in a TTC solution in a Petri dish and incubated at 37℃ for 20 minutes. Slices are turned at the 10 minutes mark to ensure staining of both surfaces.
Immunohistochemistry
To facilitate immunohistochemical analysis, brain tissues are fixed by transcardiac infusion of 4% paraformaldehyde in a phosphate buffer solution (PBS) of pH 7.4. Brains are then removed and embedded in paraffin. Serial, 5 µm coronal sections are obtained at the level of the dorsal third ventricle (Bregma -2.30 mm) (n=6). Following deparaffinization, sections are stained using a Dako kit (Dako, Glostrup, Denmark), treated with 3% H2O2 for 5 minutes to block endogenous peroxidase activity, and then reacted with a rat anti-immunoglobin G (IgG) monoclonal antibody (1 : 1,000, Vector, Burlingame, CA, USA) overnight at 4℃. Brains are then washed, incubated with biotinylated universal anti-mouse, -goat, and -rabbit immunoglobulins in PBS for 30 minutes, incubated with streptavidin conjugated to horseradish peroxidase (HRP) in PBS for 30 minutes, and finally, treated with a solution containing diaminobenzidine and hydrogen peroxide (0.001%) before being counterstained with Mayer's hematoxylin.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting
Homogenates of the choroid plexuses and peri-infarcted HFs of control (or sham-operated) (n=3 or 4, respectively) or SH (or pMCAO) rats (n=8, respectively) are prepared in ten volumes of homogenizing buffer in a Polytron for 10 seconds. The homogenizing buffer consists of 0.32 mM sucrose, 25 mM imidazole, 1 mM EDTA, pH 7.2 containing 8.5 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Aliquots are stored at -70℃. Samples of homogenates are run on 9-15% polyacrylamide minigels (Bio-Rad Mini Protean, Bio-Rad Laboratories, Hercules, CA, USA). Gels are run in duplicate, one gel is Coomassie (Coomassie brilliant blue 0.3 g, 2-propanol 200 ml, acetic acid 80 ml, H2O 640 ml) stained to ensure identical loadings, and the other is immunoblotted.
Proteins are transferred to nitrocellulose membranes over 2 hours at 400 mA and 120 V using a Bio-Rad transblot system with a buffer containing 50 mM Tris-base, 380 mM glycine, and 20% methanol. After transfer, protein bands are identified by Ponceau S (0.1% [w/v] Ponceau S, 0.1% acetic acid) staining, and then destained with distilled water. Nitrocellulose sheets were washed for 4×10 minutes each in PBST (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween-20, pH 7.5). Sheet are then incubated with rabbit anti-rat AQP1- and AQP4-affinity purified polyclonal antibodies (1 : 18,000 and 1 : 500, respectively, Chemicon, Temecula, CA, USA), rabbit anti-eNOS affinity purified polyclonal antibodies (1 : 1,000, Chemicon), and rabbit anti-VEGF affinity purified polyclonal antibodies (1 : 2,500, Santa Cruz Biotechnology, Delaware, CA, USA) overnight at 4℃ and then washed twice for 10 minutes in PBST. Labeling is achieved with HRP-conjugated secondary antibodies (1 : 3,000, Dako) using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Buckinghamshire, UK). The resulting immunoblot signals are quantified using Scion Image software ver. 1.59 (Scion Corp., Frederick, MD, USA). Values are presented as means±SEs. Groups are compared using the unpaired t-test, and P-values of <0.01 are considered significant.
Results
Establishment of SH
The average serum Na+ concentration in normonatremic controls identified is 140±5 mM (n=8), while it is seen to be 111±5 mM (n=8) in rats after 24 hours of SH. In addition, serum osmolarity is significantly reduced from 296±5 mOsm/L (normonatremic control) to 240±13 mOsm/L after 24 hours of SH. This indicates that a substantial reduction in intravascular osmolality promotes water transport from plasma into the brain, presumably causing edema.
Choroid plexus and HF after 24 hours of SH
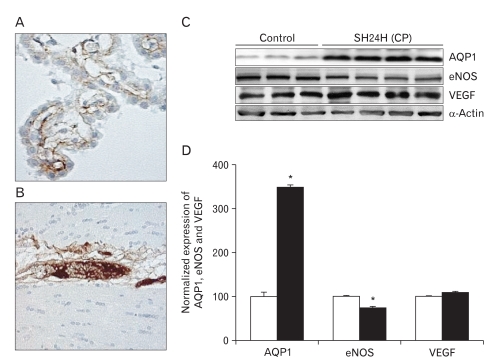
In the SH, blood-CSF barrier and BBB integrity are assessed by analyzing plasma IgG extravasation. No plasma IgG extravasation is observed in the choroid plexus of the third and lateral ventricles after 24 hours of SH (Fig. 1A, B). The expression of AQP1 in the choroid plexus epithelium of the third and lateral ventricles is determined by quantitative immunoblot analysis after 24 hours of SH. Immunoblotting reveals a strong band at 28 kDa (Fig. 1C), corresponding to the non-glycosylated isoform of AQP1. Densitometric analyses of AQP1 reveals that normalized AQP1 expression is significantly increased by 329.86±10.2% (n=4, P<0.01) after 24 hours of SH (Fig. 1D). Immunoblotting for eNOS and VEGF reveals strong bands at 130 kDa and 49 kDa (Fig. 1C), respectively. A significant decrease in the normalized expression of eNOS is seen after 24 hours of SH (75±3%, n=4, P<0.01), whereas VEGF does not increase (Fig. 1D).
Fig. 1.
Extravasation of plasma immuno globulin G (IgG) and the expressions of aquaporin 1 (AQP1), endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) in choroid plexus after 24 h of systemic hyponatremia (SH). (A, B) No plasma IgG extravasation is observed in the choroid plexus of third and lateral ventricles after 24 h of SH (×20). (C) Immunoblots are reacted with anti-AQP1, -eNOS, and -VEGF antibodies to reveal 28, 130, and 49 kDa products. α-Actin is used as an internal control. (D) Densitometric analysis reveals that SH significantly increases AQP1 expression in choroid plexus (329.86±10.2%, n=4, *P<0.01). In contrast, the expression of eNOS at 24 h after SH is significantly lower than that of control rats (75±3%, n=4, *P<0.01).
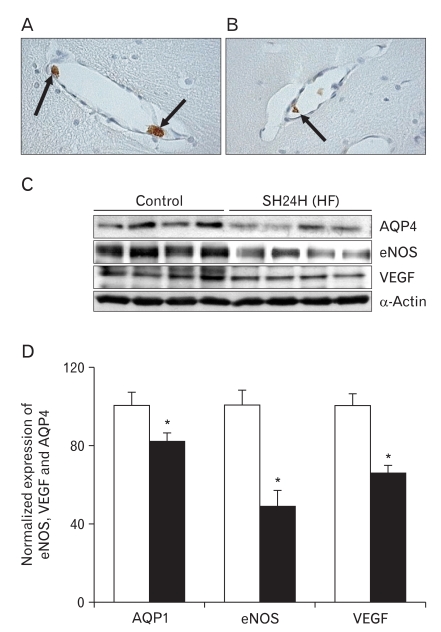
Plasma IgG is trapped solely in the luminal sides of capillaries and/or venules in the HF after 24 hours of SH (Fig. 2A, B). This indicates that the brain's BBB remains intact after 24 hours of SH. This type of edema has been categorized in immunohistochemical and immune blotting studies as "hydrocephalic edema." It is characterized by intactness of the blood-CSF barrier and the BBB as well as CSF overproduction caused by increases in AQP1. Immunoblotting for AQP4 in HF reveals a significant strong band at 32 kDa (Fig. 2C). In contrast with AQP1 expression, densitometric analyses of AQP4 reveals that the expression of AQP4 is diminished after 24 hours of SH (82±4%, n=4, P<0.01). Moreover, the expressions of eNOS and VEGF simultaneously decrease (eNOS, 49±8%; VEGF, 66±4%, n=4, P<0.01) after 24 hours of SH as compared with the levels of control subjects (Fig. 2D). α-Actin is used as an internal control for confirming equal loading of proteins.
Fig. 2.
Extravasation of plasma immunoglobulin G (IgG) and the expressions of aquaporin4 (AQP4), endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) in the hippocampal formation (HF) after 24 h of systemic hyponatremia (SH). (A, B) Plasma IgG (arrows) is trapped solely in the luminal side of capillaries and/or venules in HF after 24 h of SH (×40). (C) Immunoblot with affinity purified anti-AQP4, -eNOS, and -VEGF antibodies, revealing 32, 130, and 49 kDa products. α-Actin is used as an internal control. (D) Densitometric analysis reveals that SH significantly decrease AQP4, eNOS, and VEGF expressions in HF compared with those of control rats (AQP4, 82±4%; eNOS, 49±8%; VEGF, 66±4%, n=4, *P<0.01).
Peri-infarcted HF after 24 hours of pMCAO
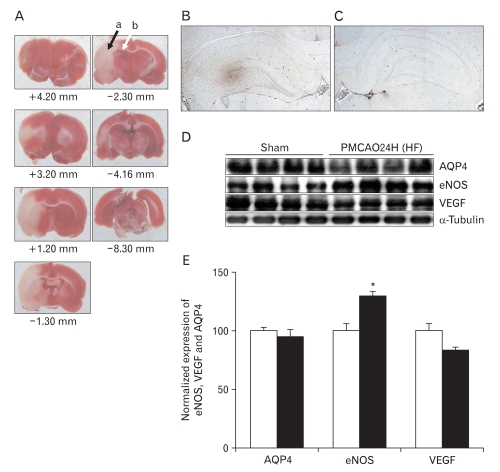
Focal cerebral ischemia is induced in rats by 24 hours of pMCAO. Fig. 3A shows the infarction zone, marked with a black arrow (a in Fig. 3A). The peri-infarcted HF, closer to the cortical infarction area, is marked with a white arrow in the ipsilateral hemisphere (b in Fig. 3A). The + and - numbers represent distances from bregma. Extensive positive staining of extravasated plasma IgG is seen in the peri-infarcted HF 24 hours of pMCAO (Fig. 3B). However, no positive staining is identified in the contralateral cortex (Fig. 3C). This result indicates that the BBB is impaired by pMCAO, which suggests that pMCAO causes vasogenic brain edema. Immunoblotting analysis reveals that pMCAO results in a significant increase in eNOS expression (130±3%, n=4, P<0.01) in peri-infarcted HF (Fig. 3D, E). However, the expressions of AQP4 and VEGF are unchanged in peri-infarcted HF, as compared with those of sham-operated controls (Fig. 3D, E). β-tublin is used as an internal control for confirming equal loading of proteins.
Fig. 3.
Extravasation of plasma immunoglobulin G (IgG) and the expressions of aquaporin4 (AQP4), endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) in peri-infarcted hippocampal formation (HF) after 24 h of permanent middle cerebral artery occlusion (pMCAO). (A) 2,3,5-Triphenyltetrazolium chloride staining of brain slices from bregma +4.20 to -8.30 mm. Peri-infarcted HF (b, marked with a white arrow) represented by the red zone near the infarction zone (a, marked with black arrow) in the ipsilateral hemisphere over a series of brain sections. (B, C) Extensive positive staining of extravasated plasma IgG is seen at the ipsilateral peri-infarcted HF. However, no prominent positive profiles are identified in contralateral HF (×4). (D) This immunoblot is reacted with affinity purified anti-AQP4, -eNOS and -VEGF antibodies to reveal 32, 130, and 49 kDa products. β-Tubulin is used as an internal control. (E) Densitometric analysis reveals that pMCAO significantly increases eNOS (130±3%, n=4, *P<0.01) in peri-infarcted HF as compared with sham-operated controls. However, the expressions of AQP4 and VEGF are not changed after pMCAO.
Discussion
Altered expressions of AQP1 and eNOS in choroid plexus after 24 hours of SH
In the present study, increases in AQP1 are found to enhance osmotically driven water permeability across the choroid epithelium. Current models of CSF production support the roles of the apical localizations of Na+-K+ ATPase and AQP1 at the choroid plexus as the most significant molecular features concerning water transport to ventricles [20]. Previous studies have noted that a 1% decrease in plasma osmolarity results in a 6.7% increase in CSF production [21]. Taking this to be true, the 15% reduction in plasma osmolarity may result in a doubling of CSF production. However, one study claims that the osmotic water permeability of choroidal epithelium is found to be reduced fivefold in AQP1 knockout mice [9], and another suggests that the choroid plexus may account for only 25-50% of total CSF production [22, 23]. Thus, it is expected that the increase in AQP1 expression could contribute to an increase of approximately 10% in total CSF production. Thus, it appears reasonable that the over-expression of choroidal AQP1 water channels is likely to result in CSF overproduction. The increase of CSF in ventricles can enhance the trans-ependymal CSF flow into the interstitial space of the brain [24].
A small amount of water accumulation in the brain can have serious consequences, due to restricted intracranial space. These consequences include an increase in ICP and subsequent cerebral hypo-perfusion caused by vascular compression, which can lead to life-threatening neurological disorders [25]. The results presented here provide evidence of the importance of elevation of ICP via CSF overproduction caused by increased AQP1 in the choroid plexus during hydrocephalic edema formation. It has been previously demon strated that AQP1 knockouts exhibit a 56% reduction in ICP vs. wild-type mice under isomolar conditions [8].
eNOS expression at the choroid plexus may be related to the secretary activities of epithelial cells of the choroid plexus. In relation to this, NO has been reported to modulate the permeabilities of epithelial cells in the small intestine [26]. It is also well known that NO, a potent vasodilator, is implicated in the regulation of BBB permeability [27]. Leakage of serum-derived substances through dilated blood vessels may lead to tissue damage in the choroid plexus. Altered structural features of the choroid plexus epithelial cells and increased levels of NO have, in fact, been observed in the CSFs of newborn infants suffering from hypoxic-ischemic encephalopathy [28]. However, in the present study, decreased expression of eNOS in choroid plexus suggests that AQP1-mediated CSF overproduction does not require structural modifications or breakdown of the blood-CSF barrier.
Decreases of AQP4, eNOS, and VEGF expression in the HF after 24 hours of SH
In the present study, decreases in the expressions of AQP4, eNOS, and VEGF in HF are observed post-SH. This suggests that the expression of these proteins is changed to maintain water homeostasis in the brain in response to SH. Thus, it can be deduced that decreased water conductance in the brain after SH would act to prevent a considerable increase in brain volume.
Since AQP4 is expressed at brain-CSF and blood-brain interfaces, it has recently been proposed that AQP4-mediated trans-ependymal CSF absorption is diminished in the hydrocephalus [29]. The protective role of AQP4 may also be explained by recent reports, which suggest that decreased AQP4 expression may reflect an endogenous protective mechanism that further reduces glial water accumulation. This would counteract the evolution of cell swelling in rats after either traumatic brain injury [30] or hyponatremia combined with brain contusion [31]. Therefore, in the present study, the decrease in AQP4 water channels observed post-SH represents the rate-limiting step that prevents osmotic water influx across the intact BBB.
eNOS expression in the HF is also significantly decreased post-SH. Decreased levels of NO synthesis resulting from a decrease in eNOS after SH might be a factor of reduced microvascular permeability, due to the fact that NO mediates vasodilatation. Recent studies have demonstrated an increased eNOS expression in perilesional blood vessels after rat cortical cold injury closely associated with BBB breakdown [32]. If this is true, the expressional downregulation of eNOS post-SH may maintain the selective vascular permeability of endothelial cells of the BBB and prevent their breakdown. This also suggests that eNOS undergoes regulatory changes to prevent BBB breakdown and maintain water homeostasis in the brain.
VEGF is a potent mediator of endothelial permeability, and has been proposed to participate in the opening of endo thelial tight junctions, the changing of the endothelial phenotype, and even in the alteration of pinocytotic transport through endothelial cells [33]. In addition, the up-regulation of VEGF has been shown to cause the breakdown of the bloodretinal barrier in cases of diabetes [34] and to promote the formation of edema after cerebral ischemia [35]. However, the present study shows that the decreased VEGF expression in the endothelium of cerebral blood vessels in response to SH may result in inhibition of the endothelial tight junction opening, fenestrae formation, and vascular leakage. Therefore, the findings of the present study suggest that VEGF, which appears to be decreased in brain parenchyma after SH, decreases the permeability of the BBB by maintaining endo thelial barriers.
Taken together, overproduction of CSF caused by increased AQP1 in the choroid plexus epithelium post-SH is likely to play a key role in the formation of hydrocephalic brain edema and increasing ICP. However, the regulation of interactions between AQP4 in glial cells and eNOS/VEGF in BBB endothelium may provide another means of protecting the brain from extreme volume changes.
Increased eNOS expression in peri-infarcted HF after 24 hours of pMCAO
Endothelium can regulate vascular tone by releasing multiple factors that relax underlying vascular muscle. These endothelium-derived relaxing factors (EDRF) include NO, the endothelium-derived hyperpolarizing factor, prostacyclin, and reactive oxygen species. Most studies of cerebral circulation indicate that NO is the predominant EDRF [36]. NO mediates the dilatory response in human and mouse cerebral arteries to acetylcholine [37], as well as responses to some other receptor-mediated agonists and to increased shear stress [38]. Zhang et al. [39] report that eNOS protein is up-regulated as early as 1 hour after the onset of permanent focal ischemia in a rat model, peaks at 24 hours, and remains up-regulated for at least seven days. Similarly, findings of this paper indicate that eNOS expression is significantly increased in peri-infarcted HF after 24 hours of pMCAO. In another study, eNOS expression is seen to increase 6 hours after transient focal ischemia, and to be up-regulated after 24 to 168 hours of reperfusion [39]. Differences between experimental paradigms, such as the permanent ischemia used in the present study and transient ischemia used by Veltkamp et al. [40] may account for different latencies of eNOS up-regulation. Therefore, increased NO synthesis by cerebral vessels can be seen to contribute to microvascular permeability when blood flow is reduced after focal cerebral ischemia. However, results of the present study do not indicate sustained eNOS up-regulation in preexisting and/or newly synthesized cerebral vessels. Other studies have shown that little or no detectable angiogenesis occurs for up to 24 hours after transient or permanent ischemia in rats or man [41, 42].
A perturbed cerebral microvascular endothelial function is a crucial step in the pathogenesis of ischemia. In the present study, we suggest that elevated eNOS in peri-infarcted HF is associated with BBB breakdown to plasma IgG. Links between eNOS-derived NO and BBB breakdown have been reported in experimental strokes, in which eNOS inhibition is seen to reduce BBB breakdown [43]. Further, although excessive NO concentrations may alter the BBB directly [44], peroxynitrite formation by reaction between NO and superoxide offers an indirect mechanism by which NO could cause BBB breakdown [43]. Matrix metalloproteinase-9 (MMP-9) is another possible mechanism linking eNOS-derived NO to BBB breakdown. MMP-9 plays a key role in the degradation of some extracellular matrix components, such as fibronectin, laminin, collagen, and gelatin, which leads to remodeling of the extracellular matrix and decreased endothelial cell-extracellular matrix contact [45]. NO also up-regulates MMP-9 by multiple mechanisms, such as S-nitrosylation [46]. The results of the present study suggest that increased eNOS expres sion in peri-infarcted HF could lead to the development of vasogenic brain edema and neuronal degeneration via BBB breakdown, as well as the subsequent loss of the selective vascular permeabilities of endothelial cells.
A number of reports have examined changes in endothelial cells and/or biochemical alterations in ECFs following cerebral ischemia [47, 48]. More recently, the loss of tight junctions of choroid plexus epithelial cells and alterations of CSF electrolyte content following transient forebrain global ischemia have been examined by Johanson et al. [49]. It can be expected that damaging a large proportion of cells that make up the BBB could cause severe disruption of the composition of extracellular fluid. In the present study, the brain is thus subjected not only to a severe primary insult, (oxygen-glucose deprivation) but is also exposed to an altered extracellular fluid composition.
Taken together, the overproduction of a modified ECF caused by increased eNOS and subsequent BBB breakdown in peri-infarcted HF after 24 hours of pMCAO appears to play a key role in the formation of vasogenic brain edema and neuronal degeneration.
Acknowledgements
This work is supported by the Korea Research Foundation Grant (KRF-2009-013-E00032).
References
- 1.Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- 2.Blei AT. Pathophysiology of brain edema in fulminant hepatic failure, revisited. Metab Brain Dis. 2001;16:85–94. doi: 10.1023/a:1011670713730. [DOI] [PubMed] [Google Scholar]
- 3.Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007;59:152–159. doi: 10.1016/j.neures.2007.06.1470. [DOI] [PubMed] [Google Scholar]
- 4.Klatzo I. Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 6.Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989;261:68–74. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshio K, Song Y, Verkman AS, Manley GT. Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl. 2003;86:525–528. doi: 10.1007/978-3-7091-0651-8_107. [DOI] [PubMed] [Google Scholar]
- 9.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 10.Crone C. Modulation of solute permeability in microvascular endothelium. Fed Proc. 1986;45:77–83. [PubMed] [Google Scholar]
- 11.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007;22:778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue H, Ando K, Wakisaka N, Matsuzaki K, Aihara M, Kumagai N. Effects of nitric oxide synthase inhibitors on vascular hyperpermeability with thermal injury in mice. Nitric Oxide. 2001;5:334–342. doi: 10.1006/niox.2001.0350. [DOI] [PubMed] [Google Scholar]
- 14.Takeda M, Mori F, Yoshida A, Takamiya A, Nakagomi S, Sato E, Kiyama H. Constitutive nitric oxide synthase is associated with retinal vascular permeability in early diabetic rats. Diabetologia. 2001;44:1043–1050. doi: 10.1007/s001250100588. [DOI] [PubMed] [Google Scholar]
- 15.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267(3 Pt 1):L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- 16.Garcia JG, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J Investig Med. 1995;43:117–126. [PubMed] [Google Scholar]
- 17.Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculovacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002;50:589–598. doi: 10.1097/00006123-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expre ssed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 20.Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454:1–18. doi: 10.1007/s00424-006-0170-6. [DOI] [PubMed] [Google Scholar]
- 21.DiMattio J, Hochwald GM, Malhan C, Wald A. Effects of changes in serum osmolarity on bulk flow of fluid into cerebral ventricles and on brain water content. Pflugers Arch. 1975;359:253–264. doi: 10.1007/BF00587383. [DOI] [PubMed] [Google Scholar]
- 22.Bering EA, Jr, Sato O. Hydrocephalus: changes in formation and absorption of cerebrospinal fluid within the cerebral ventricles. J Neurosurg. 1963;20:1050–1063. doi: 10.3171/jns.1963.20.12.1050. [DOI] [PubMed] [Google Scholar]
- 23.Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA. Cerebrospinal fluid production by the choroid plexus and brain. Science. 1971;173:330–332. doi: 10.1126/science.173.3994.330. [DOI] [PubMed] [Google Scholar]
- 24.Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992;3:12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- 26.Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol. 1992;262(6 Pt 1):G1138–G1142. doi: 10.1152/ajpgi.1992.262.6.G1138. [DOI] [PubMed] [Google Scholar]
- 27.Janigro D, Leaman SM, Stanness KA. Dynamic modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm Sci Technolo Today. 1999;2:7–12. doi: 10.1016/s1461-5347(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 28.Sivakumar V, Lu J, Ling EA, Kaur C. Vascular endothelial growth factor and nitric oxide production in response to hypoxia in the choroid plexus in neonatal brain. Brain Pathol. 2008;18:71–85. doi: 10.1111/j.1750-3639.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005;95:254–262. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- 30.Kiening KL, van Landeghem FK, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF. Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett. 2002;324:105–108. doi: 10.1016/s0304-3940(02)00180-5. [DOI] [PubMed] [Google Scholar]
- 31.Ke C, Poon WS, Ng HK, Lai FM, Tang NL, Pang JC. Impact of experimental acute hyponatremia on severe traumatic brain injury in rats: influences on injuries, permeability of blood-brain barrier, ultrastructural features, and aquaporin-4 expression. Exp Neurol. 2002;178:194–206. doi: 10.1006/exnr.2002.8037. [DOI] [PubMed] [Google Scholar]
- 32.Nag S, Picard P, Stewart DJ. Increased immunolocalization of nitric oxide synthases during blood-brain barrier breakdown and cerebral edema. Acta Neurochir Suppl. 2000;76:65–68. doi: 10.1007/978-3-7091-6346-7_13. [DOI] [PubMed] [Google Scholar]
- 33.Mayhan WG. Regulation of blood-brain barrier permeability. Microcirculation. 2001;8:89–104. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 34.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 35.van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 37.Elhusseiny A, Hamel E. Muscarinic--but not nicotinic--acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, Shakespeare WC, Murray RJ, Chopp M. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996;16:599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002;33:2704–2710. doi: 10.1161/01.str.0000033132.85123.6a. [DOI] [PubMed] [Google Scholar]
- 41.Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba-Bobrowicz T, Dymecki J. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol. 1999;37:264–268. [PubMed] [Google Scholar]
- 42.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han F, Shirasaki Y, Fukunaga K. Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J Neurochem. 2006;99:97–106. doi: 10.1111/j.1471-4159.2006.04048.x. [DOI] [PubMed] [Google Scholar]
- 44.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 46.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 47.Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Windmüller O, Lindauer U, Foddis M, Einhäupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005;128(Pt 9):2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- 49.Johanson CE, Palm DE, Primiano MJ, McMillan PN, Chan P, Knuckey NW, Stopa EG. Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell Mol Neurobiol. 2000;20:197–216. doi: 10.1023/a:1007097622590. [DOI] [PubMed] [Google Scholar]