Abstract
Non-invasive methods are normally preferred to conventional invasive methods when selecting suitable embryos to improve pregnancy rates after assisted reproduction techniques. One of the most recognized non-invasive methods is to examine the supernatants of embryo culture media. Soluble human leukocyte antigen, class I, G (sHLA-G) antigen is a non-classical class I molecule that has been widely considered as a marker of pregnancy failure or implantation success. In the current study of some Iranian patients, we examined the concentration of sHLA-G at different time points after intracytoplasmic sperm injection and compared the rates to the morphology and quality of the selected embryos. We showed that the concentration of sHLA-G increases over time in high-quality embryos. We conclude that there is a positive relationship between morphology, quality, and sHLA-G concentration. We suggest that this relationship can be used to increase the chance of a successful pregnancy.
Keywords: HLA antigen, Assisted reproductive techniques, Intracytoplasmic sperm injections
Introduction
One of the complex issues in human reproduction techniques is embryo implantation [1]. Little is known about this process and it has been reported that more than 70% of transferred embryos are not capable of implantation and only 14% of them are born healthy.
There are many variables that may impact the physiologic processes of implantation [2]; endometrial receptivity and good quality of the embryos are important factors in implantation. Moreover, embryo morphology study is one of the methods that is routinely practiced at infertility treatment centers to select good-quality embryos for transfer. The graduated embryo score (GES) is a rating system developed to predict blastocyst formation and pregnancy rate from cleavage-stage embryos [3]. GES is based on the features of the early stages of embryonic development, including the degree of fragmentation, blastomere number, and cleavage. However, implantation rate is only 39% for transferred embryos with high GES [3]. Furthermore, pre-implantation genetic diagnosis (PGD) has shown that embryos with good morphology may also have chromosomal and genetical disorders, indicating that the morphology studies cannot predict the rate of implantation of embryos of high quality. Thus, morphology analysis alone cannot determine the fate of the embryo. On the other hand, PGD techniques require invasive biopsies and can disturb embryo growth. Moreover, based on previous observations, early miscarriage is associated with chromosomal abnormalities such as aneuploidy [4]. Fluorescence in situ hybridization assays are normally used to detect such abnormalities, but this method also requires biopsies.
A non-invasive method to select suitable embryos is to examine the supernatants of embryo culture media to improve pregnancy rates after assisted reproduction techniques [5]. Soluble human histocompatibility leukocyte antigen, class I, G (sHLA-G) antigen is a known non-classical class I molecule [6] secreted from the embryo culture and is a marker of pregnancy failure or implantation success. In general, the HLA-G gene produces seven isoforms, four of which are membrane-bound and the other three are soluble [7]. Both membrane-bound and soluble isoforms are inhibitors of the immune system.
Although embryos secreting sHLA-G may predict successful pregnancy, sHLA-G-negative embryos can also lead to pregnancy [8]. However, the chance of pregnancy in sHLAG-positive embryos is higher than that of sHLA-G-negative embryos. In addition, the abortion rate increases threefold in sHLA-G negative embryos. Therefore, sHLA-G can be used to predict the chance of pregnancy in women who have been treated for infertility and it can be used as a marker in non-invasive approaches. For example, pregnancy and implantation rates can be maximized when specific embryos are selected for transfer based on their individual sHLA-G expression [9].
During the process of embryo implantation, the embryo must not be rejected by the mother's immune system [10-12]. T helper lymphocyte is an immune system factor that plays an important role in the attachment of embryonic cells to decidua [13, 14]. In addition, natural killer (NK) cells are actively involved in the endometrial luteal phase and the early stages of decidua formation. These cells contain receptors for HLA-G expressed by trophoblast interstitial cells [6, 15] at the mother-fetus contact site [16, 17]. Endocytosis of HLA-G by these receptors stimulates specific cytokines and proinflammatory/proangiogenic agents which in turn assist successful implantation [8]. Cells expressing HLA-G are protected against NK cells [18].
Whether there is any positive relation between sHLA-G and morphology of the embryo is still under debate. For example, it has been shown that there is no direct correlation between embryo morphology and the level of sHLA-G expression [1]. On the other hand, it has been reported that in the supernatant of single-embryo cultures, the sHLA-G levels were positively correlated to embryo quality and that the presence of sHLA-G was significantly associated with clinical pregnancy after intracytoplasmatic sperm injections (ICSI) [8]. However, the correlation between sHLA-G and embryo morphology at different days post-transfer was not investigated.
Here, we studied the correlation between the level of expression of sHLA-G and embryo morphology in single-embryo cultures 48-72 h post-fertilization.
Materials and Methods
Sample collection
After approval of ethics committee, seventy five embryonic samples were collected at the IVF Center of Taleghani Hospital between June and July of the year 2010. All patients (aged 24-34) agreed with the experiments and were screened for infertility related illnesses such as endometriosis and polycystic ovarian syndrome (PCO).
Ovarian stimulation
Ovarian stimulation was performed by a specialist physician at the Taleghani Hospital, Velenjak, Tehran, Iran.
Embryo culture
After collection of the egg and sperm from the couples, in vitro fertilization was done by ICSI into the oocyte. Each embryo was cultured in a single droplet of SAGE Quinn's Advantage Cleavage media (CooperSurgical, Trumbull, CT, USA) supplemented with 10% serum protein substitute (SPS) from day 1 to day 3. At day 3, the fertilized egg was checked under an inverted microscope and then it was transferred into SAGE Quinn's Advantage Blastocyst media supplemented with 10% SPS until day 5/6.
The patient groups
All cultured media were divided into three groups (A, B, and C) according to day of transfer (48 to 72 hour postfertilization) and embryonic cleavage (Table 1). Group A embryos were examined 48 hour post-fertilization. Groups B and C embryos were examined 72 hour post-fertilization. Group B em bryos had reached the 8-cell stage and group C embryos had reached the morula stage. In addition, each group was subdivided into two subgroups according to the quality of the embryos based on the microscopic appearance. The percentage of the total embryo volume that was replaced by fragments was estimated and embryos with a lower degree of fragmentation (<20%) were considered as high-quality embryos. The remaining embryos were considered as low-quality. In addition, blastomeres with different sizes, and cells with the presence of multinucleation and vacuoles were factors of low-quality. Therefore, subgroup 1 had a higher quality compared to subgroup 2 for each group. The culture medium itself was used as the negative control.
Table 1.
Grouping of the culture media
The culture media were divided according to the day of transfer after intracytoplasmic sperm injection and the embryonic cleavage. Each group was subdivided into two subgroups according to the quality of the embryos. a)Quality of the embryos was based on the microscopic appearance and the subgroup 1 had a higher quality compared to subgroup 2.
sHLA-G analysis
The culture medium was examined for the expression and secretion of sHLA-G with a Sandwich enzyme-linked immunosorbent assay (ELISA) kit (BioVendor, Heidelberg, Germany) according to the manufacturer's instruction. Briefly, the standard calibrators (n=6) and samples (n=75) were added to an anti-sHLA-G monoclonal antibody coated 96-well plate. The plate was then incubated at 2-8℃ for 16-20 hours. After washing 5 times, the monoclonal antihuman b2-microglobulin antibody labeled with horseradish peroxidase (HRP) was added and the plate was incubated at room temperature for 1 hour. Following another washing step, the substrate solution (TMP) was added to react with the remaining HRP conjugate and incubated in the dark for 30 minutes. To stop the reaction, an acidic solution was added. The absorbance of the yellowish product was immediately measured by an ELISA reader at 450 nm. The absorbance is proportional to the concentration of the sHLA-G. Sample concentrations were quantified according to the standard curve (unit/ml).
Statistical analysis
Data analysis was performed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA) software. Student's t-test was used to compare the data of two different groups. To compare three or more groups, ANOVA was used (P≤0.05, P≤0.01).
Results
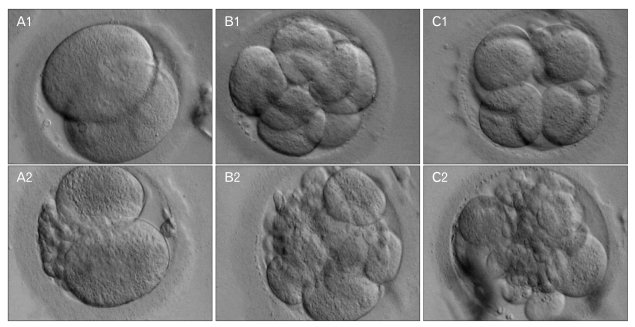
After the IVF of all sperms and eggs by the ICSI method, the culture media were grouped according to quality, embryonic cleavage, and day of transfer. The general appearance and morphology of each group is presented in Fig. 1. Some embryos showed blastomeres with different sizes (A2, B2, and C2) and therefore they were considered as low quality. Overall, there were more high-quality embryos than low-quality embryos in our samples (A1 [n=20], A2 [n=19], B1 [n=15], B2 [n=7], C1 [n=7], C2 [n=7]). As shown in Fig. 1, the area around blastomeres of subgroup 1 was not occupied with granules, unlike that of the subgroup 2.
Fig. 1.
The morphology of embryos at different time points post-intracytoplasmic sperm injection (ICSI) (×400). (A1) An embryo 48 h post-ICSI. (A2). An embryo 48 h post-ICSI, although the quality was lower compared to A1. (B1) An embryo 72 h post-ICSI. (B2) An embryo 72 h post-ICSI, although the quality was lower compared to B1. (C1) An embryo 72 h post-ICSI at morula stage. (C2) An embryo 72 h post-ICSI at morula stage, although the quality was lower compared to C1.
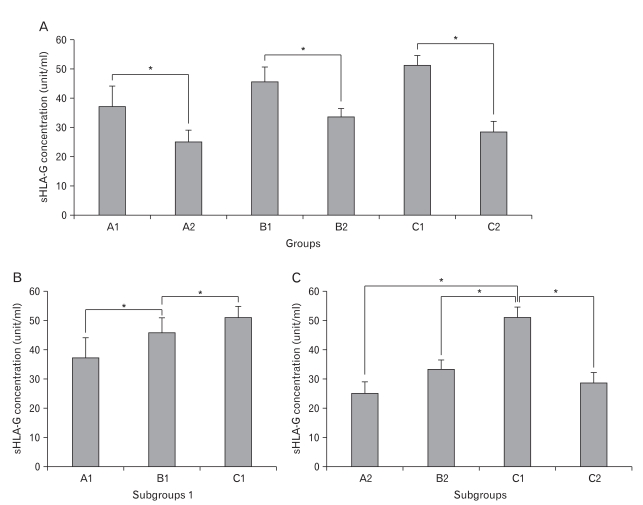
Using ELISA, the optical density of each sample was measured and concentration of sHLA-G was calculated. The corresponding data of each group/subgroup were compared (Fig. 2). Within each group, the concentration of sHLA-G of subgroup 2 was significantly lower than subgroup 1 (Fig. 2A); A2 was 12.02 unit/ml lower than A1, B2 was 12.18 unit/ml lower than B1, and C2 was 22.68 unit/ml lower than C1.
Fig. 2.
The concentration of sHLA-G in each group measured by enzyme-linked immunosorbent assay. (A) The concentration in all groups/subgroups. (B) Comparison of the concentration between each subgroup 1. Note that the concentration increased in each group compared to the previous group. (C) Comparison of the concentration between each subgroup 2. Addition of C1 in this figure was to show there was no statistically significant change between subgroups 2. Each data point represents three independent measurements and the error bars indicate the standard error of the mean. sHLA-G, soluble human leukocyte antigen, class I, G. *P<0.05 were referred to as statistically significant differences.
The concentration of sHLA-G in every subgroup 1 was significantly higher than subgroup 1 of the previous group; C1 was 5.5 unit/ml higher than B1, and B1 was 8.54 unit/ml higher than A1 (Fig. 2B). In contrast, the difference between C2 with A2 and B2 was not statistically significant (Fig. 2C). However, in general, the total concentration of sHLA-G in each group (subgroups 1 and 2 combined) was higher than the previous group meaning the concentration increased each day as the cells divided.
Discussion
Non-invasive embryo selection methods are being improved to distinguish good-quality from poor-quality embryos. One of such laboratory methods is to examine the secretion of embryonic factors into the culture media [19]. In this study, the expression of sHLA-G protein in all post-fertilized culture media samples was measured by ELISA. The main objective was to confirm if there is any correlation between the quality of embryos and the concentration of sHLA-G in Iranian patients. Here we show that in the culture media with considerable embryo quality after ICSI, the concentration of sHLA-G in the same group increases compared to the previous day.
On the other hand, the first embryonic cell divisions in humans are controlled by the mother's genome. Subsequently, activation of the embryo genome occurs between the 4-cell to 8-cell divisions after fertilization. In this study therefore, our focus was at the same stage of cell division. Unlike previous data obtained from Iranian patients [20], we showed that it is clearly possible to determine the concentration of sHLA-G protein two to three days post-ICSI. We also showed that we are able to select embryos that have a better genome, as the higher the sHLA-G concentration, the higher the quality of embryos and the chance of survival. Although the concentration of sHLA-G in C2 was lower than in B2, the concentration of C1 (which has relatively more high-quality embryos) was higher than the rest of subgroups 1. Perhaps the lower number of samples with lower quality resulted in lower concentration of sHLA-G in subgroup C2. Therefore, the conclusions of this study are based more on the data obtained from subgroups 1.
We showed that the expression of sHLA-G was higher in the morula-stage and in 8-cell embryos compared to that of the 4-cell embryos. Normally, the increase of this protein in culture media is proportional to a variety of factors including the rate and the number of division and duration of culture [5]. It has also been shown that the higher the amount of sHLA-G, the higher the chance of successful implantation and pregnancy [15]. Here, we showed that the expression of sHLA-G is relative to the morphology of the embryo and that the more normal the embryo is according to the GES system, the higher the expression of sHLA-G. It is common that during cleavage, some portions of the cells break off and separate from the nucleated area. As shown here, higher expression of sHLA-G was observed in samples with lower fragmentation scores.
Our finding was in contrast with previously published data that claimed detectable levels of sHLA-G is not produced by day 2 embryos [20]. Not only we were able to successfully detect sHLA-G, but also we showed that the concentration of sHLA-G increased daily after ICSI in samples categorized as subgroup 1. Moreover, it was previously claimed that such a measurement may not provide reliable information for embryo selection and estimation of pregnancy success [20]. According to our data however, the concentration of sHLA-G is correlated to the quality of the embryo.
In conclusion and in agreement with previous findings [8, 19], our study further suggests that sHLA-G concentration and quality of embryos are two important factors that have a direct and positive relation. We further suggest that these factors should be seriously taken into consideration in IVF trials. However, in the future experiments it would be interesting to continue this research with more samples to get results that are more accurate. This way, we will be able to establish relatively more precise standard data between highquality groups at different days post-ICSI in order to compare all of our future data with.
Acknowledgements
The authors would like to thank the IVF center of Taleghani Hospital, Shahid Beheshti Medical University for their technical assistance.
References
- 1.Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, Biagiotti R, Pellegrini S, Menicucci A, Baricordi OR. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod. 2005;20:138–146. doi: 10.1093/humrep/deh572. [DOI] [PubMed] [Google Scholar]
- 2.Lessey BA. Embryo quality and endometrial receptivity: lessons learned from the ART experience. J Assist Reprod Genet. 1998;15:173–176. doi: 10.1023/A:1023087900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated Embryo Score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod. 2001;16:1970–1975. doi: 10.1093/humrep/16.9.1970. [DOI] [PubMed] [Google Scholar]
- 4.Suzumori N, Sugiura-Ogasawara M. Genetic factors as a cause of miscarriage. Curr Med Chem. 2010;17:3431–3437. doi: 10.2174/092986710793176302. [DOI] [PubMed] [Google Scholar]
- 5.Rebmann V, Switala M, Eue I, Grosse-Wilde H. Soluble HLA-G is an independent factor for the prediction of pregnancy outcome after ART: a German multi-centre study. Hum Reprod. 2010;25:1691–1698. doi: 10.1093/humrep/deq120. [DOI] [PubMed] [Google Scholar]
- 6.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol. 1990;144:731–735. [PubMed] [Google Scholar]
- 7.Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Quiles RM, Bermond F, Dausset J, Carosella ED. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61:1138–1149. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 8.Rebmann V, Switala M, Eue I, Schwahn E, Merzenich M, Grosse-Wilde H. Rapid evaluation of soluble HLA-G levels in supernatants of in vitro fertilized embryos. Hum Immunol. 2007;68:251–258. doi: 10.1016/j.humimm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Sher G, Keskintepe L, Nouriani M, Roussev R, Batzofin J. Expression of sHLA-G in supernatants of individually cultured 46-h embryos: a potentially valuable indicator of 'embryo competency' and IVF outcome. Reprod Biomed Online. 2004;9:74–78. doi: 10.1016/s1472-6483(10)62113-x. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 11.Hviid TV, Hylenius S, Lindhard A, Christiansen OB. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigens. 2004;64:66–69. doi: 10.1111/j.1399-0039.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, Paul P, Rouas-Freiss N, Kirszenbaum M, Dausset J, Carosella ED. Molecular and immunologic aspects of the nonclassical HLA class I antigen HLA-G: evidence for an important role in the maternal tolerance of the fetal allograft. Am J Reprod Immunol. 1998;40:136–144. doi: 10.1111/j.1600-0897.1998.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 13.Ng ST, Chang TH, Wu TC. Prediction of the rates of fertilization, cleavage, and pregnancy success by cumulus-coronal morphology in an in vitro fertilization program. Fertil Steril. 1999;72:412–417. doi: 10.1016/s0015-0282(99)00290-3. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury SR, Knapp LA. Human reproductive failure II: immunogenetic and interacting factors. Hum Reprod Update. 2001;7:135–160. doi: 10.1093/humupd/7.2.135. [DOI] [PubMed] [Google Scholar]
- 15.Menicucci A, Noci I, Fuzzi B, Criscuoli L, Scarselli G, Baricordi O, Mattiuz PL. Non-classic sHLA class I in human oocyte culture medium. Hum Immunol. 1999;60:1054–1057. doi: 10.1016/s0198-8859(99)00108-1. [DOI] [PubMed] [Google Scholar]
- 16.Bamberger AM, Jenatschke S, Schulte HM, Löning T, Bamberger MC. Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 choriocarcinoma cells. J Clin Endocrinol Metab. 2000;85:3932–3936. doi: 10.1210/jcem.85.10.6849. [DOI] [PubMed] [Google Scholar]
- 17.Margreiter M, Weghofer A, Kogosowski A, Mahmoud KZ, Feichtinger W. A prospective randomized multicenter study to evaluate the best day for embryo transfer: does the outcome justify prolonged embryo culture? J Assist Reprod Genet. 2003;20:91–94. doi: 10.1023/A:1021744209193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001;166:5018–5026. doi: 10.4049/jimmunol.166.8.5018. [DOI] [PubMed] [Google Scholar]
- 19.Kotze DJ, Hansen P, Keskintepe L, Snowden E, Sher G, Kruger T. Embryo selection criteria based on morphology VERSUS the expression of a biochemical marker (sHLA-G) and a graduated embryo score: prediction of pregnancy outcome. J Assist Reprod Genet. 2010;27:309–316. doi: 10.1007/s10815-010-9403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alinejad Z, Jafari Shakib R, Forghan-Parast K, Zahiri Z, Sadri H, Nagafi F, Roushan Z. In vitro fertilized embryos do not secrete detectable HLA-G on day two. Iran J Immunol. 2009;6:195–201. [PubMed] [Google Scholar]