Abstract
Purpose
To validate the Dinamap ProCare 200 blood pressure (BP) monitor against a mercury sphygmomanometer in children 7 to 18 years old in accordance with the 2010 International Protocol of European Society of Hypertension (ESH-IP2) and the British Hypertension Society (BHS) protocol.
Methods
Forty-five children were recruited for the study. A validation procedure was performed following the protocol based on the ESH-IP2 and BHS protocols for children and adolescents. Each subject underwent 7 sequential BP measurements alternatively with a mercury sphygmomanometer and the test device by trained nurses. The results were analyzed according to the validation criteria of ESH-IP2.
Results
The mean (±SD) difference in the absolute BP values between test device and mercury sphygmomanometer readings was 1.85±1.65 mmHg for systolic BP (SBP) and 4.41±3.53 mmHg for diastolic BP (DBP). These results fulfilled the Association for the Advancement of Medical Instrumentation criterion of a mean±SD below 5±8 mmHg for both SBP and DBP. The percentages of test device-observer mercury sphygmomanometer BP differences within 5, 10, and 15 mmHg were 96%, 100%, and 100% for SBP, and 69%, 92%, and 100% for DBP, respectively, in the part 1 analysis; both SBP and DBP passed the part 1 criteria. In the part 2 analysis, SBP passed the criteria but DBP failed.
Conclusion
Although the Dinamap ProCare 200 BP monitor failed an adapted ESH-IP2, SBP passed. When comparing BP readings measured by oscillometers and mercury sphygmomanometers, one has to consider the differences between them, particularly in DBP, because DBP can be underestimated.
Keywords: Blood pressure, Oscillometric device, Dinamap, Validation studies, International protocol
Introduction
Measurement of blood pressure (BP) is an essential part of physical examinations in clinical practice, even for children, and noninvasive BP measurements are usually used for routine clinical measurements. While the mercury sphygmomanometer has been the gold standard for BP measurement, this manual auscultatory technique has presented a lot of difficulties when performed on children. This method requires a quiet environment, which is not easy to maintain, and it can be quite difficult to detect diastolic pressure by hearing the 4th or 5th Korotkoff sound, especially in children1,2). Furthermore, medical instruments containing mercury, including sphygmomanometers, are being phased out in the clinical field because of environmental concerns about the toxicity of mercury2,3). Thus, clinicians need alternatives to the mercury sphygmomanometer. In general, there are two types of monitors that can be adopted: those that work on some variation of the auscultatory technique, and those that use the oscillometric technique. The aneroid device, where the mercury pressure gauge is replaced by a mechanical spring, is the most popular alternative device for the auscultatory technique. However, it is not strongly recommended, due to considerable variations in its accuracy, varying from 0 to 35%4). On the other hand, automatic oscillometric devices have gained increasing popularity and acceptance in the medical field, as they are easy to use and do not need much observer expertise. As such, they can be used for BP measurements in children without observer errors3). The basis of the oscillometric method is that it can accurately detect mean arterial pressure; however, the estimation of systolic and diastolic is indirect, and the algorithms used by different manufacturers vary and are never publicly disclosed5). The big disadvantage is that the oscillometric method is quite different from the auscultatory method, and the correlation between the two is not always close. Another factor that needs to be considered is that most oscillometric devices are manufactured for adults, whose arteries are stiffer than children's arteries. Their accuracy and performance, therefore, must be verified using a mercury sphygmomanometer as the gold standard in a separate group of adults and children.
In 1987, The Association for the Advancement of Medical Instrumentation (AAMI)6), as well as the British Hypertension Society (BHS)7) in 1993, set standard criteria for validating these devices against the mercury sphygmomanometer. When the BHS dissolved its Working Party on BP measurement, the Working Group on Blood Pressure Monitoring of the European Society of Hypertension (ESH) undertook to produce an updated protocol, named the International Protocol (ESH-IP) in 2002, which simplified the validation procedure without sacrificing accuracy8). In 2010, the ESH-IP protocol was revised and simplified further (European Society of Hypertension International Protocol revision 2010, ESH-IP2)9). All those validation protocols, however, were confined to adults over the age of 25 years, and were not applicable to children and adolescents.
Therefore, we first prepared a validation protocol for children and adolescents according to the ESH-IP2 and BHS protocols, and then we assessed the accuracy of an oscillometric BP device, the Dinamap ProCare 200, in children aged 7 to 18 years, by comparing it with manual mercury sphygmomanometer readings according to the protocol.
Materials and methods
The oscillometric BP device model chosen for our validation study was the Dinamap ProCare 200 (GE Medical Systems, Milwaukee, WI, USA), which was used for setting the 2007 normal blood pressure centiles for Korean children and adolescents10). The Baumanometer Mercury Gravity Sphygmomanometer (W.A. Baum Co., Copiague, NY, USA) was used as the standard against the test oscillometric BP device.
A validation procedure was performed on the basis of the ESH-IP29). Although the ESH-IP2 was originally limited to adults over the age of 25 years, it referred to selection of pediatric subjects. It recommended that larger samples should be analyzed proportionally to the original 33-subject sample when the validation study for children and adolescents is planned in accordance with ESH-IP2, as they have a wide range of body size and blood pressure levels. The exact number of subjects, however, are not denoted on the ESH-IP2, we referred to the BHS protocol7) as well in determining the number of subjects and the BP range. As such, we recruited 45 children and adolescents, 15 each from the following age groups: 7 to 10 years of age, 11 to 14 years of age, and 15 to 18 years of age.
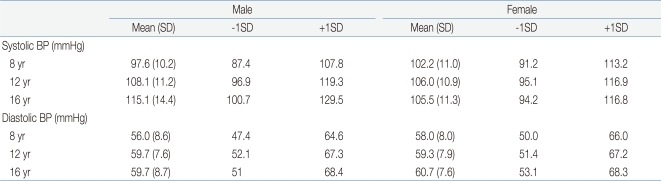
To ensure a uniform distribution of test BPs, The BHS protocol7) recommended age-specific mean and standard deviation to define the criteria of the high, mean, and low BP ranges (high BP group, >mean +1 SD; mean BP group, mean -1 SD <BP< mean +1 SD; low BP group, <mean -1 SD). For our study, BPs of mean±1 SD within each age group were derived from BP values for boys and girls with average ages and heights in the 2007 Korean Blood Pressure Tables (Table 1)10). In addition, according to the BHS protocol7), the number of subjects across a representative range, high and low BPs, should account for at least 17%, which was 8 subject each in this study.
Table 1.
Means and SDs of Blood Pressures by Sex, Age, and 50 Percentile of Height among Korean Children and Adolescents
SD, standard deviation; BP, blood pressure.
All the recruited subjects were stable pediatric patients attending regular follow-up examinations at the pediatric clinic of Inje University Ilsan Paik Hospital for minor urinary abnormalities. Some of the patients were taking antihypertensive drugs, but children with arrhythmias were excluded.
Demographic data were collected from the subjects, including age, sex, height, weight, and right upper arm circumference. A series of nine sequential measurements was obtained for each child.
The study protocol was approved by the Institutional Review Board (IRB) of Inje University Ilsan Paik Hospital.
1. Validation procedures9)
After we obtained their demographic data, the children underwent nine sequential BP measurements as follows:
1) Entry blood pressure A (BPA) with mercury sphygmomanometer by observers 1 and 2 (used to separate the subjects into appropriate BP range groups).
2) Entry blood pressure B (BPB) with test device by the supervisor (a doctor).
3) BP1 by observers 1 and 2 with mercury sphygmomanometer.
4) BP2 by the supervisor with test device.
5) BP3 by observers 1 and 2 with mercury sphygmomanometer.
6) BP4 by the supervisor with test device.
7) BP5 by observers 1 and 2 with mercury sphygmomanometer.
8) BP6 by the supervisor with test device.
9) BP7 by observers 1 and 2 with mercury sphygmomanometer.
Observers 1 and 2, who were trained nurses, sat opposite each other so that they were each blind to the other's manometer and recordings. They checked BPs simultaneously by dual head stethoscope with a manual mercury sphygmomanometer. After the initial two auscultatory readings to determine the range of BP by their mean, one reading was taken with the test device to make it familiar to the child. These initial three readings were not used in the analysis. Seven same-arm sequential measurements were taken, alternating between mercury sphygmomanometer readings simultaneously taken by observers 1 and 2 (four readings) and the test device (three readings). If the difference in readings by observers 1 and 2 was more than 4 mmHg, the measurement was repeated. Intervals between measurements were between 30 seconds and 1 minute. The readings from the test device were compared with the mean of the two measurements taken by observers 1 and 2 immediately preceding and following the test device measurements: BP2 was compared with BP1 and BP3, BP4 was compared with BP3 and BP5, and so on. The smaller absolute value of the two differences was taken. Thus, for each child, three pairs of BP comparisons were obtained.
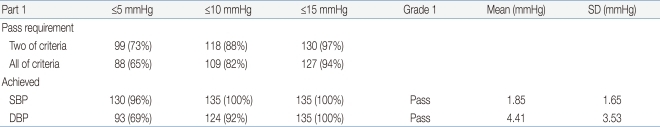
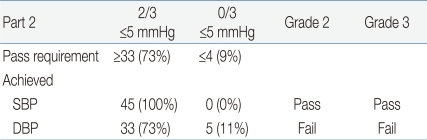
For the accuracy requirements, the percentages of BP pairs whose differences fell within 5, 10, 15, and more than 15 mmHg (corresponding to A, B, C, and D, respectively) were calculated for the device and compared with the requirements of the ESH-IP29) for the 45 children. The requirements for passing were that two of three criteria-73 for A, 87 for B, and 96% for C-or all of three criteria-65 for A, 81 for B and 93% for C-should be satisfied. In addition, subjects with more than two As of three BP pairs having differences within 5 mmHg had to be over 33 out of the 45, and those with zero As of three BP pairs having differences within 5mmHg had to be less than 4 out of the 45.
The mean±SD of BP differences (test device minus mercury auscultatory readings) were used to check whether those differences satisfied the requirement of AAMI, which was less than 5±8 mmHg6).
Results
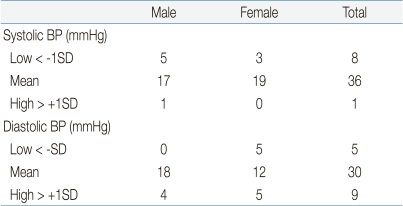
A total of 59 consecutive children and adolescents were recruited in order to achieve an adequate number in all age groups, and 14 children were excluded. Eight were excluded because the target number was fulfilled, and six showed a wide range of differences between the test device and the standard mercury sphygmomanometer. The number of subjects in each recruitment blood pressure range is provided in Table 2. We encountered difficulty in recruiting subjects with a high range of systolic BP and low range of diastolic BP. Only one child with systolic BP in the high range was recruited, and five in the low range of diastolic BP were recruited.
Table 2.
Distribution of Subjects by Range of Blood Pressure
BP, blood pressure, SD, standard deviation.
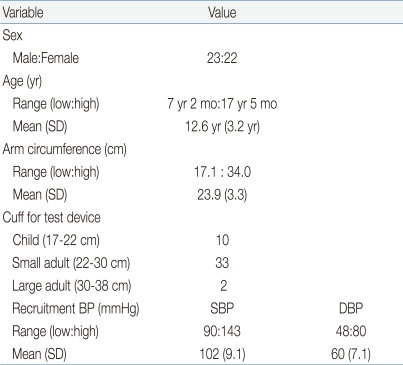
Demographic information about the 45 recruited subjects is shown in Table 3. The cuffs of the tested device and the standard mercury sphygmomanometer were used according to the manufacturers' instructions to fit the right upper arm mid-circumference, and they were compatible with the National Health and Nutrition Examination Survey recommendation1). Small adult cuffs were used for 33 (73%) participants, and large adult cuffs were required for 2 (4%) participants.
Table 3.
Demographic Details of Subjects
SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic BP.
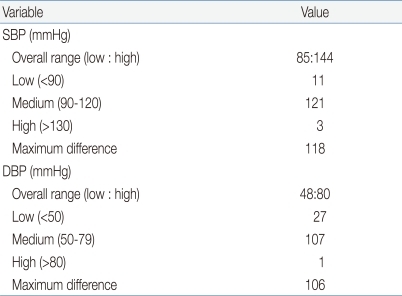
The distribution of observer BP measurements is provided in Table 4. Most of the BP measurements are in the medium ranges of systolic and diastolic BP.
Table 4.
Observer Measurements in Each Recruitment Range
SBP, systolic blood pressure; DBP, diastolic BP.
In most instances, the differences between observers 1 and 2 were within the required level of agreement, less than 4 mmHg. The mean differences in systolic and diastolic BP measurements between observers 1 and 2 were 1±1.2 mmHg and 1±0.9 mmHg, respectively (Table 5).
Table 5.
Observer Differences
SBP, systolic blood pressure; DBP, diastolic BP; SD, standard deviation.
1. Validation results
The validation criteria of part 1 and part 2 of the ESH-IP2 modified for the number of child and adolescent subjects and the results of the validation analysis are presented in Table 6 and Table 7.
Table 6.
Results of the Validation Analysis of Blood Pressure Readings (n=135)
SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic BP.
Table 7.
Results of the Validation Analysis of Individual Children (n=45)
SBP, systolic blood pressure; DBP, diastolic BP.
The test device satisfied all of the six criteria of part 1 of ESH-IP2 for both systolic and diastolic BP (Table 6).
The mean BP (±SD) difference between the test device and the standard mercury sphygmomanometer in all of the 45 subjects was 1.85±1.65 mmHg for systolic BP (SBP) and 4.41±3.53 mmHg for diastolic BP (DBP). These results fulfilled the AAMI criterion of mean±SD below 5±8 mmHg for both systolic and diastolic BP (Table 6).
With regard to the part 2 criteria of ESH-IP2, the test device passed both of two criteria for SBP, but it satisfied only one of two criteria for DBP (Table 7).
Although the test device passed the grade 1 and AAMI criteria, it failed in grades 2 and 3 for the validation test (Tables 6, 7).
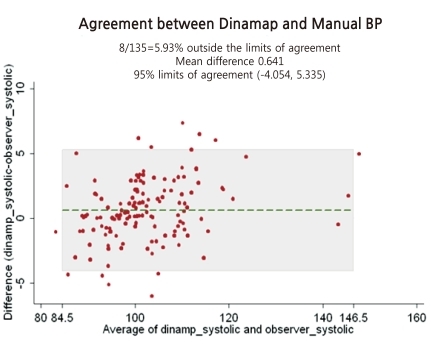
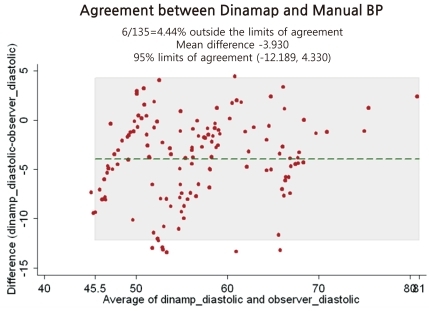
The differences in BP between the tested device and the observers' readings (135 readings) are presented in Fig. 1 for SBP and Fig. 2 for DBP. These Bland-Altman plots are mean-difference plots to compare two different methods11). The x-axis of these plots represents BP in the 80 to 160 mmHg systolic range and the 40 to 80 mmHg diastolic range. The y-axis represents errors from -15 to +15 mmHg. The mean of each test device pressure and its corresponding observer pressure is plotted against their difference with a point. Plotted dots are scattered around within horizontal reference lines of -5 mmHg and +5 mmHg for SBP (Fig. 1) and reference lines of -15 mmHg and +5 mmHg for diastolic BP (Fig. 2). Dots outside the A (errors from -5 to +5 mmHg) are more prominent in a mean BP range for SBP, but more prominent in a low BP range for DBP.
Fig. 1.
Bland-Altman plots presenting differences in systolic blood pressure (BP) between the tested device (Dinamap ProCare 200) and standard auscultatory mercury sphygmomanometer readings.
Fig. 2.
Bland-Altman plots presenting differences in diastolic blood pressure (BP) between the tested device (Dinamap ProCare 200) and standard auscultatory mercury sphygmomanometer readings.
Discussion
The present study attempted to test the accuracy, in children, of an oscillometric BP device, Dinamap ProCare 200, by comparing it with a standard auscultatory mercury sphygmomanometer. We used the International Protocol of European Society of Hypertension revised in 20109), which simplified the testing procedures without sacrificing accuracy, as compared to the previous one. The AAMI6), the BHS7), and the European Society of Hypertension Working Group on Blood pressure Monitoring8) have developed validation protocols for BP monitors. However, those protocols were limited mainly to adults; no protocols have been specifically developed for device validation in children, and experiences with validation studies in children are very limited12,13).
One of the most well recognized protocols is that of BHS, which requires 85 participants with a wide range of blood pressures7). More recently, the ESH published an international protocol requiring only 33 participants, but with more stringent grading criteria8,9). When we prepared the protocol on the basis of the International Protocol of European Society of Hypertension revised in 20109), there were concerns regarding the statistical basis of the number of recruited children and adolescents and their range of blood pressures, as there was no reference to the required distribution of subjects with reference to age and BP in the ESH-IP2. Therefore, in our subject selection, we followed the BHS protocol for special groups5), namely, children and adolescents aged 7 to 18 years old. This protocol permits a reduction in the number of subjects when a validation study of the device being tested has already been completed for adults. The Dinamap ProCare 200 Monitor had already fulfilled all the requirements of the International Protocol and the AAMI criteria, but for use in the adult population14). Following this guideline, although only 30 subjects were required, we recruited 45 children and adolescents in order to attempt to acquire the necessary numbers in the low and high BP subgroups. Because the BP of children is age and height related, the ranges are specified in relation to age- and height-specific mean and standard deviation, as shown in Table 1. According to the BHS recommendations, subjects with low or high BP should account for at least 17% (8/45). In our study, the distribution of subjects with the required BPs was not fully satisfied, due to the limitations of recruiting children and adolescents with abnormal BP.
Although the ESH-IP2 is primarily intended for studies in adults, in similar studies it is reasonable to adopt the same procedures for children. We simply modified the number of subjects recruited and the range of blood pressure of the subjects, and we then performed the procedure and analysis on the principles of ESH-IP29).
Although the test device, the Dinamap ProCare 200 Monitor, passed the AAMI standards (mean±SD test device-standard observer difference below 5±8 mmHg), it failed to fulfill all the validation requirements of the ESH-IP2 regarding the accuracy of BP readings. It passed the SBP standards, but it did not pass the DBP standards of the ESH-IP2. A previous study on the Dinamap 8100 showed significant overestimation of SBP and DBP115). In our study, the Dinamap ProCare 200 proved to be accurate in SBP measurement within the accepted levels of the validation criteria of the ESH-IP2, but it underestimated DB and failed the validation criteria of the ESH-IP2 in children and adolescents (Table 7; Figs. 1, 2). Even though the subjects with low BP range lacked the targeted number, 8 for diastolic BP, for the validation study, the result dose not seem to be changed because dots outside the A in the Fig. 2 for DBP validation study are more prominent in the range of low BP.
When the BHS published the protocol of the validation study for special groups, including children and adolescents, they asked that the proposals be regarded as somewhat tentative, and not to analyze the results to be passed or failed. Furthermore, they recommended that grading should not be attempted, but rather that the results should be stated as the mean difference and standard deviation between the test device and the standard mercury sphygmomanometer7). Figs. 1 and 2 are showing that mean differences of systolic and diastolic BP between the test device and mercury sphygmomanometer are 0.6 mmHg and -3.9 mmHg, respectively. In other words, DBP readings by an automatic Dinamap Procare 200 BP monitor seems to be lower than by mercury sphygmomanometer. From that point of view, when we measure BP using the Dinamap ProCare 200 Monitor, we have to consider the fact that DBP can be underestimated.
In summary, although this study had several limitations because of lack of a proper validation study protocol and statistical basis of number of recruited subjects, we can see the characteristics of the Dinamap ProCare 200 Monitor on children and adolescents. We are able to use the Dinamap ProCare 200 Monitor on children and adolescents for measurements of BP, but under measurements of diastolic BP as compared to mercury sphygmomanometer should be considered in assessing the BP readings. Automatic oscillometric BP measurement devices which passed the validation study in adult can be recommended for use on children and adolescents, as in the adult population, but those devices should be referenced to a standard mercury sphygmomanometer to demonstrate accuracy and characteristics before clinical use on children and adolescents.
Acknowledgements
This research was completed with financial support from 2010, a scientific research service work of the Korea Center for Disease and Prevention (KCDC).
References
- 1.Centers for Disease Control (CDC) National Health and Nutrition Examination Survey (NHANES) Health Tech / Blood Pressure Procedure Manual. Hyattsville: CDC; 2009. [Google Scholar]
- 2.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 3.Pickering TG. What will replace the mercury sphygmomanometer? Blood Press Monit. 2003;8:23–25. doi: 10.1097/00126097-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Canzanello VJ, Jensen PL, Schwartz GL. Are aneroid sphygmomanometers accurate in hospital and clinic settings? Arch Intern Med. 2001;161:729–731. doi: 10.1001/archinte.161.5.729. [DOI] [PubMed] [Google Scholar]
- 5.van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287–290. doi: 10.1097/00126097-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 6.American National Standards Institute; Association for the Advancement of Medical Instrumentation. American national standard for electronic or automated sphygmomanometers. Arlington: AAMI; c1987. [Google Scholar]
- 7.O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:677–679. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 10.Lee CG, Moon JS, Choi JM, Nam CM, Lee SY, Oh K, et al. Normative blood pressure references for Korean children and adolescents. Korean J Pediatr. 2008;51:33–41. [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 12.Wong SN, Tz Sung RY, Leung LC. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit. 2006;11:281–291. doi: 10.1097/01.mbp.0000209082.09623.b4. [DOI] [PubMed] [Google Scholar]
- 13.Narogan MV, Narogan MI, Syutkina EV. Validation of A&D UA-778 blood pressure monitor in children. Blood Press Monit. 2009;14:228–231. doi: 10.1097/mbp.0b013e328330eeb2. [DOI] [PubMed] [Google Scholar]
- 14.Reinders A, Reggiori F, Shennan AH. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Press Monit. 2006;11:293–296. doi: 10.1097/01.mbp.0000217998.96967.fb. [DOI] [PubMed] [Google Scholar]
- 15.Park MK, Menard SW, Yuan C. Comparison of auscultatory and oscillometric blood pressures. Arch Pediatr Adolesc Med. 2001;155:50–53. doi: 10.1001/archpedi.155.1.50. [DOI] [PubMed] [Google Scholar]