Abstract
Introduction
An evidenced based approach to detecting and treating dysphagia needs to be informed by costs and risks associated with pneumonia. In this study the cost of pneumonia during hospitalization after stroke and the effect of pneumonia on mortality were estimated. The effect of pneumonia on mortality and cost for different levels of risk were also analyzed.
Methods
The data come from the 2005 and 2006 Nationwide Inpatient Sample. Regression models, including the propensity for pneumonia, were used to estimate the in hospital mortality associated pneumonia, and the marginal cost of pneumonia on the hospitalization. A stratified analysis based on quintile of propensity for pneumonia was also undertaken.
Results
There were 183, 976 hospitalizations for stroke in the sample. The adjusted relative risk of death associated with pneumonia was 2.0 (95% CI 1.9–2.1). The average marginal cost of pneumonia on the hospitalization was $27,633 (95% CI $27,078–$27,988). The quintile of hospitalizations with the highest propensity for pneumonia had the highest average marginal cost associated with pneumonia and the lowest adjusted relative risk of death. There was an inverse relationship between adjusted relative risk of death and propensity for pneumonia.
Conclusions
Pneumonia after stroke is associated with higher mortality and hospitalization costs. Patients with the lowest risk for pneumonia have the highest risk for death associated with pneumonia. Screening is important at all levels of risk.
Introduction
Pneumonia is a common occurrence after stroke and contributes to the morbidity and mortality experienced by stroke survivors. It is estimated that 6–22% of stroke survivors will experience pneumonia.1–4 The primary risk factor for pneumonia after stroke is thought to be dysphagia that allows aspiration of ingested food, liquids, or oral secretions. There is evidence that treatment of dysphagia is associated with a reduction in the incidence of pneumonia.5 Dysphagia screening is part of stroke care guidelines6–9 though it is not clear which form of screening should be utilized.
There are tradeoffs between the many approaches to screening for dysphagia after stroke. Sensitive screening approaches such as the videofluoroscopic swallowing study (aka the modified barium swallow study) are more costly while clinical bedside evaluations, tend to be less sensitive but nearly free of cost.11,12 It has been suggested that some type of risk assessment would allow practitioners to determine the ideal screening method for each patient so that only those who would benefit would undergo tests that are more costly, less convenient, or potentially harmful than other available alternatives.13 At this point it is not clear which patients would benefit from different methods of swallowing evaluation.
An evidenced based approach to detecting and treating dysphagia while taking cost into consideration is limited by current estimates in the literature. There have been innovative studies that have provided estimates on the effects of pneumonia on mortality 4,14,15 and an estimate of the marginal cost of pneumonia on hospitalization.10 These studies report differences in the mortality risk due to pneumonia (adjusted relative risk 1.9–6.0), and the cost estimate was limited to Medicare patients in a small geographic area.
The purposes of this study are twofold. The first is to determine the marginal cost of pneumonia during the acute hospitalization after stroke in a large, national sample that is more generalizable than samples used in prior studies. The second purpose is to determine the effect of pneumonia on inpatient mortality among patients of differing risk for pneumonia to test the hypothesis that the effect of pneumonia on mortality is the greatest in patients with the greatest risk for pneumonia. Identifying those stroke survivors who incur the greatest risk of death associated with pneumonia will provide an initial step in determining which patients would benefit the most from screening for dysphagia after stroke.
METHODS
This study was determined to be of exempt status by the local institutional review board of the author.
Data
These data come from the 2005 and 2006 Nationwide Inpatient Sample (NIS). The NIS contains data on inpatient stays from states that participate in the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ). The project is a stratified probability sample of acute-care hospitals in the United States. The 2005 and 2006 NIS datasets contain information on approximately eight million discharges each from nearly 1000 hospitals across the nation. The NIS contains information regarding the hospital inpatient stay, including admission diagnosis, secondary diagnoses, age, gender, disposition, as well as information regarding the hospitals themselves (e.g., location, teaching status, etc.). The unit of analysis in the NIS is the hospital episode that precludes longitudinal person-level analysis.
Subjects
Hospitalizations for stroke were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for ischemic and hemorrhagic stroke (430.xx, 431.xx, 432.9, 433.x1, 434.xx, 436.xx, and 997.02) as the primary diagnosis for hospitalization. As has been done elsewhere, hospitalized patients who died within three days of admission were excluded to limit analyses to those patients who would benefit the most from efforts to reduce pneumonia.4 Hospitalizations were also excluded for patients with age less than 18 years and who had missing values. All hospitalizations in Texas in the year 2005 were excluded because of lack of cost-to-charge ratio data.
Pneumonia
Occurrence of pneumonia during the hospitalization was identified using ICD-9-CM codes of secondary diagnoses (481.xx 482.xx, 485.xx, 486.xx, 507.x, and 997.3). Up to 14 secondary diagnoses could be coded within the 2005 and 2006 NIS.
Cost Model
A regression model was used to isolate the effect of pneumonia on the cost of hospitalization from other factors that could influence costs. Covariates within the model included nineteen comorbid illnesses, a score of the propensity for pneumonia (see below), a disease specific severity of illness measure (Disease Mortality Scale), patient age and gender, admission from the emergency department, and hospital factors of teaching status and rural/urban status. Comorbid diseases were identified using software that assigns variables in hospital discharge records using the diagnosis coding of ICD-9-CM codes based on comorbidity measures reported by Elixhauser, et al.16 The Disease Mortality Scale (DMS) is a disease specific severity of illness measure that was included to isolate the effects of greater illness burden on the cost of hospitalization. The DMS is a scale of the predicted likelihood of death resulting from disease progression and is independent of the treatment process.17
Mortality Model
A regression model similar to that used for cost was also used to isolate the effect of pneumonia on mortality during the hospital stay. Covariates included twenty-three comorbid illnesses, a score for the propensity for pneumonia, patient age and gender, hospital factors of teaching status and rural/urban status, and if the patient was on mechanical ventilation during the hospitalization.
Propensity for pneumonia
The patients with pneumonia are likely different in ways that influence the probability of contracting pneumonia, and thus may provide a biased estimate of the effect of pneumonia on outcomes. A propensity model was used to reduce the bias that may exist between the patients with and without pneumonia. The propensity model was based on an established model and known risk factors for pneumonia.4,14,18 The propensity for pneumonia was predicted using logistic regression with a diagnosis of pneumonia as an outcome, and covariates of patient age, gender, admission from skilled nursing facility, secondary diagnosis of dysphagia, paralysis, placement of a gastrostomy or nasogastric tube, any occurrence of parenteral or enteral feeding, comorbid diagnoses of acquired immune deficiency syndrome, rheumatoid arthritis/collagen vascular disorders, chronic lung disease, congestive heart failure, diabetes mellitus, liver disease, metastatic disease or solid tumor, pulmonary circulation disorders, renal failure, peptic ulcer disease, paralysis, or mechanical ventilation.
The ability of the propensity model to balance covariates between stroke survivors with and without pneumonia was evaluated by examining the balance of covariates within quintiles of the propensity score.
Statistical Methods
The NIS includes all discharges from sampled hospitals which are stratified by region, location/teaching status, bed size category, and ownership. All analyses and models take into account the sampling design and sample discharge weights within the SAS 9.2 softwarea and R 2.9.1 softwareb.
Cost of pneumonia
To obtain the cost for each hospitalization, total charges were multiplied by the hospital specific cost to charge ratio (CCR), when available, or group average CCRs when the hospital specific CCR wasn’t available (approximately 25% of the time). Group average CCRs are based by state, urban/rural, investor-owned/other, and number of beds for the hospitals for which CCRs were unavailable. Costs were adjusted to 2009 dollars using the Medical Component of the Consumer Price Index.
The average incremental cost of pneumonia was estimated using a generalized linear model.19 To adjust for non-normality of the cost data and differences of the variances a gamma distribution with log link was applied to the model.20 The model adjusted for patient covariates as outlined above. The average incremental cost was the average difference in the predicted cost for those with pneumonia and those without. A 95% confidence interval was established using a bootstrap method of 1000 iterations.
Mortality due to pneumonia
The adjusted relative risk of mortality due to pneumonia was examined using a generalized linear model with poisson regression and log link21,22 predicting in-hospital death with pneumonia as the independent variable and with covariates of age, gender, propensity for pneumonia, medical comorbidities, and hospital characteristics of teaching status and urban/rural status.
Risk stratified cost and mortality
The cost and mortality models were analyzed by utilizing domain analyses by propensity quintile. Domain analyses allow computation of statistics on subpopulations while taking into account the variance of the entire sample. The mortality model was also run with acute myocardial infarction (ICD-9 code 410.xx) as the independent variable for comparison with the effects of pneumonia on mortality within each propensity quintile.
RESULTS
There were 207,718 hospitalizations with the primary diagnosis of stroke in the 2005 and 2006 NIS. Of those, 652 were excluded for age less than 18 years, 11,071 died within the first three days of admission, 8,056 were excluded from Texas in 2005, and 3,963 were excluded for other missing information. The final sample consisted of 183,976 admissions with the primary diagnosis of stroke. A secondary diagnosis of pneumonia was established in 8.1% (95% CI 7.8%–8.3%) of hospitalizations. The in-hospital mortality rate for those with pneumonia was 20.0% (95% CI 19.4%–20.8%) compared to 3.5% (95% CI 3.4%–3.7%) in those without pneumonia (Relative Risk 5.7, 95% CI 5.4–6.0). Many differences exist in between the group with pneumonia and those without (Table 1).
Table 1.
Demographic information and comorbid illnesses
| Pneumonia − | Pneumonia + | pval | |
|---|---|---|---|
| n, unweighted | 169,243 | 14,870 | |
| n, weighteda | 827,155 | 72,693 | |
| Age (years) | 70.6 | 73.3 | p<0.0001 |
| Died | 0.035 | 0.200 | p<0.0001 |
| Female | 0.546 | 0.494 | p<0.0001 |
| Admitted from | |||
| SNFb | 0.008 | 0.014 | p<0.0001 |
| EDc | 0.773 | 0.745 | p<0.0001 |
| Comorbid Conditions | |||
| AIDSd | 0.002 | 0.002 | p=0.07 |
| Alcoholism | 0.036 | 0.054 | p<0.0001 |
| Anemia | 0.088 | 0.139 | p<0.0001 |
| Arthritis | 0.021 | 0.017 | p<0.001 |
| Bloodloss | 0.005 | 0.013 | p<0.0001 |
| CHFe | 0.120 | 0.260 | p<0.0001 |
| Chronic Lung Disease | 0.136 | 0.210 | p<0.0001 |
| Coagulopathy | 0.022 | 0.054 | p<0.0001 |
| Depression | 0.078 | 0.051 | p<0.0001 |
| Diabetes Mellitus | 0.295 | 0.245 | p<0.0001 |
| Drug Abuse | 0.019 | 0.023 | p=0.0015 |
| Hypertension | 0.742 | 0.630 | p<0.0001 |
| Hypothyroidism | 0.104 | 0.077 | p<0.0001 |
| Liver Disease | 0.009 | 0.014 | p<0.0001 |
| Lymphoma | 0.004 | 0.004 | p=0.99 |
| Metastatic Cancer | 0.013 | 0.018 | p<0.0001 |
| Other Neurologic | |||
| Disease | 0.004 | 0.055 | p<0.0001 |
| Obesity | 0.046 | 0.029 | p<0.0001 |
| Paralysis | 0.246 | 0.296 | p<0.0001 |
| Mechanical Ventilation | 0.040 | 0.330 | p<0.0001 |
| Peripheral Vascular | |||
| Disease | 0.068 | 0.053 | p<0.0001 |
| Psychiatric Disease | 0.024 | 0.020 | p<0.001 |
| Pulmonary Circulation | |||
| Disease | 0.013 | 0.014 | p=0.28 |
| Renal Failure | 0.074 | 0.113 | p<0.0001 |
| Tumor | 0.015 | 0.019 | p<0.001 |
| Peptic Ulcer | <0.001 | <0.001 | p=.4 |
| Valvular Disease | 0.096 | 0.094 | p=.36 |
| Weightloss | 0.018 | 0.091 | p<0.0001 |
| Dysphagiaf | 0.115 | 0.429 | p<0.0001 |
| Gastrostomy Tube | 0.037 | 0.265 | p<0.0001 |
| Nasogastric Tube | 0.002 | 0.014 | p<0.0001 |
| Enteral/Parenteral | |||
| Nutrition | 0.024 | 0.154 | p<0.0001 |
| Insurance | |||
| Medicare | 0.663 | 0.715 | p<0.0001 |
| Medicaid | 0.064 | 0.075 | p<0.0001 |
| Private | 0.203 | 0.153 | p<0.0001 |
| Other | 0.070 | 0.055 | p<0.0001 |
| Hospital | |||
| Urban | 0.856 | 0.879 | p<0.0001 |
| Teaching | 0.411 | 0.462 | p<0.0001 |
All analyses take into account the weighted sample.
Skilled Nursing Facility
Emergency Department
Acquired Immune Deficiency Syndrome
Congestive Heart Failure
Dysphagia- derived from ICD-9 code (438.82, 787.2) and other clinical codes associated with dysphagia
Multivariable Regression of mortality due to pneumonia
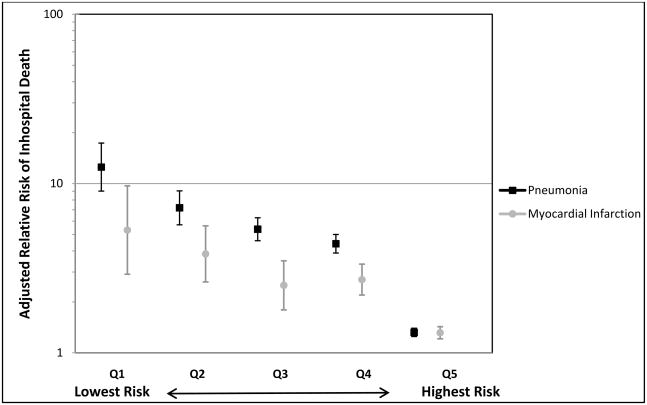
The adjusted relative risk for mortality in stroke survivors with pneumonia was 2.0 (95% CI 1.9–2.1). Stratifying by quintile of propensity for pneumonia showed that the group with the lowest risk of pneumonia (quintile 1) had the highest adjusted relative risk for death when a diagnosis of pneumonia was present (Figure 1), with a decreasing adjusted relative risk for death as the risk of pneumonia increased. This pattern is similar to the effect of a diagnosis of acute myocardial infarction on mortality; however, there was a greater risk of mortality due to pneumonia than acute myocardial infarction among the quintiles with a lower risk for pneumonia.
Figure 1.
Adjusted relative risk of inhospital death by quintile of propensity for pneumonia. The error bars represent 95% confidence intervals. Q1= Quintile 1, Q2=Quintile 2, Q3= Quintile 3, Q4=Quintile 4, Q5=Quintile 5.
Cost of pneumonia
The average unadjusted cost per stroke hospitalization with pneumonia was $34,706 (95% CI $32,685–$36,727) while the average unadjusted cost per stroke hospitalization without pneumonia was $11,604 (95% CI $11,154–$12,053). The average unadjusted incremental cost in those with pneumonia was $23,102. The average adjusted incremental cost in those with pneumonia was $27,633 (95% CI $27,078–$27,988). The average adjusted incremental cost of pneumonia in the quintile of highest risk was $34,057 (quintile 5) compared to $10,815 in the quintile of lowest risk (quintile 1, see Table 2). Quintiles 2–4 had the lowest average adjusted incremental costs of pneumonia of $6,748–$7,868.
Table 2.
Demographic, covariate, and outcome information by quintile of propensity for pneumonia.
| Quintile | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pneumonia | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| n, unweighted | 36,117 | 593 | 35,845 | 936 | 35,451 | 1,465 | 34,346 | 2,431 | 27,367 | 9,425 |
| n, weighteda | 175,936 | 2,894 | 175,375 | 4,586 | 173,554 | 7,181 | 168,364 | 11,934 | 133,925 | 46,098 |
| Age (years) | 53.08 | 52.59 | 69.06 | 69.90 | 77.43 | 78.48 | 78.55 | 79.69 | 76.80 | 72.54 |
| Female | 0.656 | 0.638 | 0.570 | 0.627 | 0.554 | 0.626 | 0.420 | 0.467 | 0.516 | 0.459 |
| Admitted from SNFb | 0.001 | 0.002 | 0.003 | 0.004 | 0.006 | 0.011 | 0.014 | 0.020 | 0.021 | 0.014 |
| AIDSc | < 0.001 | 0.002 | 0.001 | 0.004 | 0.002 | 0.003 | 0.003 | 0.002 | 0.002 | 0.002 |
| Arthritis | 0.024 | 0.019 | 0.024 | 0.037 | 0.020 | 0.021 | 0.018 | 0.020 | 0.017 | 0.013 |
| CHFd | 0.002 | 0.009 | 0.009 | 0.016 | 0.029 | 0.039 | 0.228 | 0.279 | 0.405 | 0.331 |
| Chronic Lung Disease | 0.024 | 0.036 | 0.058 | 0.068 | 0.102 | 0.113 | 0.274 | 0.274 | 0.255 | 0.233 |
| Diabetes Mellitus | 0.370 | 0.311 | 0.314 | 0.354 | 0.168 | 0.193 | 0.190 | 0.211 | 0.181 | 0.188 |
| Liver Disease | 0.004 | 0.003 | 0.008 | 0.008 | 0.009 | 0.011 | 0.012 | 0.012 | 0.015 | 0.017 |
| Metastatic Cancer | 0.001 | 0.002 | 0.006 | 0.007 | 0.011 | 0.020 | 0.029 | 0.030 | 0.022 | 0.016 |
| Pulmonary/Circulatory Disorders | 0.012 | 0013 | 0.013 | 0.026 | 0.009 | 0.011 | 0.016 | 0.017 | 0.015 | 0.013 |
| Renal Failure | 0.033 | 0.059 | 0.046 | 0.069 | 0.057 | 0.070 | 0.112 | 0.113 | 0.137 | 0.127 |
| Tumor | 0.003 | 0.012 | 0.008 | 0.008 | 0.014 | 0.020 | 0.032 | 0.031 | 0.023 | 0.018 |
| Peptic Ulcer | < 0.001 | 0 | < 0.001 | 0 | < 0.001 | 0.001 | 0.001 | 0 | 0.001 | 0.001 |
| Paralysis | 0.195 | 0.192 | 0.216 | 0.238 | 0.225 | 0.238 | 0.272 | 0.293 | 0.347 | 0.319 |
| Mechanical Ventilaiton | 0 | 0 | 0 | 0 | 0 | 0 | < 0.001 | 0 | 0.246 | 0.520 |
| Dysphagiae | < 0.001 | 0.002 | 0.003 | 0.006 | 0.012 | 0.008 | 0.116 | 0.116 | 0.541 | 0.645 |
| Gastrostomy Tube | 0 | 0 | 0 | 0 | 0 | 0 | 0.001 | < 0.001 | 0.227 | 0.417 |
| Nasogastric Tube | 0 | 0 | < 0.001 | 0 | < 0.001 | 0 | 0.001 | 0.001 | 0.014 | 0.022 |
| Enteral/Parenteral Nutrition | 0 | 0 | < 0.001 | 0.003 | 0.001 | 0.001 | 0.009 | 0.015 | 0.142 | 0.244 |
| Died | 0.005 | 0.086 | 0.012 | 0.118 | 0.022 | 0.142 | 0.029 | 0.152 | 0.131 | 0.237 |
| Adjusted Relative Risk Death | 1 | 12.511 | 1 | 7.1863 | 1 | 5.37102 | 1 | 4.41061 | 1 | 1.3208 |
| Cost of Hospitalization | $12,722 | $23,537 | $9,926 | $17,794 | $9,097 | $15,950 | $9,330 | $16,078 | $17,745 | $51,802 |
| NNTf | 13 | 10 | 9 | 9 | 10 | |||||
Quintile 1 is the lowest risk for pneumonia, Quintile 5 has the highest risk for pneumonia.
All analyses take into account the weighted sample.
Skilled Nursing Facility
Acquired Immune Deficiency Syndrome
Congestive Heart Failure
Dysphagia- derived from ICD-9 code (438.82, 787.2) and other clinical codes associated with dysphagia
Number of pneumonia cases that would need to be prevented to prevent one death. Calculated as the reciprocal of the absolute difference in death rounded up to the nearest whole integer.
DISCUSSION
This study confirms that the cost of pneumonia after stroke is not trivial and that the average adjusted cost may be higher than previously estimated. Katzan, et al. estimated the cost of pneumonia to be $21,338 (95% CI $20,762–21,913), inflation-adjusted in 2009 US dollars, about $6,300 less than the estimate from this study.10 If this study were limited to Medicare recipients, as in Katzan’s study, the average adjusted incremental cost of pneumonia would be $23,292, within $2,000 of the inflation-adjusted cost estimated previously.
This study also confirms that pneumonia after stroke is associated with a higher relative risk of in-hospital death.4,14,15, 23–25 The unadjusted relative risk for death in the hospital due to pneumonia in this study (5.7, 95% CI 5.4–6.0) is similar to that found by Ovbiagele14(6.4, 95% CI 3.8–10.6) and to the 30-day mortality risks found by Katzan4 (5.9, 95% CI 5.1–6.8) and Saposnik15 (5.18, 95% CI 3.9–6.9). After adjustment the relative risk in this study (2.0, 95% CI 1.9–2.1) is similar to that of Saposnik15 (1.9, 95% CI 1.23–2.95) and lower than the studies by Katzan4 (3.0, 95% CI 2.4–3.7) and Ovbiagale14 (6.0,95% CI 3.0–11.7). Because these differences are more pronounced after adjustment they likely arise because of differences between the samples, models, and methods used.
The finding that the adjusted relative risk for death associated with pneumonia is inversely related to propensity for pneumonia did not support the initial hypothesis. One potential reason can be seen in Table 2 where the propensity for pneumonia is associated with greater burden of illness. It is possible that the marginal risk for mortality associated with pneumonia in those with the greatest illness burden is blunted by the higher mortality risk that is shared within propensity quintile. Mortality increases as the propensity for pneumonia increases even among those who did not have a diagnosis of pneumonia. A similar phenomenon is seen with a secondary diagnosis of acute myocardial infarction. It is possible that the greater burden of illness in the highest risk group also accounts for the greater incremental cost of hospitalization associated with pneumonia that is greater than the other quintiles in both regardless of diagnosis of pneumonia. The greater proportion of hospitalizations with mechanical ventilation and enteral or parenteral nutrition in those with pneumonia in the highest risk group provides some insight into the additional burden of illness and required level of care that likely contributes to the costs.
The inverse relationship of propensity for pneumonia and the magnitude of the relative risk for death is an important finding when considering how to approach screening for dysphagia in stroke survivors. This study suggests that screening for dysphagia is important regardless of the apparent risks for pneumonia. The number of cases of pneumonia that need to be prevented to prevent one death is similar for the quintiles two through five, accounting for 80% of the hospitalizations. The idea that patient selection can offer the key to utilization of appropriate screening techniques may be more difficult to apply than previously thought, if not misguided altogether. It is possible that a better choice would be to screen all stroke survivors by the most sensitive test available. Pneumonia confers a greater monetary cost for those patients with the highest risk of pneumonia yet greater risk of mortality in those patients who appear to have the lowest risk of pneumonia.
There are several limitations to this study. First, secondary data of this sort have inherent problem of relying on physicians or coders to correctly document diagnoses. It is likely that some diagnoses that may not seem salient to the hospitalization at hand are not recorded yet may have improved the estimates from these analyses had they been. While it would be preferable to have the ability to perform a quality-check on the diagnostic codes within the dataset it is not possible within this dataset. Second, the unit of analysis for the NIS is the hospitalization and not the individual and multiple hospitalizations by the same person could be present within the data. There is no way to estimate how often repeat admissions occur within the data. The results are still representative of hospitalizations occurring within this group and thus provide insight as to where efforts might be best spent for prevention of pneumonia. Finally, risk in this study is based on statistical methods of a limited number of after the fact diagnoses that may not accurately represent the risk that practitioners are able to estimate by clinical examination. It would have been ideal to have such information to include in this study but that was not possible with the data sources utilized for this study.
Many questions remain to be answered about pneumonia prevention after stroke. Foremost, it is not clear how well dysphagia treatments prevent pneumonia. In practice, multiple treatments and strategies are utilized in various combinations in attempt to decrease aspiration, and treatments are often tailored to the individual. One study evaluated the incidence of pneumonia in a population with stroke after implementation of a comprehensive dysphagia treatment program resulted in a 3 month incidence of pneumonia of 1.8%, considerably lower than the 8.1% prevalence found in this study.11 Two studies have shown that a formal dysphagia screening program reduces aspiration pneumonia, and one completely eliminated it, though the diagnostic criteria for aspiration pneumonia are not provided. 24, 26 It is also not clear, if it is presumed that dysphagia treatment is effective, which screening method is best. While the videofluoroscopic swallowing study is considered the best instrument for diagnosing dysphagia and determining the best treatment the examination protocol has not been standardized and thus effectiveness is likely to vary.27 It’s also not clear whether using such a method for dysphagia screening in all stroke survivors would be cost-effective.
Conclusion
This study of a large, nationally representative sample confirms that pneumonia after stroke is associated with higher mortality and hospitalization costs. The marginal cost of pneumonia is higher for those who have the highest risk of pneumonia while the risk of mortality associated with pneumonia is highest in those who are the least likely to be diagnosed with pneumonia. Preventing pneumonia after stroke should be a top priority at all levels of risk.
Acknowledgments
I greatly appreciate the assistance and guidance of Mark Votruba, Ph.D. with the economic analyses of this study and manuscript review and advice of John Chae, MD.
Financial Support:
Dr. Wilson was partially supported by NIH grant K12-HD01097, Rehabilitation Medicine Scientist Training Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- 2.Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29:447–453. doi: 10.1161/01.str.29.2.447. [DOI] [PubMed] [Google Scholar]
- 3.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 4.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 5.Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post-stroke: a systematic review of randomized controlled trials. Age and Ageing. 2008;37:258–264. doi: 10.1093/ageing/afn064. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association Medical Position Statement On Management Of Oropharyngeal Dysphagia. Gastroenterology. 1999;116:452–454. doi: 10.1016/s0016-5085(99)70143-5. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, Jr, Adams RJ, Brott T, et al. Stroke Council of the American Stroke Association. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 8.Smith Hammond CA, Goldstein LB. Cough and Aspiration of Food and Liquids Due to Oral-Pharyngeal Dysphagia: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006;129:154–168. doi: 10.1378/chest.129.1_suppl.154S. [DOI] [PubMed] [Google Scholar]
- 9.Joint Commission, 2006 Joint Commission. Stroke Disease Specific Care Performance Measures [PDF] 2006 http://www.jointcommission.org/NR/rdonlyres/290C2DD7-8A8A-4854-82CC-CE6F6FD692E7/0/F_Section4_Oct04.pdfRetrieved November 14, 2008.
- 10.Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD. The cost of pneumonia after acute stroke. Neurology. 2007 May 29;68(22):1938–43. doi: 10.1212/01.wnl.0000263187.08969.45. [DOI] [PubMed] [Google Scholar]
- 11.Daniels SK, Brailey K, Priestly DH, et al. Aspiration in patents with acute stroke. Arch Phys Med Rehabil. 1998;115:1104–1112. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- 12.Smith CH, Logemann JA, Colangelo LA, Rademaker AW, Pauloski BR. Incidence and Patient Characteristics Associated with Silent Aspiration in the Acute Care Setting. Dysphagia. 1999;14:1–7. doi: 10.1007/PL00009579. [DOI] [PubMed] [Google Scholar]
- 13.ECRI. Evidence report/technology assessment, number 8. Rockville, MD: Agency for Health Care Policy and Research; 1999. Diagnosis and treatment of swallowing disorders (dysphagia) in acute-care sftroke patients. 99-E024. [PMC free article] [PubMed] [Google Scholar]
- 14.Ovbiagele B, Hills NK, Saver JL, Johnston SC. Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J Stroke Cerebrovasc Dis. 2006;15(5):209–13. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saposnik G, Hill MD, O’Donnell M, et al. Variables Associated with 7-Day, 30-Day, and 1-Year Fatality After Ischemic Stroke. Stroke. 2008;39:2318–2324. doi: 10.1161/STROKEAHA.107.510362. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Disease Staging Software User Guide. Ann Arbor, MI: Medstat Group, Inc; 2001. [Google Scholar]
- 18.Vinogradova Y, Hippisly-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59(567):e329–38. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 20.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465–88. doi: 10.1016/j.jhealeco.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNutt L, Wu C, Xue X, Hafner JP. Estimating the Relative Risk in Cohort Studies and Clinical Trials of Common Outcomes. Am J Epidem. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 23.Hilker R, Poetter C, Findeisen N, et al. Nosocomial Pneumonia After Acute Stroke: Implications for Neurological Intensive Care Medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 24.Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005 Sep;36(9):1972–6. doi: 10.1161/01.STR.0000177529.86868.8d. [DOI] [PubMed] [Google Scholar]
- 25.Vermeij FH, Scholte op Reimer WJ, de Man P, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 2009;27(5):465–71. doi: 10.1159/000210093. [DOI] [PubMed] [Google Scholar]
- 26.Odderson IR, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995 Dec;76(12):1130–3. doi: 10.1016/s0003-9993(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Harris B, Jones B. The Videofluorographic Swallowing Study. Phys Med Rehabil Clin N Am. 2008;19(4):769–85. doi: 10.1016/j.pmr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a.[SAS/STAT] software, Version 9.2. SAS Institute Inc; Cary, NC, USA: [Google Scholar]
- b.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2009. URL http://www.R-project.org. [Google Scholar]