Abstract
The functional integrity of the dorsolateral prefrontal cortex (DLPFC) is altered in schizophrenia leading to profound deficits in working memory and cognition. Growing evidence indicates that dysregulation of glutamate signaling may be a significant contributor to the pathophysiology mediating these effects; however, the contribution of NMDA and AMPA receptors in the mediation of this deficit remains unclear. The equivocality of data regarding ionotropic glutamate receptor alterations of subunit expression in the DLPFC of schizophrenics is likely reflective of subtle alterations in the cellular and molecular composition of specific neuronal populations within the region. Given previous evidence of Layer II/III and V pyramidal cell alterations in schizophrenia and the significant influence of subunit composition on NMDA and AMPA receptor function, laser capture microdissection combined with quantitative PCR was used to examine the expression of AMPA (GRIA1-4) and NMDA (GRIN1, 2A and 2B) subunit mRNA levels in Layer II/III and Layer V pyramidal cells in the DLPFC. Comparisons were made between individuals diagnosed with schizophrenia, bipolar disorder, major depressive disorder and controls (n=15/group). All subunits were expressed at detectable levels in both cell populations for all diseases as well as for the control group. Interestingly, GRIA1 mRNA was significantly increased in both cell types in the schizophrenia group compare to controls, while similar trends were observed in major depressive disorder (Layers II/III and V) and bipolar disorder (Layer V). These data suggest that increased GRIA1 subunit expression may contribute to schizophrenia pathology.
Keywords: AMPA, NMDA, Pyramidal cell, Schizophrenia, Dorsolateral prefrontal cortex, Laser capture microdissection, Quantitative PCR
1. Introduction
Converging evidence from human postmortem, neuropsychological and imaging studies implicate the dorsolateral prefrontal cortex (DLPFC) as an important brain region in schizophrenia (Bunney and Bunney, 2000; Callicott et al., 2000; Castner et al., 2004; Goldman-Rakic, 1994; Goldman-Rakic and Selemon, 1997; Lewis, 1995; Weinberger et al., 1994; Weinberger et al., 1986). Cognitive dysfunction is a defining feature of schizophrenia (Goldberg et al., 1993; Harvey et al., 2001; Weinberger and Gallhofer, 1997) and is associated with deficits in DLPFC functioning (Goldman-Rakic, 1994; Goldman-Rakic and Selemon, 1997; Lewis and Gonzalez-Burgos, 2000; Weinberger et al., 2001).
The subtlety of structural changes in the DLPFC of individuals diagnosed with schizophrenia (Goldstein et al., 1999; Schlaepfer et al., 1994; Sullivan et al., 1998) suggests that dysfunction in this region may result from alterations in circuitry and cellular connectivity versus gross structural abnormalities. Within DLPFC, Layer III pyramidal cells exhibit decreased cell size (Pierri et al., 2001; Rajkowska et al., 1998), spine density (Glantz and Lewis, 2000; Hill et al., 2006), dendritic arborization and complexity (Kalus et al., 2000), synaptic connectivity (Mirnics et al., 2001; Selemon and Goldman-Rakic, 1999), and increased neuronal density (Selemon et al., 1995; Selemon et al., 1998). Similarly, Layer V pyramidal cells exhibit decreased somal size (Cotter et al., 2002) and dendritic spine density (Black et al., 2004). The involvement of DLPFC Layer II/III and V pyramidal neurons in intrinsic and extrinsic circuitry and the aforementioned anatomical and morphological disruptions of these cells make these cells prime candidates for probing disease-related differences in gene expression associated with schizophrenia.
Substantial evidence indicates a hypofunction of NMDA (see for review (Coyle, 2006)) and AMPA receptors in the brains of individuals diagnosed with schizophrenia (Goff and Coyle, 2001; Goff et al., 2001; Lynch, 2004; O’Neill et al., 2004). NMDA and AMPA receptors are highly abundant in pyramidal cells in the DLPFC (Beneyto and Meador-Woodruff, 2004; Conti et al., 1999; Conti et al., 1994; Huntley et al., 1997; Vickers et al., 1995); however, little is known about the alterations of subunit composition of ionotropic glutamate receptors within this region in individuals diagnosed with schizophrenia. The subunit stoichiometry of NMDA and AMPA receptors determines several of the kinetic and pharmacological properties of the receptor (Cull-Candy et al., 2001; Dingledine et al., 1999). Determining alterations in subunit composition in the schizophrenic brain may provide insight into dysfunctional glutamate signaling in the disease.
Previous studies have examined the expression of NMDA and AMPA receptor subunits in the DLPFC and report increased NR1 (Dracheva et al., 2001), NR2D (Akbarian et al., 1996), and GluR1, decreased GluR2 (Beneyto and Meador-Woodruff, 2006; Vawter et al., 2002), and increased (Dracheva et al., 2005) and/or decreased (Beneyto and Meador-Woodruff, 2006) GluR4 subunit mRNA expression in the DLPFC of schizophrenics. Other studies report no change in NMDA receptor mRNA or protein (Kristiansen et al., 2006) or AMPA receptor subunit mRNA (Healy et al., 1998; O’Connor et al., 2007).
While regional assessments of gene expression create an informative mosaic of expression level changes, the molecular pathology of schizophrenia is likely attributable to dysfunction of discrete components within neuronal circuits in affected brain regions. However, reliance on regional assessment emphasizes gene expression contained in the majority of cells of the neuronal population and/or those genes in highest abundance in the region, which may not adequately reflect alterations in gene expression in target neurons. Microdissection of discrete cell populations, for example by laser capture microdissection (LCM), allows the quantification of multiple transcripts within specific neuronal populations when combined with quantitative gene expression strategies (Backes and Hemby, 2003; Fasulo and Hemby, 2003; Ginsberg et al., 2000; Hemby, 2004; Hemby et al., 2002, 2003; Kamme and Erlander, 2003; Kamme et al., 2003).
To this end, the combination of LCM and quantitative PCR (qPCR) was used to compare the relative expression levels of NMDA and AMPA subunits in Layers II/III and V pyramidal neurons of the DLPFC in individuals diagnosed with schizophrenia (SCZ), bipolar disorder (BPD), major depressive disorder (MDD) and nonpsychiatric control (CRTL) to determine the specificity of transcriptional changes in NMDA and/or AMPA receptor subunits across these spectrum disorders.
2. Materials and methods
2.1. Postmortem tissue
All tissue used for this study was obtained from the Stanley Medical Research Institute’s Neuropathology Consortium Collection [SCZ (n=15), BPD (n=15), MDD (n=15), and CTRL (n=15)] (Torrey et al., 2000).
Tissue blocks containing the frontal pole were dissected at autopsy and immediately frozen in a mixture of isopentane and dry ice (−70 °C). Blocks containing the superior frontal gyrus were sectioned on a cryostat (14 μm) and the resultant sections were immediately stored at −80 °C. Upon request, sections were shipped on dry ice to the Hemby lab and upon arrival were stored at −80 °C.
Demographic information for these subjects is included in Table 1. Additional information regarding clinical diagnosis, tissue acquisition methods and demographic information is provided in a previously published report (Torrey et al., 2000).
Table 1.
Demographic information of human subjects
| N | Gender (M:F) |
PMI (h±S.E.M.) |
Age at death (years±S.E.M.) |
Brain weight (g±S.E.M.) |
Brain pH | Duration of illness (years±S.E.M.) |
|
|---|---|---|---|---|---|---|---|
| Normal controls | 15 | 9:6 | 23.7±2.7 | 48.1±2.1 | 1501±43.9 | 6.3±0.1 | n/a |
| Schizophrenia | 15 | 9:6 | 33.7±3.9 | 44.5±3.5 | 1471.7±28.9 | 6.2±0.1 | 21.3±3.1 |
| Bipolar disorder | 15 | 9:6 | 32.5±4.3 | 42.3±3.1 | 1441.2±45.8 | 6.2±0.1 | 20.1±2.6 |
| Major depressive disorder | 15 | 9:6 | 27.5±2.9 | 46.5±2.5 | 1462±38 | 6.2±0.1 | 12.7±3.0 |
Values represent mean±standard error of the mean.
2.2. Laser capture microdissection (LCM)
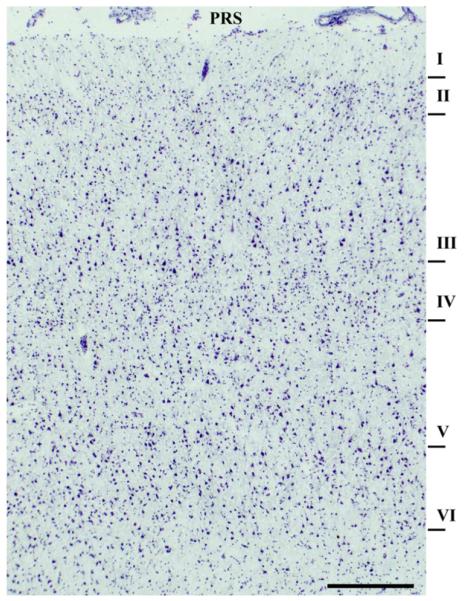
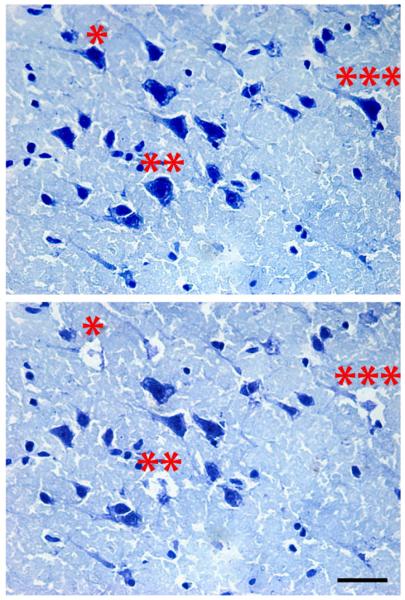
One to three sections per subject were warmed to approximately 22 °C and incubated in 1% cresyl violet acetate for 1–2 min. Sections selected for the LCM were adjacent to those used for regional analysis published previously (O’Connor et al., 2007). Slides were sub-merged in 95% ethanol (2×), and 100% EtOH for 30 s each, followed by submersion in xylene for 5 min and air dried. Slides were transported in a desiccator to prevent hydration. LCM was performed (Arcturus PixCell II, Arcturus, Mountain View, CA) using the following parameters: spot size=7 μM, duration=1000 μs, power=35–55 mW, duration 1.0–1.5 ms. Layer II/III and Layer V pyramidal cells were identified based on anatomical arrangement and cell morphology and micro-dissected from the dorsal and ventral banks of the principal sulcus (Fig. 1). Only cells triangular in shape with a clearly visible apical dendrite within these layers were dissected (300–500 cells per layer per subject;Fig. 2). Following, caps were incubated for 30 min at 42 °C in PicoPure Extraction buffer and the resulting cell lysate was used for subsequent RNA extraction.
Fig. 1.
Photomicrograph of area 9/46 at the level of the principal sulcus in human frontal lobe. Numerals indicate cortical layers and principal sulcus is designated as PRS (scale bar=50 μm).
Fig. 2.
Photomicrograph of pyramidal cells from Layer V from ventral bank of the principal sulcus of the DLPFC. (Top panel) Representative pyramidal neurons selected for microdissection based on morphology and cortical location. (Bottom panel) Same tissue section shown in A illustrating the areas of microdissection. Note the specificity of the dissection and the minimal disruption of surrounding neuropil. Asterisks indicate cells dissected from a representative section (scale bar=500 μm).
2.3. RNA isolation and cDNA synthesis
RNA was isolated from cell lysate using Pico Pure RNA Isolation Kits (Arcturus, #KIT0204) from Layer II/III and V, respectively, for each subject. Purified total RNA was eluted in 15–30 μl of elution buffer. Resulting RNA was quantified using the Quant-iT Ribo Green RNA Assay Kit (Invitrogen #R11490). RNA quality was assessed for each subject from sections adjacent to those used in this study using the Agilent 2100 Bioanalyzer with RNA 6000 Nano Lab Chips (O’Connor et al., 2007). Reverse transcription separately on total RNA (75 ng) for Layer II/III and Layer V captured pyramidal cells for each subject using random hexamers and SuperScript III (Invitrogen, Carlsbad, CA). In addition, aliquots of RNA from each subject for eachlayer were pooled prior to reverse transcription for subsequent use as standards. Resulting complimentary DNA (cDNA) was diluted 1:20 for qPCR and standards were generated from the aforementioned pooled cDNA and serially diluted in two fold dilutions from 1:5–1:160. Due to the low abundance of GRIA4 mRNA, cDNA for analysis of this subunit was diluted 1:5 for each sample and standards were generated from the pooled cDNA serially diluted in two fold dilutions 1:3–1:96.
2.4. qPCR
Methods for qPCR have been described previously (O’Connor et al., 2007). Standard Taqman assays were purchased for measurement of AMPA and NMDA subunit levels and endogenous controls (Applied Biosystems; GRIA1, Hs00181348_m1; GRIA2, Hs00181331_m1; GRIA3, Hs00241485_m1; GRIA4, Hs00168163_m1; phosphoglycerate kinase 1: PGK1, Hs99999906_m1; peptidylprolyl isomerase A (cyclophilin A): PPIA, Hs99999904_m1; glyceraldehyde-3-phosphate dehydrogenase: GAPDH, Hs99999905_m1; TATA box binding protein, Hs99999910_m1; β-2 microglobulin; Hs99999907_m1; ribosomal protein, large, PO; Hs99999902_m1; ribosomal protein 18 S, Hs99999901_m1; β-actin, Hs99999903_m1; hypoxanthine phosphoribosyltransferase 1, Hs99999909_m1; transferrin receptor, Hs99999911_m1; β-glucuronidase, Hs99999908_m1).
Using a 384 well format with the ABI Prism 7900HTS real-time detector, 0.5 μl aliquots of Taqman Expression Assay (20×), 5.0 μl 2× Absolute QPCR ROX PCR Mastermix (Abgene), and 4.5 μl dilutedc DNA (either sample or pooled standard) were mixed together and pipetted into single wells of the PCR plate. For no template controls (NTC) for each gene tested, water was added in lieu of cDNA. Each sample, including NTC was run in triplicate. Thermocycling conditions: 1) one cycle 2 min at 50 °C, 2) one cycle 15 min at 95 °C, and 3) 40 cycles 15 s at 95 °C and 1 min at 60 °C. Fluorescence was measured during the 60 °C step for each cycle. Reactions were quantified by the standard curve method using SDS2.1 software (as described in User Bulletin #2, Applied Biosystems) generating a mean quantity value (Qty mean) for each sample from the triplicates of that sample for each gene of interest. Endogenous controls were selected for each experiment from the set of candidate reference transcripts noted above.
For the present experiments, PGK1, PPIA, and GAPDH were selected as endogenous controls in both Layer II/III and Layer V studies, using the geNorm algorithm (Vandesompele et al., 2002). Data for each gene of interest were expressed as Qty mean/geometric mean of Qty mean values for the selected endogenous control genes as described previously (O’Connor et al., 2007). Normalized values were expressed as a percent control.
2.5. Data analysis
Experiments determining relative gene expression for each candidate gene were run independently. Likewise, experiments for Layer II/III cells and Layer V cells were run independently. A one way analysis of variance comparing group means for controls, SCZ, BPD, and MDD was performed to determine differences in expression levels for each subunit in each layer. The null hypothesis was rejected if p<0.05. Data for GRIA1, GRIA2, GRIA4, GRIN1, GRIN2A, and GRIN2B failed tests of normality and equal variance, so Kruskal–Wallis one way analysis of variance on ranks was performed. The Dunn’s Method was used for post hoc comparisons to control, and the null hypothesis was rejected if p<0.05.
3. Results
3.1. Demographic data
Data from regional dissections of adjacent sections have been previously published (O’Connor et al., 2007). As reported previously, there were no significant differences between disease groups in age, postmortem interval (PMI), pH, or brain weight; however, there was a significant difference between the groups in number of days the tissue had been stored in the freezer (F=5.35, p=0.003). Bonferroni post hoc analysis confirmed significant differences between storage time for CTRL and SCZ groups (p=0.005) and CTRL and BP groups (p=0.005), but not CTRL and MDD groups (p=0.8). It is possible that increased storage time could negatively affect tissue quality and overall RNA quality. As reported previously (O’Connor et al., 2007), RNA quality was not significantly different between the groups as indicated by the RNA Integration Number (RIN) obtained from the Agilent Bioanalyzer RNA Nano Chip (CTRL: 7.74±0.10; SCZ: 7.15±0.20; BP: 7.42±0.28; MDD: 7.12±0.36; p=0.148). Further, there was no significant correlation between storage time and gene expression for any of the NMDA or AMPA receptor subunit mRNAs (R2 between −0.12 and 0.18, p>0.05 for all transcripts).
3.2. NMDA receptor subunit mRNA expression in Layer II/III and Layer V cells in DLPFC
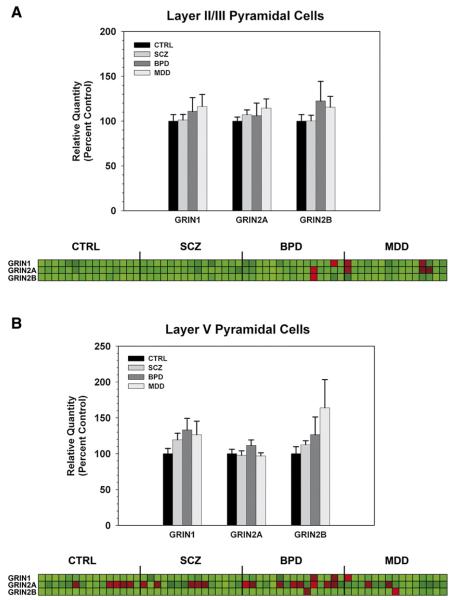
cDNA from Layer II/III pyramidal cells of the DLPFC was subjected to qPCR, and expression of GRIN1, GRIN2A, or GRIN2B subunit mRNA was measured relative to the geometric mean of the endogenous controls. Outliers≥two standard deviations from the mean were excluded from subsequent analysis. There was no significant effect of diagnosis on expression of GRIN1 (p=0.927), GRIN2A (p=0.552), or GRIN2B (p=0.934) mRNA expression in Layer II/III pyramidal cells (Fig. 3A). Similarly, there was no significant effect of diagnosis on expression of GRIN1 (p=0.209), GRIN2A (p=0.296), or GRIN2B (p=0.477) mRNA expression in Layer V pyramidal cells (Fig. 3B).
Fig. 3.
NMDA receptor subunit mRNA levels in Layer II/III (A) and Layer V (B) pyramidal cells of the DLPFC in SCZ, BPD, MDD, and controls (CTRL). Bar graphs represent data expressed as mean (±S.E.M.) of the percent control values for normalized relative quantities. There were no significant differences compared to control subjects (p<0.05). The heatmap illustrates expression profiles of each NMDA subunit (rows) by subject (columns). Values were normalized for each subunit across all subjects and displayed from low (green) to high (red).
3.3. AMPA receptor subunit mRNA expression in Layer II/III and Layer V cells in DLPFC
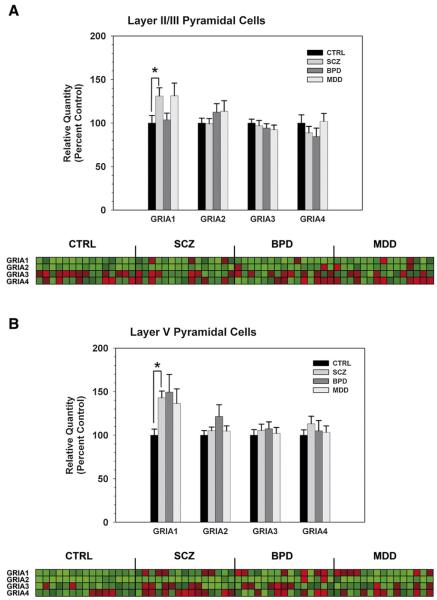
Given that AMPA receptor subunit expression could also underlie SCZ pathology, differential expression of GRIA1-4 subunit mRNAs was determined in Layers II/III and V of the DLPFC. While there was no significant effect of diagnosis on expression of GRIA2 (p=0.830), GRIA3 (p=0.73), or GRIA4 (p=0.48) mRNA, there was a significant effect of diagnosis on GRIA1 mRNA expression (p=0.019) in Layer II / III pyramidal cells of the DLPFC. Post hoc analysis confirmed a significant increase in GRIA1 subunit mRNA in SCZ versus CTRL (p<0.05), but not for BPD or MDD versus CTRL (Fig. 4A). Similar to data from Layer II/III cells, there was no significant effect of diagnosis on expression of GRIA2 (p=0.588), GRIA3 (p=0.871), or GRIA4 (p=0.640) mRNA, but there was a significant effect of diagnosis on GRIA1 mRNA expression (p=0.034) in Layer V pyramidal cells. Post hoc analysis confirmed a significant increase in GRIA1 subunit mRNA in SCZ versus CTRL (p<0.05), but not for BPD or MDD versus CTRL (Fig. 4B).
Fig. 4.
AMPA receptor subunit mRNA levels in Layer II/III (A) and Layer V (B) pyramidal cells of the DLPFC in SCZ, BPD, MDD, and CTRL. Bar graphs represent data expressed as mean (±S.E.M.) of the percent control values for normalized relative quantities. There was a significant (p<0.05) increase in GRIA1 subunit mRNA in the SCZ versus CTRL groups in both Layer II/III (A) and Layer V (B) DLPFC cells. The heatmap illustrates expression profiles of each NMDA subunit (rows) by subject (columns). Values were normalized for each subunit across all subjects and displayed from low (green) to high (red).
Given that SCZ subjects received antipsychotic drug (APD) treatment in their lifetime, it is possible that changes in gene expression may be attributable to the effects of these drugs. Correlative analysis of GRIA1 subunit mRNAs and lifetime dose of APD (reported as fluphenazine equivalent dose) did not reveal a significant relationship between these two measures in the SCZ group (R2 =0.02, p=0.904 and R2 =−0.132, p=0.495 for Layers II/III and V respectively) suggesting that APD treatment did not influence the expression of GRIA1 in these subjects. Table 2 includes APD information for all subjects in this study.
Table 2.
Lifetime exposure to antipsychotic medication: fluphenazine or equivalent (mg)
| Schizophrenia group |
Bipolar disorder group |
||
|---|---|---|---|
| Subject | Lifetime qty fluphenazine or equivalent (mg) |
Subject | Lifetime qty fluphenazine or equivalent (mg) |
| CZ 1 | 4000 | BPD 1 | 32,000 |
| SCZ 2 | 50,000 | BPD 2 | 0 |
| SCZ 3 | 80,000 | BPD 3 | 60,000 |
| SCZ 4 | 1000 | BPD 4 | 200 |
| SCZ 5 | 50,000 | BPD 5 | 60,000 |
| SCZ 6 | 0 | BPD 6 | 12,000 |
| SCZ 7 | 6000 | BPD 7 | 30,000 |
| SCZ 8 | 130,000 | BPD 8 | 7500 |
| SCZ 9 | 9000 | BPD 9 | 40,000 |
| SCZ 10 | 200,000 | BPD 10 | 0 |
| SCZ 11 | 4000 | BPD 11 | 0 |
| SCZ 12 | 150,000 | BPD 12 | 1200 |
| SCZ 13 | 50,000 | BPD 13 | 7000 |
| SCZ 14 | 15,000 | BPD 14 | 2500 |
| SCZ 15 | 35,000 | BPD 15 | 60,000 |
4. Discussion
In the present study, a combination of LCM and qPCR were used to examine AMPA and NMDA subunit mRNA expression in Layer II/III and Layer V pyramidal cells of the DLPFC of postmortem tissue from individuals diagnosed with SCZ, BPD or MDD. When compared to CTRL, GRIA1 subunit mRNA expression was significantly increased in pyramidal cells in both Layers II/III and V in the SCZ group; however, there was no change in expression of NMDA receptor subunits (NR1, NR2A-2B) or AMPA receptor subunits GRIA2-4 in either layer. Increased levels of GRIA1 subunit mRNA were also observed in the MDD in Layer II/III cells, and in BPD and MDD in Layer V cells; however, these increases were not statistically significant. Nonetheless, the present study is the first to explore NMDA and AMPA receptor subunit expression variation in discrete cells within the DLPFC using LCM and to identify increased GRIA1 subunit mRNA in Layer II/III and V pyramidal cells in this region in individuals diagnosed with SCZ.
Increased expression of GRIA1 subunit mRNA may lead to increased receptor levels and possibly increased excitability of DLPFC pyramidal cells in SCZ. Layer III pyramidal cells provide reciprocal connections with other cortical regions (Lewis, 1995; Lewis and Anderson, 1995) as well as intrinsic circuitry within the DLPFC, wherein these cells provide local and long-range axon collaterals that arborize in stripe-like clusters (Levitt et al., 1993; Lewis et al., 2003; Pucak et al., 1996). Long-range collaterals target dendritic spines of other pyramidal cells, while local axon collaterals target both the dendritic shaft of parvalbumin-containing interneurons and dendritic spines of pyramidal cells (Melchitzky et al., 2001; Melchitzky and Lewis, 2003; Melchitzky et al., 1998). Furthermore, deep layer III cells are involved in cortico-cortico and cortico-thalamic circuitry (Lewis et al., 2003) and evidence suggests disruptions in the functional integrity of both circuits in SCZ (Bunney and Bunney, 2000; Tekin and Cummings, 2002). Alterations in GRIA1 subunit mRNA expression in Layer III pyramidal cell populations may disrupt the intrinsic and projection circuitry of the DLPFC which regulates critical elements of working memory (Fuster et al., 1982; Goldman-Rakic, 1990; Goldman-Rakic, 1995; Kubota and Niki, 1971).
Similar increases in GRIA1 expression were observed in Layer V pyramidal neurons in SCZ compared to controls. Interestingly, these cells project primarily to the striatum and are a critical component of the cortico-striato-thalamic circuit implicated in SCZ (Tekin and Cummings, 2002). Dysregulation of this circuit in SCZ is presented as prefrontal hypofunction with consequential enhanced subcortical dopamine responsivity to stress (Tekin and Cummings, 2002). Elevated GRIA1 mRNA levels in the cells suggest a possible hyperactivation of these cells which is inconsistent with the aforementioned model of hypofrontality in SCZ.
Cortical pyramidal cells are traditionally considered a relatively homogenous cell population, unlike GABAergic interneurons which can be divided into functionally distinct subtypes based on their immunoreactivity for calcium binding proteins, (Lewis et al., 2003). However, differentiation may be possible in that pyramidal cells with larger cell bodies and higher expression of nonphosphorylated neurofilament are more likely pyramidal cells involved in long-range projections to other cortical regions (Hof et al., 1996). A number of molecular markers have now been identified to classify distinct subpopulations of pyramidal cells (Hevner et al., 2003; Lewis et al., 2003; Molnar and Cheung, 2006; Pierri et al., 2003; Voelker et al., 2004); however, the functional relevance of these subpopulations remains incomplete. Future studies which take into account the functional importance of subclasses of pyramidal cells will be important to future research investigating the molecular alterations of the DLPFC circuitry.
It is important to view the observations of this study in relation to regional studies in the DLPFC of SCZ subjects. While one previous study reported increases in GRIA1 subunit expression at a regional level (Dracheva et al., 2005), other studies showed no change in GRIA1 subunit mRNA expression (Healy et al., 1998; O’Connor et al., 2007). Discrepancies in findings between studies examining NMDA and AMPA receptor subunit expression have been attributed to clinicopathic differences between cohorts, technique sensitivity, and/or antipsychotic drug exposure. Interestingly, the present data describing increased GRIA1 levels in discrete cell populations of the DLPFC were generated from the same cohort and technique used for the regional analysis of AMPA subunits from the same subjects in which no changes in GRIA1 subunit mRNA levels were observed (O’Connor et al., 2007). As noted previously, regional assessment of gene expression reflects transcripts contained in the majority of neuronal and glial populations but may not adequately reflect alterations in gene expression in target neuronal population. LCM combined with gene expression methodologies enables precise localization of coordinate changes in gene expression within defined cell types (Eberwine et al., 1992; Hemby et al., 2002, 2003; Kamme et al., 2003).
Data from the present study do not support a role for altered NMDA receptor subunit expression in Layer II/III or V pyramidal cells of the DLPFC as a mechanism for SCZ pathology; however, these findings do not negate the possibility that NMDA receptor subunit gene expression may be altered in different cell populations within the DLPFC. As mentioned above, current theories of NMDA receptor hypofunction suggest that NMDA receptor hypofunction on GABAergic interneurons may contribute to SCZ pathology. Within the DLPFC, there is strong evidence for decreased inhibition from parvalbumin-containing chandelier neurons within Layer III of the DLPFC circuitry in SCZ (Lewis et al., 2001, 1999). Studies combining immunohisto-chemistry, laser capture microscopy, and quantitative PCR in distinct GABAergic cell populations within the DLPFC may provide information as to the location of the hypothesized NMDA receptor hypofunction in this region.
There are several caveats of the present experiment and the related findings that warrant discussion. First, individuals diagnosed with SCZ and a portion diagnosed with BPD were administered antipsychotic drugs during their illnesses. Therefore, there is the possibility that the observed changes are due in part to antipsychotic drug administration; however, correlational analyses indicate that the changes are independent of lifetime exposure to antipsychotic drug administration. Second, a variety of subclasses of pyramidal neurons exist in the DLPFC; the current approach of Nissl staining and identification of pyramidal cells based on morphology does not allow the differentiation of these subclasses. In the present study, the possibility exists that the pyramidal cells “captured” for downstream analysis were biased towards larger pyramidal cells within Layers III and V since the morphological characteristics of these cells were more readily apparent. Thirdly, we cannot be certain that the changes observed in the DLPFC are specific to this region as other brain regions were not examined. Previously, we have reported that GRIN1 is increased and GRIA1 is not changed in entorhinal cortical Layer II stellate cells in a different cohort of individuals diagnosed with SCZ (Hemby et al., 2002) suggesting that the observed changes may be specific to the DLPFC. Studies are currently underway to assess NMDA and AMPA receptor subunit mRNA expression in the entorhinal cortex of individuals from the Neuropathology Consortium collection of the Stanley Medical Research Institute as well as a separate cohort. Finally, a number of studies have reported regional and laminar alterations in NMDA and AMPA receptor trafficking proteins in the DLPFC (Beneyto and Meador-Woodruff, 2006; Dracheva et al., 2001; Dracheva et al., 2005), providing insight into additional mechanism of glutamate dysregulation in this region. Due to limitations in resource material, we were unable to assess alterations in the mRNAs encoding glutamate receptor trafficking proteins in these cells. Given the interaction between GRIA1 and synapse associated protein 97 (SAP97) (Leonard et al., 1998) and the presumed role of SAP97 in trafficking of GRIA1 to the membrane (Rumbaugh et al., 2003; Sans et al., 2001), future studies investigating the expression of SAP97 and other binding proteins may provide additional insight into biochemical correlates of increased GRIA1 subunit expression in Layer III and V pyramidal in the DLPFC.
In summary, this is the first study to our knowledge to examine alterations in NMDA and AMPA receptor subunit expression in discrete cell populations within the DLPFC of individuals diagnosed with SCZ. While these data do not support a role for altered NMDA receptor subunit expression in pyramidal cells of the DLPFC, increased GRIA1 subunit mRNA expression in Layer II/III and V pyramidal cells within the SCZ DLPFC supports a role for altered AMPA receptor subunit composition in SCZ pathology. These data are consistent with current theories of SCZ that suggest a hyperactivation of glutamatergic cells through increased activity at non-NMDA receptors. The functional consequences of increased GRIA1 subunit mRNA in Layer II/III and V pyramidal cells in SCZ DLPFC are yet to be determined; however, these data provide insight into a potential mechanism of glutamatergic dysregulation within the DLPFC and provide additional targets for medication development that may be relevant to cognitive disturbances associated with SCZ.
Acknowledgements
The authors acknowledge and appreciate the technical assistance of Brian Horman in the preparation of the photomicrographs. Postmortem brain tissue was donated by the Stanley Medical Research Institute’s Neuropathology Consortium. We are indebted to the altruism and support of the individuals and families for the donation of tissue for research.
Role of funding source The research was funded in part by grants from the NIHMH074313 (SEH) and the Stanley Medical Research Institute (SEH).
Abbreviations
- APD
antipsychotic drug
- DLPFC
dorsolateral prefrontal cortex
- qPCR
quantitative polymerase chain reaction
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartic acid
- GRIA
glutamate receptor ionotropic AMPA
- B2M
β-2 microglobulin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RPLPO
ribosomal protein, large
- ACTB
β-actin
- 18S
ribosomal protein 18S
- TABP
TATA box binding protein
- HPRT1
hypoxanthine phosphoribosyltransferase 1
- TFRC
transferrin receptor
- PPIA
peptidylprolyl isomerase A (cyclophilin A)
- GUSB
β-glucuronidase
- PKG1
phosphoglycerate kinase 1
- cDNA
complimentary DNA
Footnotes
Contributors Dr. Hemby conceptualized the scientific question, provided the scientific infrastructure for the study, worked in conjunction with Dr. O’Connor in the design of the study, data analysis and writing of the manuscript.
Dr. O’Connor worked in conjunction with Dr. Hemby in the design of the study, data analysis and writing of the manuscript. Dr. O’Connor conducted the sample preparation and qPCR analysis.
Conflict of interest Dr. Hemby has served as a consultant for Ortho-McNeil Janssen Scientific Affairs, Johnson and Johnson Pharmaceuticals and Amgen, Inc. None of these consultancies have influence this research. Dr. O’Connor does not have conflicts of interest.
References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr., Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J. Neurosci. 1996;16(1):19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes E, Hemby SE. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J. Pharmacol. Exp. Ther. 2003;307(2):450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J. Comp. Neurol. 2004;468(4):530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60(8):585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am. J. Psychiatry. 2004;161(4):742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res. Brain Res. Rev. 2000;31(2–3):138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl.) 2004;174(1):111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb. Cortex. 1999;9(2):110–120. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Molnar M, Brecha NC. Cellular localization and laminar distribution of NMDAR1 mRNA in the rat cerebral cortex. J. Comp. Neurol. 1994;343(4):554–565. doi: 10.1002/cne.903430406. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex. 2002;12(4):386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and Schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-d-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am. J. Psychiatry. 2001;158(9):1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J. Neurosci. Res. 2005;79(6):868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc. Natl. Acad. Sci. U. S. A. 1992;89(7):3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasulo WH, Hemby SE. Time-dependent changes in gene expression profiles of midbrain dopamine neurons following haloperidol administration. J. Neurochem. 2003;87(1):205–219. doi: 10.1046/j.1471-4159.2003.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP. Cellular discharge in the dorsolateral prefrontal cortex of the monkey in cognitive tasks. Exp. Neurol. 1982;77(3):679–694. doi: 10.1016/0014-4886(82)90238-2. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Ann. Neurol. 2000;48(1):77–87. [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J. Clin. Psychopharmacol. 2001;21(5):484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Greenberg RD, Griffin SJ, Gold JM, Kleinman JE, Pickar D, Schulz SC, Weinberger DR. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br. J. Psychiatry. 1993;162:43–48. doi: 10.1192/bjp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog. Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr. Bull. 1997;23(3):437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Jr., Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch. Gen. Psychiatry. 1999;56(6):537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bowie CR, Friedman JI. Cognition in schizophrenia. Curr. Psychiatry Rep. 2001;3(5):423–428. doi: 10.1007/s11920-996-0038-7. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ, Meador-Woodruff JH. AMPA receptor binding and subunit mRNA expression in prefrontal cortex and striatum of elderly schizophrenics. Neuropsychopharmacology. 1998;19(4):278–286. doi: 10.1016/S0893-133X(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126(3):689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch. Gen. Psychiatry. 2002;59(7):631–640. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Trojanowski JQ, Ginsberg SD. Neuron-specific age-related decreases in dopamine receptor subtype mRNAs. J. Comp. Neurol. 2003;456(2):176–183. doi: 10.1002/cne.10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev. Neurosci. 2003;25(2–4):139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hof PR, Ungerleider LG, Webster MJ, Gattass R, Adams MM, Sailstad CA, Morrison JH. Neurofilament protein is differentially distributed in subpopulations of corticocortical projection neurons in the macaque monkey visual pathways. J. Comp. Neurol. 1996;376(1):112–127. doi: 10.1002/(SICI)1096-9861(19961202)376:1<112::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Vickers JC, Morrison JH. Quantitative localization of NMDAR1 receptor subunit immunoreactivity in inferotemporal and prefrontal association cortices of monkey and human. Brain Res. 1997;749(2):245–262. doi: 10.1016/S0006-8993(96)00847-5. [DOI] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. NeuroReport. 2000;11(16):3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kamme F, Erlander MG. Global gene expression analysis of single cells. Curr. Opin. Drug Discov. Dev. 2003;6(2):231–236. [PubMed] [Google Scholar]
- Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, Erlander M. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J. Neurosci. 2003;23(9):3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Altered NMDA receptor expression in schizophrenia. Mol. Psychiatry. 2006;11(8):705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J. Neurophysiol. 1971;34(3):337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem. 1998;273(31):19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lewis DA, Yoshioka T, Lund JS. Topography of pyramidal neuron intrinsic connections in macaque monkey prefrontal cortex (areas 9 and 46) J. Comp. Neurol. 1993;338(3):360–376. doi: 10.1002/cne.903380304. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Neural circuitry of the prefrontal cortex in schizophrenia. Arch. Gen. Psychiatry. 1995;52(4):269–273. doi: 10.1001/archpsyc.1995.03950160019004. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Anderson SA. The functional architecture of the prefrontal cortex and schizophrenia. Psychol. Med. 1995;25(5):887–894. doi: 10.1017/s0033291700037375. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am. J. Psychiatry. 2001;158(9):1411–14422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Glantz LA, Pierri JN, Sweet RA. Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann. N.Y. Acad. Sci. 2003;1003:102–112. doi: 10.1196/annals.1300.007. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res. Bull. 2000;52(5):309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry. 1999;46(5):616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lynch G. AMPA receptor modulators as cognitive enhancers. Curr. Opin. Pharmacol. 2004;4(1):4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supra-granular pyramidal neurons in monkey prefrontal cortex. J. Comp. Neurol. 2001;430(2):209–221. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb. Cortex. 2003;13(5):452–460. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J. Comp. Neurol. 1998;390(2):211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol. Psychiatry. 2001;6(3):293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci. Res. 2006;55(2):105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- O’Connor JA, Muly EC, Arnold SE, Hemby SE. AMPA receptor subunit and splice variant expression in the DLPFC of schizophrenic subjects and rhesus monkeys chronically administered antipsychotic drugs. Schizophr. Res. 2007;90(1–3):28–40. doi: 10.1016/j.schres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets CNS Neurol. Disord. 2004;3(3):181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch. Gen. Psychiatry. 2001;58(5):466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Somal size of prefrontal cortical pyramidal neurons in schizophrenia: differential effects across neuronal subpopulations. Biol. Psychiatry. 2003;54(2):111–120. doi: 10.1016/s0006-3223(03)00294-4. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J. Comp. Neurol. 1996;376(4):614–630. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry. 1998;55(3):215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J. Neurosci. 2003;23(11):4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J. Neurosci. 2001;21(19):7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD. Decreased regional cortical gray matter volume in schizophrenia. Am. J. Psychiatry. 1994;151(6):842–848. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45(1):17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–20. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J. Comp. Neurol. 1998;392(3):402–412. [PubMed] [Google Scholar]
- Sullivan EV, Lim KO, Mathalon D, Marsh L, Beal DM, Harris D, Hoff AL, Faustman WO, Pfefferbaum A. A profile of cortical gray matter volume deficits characteristic of schizophrenia. Cereb. Cortex. 1998;8(2):117–124. doi: 10.1093/cercor/8.2.117. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J. Psychosom. Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The Stanley foundation brain collection and neuropathology consortium. Schizophr. Res. 2000;44(2):151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr. Res. 2002;58(1):11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Vickers JC, Huntley GW, Hof PR, Bederson J, DeFelipe J, Morrison JH. Immunocytochemical localization of non-NMDA ionotropic excitatory amino acid receptor subunits in human neocortex. Brain Res. 1995;671(1):175–180. doi: 10.1016/0006-8993(94)01372-o. [DOI] [PubMed] [Google Scholar]
- Voelker CC, Garin N, Taylor JS, Gahwiler BH, Hornung JP, Molnar Z. Selective neurofilament (SMI-32, FNP-7 and N200) expression in subpopulations of layer V pyramidal neurons in vivo and in vitro. Cereb. Cortex. 2004;14(11):1276–1286. doi: 10.1093/cercor/bhh089. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Aloia MS, Goldberg TE, Berman KF. The frontal lobes and schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6(4):419–427. doi: 10.1176/jnp.6.4.419. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. Int. Clin. Psychopharmacol. 1997;12(Suppl 4):S29–S36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]