Abstract
A major focus of our laboratory has been an in-depth evaluation as to how estrogens exert a pronounced protective effect on clinical and histological disease in the animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE). An important issue regarding their therapeutic application has been the undesirable estrogenic side effects thought to be mediated primarily through 17β-estradiol (E2) binding to intracellular estrogen receptor alpha (ERα). With the discovery and characterization of GPR30 as the putative membrane estrogen receptor, we sought to study whether signaling through GPR30 was sufficient to mediate protection against EAE without engagement of ERα. Treatment of EAE in WT mice with G-1, a selective GPR30 agonist, retained estradiol’s ability to protect against clinical and histological EAE without estrogenic side effects. G-1 treatment deviated cytokine profiles and enhanced suppressive activity of CD4+Foxp3+ Treg cells through a GPR30- and programmed death 1 (PD-1)-dependent mechanism. This novel finding was indicative of the protective effect of GPR30 activation in EAE and provides a strong foundation for the clinical application of GPR30 agonists such as G-1 in MS. However, future studies are needed to elucidate cross-signaling and evaluate possible additive effects of combined signaling through both GPR30 and ER-α. Deciphering the possible mechanism of involvement of GPR30 in estrogen-mediated protection against EAE may result in lowering treatment doses of E2 and GPR30 agonists that could minimize risks and maximize immunoregulation and therapeutic effects in MS. Alternatively, one might envision using E2 derivatives with reduced estrogenic activity alone or in combination with GPR30 agonists as therapies for both male and female MS patients.
Keywords: EAE, Estrogen, ER-α, ER-β, GPR30, G-1, G-15, 17β-estradiol, encephalitogenic T cells, Tregs, dendritic cells, inflammation, IL-10, IL-17, MS
INTRODUCTION
Human autoimmune diseases exhibit a distinct female predominance of incidence in a long list of diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), scleroderma, Sjogren’s syndrome, Graves disease and multiple sclerosis (MS) [1]. This differential gender susceptibility has also been observed in many experimental animal models of autoimmune disease [1], including lupus, insulin-dependent diabetes mellitus (IDDM), chronic thyroiditis (CLT), myasthenia gravis (MG), collagen- or adjuvant-induced arthritis (CIA or AA), and EAE [2, 3]. Recent evidence suggests that sex hormones play a central role in gender dimorphism. Gender differences in immune response and susceptibility to autoimmune diseases usually become apparent after sexual maturity [4]. Furthermore, the increased levels of sex hormones produced during pregnancy are reported to reduce the clinical symptoms of RA and MS but to exacerbate symptoms of SLE [5–7]. Interestingly, the clinical symptoms of RA and MS worsen postpartum, which is marked by reduced sex hormone levels [7, 8]. The importance of sex steroids on pathogenic autoimmune disease has also been demonstrated in experimental animal models by altering their hormone levels. Estrogens enhance the incidence and severity of experimental murine lupus [9]. Estrogens are capable of suppressing CIA and AA in rats [10, 11] and estrogen or synthetic estrogen analogues have also shown to suppress EAE in both mice and rats[12, 13]. In summary, females have a stronger immune response than males, which is likely responsible for their increased incidence of autoimmune disease. In females, estrogens can enhance autoantibody production, resulting in increased severity of antibody-mediated diseases such as lupus, but, at higher concentrations, estrogens have also been shown to suppress experimental diseases mediated by T cells.
Multiple sclerosis (MS) a debilitating neurological and inflammatory autoimmune disease finds itself in the list of the many other human autoimmune disorders that have a distinct female predominance. The pathogenesis for MS is believed to involve activation or dysregulation of T cells that recognize myelin protein antigens (Ag) in genetically susceptible individuals. However, the cause of activation of T cells against self-Ag remains yet unidentified. Subjects with MS often show clinical improvement during pregnancy, followed by temporary post-partum exacerbations [7, 14]. Levels of sex steroids mirror these improvements during pregnancy, suggesting an immunoregulatory role. Treatment with pregnancy level estriol (E3) reduces central nervous system (CNS) lesions [15]. Experimental autoimmune encephalomyelitis (EAE) is a T cell–mediated disease directed at CNS myelin proteins, including myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG) [16] and shares a number of immunological similarities with the human demyelinating disease MS [17]. These myelin proteins are widely encephalitogenic among mammalian species and strains when injected with adjuvants, induce a spectrum of clinical and histological sequelae that mimic various aspects of MS. As in humans with MS, EAE severity in guinea pigs, rats and rabbits improves during pregnancy [14]. We have previously demonstrated that relatively low doses of 17β-estradiol (E2) and E3 confer potent protection against clinical and histological signs of EAE [18, 19]. Although sex hormones are clearly involved in regulating the immune response, the mechanisms involved are complex and poorly understood. Studies demonstrate direct effects of sex hormones on immunocompetent cells. In vitro studies have shown that high doses of E2 can suppress mitogen- and antigen-induced proliferation of T cells, and depress suppressor T cell activity [20, 21]. Sex hormones have also been shown to influence the production of a number of inflammatory Th1 cytokines, including IFN-γ, TNF-α, IL-1, and IL-6, as well as anti-inflammatory Th2 cytokines IL-4, IL-5, and TGF-β [1].
Hormones, including steroids, play an important role in the regulation of normal physiology and in the treatment of several human diseases, ranging from various cancers to many autoimmune diseases. The cellular responses to hormones initiate a complex array of events within the cell upon ligand binding to specific hormone receptors. The cellular responses that hormones elicit are broadly classified as i) genomic responses characterized by changes in gene transcription requiring hours to days and ii) rapid signaling events occurring within seconds to minutes of cell stimulation.
The most thoroughly characterized steroid-induced cellular responses are mediated by estrogen. Estrogen (E2) is a critical hormone in the human body, regulating functionally dissimilar processes in numerous tissues. Estrogen is one member of the family of steroid hormones, which also includes progesterone, testosterone, cortisol/glucocorticoids and aldosterone/mineralocorticoids that control many aspects of mammalian physiology. Additional estrogen-based steroids, estrone and estriol are also known to mediate biological functions. Estrogen is involved in diverse functions such as regulating growth, development and homeostasis of numerous tissues [22]. The best understood of these are mammalian female reproduction and breast development [23]. In addition, estrogen regulates skeletal physiology [24], cardiovascular function [25] and the central nervous system [26] as well as the immune system [27]. In addition to its well-characterized role in sexual differentiation and regulation of reproductive neuroendocrine function, E2 also demonstrates important neuroprotective effects in the central nervous system [28, 29]. E2 promotes the growth and differentiation of neurons in the developing forebrain through high-affinity interactions with E2 receptors [30]. E2 acts in concert with neurotrophins (e.g., nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophins 3 and 4/5) that also have well-documented effects on the development, survival, plasticity, and aging of neurons that contribute to the reproductive and cognitive functions in the mammalian forebrain [30].
Until recently, the classical estrogen receptors (ER-α and ER-β) were considered to be the sole contributors of both the genomic and rapid signaling responses initiated by estrogen. However, during the past few years, a member of the 7-transmembrane (7-TM) G-protein coupled receptor (GPCR) family, GPR30, has been characterized to be capable of mediating both genomic and rapid events in response to estrogen [31]. The immunosuppressive effects of E2 are mediated via specific receptors and depend on the regulated expression and cellular distribution of these receptors [32], particularly estrogen receptor-α (ER-α) that mediates the major therapeutic effects of E2 [33]. The expression of ERs on various immune cells, including T and B lymphocytes and myeloid antigen presenting cells (APCs), suggests that therapeutic effects of E2 may involve direct receptor binding. Neurons in the forebrain co-express E2 and neurotrophin receptors [34] and also are abundant producers of E2 [35] and neurotrophin. E2 and neurotrophins appear to reciprocally regulate each other’s actions by cross-coupling of their signaling pathways, which eventually lead to the induction of same set of genes involved in neurite outgrowth and differentiation. Studies demonstrating the presence of membrane associated estrogen receptor (mER) in the oligodendrocyte plasma membrane and within the myelin sheath [36] indicate the importance of estrogen-mediated signaling via its various receptors in the neurological compartment as well. These data suggest that estrogen could act directly on myelin as well and contribute to its preservation and function. The striking protective effects of E2 in EAE raise the possibility that some form of estrogen might be beneficial as a therapy for MS. Moreover, the neuroprotective effects of estrogen might have a particular value in preserving the nerve viability and myelin function in the CNS that is gradually lost during the progression of MS. Rhonda Voskuhl and colleagues have now completed a phase I clinical trial of estriol to treat 10 female MS patients [37]. Their study demonstrated that at a dose of 8 mg/day given orally for 6 months (producing serum levels of about 4 ng/mL), the patients had decreased delayed-type hypersensitivity reactions to tetanus recall antigen, decreased IFN-γ levels in blood mononuclear cells, and reduced numbers and volumes of gadolinium enhancing lesions. Moreover, there was a significant improvement in cognitive scores using the paced auditory serial addition (PASAT) test in women with relapsing–remitting MS, but not secondary progressive MS. These are encouraging results that suggest a role for sex hormones in the treatment of MS. It is of particular importance that the preclinical studies in EAE involved a treatment regimen in which estriol was given 10–14 days prior to the transfer of encephalitogenic T cells, resulting in disease reduction but not complete protection [38]. This treatment regime has some similarity to the fully protective regime we used routinely with low doses of E2 to prevent EAE. The studies carried out in our lab makes use of estrogen pellets within the range: 0.36mg pellet providing 0.15–0.2 ng/ml concentrations of serum estrogen (estrus levels); 2.5mg pellet providing 1.5–2.0 ng/ml of estrogen (one-fifth of pregnancy level) and 15mg pellet providing 9.0–10.0 ng/ml serum estrogen levels (pregnancy level). Thus, indicating that results obtained in our E2 pretreatment studies might also be translatable to MS patients.
In this review, we take into consideration the various controversies regarding GPR30, some of them even questioning the very existence of GPR30 as a membrane ER. We are aware of the fact that even as more studies recognize GPR30 as an estrogen receptor, many questions pertaining to its function perhaps complementing the classical ERs and participating in the non-genomic effects induced by estradiol, still remain unanswered. Some of these unanswered questions include the physiological function of GPR30 in normal as well as diseased tissues and discerning the overlapping or distinct functions of GPR30 with respect to the classical ERs, ER-α and ER-β. We make use of the recent literature in understanding this novel estrogen receptor and attempt to discuss the association of GPR30 with EAE. We are aware that our understanding of GPR30 at both the molecular and physiological level is very much in its infancy, and role of GPR30 as one of the ER and its subsequent role in EAE remain to be thoroughly investigated. As the vast literature in this field of study points towards the strengths and limitations of each of the mouse models used to study GPR30, this review focuses on recent findings of GPR30 and its agonist G-1 and compares its functional and physiological properties with those of classical estrogen receptors ER-α and ER-β in EAE, as an attempt to characterize a putative therapeutic agent against MS.
ROLE OF ESTROGEN IN EAE
Since, subjects with MS are more females than males [1] and since it has been established that the clinical signs of MS often show clinical improvement during pregnancy, followed by temporary post-partum exacerbations [1, 7, 14], much interest has been generated on the role of sex hormones especially estrogen and estriol on regulation of immune responses as well as repair and protection of neurologic parameters. Extensive literature and work from our laboratory demonstrate numerous processes impacted by estrogen (17β-estradiol) and its derivatives, to inhibit the clinical and histological signs in EAE in mice.
EAE, a mouse model to study the clinical symptoms of MS, is a chronic disease that is induced upon injection of MOG35–55 peptide/CFA/pertussis toxin. The initial immune response in EAE is inflammatory and is mediated by cytokines such as IFN-γ, TNF-α, IL-1, IL-2, IL-6 and IL-8. In recent studies the pro-inflammatory cytokine, IL-17, has been indicated to take center stage for induction of EAE since it is responsible for activation of T cells leading to production of a variety of cytokines, chemokines and matrix metalloproteases [39, 40]. The recovery phase of EAE is associated with the production of Th-2 like cytokines, including IL-4, IL-5, IL-10, IL-13 and TGF-β. Although the etiology of MS remains unknown, pathogenic mechanisms are similar to EAE. Patients with MS in early phases demonstrate episodic loss of motor function, followed by a progressive neurological deficit [41]. Histologically, there are perivascular demyelinating lesions that develop into scarified plaques [42]. A common histopathological feature shared by EAE and MS is that both diseases appear to be induced by auto-reactive T cells in the CNS, with an effector phase mediated by cells of the myeloid lineage. Macrophages and CD4+ and CD8+ T cells have been identified in the active MS lesions in the CNS. Self-reactive T-cell clones persist as a part of the normal repertoire and possess the capability to induce autoimmune disease. In most normal individuals, potentially dangerous autoreactive T cells never exceed a critical threshold frequency because of efficient peripheral regulatory mechanisms or the lack of sufficient activation [43, 44].
Our studies clearly demonstrate an important regulatory role of estrogen in EAE. Endogenous ovarian hormones naturally limit EAE [45]. We found that ovariectomized (OVX) mice had significantly earlier onset and greater severity of EAE than sham operated mice, clearly demonstrating that basal levels of ovarian hormones, including E2, acted to regulate rather than facilitate EAE. Moreover, exogenous E2, given as implanted pellets a week before EAE induction, inhibited clinical signs of EAE in a dose-dependent and mouse strain–dependent manner at doses ranging from pregnancy levels (5000–10,000 pg/mL E2 in serum delivered by 15mg pellets) to diestrus levels (20–30 pg/mL E2 in serum delivered by 0.1mg pellets) in both female and male mice [18, 19, 45, 46]. Histopathological examination of spinal cords from E2-treated mice revealed a striking reduction of inflammation and demyelination. Estrogen treatment has been found not effective when initiated after onset of EAE [47], suggesting inhibition of naive rather than differentiated cells. Estrogen treatment was effective at reducing the severity of EAE in a variety of mouse strains, although the optimal dose varied. The rank order of susceptibility to E2 treatment versus EAE was SJL/J>TCR AV4/BV8S2 double transgenic (Tg)>B10.PL>C57BL/6>TCR BV8S2 single Tg mice. These data demonstrate conclusively the protective effects of E2 on EAE in five mouse strains using three different encephalitogenic peptides. Furthermore, we have established the role of various proinflammatory and regulatory cytokines in EAE susceptibility and E2 treatment of EAE using WT, OVX, and cytokine knockout mice [19, 46]. We found that TNF-α, MIP-1α, and MIP-2 were associated with increased severity of EAE. E2 treatment strongly inhibited infiltration of total cells, macrophages, and T cells into the CNS, with a relative enrichment of brain microglial cells. This marked reduction in inflammatory cells was reflected by a strong reduction in expression of RANTES, MIP-1α, MIP-2, IP-10, and MCP-1, and CCR1, 2, and 5 in CNS of both intact and OVX mice, and LT-β, TNF-α, and IFN-γ in the CNS of intact mice [46]. Moreover, E2 inhibited intracellular staining of TNF-α in CNS macrophages, microglia, and T cells at onset of EAE, and in macrophages at the peak of EAE [19]. Surprisingly, proliferation responses to encephalitogenic peptides were only modestly reduced in cultures from fully protected E2-treated B6 mice [18, 19, 45], and there were no striking changes in expression of cell surface markers from lymph node (LN) cells, including VLA-4, CD44hi, CD69, FasL, CD25, CD40L, CD28, and CD62lo [19]. We further documented a pronounced systemic effect of estrogen on TNF-α production, in which expression of TNF-α was reduced>10-fold in splenocytes from E2-treated mice compared with control mice with EAE [48]. Systemic E2 regulation of TNF-α production could account for much of the observed reduction in EAE severity by restricting the ability of encephalitogenic cells to enter the CNS, suppressing the recruitment and activation of other inflammatory cells, and/or inhibiting TNF-α-induced damage to myelin-producing oligodendrocytes. These observations, coupled with the established importance of TNF-α in the induction of EAE [49], provide strong evidence that the inhibitory effects of estrogen on TNF-α production by T cells, macrophages, and microglial cells constitutes a major regulatory pathway contributing to EAE protection. E2 treatment was demonstrated to have multiple effects on antigen-presenting cells, macrophages and dendritic cells (DCs), which contribute to downregulation in activation of Th1 cells. An E2-induced enhancement of programmed death receptor-1 (PD-1) expression was observed in DCs and macrophages indicating that E2-mediated immunomodulation could be in part via the PD-1 co-stimulatory pathway [50]. We were also able to demonstrate that E2 contributed in the upregulation of T-regulatory (Treg) cell function during pregnancy and E2 treatment regimens [51] and that functional Treg suppression was closely linked to PD-1 expression on the Treg cells [52, 53]. Thus, E2 can both upregulate Treg suppression function and inhibit the ability of antigen presenting cells to activate T cells indicating that E2 can mediate these opposing functions to curb inflammatory environments during EAE and thereby mediate protection.
ROLE OF ESTROGEN RECEPTORS IN E2-MEDIATED PROTECTION AGAINST EAE THE CLASSICAL ERs
There are two known classical intracellular estrogen receptors, ER-α and ER-β, which mediate their effects by transcription regulation, as well as a newly described membrane associated receptor GPR30 which is associated with calcium flux and other signaling events. One of our main goals was to determine which ER mediates various E2-related effects on EAE. To assess the role of intracytoplasmic estrogen receptors in mediating suppression of EAE, we studied ER-α knockout (ERKO) and ER-β knockout (BERKO) mice from Taconic Farms (Korach line). We demonstrated that the protective effect of E2 was abrogated in ERKO but not in BERKO mice [33]. The loss of E2-mediated protection from EAE in ERKO mice immunized with encephalitogenic MOG-35–55 peptide was manifested phenotypically by the development of severe acute clinical signs and histopathologic lesions, even in the presence of moderately high serum E2 levels. On the other hand, despite comparable serum levels of E2, E2 treatment in BERKO mice markedly suppressed clinical signs of EAE and abolished inflammatory lesions in the CNS. The lack of E2-mediated protection in the ERKO mice went along with an enhanced, rather than reduced secretion of TNF-α, IFN-γ and IL-6 in MOG-specific splenocytes and a lack of inhibition of message for inflammatory cytokines, chemokines, and chemokine receptors in CNS tissue. These results indicate that the immunomodulatory effects of E2 in EAE are dependent on ER-α and not ER-β signaling. Our results have been independently confirmed [54] and are highly consistent with another recent report showing that ER-α mediates anti-inflammatory effects of E2 in brain [55].
Estrogen receptors are expressed by various immune cells (e.g., T and B lymphocytes, antigen-presenting cells) suggesting that the therapeutic effect of E2 is likely mediated directly through specific receptor binding. In furthering our estrogen receptor-related studies, the pathogenic role for myelin specific T lymphocytes in EAE suggested that E2-mediated protection from EAE might be mediated through direct effects on ER-α-positive T lymphocytes. We examined this possibility by evaluating E2-protective effects in mice with EAE induced after adoptive transfer of ER-α+/+ versus ER-α−/− encephalitogenic T cells. E2 treatment protected mice from EAE and did not depend on ER-α expression by encephalitogenic myelin-specific T cells [56]. This demonstrated that the disease-initiating lymphocytes are not the primary E2-responsive cells and implicates other cell types as the primary E2-responsive cells in EAE. The clinical response to E2 was associated with decreased mononuclear cells (total cells, CD4+ and CD8+ lymphocytes, and CD11b+ macrophages and dendritic cells) in the CNS. The numbers of T cells expressing inflammatory adhesion markers VLA-4 and LFA-1 in the brain was reduced in E2-protected mice, indicating that E2 treatment blocks entry of T cells into the CNS indirectly and is independent of E2 effects on adhesion molecule expression by encephalitogenic T cells. An absence of inflammation and demyelination, pathological disease in all E2-protected mice regardless of ER-α expression by encephalitogenic T cells further suggests that encephalitogenic T cells do not directly mediate the E2 treatment response. This raises the possibility that non-lymphoid cells such as bone marrow-derived dendritic cells and macrophages are the primary E2-responsive cells in EAE.
Although a requirement for ER-β was not previously demonstrated using homozygous BERKO mice, role for ER-β could not be ruled out since the ER-β-selective ligand treatment produced a delayed therapeutic response that depended on ER-β expression and was attributed to an ER-β-mediated neuroprotective effect [57]. Therefore, we investigated ER-β in the therapeutic response to E2 using bone marrow chimeric mice lacking ER-β in the bone marrow versus non-bone marrow compartments. Our results demonstrated that ER-α signaling was not sufficient to sustain the full E2 response in the absence of ER-β in the non-bone marrow compartment, thus indicating that signaling through ER-β on non-bone marrow-derived cells (e.g. CNS) is important to the neuroprotective response to E2. ER-β signaling in bone marrow compartment did not appear to be at all necessary for the full response to E2 when signaling though ER-α was intact. The early response to E2 (corresponding to early immunosuppression) seemed to be entirely independent of a requirement for ER-β, instead depending on ER-α. ER-β was not required in the hematopoietic compartment for E2-induced up-regulation of IL-5 and IL-13, nor for the E2-induced down-regulation of IL-2, IL-4, IL-6, IL-17 and IFN-γ. However, when present with ER-α in the hematopoietic compartment, ER-β may inhibit certain ER-α mediated anti-inflammatory cytokine responses to E2 [58]. However, with the discovery of the mER, GPR30, one cannot disregard the potential multiple effects of E2 signaling through GPR30 and that additional therapeutic pathways may operate in presence and/or absence of ER-mediated effects.
GPR30 AND THE NEED FOR ESTROGEN TO HAVE MULTIPLE RECEPTORS
In 1977, Pietras and Szego provided the first evidence of membrane associated ERs in rat endometrial cells [59]. Subsequently ER-α has been shown to localize to the cell membrane in vitro and possibly is derived from the same genes as the nuclear receptor. Thus, the classical nuclear ER-α (and ER-β) have been characterized to relocate to the plasma membrane under certain circumstances and mediate extra-nuclear functions of ER [32, 60–63]. Post-translational modification of the receptor is believed to play a pivotal role in ER membrane localization, for example lipid modification, such as palmitoylation of ER [60, 64]. Localization of ER-α to specialized membrane structures enriched in signaling molecules, such as caveolae, provide an alternative mechanism for ER to communicate and cross-talk with membrane or cytoplasmic signaling cascades [64–66]. ER-α, tethered to the plasma membrane, is capable of mediating estrogen activation of MAPK and PI3K/Akt pathway [67], however it’s presence at the membrane is insufficient to activate genes and other cellular processes required for cell proliferation [68]. Moreover, mice expressing a membrane-only estrogen receptor (MOER) do not exhibit normal phenotype indicating that the nuclear function of ER-α is required for normal development and physiological functions. When co-expressed with ER-α, ER-β causes a reduction of the ER-α-mediated transcriptional activation, including cell proliferation [69, 70]. In fact, findings indicate that the plasma membrane localized ER-β is important for anti-proliferative effects of E2 [71]. Thus, despite advances in understanding of rapid extra-nuclear actions of ER-α in recent years, the molecular mechanisms by which ER-α initiates and participates in extra-nuclear signaling still remains unclear.
While the regulatory role of estrogen in controlling autoimmune diseases like EAE, have been repeatedly demonstrated and therefore widely accepted, it is believed that this effect is associate with the nuclear receptors that function as transcription factors. However, it is now also known that various events, such as calcium influx and production of nitric oxide (NO) elicited by E2 are observed within minutes of stimulation. The time course taken led to the idea that a novel membrane receptor for E2 existed on the cell surface. In the past few years a previously uncharacterized ‘orphan’ G protein coupled receptor (GPCR), GPR30 (GPR30) has been shown to be a novel mediator of estrogenic action. GPR30 variously known as G protein-linked estrogen receptor (GPER-1), CEPR, FEG-1, CMKRL2 or LyGPR is a 375 amino acid ‘class-1’ G-protein-coupled receptor was first identified by several groups in the late 1990s [72–75], however little was known regarding its possible function. In 2000, activation of MAP kinase (Erk1/2) by estrogen in breast cancer cell lines expressing GPR30 but not in cell lines lacking the receptor was demonstrated for the first time [76]. Recent studies examining the identity of the membrane-bound estrogen receptor (mER) have diverged into two schools of thought, the first being that an orphan G-protein-coupled receptor (GPCR) protein ‘GPR30’ is the mER [77, 78]. Although Filardo et al. originally described GPR30 as a plasma membrane receptor; Thomas et al. and Funakoshi et al. reported the expression of GPR30 in the plasma membrane [78, 79]. However, numerous subsequent studies have confirmed the expression of endogenously expressed GPR30 in the endoplasmic reticulum/Golgi apparatus [80–82]. Although the majority of GPCRs are expressed in the plasma membrane, it is becoming more evident that some GPCRs may be functionally expressed at intracellular sites [83], particularly, the GPCRs with lipophilic ligands. Given that the ligand for GPR30 is the lipophilic E2 that can permeate the membrane easily, an intracellular localization of the receptor is consistent with its function [22]. GPR30 was found to belong to the rhodopsin-like receptor super family and showed highest homology to the interleukin 8 receptor and the angiotensin II receptor type 1 [74, 75]. GPR30 has been found to be expressed on a wide variety of cell types including the nervous system [22, 84] and on the cell that have immune functions [85–87].
GPR30 AND THE IMMUNE SYSTEM
Estrogens are known to exert multiple effects on the development and regulation of the immune system including E2-promoted thymic atrophy during pregnancy [88]. Early findings indicated a role for GPR30 in the immune system because of its robust expression in human immune cells and immune cell-derived cell lines [79, 89]. GPR30 mRNA was also detected in peripheral B cells, circulating T cells, and in CD14+ monocytes, whereas no mRNA was detected in CD15+ cells (neutrophils and eosinophils) [89]. These observations suggest that GPR30 may participate in adaptive immune system, particularly in B and T cell maturation however similar such receptor mapping has not yet been performed in mouse. Our lab reported [89] that GPR30-deficient mice are partly resistant to thymic atrophy induced by E2. In an attempt to confirm this phenotype, we also treated wild type mice with G-1 and E2; G-1 was found to be substantially less effective in inducing thymic atrophy than E2, whereas G-1 and E2 were equally effective in inducing thymocyte apoptosis. However, Windahl et al. [90], using ovariectomized GPR30-deficient mice generated by Martensson et al. [91], were unable to reproduce our results on E2-promoted thymic atrophy. Consistent with this, Otto et al. [92] did not observe any effect of G-1 on thymic atrophy in wild-type mice. The precise reason for these conflicting observations is presently unclear but, as suggested by Otto et al., could be related to the use of intact female mice that were not controlled for the estrus cycle. However, Isensee et al. [93] reported an increased apoptosis rate for naïve T cells in their GPR30-deficient mice. Nevertheless, some results point towards a possible role of GPR30 in adaptive immune cell maturation, but it is clearly premature to specify what its role might be. Furthermore, additional studies are necessary to determine if, besides the well documented role of ERα [94], GPR30 truly contributes to E2 regulation of immune cell function and thymic atrophy [84]. Currently, there are four GPR30 mutant mouse lines available, each with diverse phenotypes generated using distinct targeting strategies and genetic backgrounds. Each mouse model has its own strengths and caveats with only partial overlap amongst the four models. The experimental purpose for which each mouse model is being used might explain the difference in observations and careful considerations must be made in order to facilitate the elucidation of the potential physiological role of GPR30 [95].
GPR30 AND EAE (THE GPR30 MOUSE MODEL AS DESCRIBED BY WANG et. al.)
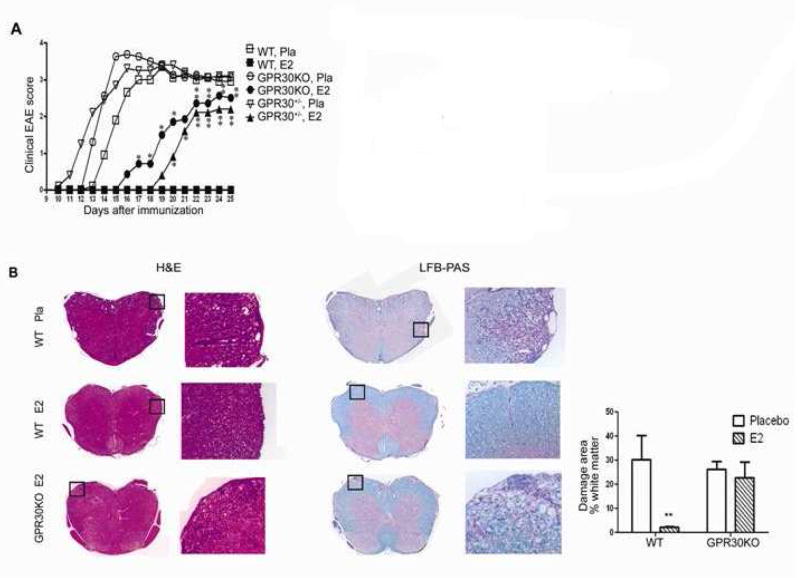
GPR30 signaling can account for several E2-induced effects such as inhibition of oxidative stress-induced apoptosis and upregulation of NGF in macrophages [31]. Recently, we reported the generation of GPR30-deficient mice (GPR30KO) [89] that possess many similarities to the mouse strain generated independently by Mårtensson et al. [91]. Mice homozygous for GPR30KO are viable, fertile, and do not display any gross physical or neurological abnormalities; however, E2-induced thymocyte apoptosis and thymic atrophy were drastically mitigated [89]. The mice used for our study were generated using a targeting strategy wherein the exon 3 was replaced by neomycin resistance cassette, with the neomycin cassette being retained in the genome. Homozygous intercrosses were set-up in C57Bl/6 mice, with the mice being backcrossed, 3 times before use using the SvEv ES cells. We used these GPR30KO mice to investigate the role of GPR30 in E2-mediated protection against EAE and to define whether GPR30 is indispensible for E2-mediated protection against EAE. As indicated in Figure 1, GPR30 can be an active contributor but not the only player in mediating the suppressive effects of E2 on EAE. This conclusion is consistent with our previous results on ER-α knockout mice [33], which demonstrated that the protective effect of E2 was mainly dependent on ER-α. The study demonstrated that, although E2-treatment delayed the disease onset for 4 days, it did not reduce the incidence, disease peak, or CDI score. Similarly, Voskuhl et al. reported complete loss of protection by estriol treatment in ERKO mice [54]. However, as shown in Figure. 1, there clearly is residual E2 protection in GPR30 KO mice, indicating contribution of other receptors, presumably ERα. Thus, it is likely that both GPR30 and ERα participate in E2-mediated protection in an additive manner.
Fig. (1). E2-induced protection against EAE is reduced in GPR30KO mice.
Age-matched GPR30KO, WT and heterozygous mice from the same colony were implanted with 2.5 mg/60 day release E2 or placebo pellets one week prior to immunization with mMOG-35–55 peptide plus CFA and PTX. The mice were scored for EAE development and euthanized 25 days after immunization. A. The protective effect of E2 was reduced but not completely abrogated in GPR30KO mice. B. E2 did not prevent CNS infiltration and demyelination in GPR30KO mice. For quantification, 5 mice randomly selected from each group were included, and similar differences were noted consistently in all mice from each group. *P < 0.05 or **P < 0.01 as compared to placebo control. The experiment was repeated 3 times with at least 7 mice in each group.
ROLE OF G-1 AS GPR30-SPECIFIC AGONIST
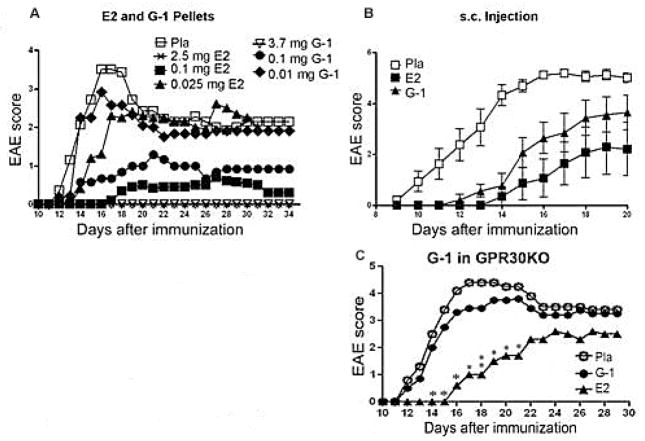
The development of selective agonists for mER made it possible to specifically activate mER without transactivation of iERs. A synthesized compound, STX, that does not exhibit any binding affinity for the nuclear receptors ER-α or ER-β, but mimics the quick effect of estrogens on neurons has been earlier described [96]. But since the molecular target of STX has not been identified and it remains to be determined whether STX indeed interacts with a mER. Besides STX, the only other reported mER ligand is G-1, which we selected for further study due to its ability to bind GPR30 with high affinity and excellent specificity [97]. Biomolecular screening was carried out using flow cytometry based on the competitive binding of the fluorescent estrogen by 17β-estradiol in GPR30-transfected cells yielding a single compound, referred to as G-1 that consistently competed for binding of the fluorescent estrogen reporter [98]. In an attempt to characterize both the binding affinity of G-1 to GPR30 and its binding specificity with respect to ER-α and ER-β, when competition binding was carried out, it was found that Ki values for 17β-estradiol binding to GPR30-expressing cells were 5.7 nM as opposed to the Ki values of 0.30 and 0.38 nM for 17β-estradiol binding to ER-α and ER-β expressing cells. Whereas Ki for G-1 in GPR30-expressing cells was 11 nM with no substantial binding of G-1, at concentrations up to 1 μM, in the ER-α and ER-β expressing cells. These results indicated that G-1 not only has a very high affinity for GPR30 but also great selectivity toward GPR30 as compared to ER-α and ER-β [98]. Similarly, using competitive binding assays, it was demonstrated that, whereas on one hand, G15 bound to GPR30 with an affinity of approximately 20 nM it displayed very little binding to ER-α or ER-β, i.e. at concentrations up to 10 μM, where estrogen competes with a Ki of approximately 0.3–0.5 nM. These results reveal that G15, like G-1, displays high affinity for GPR30 with minimal binding to ER-α and ER-β (Ki >10 mM) [99]. Since the loss of protection in GPR30KO mice indicated that GPR30 contributed to E2-mediated protection against EAE, we next determined whether activation of GPR30 alone could confer protection against EAE. Remarkably, none of the WT mice receiving the highest dose of subcutaneous G-1 pellet implants or injections showed any clinical signs of EAE (Figure 2A & B). In the absence of GPR30, the effect of G-1, but not E2 completely disappeared (Figure 2C and Figure 4). These results suggested that solo activation of GPR30 is sufficient to confer protection against EAE. The protective effects of G-1 in WT mice eliminated the possibility that the observed role of GPR30 could be due merely to an artifact of gene deletion or a compensation effect in the developing animal. However, the immediate question was that if GPR30 is only one of the receptors that mediate the protective effect of E2, how could G-1 treatment at a sufficiently high dose completely protect mice against EAE? Since G-1 did not protect the mice from EAE in GPR30KO mice, we did not believe that transactivation of ER-α by G-1 played a significant role. Also, we did not detect any significant increase in the serum levels of estradiol, corticosterone, progesterone or testosterone in G-1-treated mice, thus ruling out the possibility that upregulation of endogenous steroid hormones accounted for the clinical and neuroprotective effects of G-1 treatment (Figure 3). Thus, we concluded that ER-α and GPR30 work together additively to achieve optimal protection, and each receptor may adequately compensate the functional loss of the other.
Fig. (2). Activation of GPR30 conferred substantial protection against clinical EAE in WT B6 mice.
A. Treatment with G-1 delayed and ameliorated EAE in a dose-dependent manner. Various doses of G-1, E2 or placebo pellets were imbedded underneath the skin on the back of the mice. One week following the start of treatment, the mice were immunized with mMOG-35–55 peptide plus CFA and PTX. The mice were scored for EAE development and euthanized 34 days after immunization for ex vivo studies. P < 0.05 or 0.01 for 2.5 and 0.1 mg E2- and 3.7 mg G-1-treatment groups from Day 14 to 34 and for 0.1 mg G-1-treatment group from Day 14 to 19 and from Day 25 to 34. The experiment was repeated 2 times with 5–8 mice in each group. B. G-1 injected s.c had nominally less protection than E2 against EAE. G-1 (20 μg/mouse/day in 100 μl of 10% ethanol and 90% olive oil), E2 (1 μg/mouse/day in 100 μl of 10% ethanol and 90% olive oil) or placebo (100 μl of 10% ethanol and 90% olive oil) were daily injected underneath the neck skin of the mice one week prior to immunization. The mice were monitored for changes in clinical EAE scores and euthanized 20 days after the immunization. P < 0.05 or 0.01 for E2-treatment groups from Day 11 to 20, and for G-1-treatment group from Day 11 to 17 as indicated by One-way ANOVA followed by Newman-Kuels multiple comparisons test. C. G-1- but not E2-indcued protection against EAE was abrogated in GPR30KO mice. Neither G-1 nor E2 affected T cell proliferation to mMOG-35–55. GPR30KO mice were immunized one week after implantation with 1.8 mg/40 day release G-1 or 2.5 mg/60 day release E2 pellets for a week. The experiment was concluded 29 days after immunization for ex vivo experiments. *P < 0.05 or **P < 0.01 compared to placebo control. The experiment was repeated 2 times with 7–10 mice in each group.
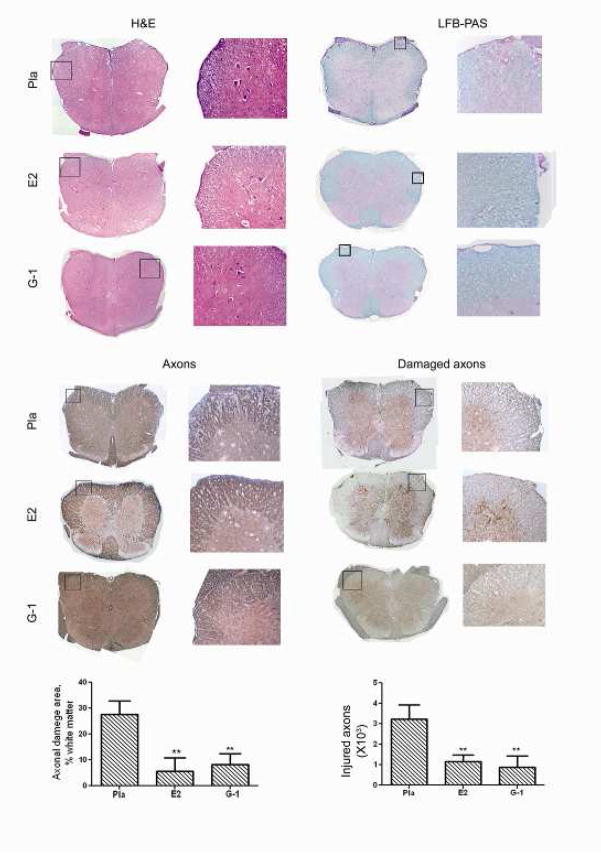
Fig. (4). G-1 treatment reduced CNS infiltration (H&E), demyelination (LFB-PAS), axonal loss (NFLs) and ongoing axonal damage (dephosphorylated NFLs).
The mice from the clinical experiment shown in Fig. 2a were euthanized at the end of the experiment and spinal cords from ≥3 mice from each group were dissected for histology. Immune cell infiltration and demyelination of CNS were examined with H&E and LFB-PAS staining. Total and damaged axons were examined with immunohistological staining for neurofilaments (NFLs) or dephosphorylated NFLs.
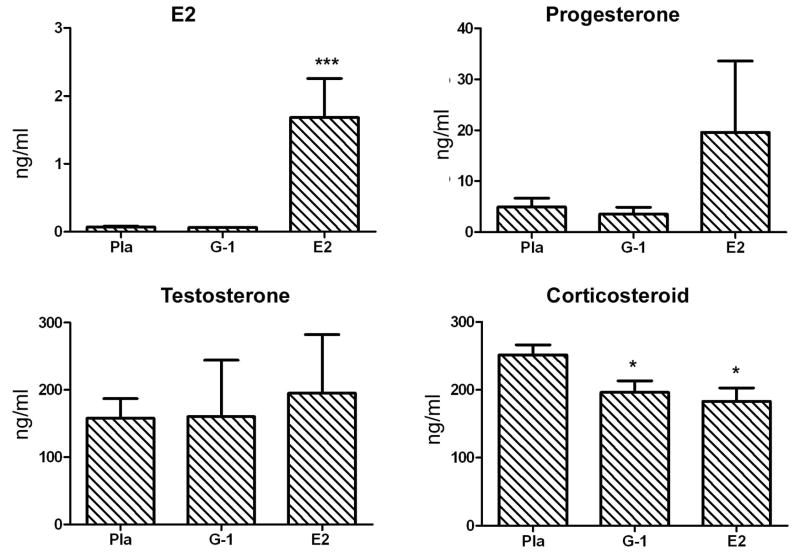
Fig. (3). G-1 treatment did not change the serum levels of E2, progesterone or testosterone and only slightly lowered corticosteroid.
Steroid levels were measured in sera collected from placebo-, 3.7 mg G-1- and 2.5 mg E2-treated mice used in clinical experiments shown in Fig. 2A. *P < 0.05 or ***P < 0.001.
Mechanistically, we observed that G-1 treatment did not directly suppress pathogenic T cell proliferation, nor did it increase the percentage of CD4+FoxP3+ Treg cells. Nevertheless, both E2 and G-1 markedly increased the level of PD-1 expression in CD4+FoxP3+ Treg cells. PD-1 has been found to play a crucial role in the development and maintenance of peripheral tolerance [100]. Moreover, the therapeutic effect of G-1, but not E2, disappeared completely in PD-1KO mice. Thus, it seemed that G-1 was exclusively dependent on the presence of PD-1 to function, but the effects of E2 could be mediated by alternative pathways in the absence of PD-1. Taken together, these results suggested that G-1 may suppress EAE by upregulation of the PD-1 signaling pathway in CD4+FoxP3+ cells. Also, supporting the role of PD-1 in G-1-mediated stimulation of GPR30 was the fact that both E2 and G-1 decreased IL-17 in vivo, since this cytokine is closely linked to the development of EAE. However, since G-1 failed to reduce IL-17 production in PD-1 gene-deficient mice it strongly supported the idea that G-1 regulated IL-17 production via manipulation of PD-1.
Most importantly, our results showed that treatment with G-1 in vivo lacked some of the prominent “estrogenic effects” of E2 and did not cause any abnormalities in liver, eyes, heart, mammary gland, brain, spleen, kidney, muscle or lung. Although we believe that the use GPR30 ligands will avoid some of the side effects mediated by ER-α, it remains to be seen whether or not GPR30 agonists represent a “safer” alternative to estrogen treatment. Nevertheless, our study demonstrated that it is possible to avoid some of the side effects mediated via intracellular ERs (iERs) by specifically targeting the membrane receptor, while retaining much of the therapeutic efficacy of estradiol, at least in EAE. Taken together, we showed that the putative mER, GPR30, is sufficient, yet not exclusively responsible for full E2-mediated protection against EAE. Treatment with G-1 that specifically targets GPR30 suppressed clinical and histological EAE and the production of IL-17 by upregulation of PD-1 expression in regulatory T cells. This study was the first to evaluate the contribution of a membrane steroid receptor in suppression of autoimmune disease in an animal model, and may provide the necessary foundation for the clinical application of membrane steroid receptor agonists such as G-1 in human subjects.
Additionally, our group was able to demonstrate the neuroprotective and immunosuppressive role of G1 in a stroke model. The reduced risk and severity of stroke in adult females is thought to depend on normal endogenous levels of estrogen, a well-known neuroprotectant and immunomodulator. In male mice, experimental stroke induces immunosuppression of the peripheral immune system, characterized by a reduction in spleen size and cell numbers and decreased cytokine and chemokine expression. To test the hypothesis that estradiol (E2) deficiency exacerbates immunosuppression after focal stroke in females, we evaluated the effect of middle cerebral artery occlusion (MCAO) on infarct size and peripheral and CNS immune responses in ovariectomized mice with or without sustained, controlled levels of 17β-estradiol administered by subcutaneous implant or the putative membrane estrogen receptor agonist, G-1. Both E2- and G-1-replacement decreased infarct volume and partially restored splenocyte numbers [101]. In addition to the classical estrogen receptors, ER-α and ER-β, that are known to contribute to protection in the brain after MCAO [102, 103], the protective ability of G-1 renders it a potential candidate for therapeutic intervention against ischemic brain injury because it lacks undesired effects on reproductive organs.
Elsewhere, in an attempt to mimic an infectious disease model, it was demonstrated that E2 or GPR30-specific agonist decreased TLR4 expression on macrophages within 10–60min and such effects were abolished following GPR30 knockdown [87]. Based on these findings, it was suggested that estrogen may utilize GPR30 to limit potentially lethal acute inflammatory responses without compromising long- term host defense. This adds on to the capability of G1 in effectively limiting inflammatory responses, in concert with the finding that G-1 can also reduce disease severity and CNS inflammation in both active and passive EAE models of multiple sclerosis in SJL mice [85], raising an exciting possibility of its therapeutic potential.
ETHINYL ESTRADIOL AND GPR30 IN EAE
We have earlier shown that 17β-estradiol can protect against the development of EAE when administered subcutaneously prior to immunization with myelin peptide, but it cannot treat the disease (Offner unpublished data; [47]). Conversely ethinyl estradiol (EE), a synthetic estrogen, frequently used in oral contraceptives, can reduce disease severity when given orally at the onset of the disease symptoms [104]. EE is able to maintain its bioavailability in the host system after oral dosing, unlike E2 [105]. In addition to the differences in the bioavailability, it is possible that differential activation of estrogen receptors may be involved in the treatment ability of EE compared to the ineffectiveness of E2. We addressed this issue by using mice lacking ER-α and GPR30 to explore their role in the ability of EE to treat EAE. Expression of GPR30 seemed to be crucial in the ability of EE to reduce disease, as no treatment effect was observed in GPR30KO mice while ERKO mice maintained their ability to respond to EE treatment of EAE. When the IL-10 and IL-17, cytokine production by splenocytes in response to antigen, was examined in an effort to discern the specific changes responsible for the disease inhibition in EE-ERKO but not EE-GPR30KO mice, it was found that the secretion of IL-10 was increased in EE-ERKO mice compared to controls, but decreased in EE-GPR30KO mice. No change in IL-17 secretion as well as percentage of Foxp3+PD-1+ Treg cells was seen. This difference in IL-10 production may be an important contributor to the disease reduction in EE-ERKO mice compared to the GPR30KO mice which did not improve with EE treatment. However, the subset of cells responsible for this differential secretion of IL-10 in these studies was unclear and warrants further investigation [106].
Thus, based on our above mentioned results, it is likely that both GPR30 and ER-α participate in E2-mediated protection in an additive manner. However, based on the difference in residual protection in ERKO vs. GPR30KO mice treated with E2, we think that there is a lesser contribution of GPR30 to protection when compared to ER-α. We cannot yet conclude that E2-induced protection against EAE is mediated exclusively by ER-α plus GPR30, but GPR30KO and ERKO double-gene-deficient mice are currently being constructed to address this issue.
FUTURE DIRECTIONS AND CONSIDERATIONS
As GPR30 becomes recognized as an estrogen receptor participating in the non-genomic rapid effects induced by estradiol, the findings open a can of worms whose full impact has yet to be understood. The questions yet unanswered not only include the physiological role of GPR30 in normal and diseases states, but also understanding the distinct and/or overlapping functions of GPR30 with respect to ER-α and ER-β. Despite several groups having shown that GPR30 mRNA is ubiquitously expressed in both humans and rodents, attempts to detect the receptor protein in tissues have met variable success. The quest for deciphering the physiological role for GPR30 should take into consideration: a) receptor expression patterns, b) phenotypes of receptor-deficient mouse models and c) earlier data on non-genomic estrogen signaling. Also, although human and mouse GPR30 bear a 87% homology, the fact that the remaining 13% could potentially impact the interpretation of GPR30 function and pharmacological differences between the species should be taken into consideration. It is slowly becoming evident that GPR30 does not participate in some of the many and well-known estrogenic responses in vivo. Even if GPR30 does not involve in the well-characterized estrogenic responses like regulation of reproduction, fat mass and bone mineral density, its role has become evident as an estrogen receptor in some of the other estrogenic responses where estrogen conditions the cells and tissues. Over time as more is being unraveled, it appears that under certain circumstances both GPR30 and the iERs are required, whereas in others, GPR30 can act in the absence of iERs to mediate estrogen-dependent effects. Discovering the biological functions of GPR30 needs further investigation and for more clearer results a consideration of the use of a combination of genetic (for example, mouse knockouts in controlled genetic backgrounds, RNA interference and gene transfer), pharmacological (synthetic agonists and antagonists) and biochemical (highly specific and validated antibodies and other types of probes) tools becomes a necessity. However with the use of selective ligands (agonist G-1 and the antagonist G15), siRNA approach and knockout mouse models, an enhanced understanding of the role of GPR30 in controlling normal and diseased conditions, stands firm for the future. Based on our studies, we believe that the membrane steroid receptor, GPR30, contributes to the suppression of EAE and future studies may provide the necessary foundation for clinical application of GPR30 agonists such as G-1 in treating human subjects with minimal side effects.
Acknowledgments
The authors wish to thank Ms. Eva Niehaus for assistance with manuscript preparation. This work was supported by NIH grant NS45445; National Multiple Sclerosis Society grant RG3405-C-6; this material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
LIST OF ABBREVIATIONS
- MS
Multiple sclerosis
- EAE
Experimental autoimmune encephalomyelitis
- MBP
Myelin basic protein
- PLP
Proteolipid protein
- MOG
Myelin oligodendrocyte glycoprotein
- CNS
Central nervous system
- E2
17β-estradiol
- E3
Estriol
- APCs
Antigen presenting cells
- DCs
Dendritic cells
- OVX
Ovariectomized
- ERKO
ER-α knockout
- BERKO
ER-β knockout
- mER
Membrane associated estrogen receptor
- iERs
Intrinsic estrogen receptors
- NGF
Nerve growth factor
- BDNF
Brain-derived neurotrophic factor
- MCAO
Middle cerebral artery occlusion
- EE
Ethinyl estradiol
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFEREENCES
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139–151. J Neurosci Res. 1996;45(6):680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol. 1996;39(6):724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 4.Blazkovec AA, Orsini MW. Ontogenetic aspects of sexual dimorphism and the primary immune response to sheep erythrocytes in hamsters from prepuberty through senescence. Int Arch Allergy Appl Immunol. 1976;50(1):55–67. doi: 10.1159/000231480. [DOI] [PubMed] [Google Scholar]
- 5.Garsenstein M, Pollak VE, Kark RM. Systemic lupus erythematosus and pregnancy. N Engl J Med. 1962;267:165–169. doi: 10.1056/NEJM196207262670401. [DOI] [PubMed] [Google Scholar]
- 6.Oka M, Vainio U. Effect of pregnancy on the prognosis and serology of rheumatoid arthritis. Acta Rheumatol Scand. 1966;12(1):47–52. doi: 10.3109/rhe1.1966.12.issue-1-4.06. [DOI] [PubMed] [Google Scholar]
- 7.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 8.Ostensen M. Piroxicam in human breast milk. Eur J Clin Pharmacol. 1983;25(6):829–830. doi: 10.1007/BF00542529. [DOI] [PubMed] [Google Scholar]
- 9.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59(6):1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toivanen P, Siikala H, Laiho P, Paavilainen T. Suppression of adjuvant arthritis by estrone in adrenalectomized and ovariectomized rats. Experientia. 1967;23(7):560–561. doi: 10.1007/BF02137970. [DOI] [PubMed] [Google Scholar]
- 11.Larsson P, Holmdahl R. Oestrogen-induced suppression of collagen arthritis. II. Treatment of rats suppresses development of arthritis but does not affect the anti-type II collagen humoral response. Scand J Immunol. 1987;26(5):579–583. doi: 10.1111/j.1365-3083.1987.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 12.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol. 1994;53(2):203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 13.Trooster WJ, Teelken AW, Kampinga J, Loof JG, Nieuwenhuis P, Minderhoud JM. Suppression of acute experimental allergic encephalomyelitis by the synthetic sex hormone 17-alpha-ethinylestradiol: an immunological study in the Lewis rat. Int Arch Allergy Immunol. 1993;102(2):133–140. doi: 10.1159/000236563. [DOI] [PubMed] [Google Scholar]
- 14.Abramsky O. Pregnancy and multiple sclerosis. Ann Neurol. 1994;36 (Suppl):S38–41. doi: 10.1002/ana.410360712. [DOI] [PubMed] [Google Scholar]
- 15.Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171(11):6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 16.Olsson T. Critical influences of the cytokine orchestration on the outcome of myelin antigen-specific T-cell autoimmunity in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev. 1995;144:245–268. doi: 10.1111/j.1600-065x.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Paterson PY. Multiple sclerosis: an immunologic reassessment. J Chronic Dis. 1973;26(3):119–126. doi: 10.1016/0021-9681(73)90085-4. [DOI] [PubMed] [Google Scholar]
- 18.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 19.Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167(1):542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 20.Paavonen T, Andersson LC, Adlercreutz H. Sex hormone regulation of in vitro immune response. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med. 1981;154(6):1935–1945. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stimson WH, Hunter IC. Proceedings: An investigation into the immunosuppressive properties of oestrogen. J Endocrinol. 1976;69(3):42P–43P. [PubMed] [Google Scholar]
- 22.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109(3–5):350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 24.Frank GR. Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol. 2003;41(3):217–221. doi: 10.1002/mpo.10340. [DOI] [PubMed] [Google Scholar]
- 25.Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, Tsai B, Hile K, Brown JW, Meldrum DR. The role of estrogen in cardiovascular disease. J Surg Res. 2003;115(2):325–344. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 26.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145(3):1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol. 2002;193(1–2):129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 28.Czlonkowska A, Ciesielska A, Joniec I. Influence of estrogens on neurodegenerative processes. Med Sci Monit. 2003;9(10):RA247–256. [PubMed] [Google Scholar]
- 29.McEwen BS. Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 30.Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20(2):97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 31.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 32.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67(6):471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, Teuscher C, Vandenbark AA, Offner H. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163(4):1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci US A. 1993;90(14):6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140(8):3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- 36.Arvanitis DN, Wang H, Bagshaw RD, Callahan JW, Boggs JM. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J Neurosci Res. 2004;75(5):603–613. doi: 10.1002/jnr.20017. [DOI] [PubMed] [Google Scholar]
- 37.Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, Wu TC, Voskuhl RR. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52(4):421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 38.Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7(3):258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 40.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 41.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2(9):762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 42.Raine CS, Scheinberg LC. On the immunopathology of plaque development and repair in multiple sclerosis. J Neuroimmunol. 1988;20(2–3):189–201. doi: 10.1016/0165-5728(88)90160-9. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 44.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280(5361):243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 45.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105(10):1465–1472. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65(6):529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 47.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 48.Matejuk A, Dwyer J, Zamora A, Vandenbark AA, Offner H. Evaluation of the effects of 17beta-estradiol (17beta-e2) on gene expression in experimental autoimmune encephalomyelitis using DNA microarray. Endocrinology. 2002;143(1):313–319. doi: 10.1210/endo.143.1.8571. [DOI] [PubMed] [Google Scholar]
- 49.Klinkert WE, Kojima K, Lesslauer W, Rinner W, Lassmann H, Wekerle H. TNF-alpha receptor fusion protein prevents experimental auto-immune encephalomyelitis and demyelination in Lewis rats: an overview. J Neuroimmunol. 1997;72(2):163–168. doi: 10.1016/s0165-5728(96)00183-x. [DOI] [PubMed] [Google Scholar]
- 50.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84(2):370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 51.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170(1–2):85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19(3):337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126(3):329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen’s immune protection in autoimmune disease. J Immunol. 2003;171(12):6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 55.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci US A. 2003;100(16):9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, Cooke P, Vandenbark AA, Offner H. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am J Pathol. 2004;165(6):2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci US A. 2007;104(37):14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones RE, Kaler L, Murphy S, Offner H. Tissue-dependent expression of estrogen receptor beta in 17beta-estradiol-mediated attenuation of autoimmune CNS inflammation. Open Autoimmunity Journal. 2010;2:197–204. doi: 10.2174/1876894601002010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265(5589):69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 60.Evinger AJ, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70(5–7):361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18(12):2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 62.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9(5):404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 63.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 64.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23(5):1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73(9–10):853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19(6):1606–1617. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- 68.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22(9):2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3(5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 70.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun. 1998;243(1):122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- 71.Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005;203(1):193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- 72.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45(3):607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 73.O’Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, Jr, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47(2):310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- 74.Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228(2):285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 75.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240(3):737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 76.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 77.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 78.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 79.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346(3):904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 80.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148(12):5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 82.Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148(10):4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- 83.Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 2006;84(3–4):287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- 84.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20(8):409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214(1–2):67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanda N, Watanabe S. 17Beta-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J Invest Dermatol. 2003;121(4):771–780. doi: 10.1046/j.1523-1747.2003.12487.x. [DOI] [PubMed] [Google Scholar]
- 87.Rettew JA, McCall SHt, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328(1–2):87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17(4):369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 89.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22(3):636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296(3):E490–496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 91.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 92.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 93.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 94.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103(1):17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75(8–9):603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol. 2007;2(8):536–544. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- 98.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 99.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5(6):421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19(3):309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 101.Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184(8):4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann NY Acad Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 103.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107(1):201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170(3):1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 105.Fotherby K. Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy. Contraception. 1996;54(2):59–69. doi: 10.1016/0010-7824(96)00136-9. [DOI] [PubMed] [Google Scholar]
- 106.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunology. 2010;11(20):1–5. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]