Abstract
Normal aging causes a decline in object recognition. Importantly, lesions of the perirhinal cortex produce similar deficits and also lead to object discrimination impairments when the test objects share common features, suggesting that the perirhinal cortex participates in perceptual discrimination. The current experiments investigated the ability of young and aged animals to distinguish between objects that shared features with tasks with limited mnemonic demands. In the first experiment, young and old rats performed a variant of the spontaneous object recognition task in which there was a minimal delay between the sample and the test phase. When the test objects did not share any features (‘Easy’ perceptual discrimination) both young and aged rats correctly identified the novel object. When the test objects contained overlapping features, however, only the young rats showed an exploratory preference for the novel object. In Experiment 2, young and aged monkeys were tested on an object discrimination task. When the object pairs were dissimilar, both the young and aged monkeys learned to select the rewarded object quickly. In contrast, when LEGOs® were used to create object pairs with overlapping features, the aged monkeys took significantly longer than did the young animals to learn to discriminate between the rewarded and the unrewarded object. Together, these data indicate that behaviors requiring the perirhinal cortex are disrupted in advanced age, and suggest that at least some of these impairments may be explained by changes in high-level perceptual processing in advanced age.
Keywords: aging, dentate gyrus, monkey, object discrimination, rat
Introduction
In advanced age there is a decline in the ability to discriminate novel stimuli from those that have been encountered previously (Bartolini, Casamenti, & Pepeu, 1996; Bastin & Van der Linden, 2003; Burke, Wallace, Nematollahi, Uprety, & Barnes, 2010; de Lima et al., 2005). Recent data have indicated that this age-associated deficit arises from the false identification of novel objects as familiar (Burke et al., 2010). Importantly, the inability to correctly classify novel stimuli as new is also observed in rats with lesions of the perirhinal cortex (McTighe, Cowell, Winters, Bussey, & Saksida, 2010). Together these data suggest that the perirhinal cortex is among the structures in the medial temporal lobe that are vulnerable to normative aging processes.
Lesion experiments indicate that animals without an intact perirhinal cortex falsely identify novel stimuli as new because of a diminished capacity to discriminate novel stimuli from extraneous sensory input encountered during delay periods in these types of tasks. For example, objects encountered during delay intervals where the animals are removed from the testing environment may share common features with the test stimuli (McTighe et al., 2010). These data suggest that the underlying deficit in animals with disrupted perirhinal cortical function is a reduced ability to uniquely represent distinct stimuli that have overlapping features. Two observations support this idea. First, young rats (Bartko, Winters, Cowell, Saksida, & Bussey, 2007a, 2007b), monkeys (Bussey, Saksida, & Murray, 2002, 2003), and humans (Barense et al., 2005; Barense, Gaffan, & Graham, 2007) with perirhinal cortical lesions cannot discriminate between similar objects even when the memory demands are limited. Second, both aged rats (Burke et al., 2010), and rats with perirhinal cortical lesions (McTighe et al., 2010) have intact object recognition memory when the objects are different (i.e., have no overlapping features) and sensory input encountered during the delay is kept minimal.
Because animals with a lesioned or a compromised perirhinal cortex have difficulty in discriminating between stimuli that share features, it has been proposed that this structure is necessary for “disambiguating” perceptually similar objects (Bussey et al., 2002, 2003; Bussey, Saksida, & Murray, 2005), a cognitive computation that could be considered analogous to “pattern separation”. This pattern separation function requires that overlapping or similar representations are transformed into less similar outputs, a neural computation that has been extensively studied in realtion to the dentate gyrus subregion of the hippocampus (e.g., McNaughton & Morris, 1987; O’Reilly & McClelland, 1994; Treves & Rolls, 1992). Although behavioral data indicate that the dentate gyrus is involved in disambiguating similar spatial locations (Gilbert, Kesner, & Lee, 2001; Goodrich-Hunsaker, Hunsaker, & Kesner, 2008; Hunsaker & Kesner, 2008), the computations necessary for pattern separation operations are likely performed by many brain regions (Aimone, Deng, & Gage, 2011). Specifically, a mechanism for representing similar inputs as unique is critical for any structure involved in the encoding complex stimuli. For the dentate gyrus, it appears that adult neurogenesis may promote a pattern separation function (Clelland et al., 2009; Sahay et al., 2011). Although this is clearly not the case for the perirhinal cortex, where there is no adult neurogenesis, behavioral data indicate that this structure does have the ability to distinguish between different sensory inputs that share common features (Bartko et al., 2007a, 2007b; Bussey et al., 2002, 2005). This ability effectively describes pattern separation in the domain of the perirhinal cortex.
The observation that aged rats are more vulnerable to interference and behave as if novel objects are familiar suggest that during aging, functional changes within the perirhinal cortex lead to difficulty in disambiguating similar stimuli. This hypothesis, however, has not been tested directly. Two experiments were used in order to assess the degree to which aged animals have difficulty with perirhinal cortex-dependent pattern separation operations. In Experiment 1, young and aged rats performed a variant of the spontaneous object recognition task with a minimal delay of 30 seconds between the familiarization and test phase. In one condition the test objects shared no features, and in the other condition the test objects were similar. In Experiment 2, young and aged monkeys performed a simple object discrimination (OD) task that does not typically show an age-associated deficit (Bachevalier et al., 1991; Lai, Moss, Killiany, Rosene, & Herndon, 1995). In this experiment the objects were constructed from LEGOs® so that the amount of feature overlap could be manipulated and the effects of aging on the ability to pattern separate similar stimuli could be measured directly.
Methods
Subjects
In Experiment 1 (spontaneous object recognition), a total of twenty-three young (7-9 months old) and twenty-seven aged (24-25 months old) male F344 rats (from the National Institute on Aging’s colony at Charles River, Wilmington, MA) participated in spontaneous object recognition (SOR) and spatial memory testing. The rats were housed individually in plexiglas guinea pig tubs and maintained on a reversed 12-hr light-dark cycle. All behavioral testing occurred during the dark phase of the rats’ light-dark cycle and each animal was given access to food and water ad libitum for the duration of these experiments.
After arriving, rats were handled by experimenters over several days for at least 5-10 minutes per day. After the animals stopped vocalizing, defecating, and appeared calm during handling, they were tested on the spatial and the visually-cued versions of the Morris swim task (see below; Morris, 1984). SOR testing was administered within one week of the completion of the visually-cued Morris swim task.
In Experiment 2 (object discrimination), five young (mean age ± SD, 11.2 ± 0.45; age range, 11-12 years) and five aged (mean age ± SD, 24.8 ± 3.4; age range, 22-29 years) female bonnet macaques (Macaca radiata) participated in a two-choice object discrimination (OD) task. The age groups significantly differed in mean age (T(9) = 4.96, p < 0.001; equal variances not assumed). The age of each monkey can be multiplied by a factor of 3 to provide an approximate comparison to human aging (Tigges, Gordon, McClure, Hall, & Peters, 1988). All monkeys were born and maternally reared in a seminaturalistic, temperature- and humidity-controlled environment at the State University of New York (SUNY), Downstate Primate Behavior Facility. After being weaned, animals were housed in social groups of 6–12. Both young and aged monkeys had at least one viable pregnancy while at SUNY, Downstate. Monkeys were moved to the University of Arizona primate facility in June, 2007 where they were paired-housed and remained in a temperature- and humidity-controlled environment with a 12-hr light-dark cycle. Health exams of all animals, including screenings for age-associated eye diseases, were performed semi-annually and no monkey showed any signs of vision problems. Additionally, the aged monkey with the worst object discrimination performance was given a dilated fundus exam by an ophthalmologist, and showed no identifiable retina pathology. All monkeys had participated in four behavioral tasks (data not shown) prior to OD testing, and were therefore already habituated to the testing apparatus and the procedure of displacing an object to retrieve a food reward. These tasks included: reinforcer devaluation (e.g., Baxter, Parker, Lindner, Izquierdo, & Murray, 2000; Malkova, Gaffan, & Murray, 1997), object reversal learning (e.g., Izquierdo & Murray, 2007), delayed response (e.g., O’Donnell, Rapp, & Hof, 1999), and delayed nonmatch to sample (e.g., Moss, Rosene, & Peters, 1988). All experimental procedures were performed in accordance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committees at the University of Arizona.
Testing Apparati and Behavioral Procedures
Experiment 1: Spatial memory testing and spontaneous object recognition in rats
The Morris swim task procedures were conducted in a large tank ~5.7 meters in circumference and 0.5 meters deep. The procedures for this task have been described in detail previously (e.g., Barnes, Rao, & McNaughton, 1996; Shen, Barnes, Wenk, & McNaughton, 1996). Briefly, during the spatial version of the Morris swim task, all animals were given 6 training trials per day over 4 consecutive days. During these trials, an escape platform was hidden below the surface of water, which was made opaque with non-toxic Sargent Art® paint. Rats were released from seven different start locations around the perimeter of the tank, and each animal performed two successive trials before the next rat was tested. The order of the release locations was pseudo-randomized for each rat such that no rat was released from the same location on two consecutive trials. Immediately following the 24 spatial trials, the rats performed a probe trial in which the platform was removed and a rat swam in the pool for 60 sec (data not shown). Following the probe trial the animals were screened for visual ability with 2 days of cued visual trials (6 trials per day) in which the escape platform was above the surface of the water but the position of the platform changed between each trial. Rats’ performance on the swim task was analyzed offline with a commercial software application (ANY-maze, Wood Dale, IL). Because different release locations and differences in swimming velocity produce variability in the latency to reach the escape platform, a corrected integrated path length (CIPL) was calculated to ensure comparability of the rats’ performance across different release locations (Gallagher, Burwell, & Burchinal, 1993). The CIPL value measures the cumulative distance over time from the escape platform corrected by an animal’s swimming velocity, and is equivalent to the cumulative search error described by Gallagher and colleagues (1993). Therefore, regardless of the release location, if the rat mostly swims towards the escape platform the CIPL value will be low. In contrast, the more time a rat spends swimming in directions away from the platform, the higher the CIPL value.
Within one week of completing the Morris swim task procedures, each rat participated in two trials of the spontaneous object recognition task. The apparatus used for this experiment was a circular arena ~201 cm in circumference with a wooden floor and 40.6 cm high walls that were constructed from stiff black poster board. Figure 1 shows a schematic diagram of this testing apparatus. An overhead camera and a video recorder were used to monitor and record the animal’s behavior for subsequent offline analysis of “exploratory behavior”, which was defined as the animal directing its nose toward the object at a distance of ~2 cm or less (Ennaceur & Delacour, 1988). Any other behavior, such as resting against the object, or rearing on the object was not considered to be exploration. Exploration was scored by an observer blind to the rat’s age. Additionally, the amount of time spent exploring objects during the test phase was scored prior to measuring the amount of exploration during the object familiarization phase. This reverse-order of analysis ensured that the scorer was blind with respect to which object was familiar and which object was novel. Moreover, the positions of the objects in the test phases were counterbalanced between the young and the aged animals. Finally, for thirteen young and eighteen aged rats the objects used for the familiarization phase and the object designated as ‘novel’ was consistent for all animals (Group 1). Because the objects were not counterbalanced between rats, this could potentially introduce an object preference confound, which could interfere with the exploratory behavior elicited by novelty. Therefore, an additional ten young and nine aged rats were tested on the SOR task in which the novel versus familiar objects were counterbalanced between rats (Group 2).
Figure 1. Schematic of the spontaneous object recognition (SOR) task apparatus and procedure used in Experiment 1.
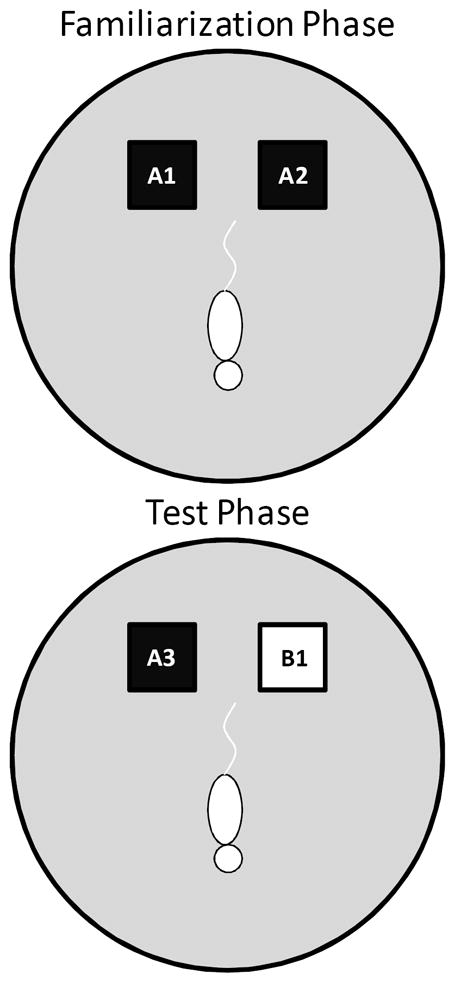
The testing arena was a circle ~201 cm in circumference with a wooden floor and 40.6 cm high walls. The black squares indicate the location in which that the objects were fixed in place during the familiarization phase (top arena). The schematic white rat is shown in the initial orientation of the rat when it is first placed in the arena. In the object familiarization phase (top arena), a rat is placed into the arena to explore duplicate copies of an object (A1 and A2). The rat is then moved from the arena and placed into a covered pot adjacent to the arena for a 30 sec delay. Following the delay, during the test phase (bottom arena), the animal is returned to the arena to explore a triplicate copy of the objects presented during the familiarization phase (A3) and a novel object (B1).
Before testing began, all rats were exposed to the empty apparatus (Figure 1) for 10 minutes on two consecutive days. Recognition testing began the day immediately following this habituation procedure. There are two components or “phases” of SOR testing: “object familiarization” and “test” phases. All rats participated in two object familiarization and test phases with 2 different levels of perceptual difficulty (‘Easy’ and ‘Difficult’). In the Easy condition the test objects did not share any common features, that is, they were different in shape, color, and texture, although they were similar in overall size. For the Difficult condition, the test objects were both cubic in shape, and 3 of the 4 sides of the objects had a similar smooth texture. Therefore, in the Difficult condition the test objects had more features in common relative to the Easy condition. A previous experiment has used objects constructed from LEGOs® to assess the effects of the feature overlap on SOR task performance in Lister hooded rats (Bartko et al., 2007a). Although LEGOs® were initially used in pilot studies, reliable recognition performance was not achieved in either age group with these stimuli, for which we have no definitive explanation (data not shown). Therefore, 3-dimensional junk objects were used as stimuli for Experiment 1, for which high levels of recognition performance could be achieved. For both the Easy and Difficult conditions rats were tested with one of two different sets of objects. Supplemental Figure 1 shows the test objects used in Experiment 1. Group 1 rats were tested with the objects shown in the left panels and Group 2 rats were tested with the objects shown in the right panels. Each rat participated in both object conditions once. The order that a rat participated in each condition and the location of the novel object (left or right) was pseudo-randomized individually for every rat. Additionally, for the Group 2 rats, the objects used for the familiarization phase and the novel object were also counterbalanced to control for possible differences in object preference.
In the object familiarization phase, duplicate copies of an object (Figure 1; A1 and A2) were placed as shown in Figure 1. The animal was placed into the arena facing the opposite direction of the objects (Figure 1; schematic white rat). The rat was then allowed a total of 4 min of exploration in the open arena. After the object familiarization phase, a 30 sec delay was imposed before exposure to the box in the test phase. During the delay, the rat was placed in a covered pot next to the apparatus. This prevented the animal from being exposed to extraneous stimuli. Rats were placed in covered pots for all transportation between the colony room and the experimental apparatus.
During the test phase (bottom arena), the animal was returned to the apparatus and placed back into the same start location as for the object familiarization phase (Figure 1; schematic white rat). Again, the rat was allowed 4 min of exploration but was presented with two different objects than had been used during the familiarization phase. One object (Figure 1; A3) was the third copy of the triplicate set of the objects used in the object familiarization phase (familiar), and the other was a novel object (Figure 1; B1). All objects and the apparatus were washed with 70% ethanol between every trial and before procedures began with another rat.
The difference in time spent exploring the novel object compared with the familiar object divided by the time spent exploring both objects was calculated to obtain the “discrimination ratio” (Dix & Aggleton, 1999). Additionally, the absolute time spent exploring the individual familiar or novel objects was compared between age groups.
Experiment 2: Two-choice object discrimination (OD) testing in monkeys
The behavioral apparatus and testing procedures used in this study have been described in detail previously (Bachevalier et al., 1991; Rapp, 1990; Rapp, Morrison, & Roberts, 2003). Briefly, a modified Wisconsin General Test Apparatus (WGTA; Harlow & Bromer, 1938) was used for all behavioral testing. The WGTA was composed of a chamber with vertical bars situated in front of a tray for stimulus presentation. The tray included three equally spaced wells. Either the left or the right well was baited with a food reward during testing, and the middle well was not used during OD testing. A wooden guillotine door, controlled by the experimenter, was used to limit the animal’s physical access to the wells and to impose 15 sec inter-trial intervals (ITIs). A one-way mirrored screen allowed the tester to remain undetected while observing the animal’s performance.
On each OD trial, subjects chose between two objects, one of which was consistently associated with reward across trials (Figure 2A), but the location of the rewarded object (left versus right) pseudo-randomly changed for each trial. The same discrimination problem was presented for 30 trials per day for successive days until the animal selected the rewarded object with 90% accuracy. If this performance criterion was achieved on the first day of testing, the animal would perform one additional day of testing with the same object pair. Following the achievement of criterion performance, or the second day of testing if this was achieved on day 1, a 48 hour delay was imposed before a final 30-trial session. Animals were tested on 12 successive discriminations problems according to this schedule. For the first four discrimination problems object pairs were visually distinct junk objects that did not share any common features (Supplemental Figure 2). The other 8 object pairs were constructed from LEGOs® so the experimenters could manipulate the amount of ‘feature overlap’ between pairs (Bartko et al., 2007b). A schematic of the pair of LEGO® objects with 86% feature overlap is shown in Figure 2B. Feature overlap was calculated by dividing the number of LEGO® bits (indicated by the black arrow in Figure 2B) that were the same between the two objects by the total number of bits in a single object. For the example shown in Figure 2B each object contained 128 LEGO® bits and the there were 96 bits in common. The percent of overlap for the 8 LEGO® objects ranged from 12.5% to 96%, and the order of presentation was pseudo-randomly shuffled for each monkey individually. Photographs of all eight LEGO® object pairs are shown in Supplemental Figure 3.
Figure 2. Schematic of the two-choice object discrimination (OD) apparatus and procedure used in Experiment 2.
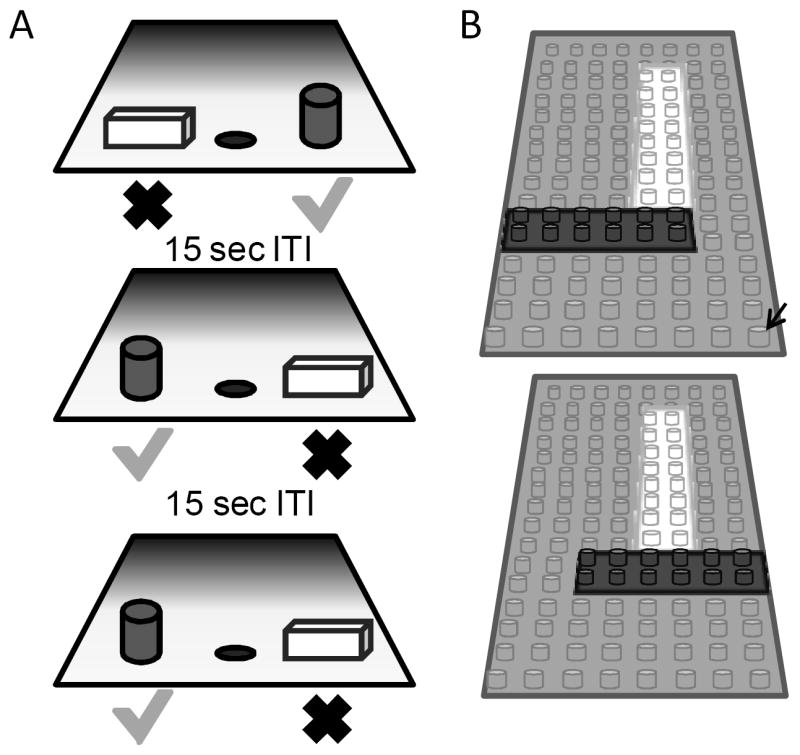
(A) Three different trials of the OD task that monkeys were tested on. For each OD problem, an object was placed over the left and right food well. The grey cylinder represents the rewarded object (green check) and the other object of the pair (rectangular cube; red X) was never rewarded. A 15 sec inter-trial interval (ITI) was imposed between successive trials, and the side of the rewarded object pseudo-randomly changed across trials. (B) A schematic of the pair of LEGO® objects with 86% feature overlap. Feature overlap was calculated by dividing the number of LEGO® bits (indicated by the black arrow) that were the same between the two objects by the total number of bits in a single object.
Data analysis
In Experiment 1, group means of four measures (the total time spent exploring objects during the familiarization phase, the discrimination ratio, the time spent exploring the novel object during the test phase, and the time spent exploring the familiar object during the test phase) were examined using repeated-measures analysis of variance (ANOVA). The within-subjects factor of ‘difficulty’ and the between-subjects factor of age group were the independent variables. For Experiment 2, percent correct on the OD task with no overlap was compared between age groups for Day 1, Day 2 and the 48 hour delay with ANOVA. Performance on the OD task with LEGO® objects was analyzed separately. For this analysis, the number of trials required to reach the 90% correct performance criterion was the dependent variable and the independent variables were percent overlap (8 levels, 12.5-92%) and age group (2 levels, young and aged). All statistical tests and p-values were calculated using SPSS 19 (Chicago, IL) and alpha was set at the 0.05 level. When the F statistic reached statistical significance, tests for individual group differences using either the post hoc Tukey HSD test or, if there was a specific hypothesis regarding the group differences, planned orthogonal contrasts were made.
Results
Morris swim task performance
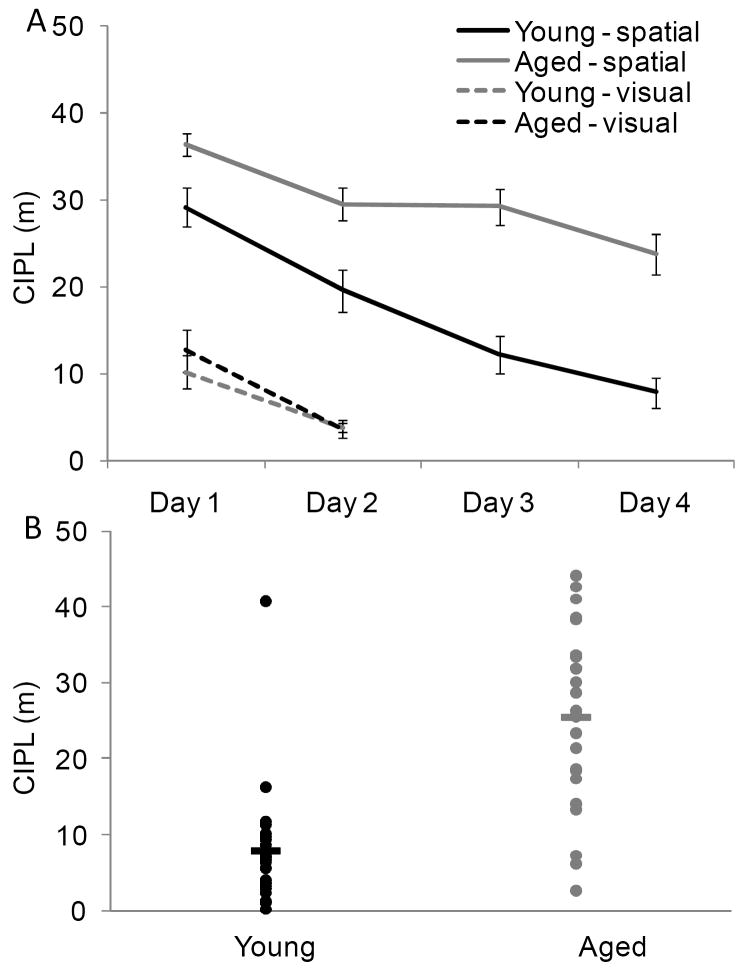
As reported previously, the aged rats had worse performance on the spatial version of the Morris swim task (e.g., Barnes, Suster, Shen, & McNaughton, 1997; Gallagher et al., 1993). Importantly, every aged rat that participated in recognition testing was able to learn the visual version of the task. Figure 3A shows the mean CIPL (Gallagher et al., 1993) of the eighteen aged rats (grey solid lines), and the thirteen young rats (black solid lines) that participated in the object recognition experiment for each day of spatial swim task testing. All animals were tested over 4 days with 6 spatial trials on each day. The old rats had significantly longer mean CIPL scores, compared with the young rats (F[1,48] = 37.34, p < 0.001). Post hoc analysis revealed that this difference was due to the aged rats having significantly longer CIPL scores on all days of spatial testing (p < 0.05; for all comparisons, equal variances not assumed; Tukey HSD). The old rats, however, did show a significant improvement in finding the hidden platform over the four days of spatial testing (F[3,78] = 15.12, p < 0.001; repeated-measures), and planned comparisons showed that the mean CIPL values of the aged rats were significantly lower on Days 2-4 relative to Day 1 (p < 0.01 for all comparisons; simple contrasts). Figure 3B shows the CIPL scores for the spatial trials on Day 4 of testing for individual rats. The horizontal lines indicate the mean CIPL for each age group. Only 5 aged rats had a CIPL score within 2 standard deviations of the mean score for the young animals. For this reason, analyses of object recognition measures only compared the young rats to the aged rats because the small number of “spatially-unimpaired” aged rats did not provide adequate statistical power to further subdivide the aged group into impaired and unimpaired.
Figure 3. Morris swim task performance in young and aged rats.
(A) The X-axis is the day of testing and the Y-axis is the mean corrected integrated path length (CIPL) score. Higher CIPL scores indicate longer path lengths to reach the escape platform. All rats completed 4 days of spatial trials (solid lines) in which the platform was hidden below the surface of the water. These spatial trials were followed by 2 days of visually-cued trials in which the platform was visible (dashed lines). During the spatial trials, the aged (grey) rats had significantly longer CIPL scores compared with the young (black) rats (F[1,48] = 37.34, p < 0.001). The performance during the visual trials, however, was not significantly different between age groups (F[1,48] = 0.05, p = 0.83). Error bars represent +/-1 standard error of the mean. (B) The mean CIPL scores of individual young (black) and aged (grey) rats on Day 4 of spatial testing. The horizontal lines indicate the mean CIPL for each age group. Only 1 aged rat had a CIPL score on Day 4 of spatial testing that was within 2 standard deviations of the young rat mean.
To test whether the spatial impairments of the aged rats were due to visual problems, and to ensure that all rats had adequate vision to participate in recognition testing, the rats also performed 12 trials (6 trials/day) on the visually-cued version of the Morris swim task. In this version of the task the platform is raised above the surface of the water, but its location changed every trial. Figure 3A shows the CIPL values for the visual trials of the Morris swim task for the trials on Day 1 and Day 2 for the aged (grey – dashed line) and the young (black – dashed line) animals. Both the aged and the young rats showed a significant decrease in the CIPL measure on the 2nd day compared with the first day (F[1,48] = 32.26, p < 0.001; repeated-measures). There was not a significant effect of age group on the mean CIPL scores during visual swim task testing (F[1,48] = 0.05, p = 0.83). When the CIPL score from Day 4 of spatial testing was compared to the visual score from Day 2, however, it was observed that the mean CIPL score for all rats was significantly less when the platform was visible relative to when it was hidden (F[1,48] = 69.68, p < 0.001; repeated-measures). Finally, there was a significant interaction effect between age group and the visual (Day 2) versus the spatial (Day 4) CIPL score (F[1,48] = 33.11, p < 0.001; repeated-measures). This indicates that, relative to the young animals, the performance of the aged rats benefitted significantly more when the escape platform was made visible. Together these data indicate that the aged rats used in the current experiment showed impairments in hippocampal-dependent spatial memory, but had adequate vision to participate in object recognition testing.
Experiment 1: The effects of feature overlap on spontaneous object recognition in rats
Figure 1 shows a schematic of the SOR task with a 30 sec delay that was used for Experiment 1, and Supplemental Figure 1 shows the test objects used for the ‘Easy’ and ‘Difficult’ recognition testing conditions for Groups 1 and 2. Supplemental Figure 4 shows the discrimination index (A), and the raw exploration times during the test phase (B) for Group 1 (left panels) and Group 2 (right panels). As evident in this figure, the data obtained from the different groups are qualitatively similar. Moreover, there was not a significant effect of Group on the discrimination index (F[1,92] = 0.02, p = 0.89), or the amount of exploration time during the test phase for the novel (F[1,92] = 2.71, p = 0.1) or the familiar objects (F[1,92] = 3.48, p = 0.07). Therefore, data from Groups 1 and 2 were combined for the remainder of the analyses.
The young and the aged rats spent a comparable amount of time exploring the two identical objects during the familiarization phases of both SOR task conditions (Table 1), as indicated by the lack of a significant main effect of age group on exploration time during the object familiarization phases (F[1,96] = 0.05, p = 0.81). Additionally, there was not a significant interaction effect between age and condition on the exploration times during the familiarization phases (F[1,96] = 0.13, p = 0.72). Together, these data suggest that differences in encoding during object familiarization cannot account for any potential effects of age on recognition performance during the test phase.
Table 1.
Exploration times during the familiarization phase of Experiment 1 ± 1 standard error of the mean
| Easy Condition | Difficult Condition | |
|---|---|---|
| Young | 16.4 ± 1.9 | 22.2 ± 2.4 |
| Aged | 16.5 ± 2.1 | 20.2 ± 3.0 |
| p value | 0.9 | 0.6 |
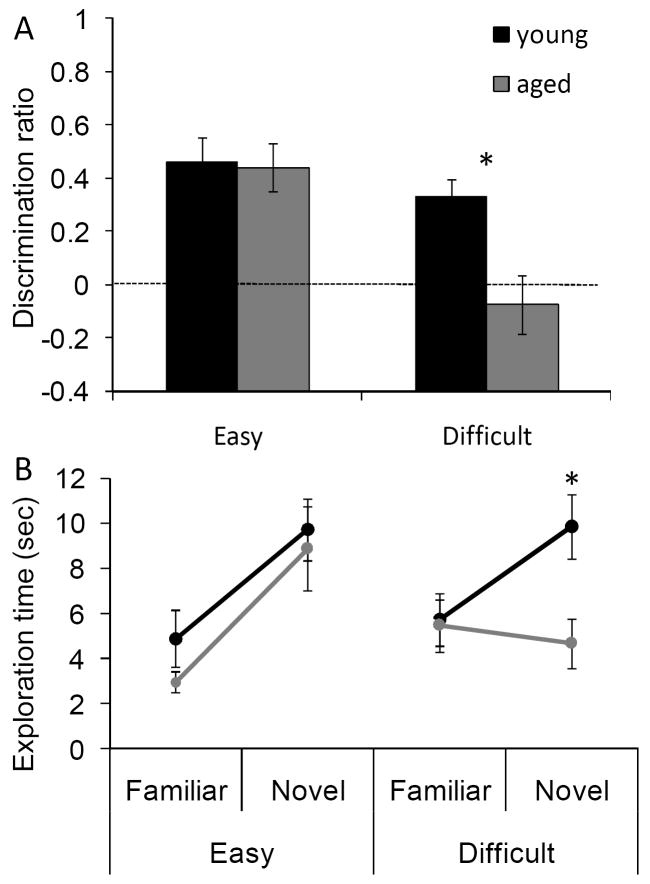
When discrimination ratios were calculated from the time spent exploring the novel versus familiar object during the test phase, the aged rats had significantly lower discrimination ratios compared to the young animals (F[1,96] = 5.01, p < 0.05). Planned comparisons revealed that the discrimination ratio was significantly less in the aged rats relative to the young rats for the Difficult condition (p < 0.01; repeated contrasts), but not the Easy condition (p = 0.76, repeated contrast). This indicates that the aged rats were able to discriminate between the novel and familiar objects at a 30 sec delay when the test objects did not share any features. Therefore, it is unlikely that the recognition impairment during the difficult condition is due to age-associated impairments in lower-level visual function, olfaction or somatosensation. Additionally, changes in the general motivation to explore objects cannot account for the current results. Figure 4A shows the discrimination ratios of the adult (black) and aged rats (grey) during the Easy and Difficult conditions.
Figure 4. Spontaneous object recognition (SOR) task performance (Experiment 1).
(A) The mean discrimination ratio of the adult (black) and the aged rats (grey) measured during the test phase following a 30 sec delay for the ‘Easy’ and ‘Difficult’ conditions. A higher discrimination ratio indicates that the animal spent more time exploring the novel object relative to the familiar object. The aged rats had significantly smaller discrimination ratios when compared to the adult rats for the Difficult condition (p < 0.01), but not for the Easy condition (p = 0.76). The dashed horizontal line indicates chance performance. (B) The mean amount of time young (black) and aged (grey) rats spent exploring the familiar and the novel object during the different testing conditions. Compared to the young animals, the aged rats spent significant less time exploring the novel object during the test phase of the Difficult condition (p < 0.05). In contrast, novel exploration between age groups was similar during the Easy condition (p = 0.87). Asterisks indicate p < 0.05 and error bars represent +/-1 standard error of the mean.
In order to determine if the reduced discrimination ratio during the Difficult condition was due to the aged rats exploring the familiar object more or to exploring the novel object less, the raw exploration times during the test phases of both SOR task conditions were compared between young and aged rats (Burke et al., 2010). This analysis of the exploration times of individual objects showed that the age-associated reduction in the discrimination ratio during the test phase of the Difficult condition was associated with a reduction in novel object exploration in the aged compared to the young rats. In contrast, the amount of time spent exploring the familiar objects was similar in both age groups. Figure 4B shows the mean time spent exploring the novel and familiar objects for the young (black), and aged rats (grey). There was a significant main effect of novel versus familiar objects on exploration time (F[1,96] = 25.14, p < 0.001; repeated-measures). Additionally, the interaction effect of age group, and object familiarity (novel versus familiar) on exploration time during the test phase was also statistically significant (F[1,96] = 4.65, p < 0.05; repeated-measure). Planned contrasts comparing the exploration times of the novel object between the young and the aged rats revealed that this interaction effect was due to a significant reduction in the old rats’ exploration of the novel object during the Difficult condition (p < 0.05), but not the Easy condition (p = 0.87; repeated contrast). In contrast to the novel objects, there were no significant differences in the time spent exploring familiar objects in the test phase between the young and the aged rats during any condition (p > 0.2 for all comparisons; repeated contrast). These data suggest that the age-associated reduction in the discrimination ratio for the Difficult condition selectively results from the aged rats exhibiting reduced exploration of the novel object when it shares features with the familiar object. The observation that there is a significant reduction in the amount of exploration time of the novel object in the test phase of the Difficult condition relative to the exploration time of a novel object in the familiarization phase (T[26] = 2.32, p < 0.05; repeated-measures) provides additional support for this hypothesis.
A previous study has shown that there was a significant correlation in object recognition memory performance between 15 min and 2 hr delay conditions, but not between the 2 min and longer delays (Burke et al., 2010). This indicates that the same brain regions support performance at these longer delays. Interestingly, although the delay was the same in the present study, there was not a significant correlation between performance on the Easy and Difficult recognition testing conditions in either the young (R[23] = 0.12, p = 0.60), or the aged rats (R[28] = -0.15, p = 0.44). Additionally, there was not a significant relationship between performance on the Easy and Difficult recognition conditions when both age groups were analyzed together (R[51] = -0.05, p = 0.71). Importantly, perirhinal cortical lesions do not typically lead to object recognition memory impairments at short delays when the two test objects do not have features in common, but deficits can be detected as the test objects become more similar (e.g., Bartko et al., 2007a). Together, these data suggest that the perirhinal cortex is not necessary for stimulus discrimination under conditions when pattern separation is not necessary, such as when the test objects are very distinct or the delay between stimulus familiarization and recognition is short. Moreover, the current data suggest that age-associated recognition impairments only become evident when the task requires the functional integrity of the perirhinal cortex.
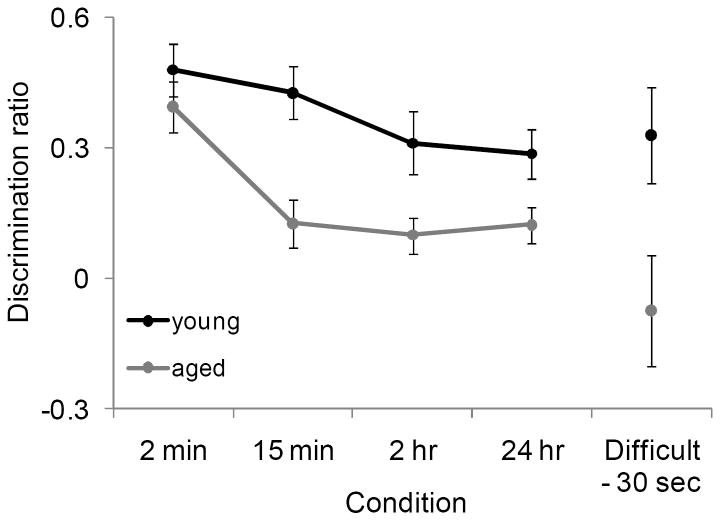
Previous studies have shown that aged rats (Burke et al., 2010), or rats with perirhinal cortical lesions (McTighe et al., 2010) behave as if a novel test object is familiar because of a reduced ability to distinguish the novel object from extraneous stimuli that are encountered during long delay periods. These observations imply that increasing the similarity between the test objects may have a similar effect on stimulus recognition as long delays between the familiarization and test phases, as both conditions require more pattern separation (Burke et al., 2010; McTighe et al., 2010). Figure 5 shows a comparison of the discrimination ratios from young and aged rats that performed the standard version of the SOR task with delays from 2 min to 24 hour (data published previously, Burke et al., 2010) and the current data from the Difficult condition.
Figure 5. Comparison of the effects of perceptual difficulty and long delays on discrimination ratios.
In a previous study, a different group of young and aged rats performed the standard version of the SOR task with delays from 2 min to 24 hours (Burke et al., 2010). The discrimination ratios from these previously published data are plotted with the current data obtained from the Difficult condition and shown on the X axis. The Y axis is the different testing conditions. In both age groups performance on the SOR task with a 24 hr delay was similar to performance on the difficult condition with a 30 sec delay (F[1,80] = 0.58, p = 0.45; ANOVA, equal variances not assumed). The interaction effect between age group and task condition was also not significant (F[1,80] = 1.55, p = 0.22; ANOVA, equal variances not assumed), which indicates that increasing the amount of similarity between the test objects is comparable to a 24 hour delay in both age groups. Error bars represent +/-1 standard error of the mean.
Data obtained from these two different tasks were compared using a factorial ANOVA for samples with unequal variances. The fixed factors were age group (young versus aged) and task condition (Difficult versus 24 hour). In both age groups performance on the SOR task with a 24 hr delay was similar to performance on the Difficult condition with a 30 sec delay, as shown by the lack of a significant main effect of task condition (24 hour delay versus difficult – 30 sec delay) on the discrimination ratio (F[1,80] = 0.58, p = 0.45; equal variances not assumed). The interaction effect between age group and task condition was also not significant (F[1,80] = 1.55, p = 0.22; equal variances not assumed), which indicates that increasing the amount of similarity between the test objects is comparable to a 24 hour delay in both age groups. Together these data indicate that with advanced age there is deficit in perirhinal-dependent pattern separation.
Experiment 2: The effects of feature overlap on object discrimination (OD) performance in monkeys
The effect of age on the ability to pattern separate distinct stimuli can also be tested by measuring the number of trials required for an animal to learn which of two stimuli is rewarded and varying the degree to which they are similar (Bussey et al., 2003). In experiment 2, young and old monkeys performed an OD task in which 8 of the 12 object pairs were constructed from LEGOs® so that the amount of feature overlap could be manipulated. The other 4 object pairs where junk items that did not share any stimulus features (0% overlap; Supplemental Figure 2). All monkeys performed the standard OD task for the 4 object pairs with 0% overlap first. A single object pair was presented for 30 trials/day for 2 successive days, or until the animal reached 90% correct. This was followed 48 hrs later by another presentation of 30 trials in order to test retention. When the object pairs did not share any features, each animal was able to reach a 90% correct performance criterion within 2 days of testing. Figure 6 shows the mean percent correct for the young (black) and aged (grey) monkeys on the OD task for these first 4 object pairs. Consistent with previous data (e.g., Bachevalier et al., 1991; Lai et al., 1995), there was not a significant difference in the mean percent correct between young and aged monkeys when the object pairs do not have overlapping features (F[1,24] = 1.91, p = 0.2). The day of testing, however, did have a significant effect on performance (F[2,24] = 53.38, p < 0.001), and post hoc analysis indicated that all monkeys performed significantly better during Day 2, and after the 48 hour delay relative to Day 1 of testing (p < 0.001 for both comparisons; Tukey HSD). This improvement following the first day of testing with a new object pair was similar between young and aged monkeys, as indicated by the lack of a significant interaction effect between day and age group (F[2,24] = 0.14, p = 0.87). These data indicate that there was no difference in the ability of young and aged monkeys to learn which of two objects was associated with reward when the objects did not contain similar features.
Figure 6. Performance on the two-choice object discrimination (OD) task when object pairs contain no feature overlap.
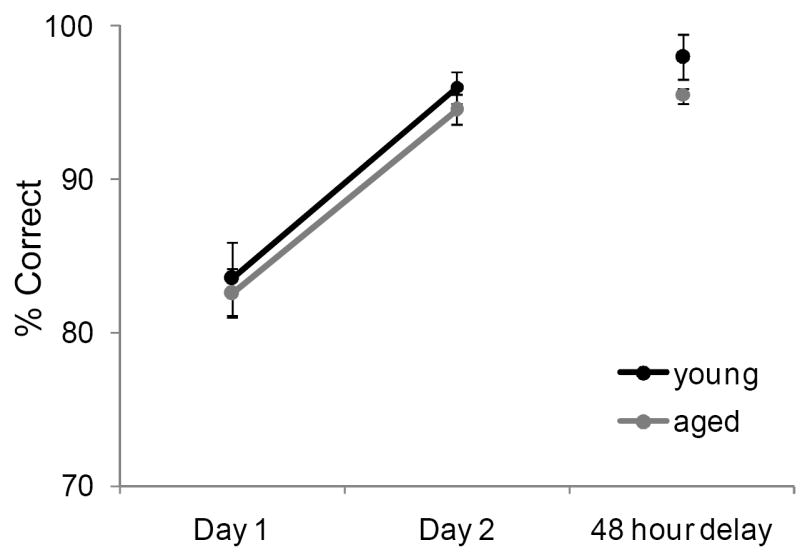
Both young (black) and aged (grey) monkeys quickly learned which item of an object pair was rewarded when the objects were different. The Y axis shows the percent correct across two successive days of testing and following a 48 hour delay (X axis). There was not a significant difference in the mean percent correct between young and aged monkeys (F[1,24] = 1.91, p = 0.2). The day of testing, however, did have a significant effect on performance (F[2,24] = 53.38, p < 0.001), and post hoc analysis indicated that all monkeys performed significantly better during day 2 and after the 48-hr delay relative to day 1 of testing (p < 0.001 for both comparisons; Tukey HSD). Error bars represent +/-1 standard error of the mean.
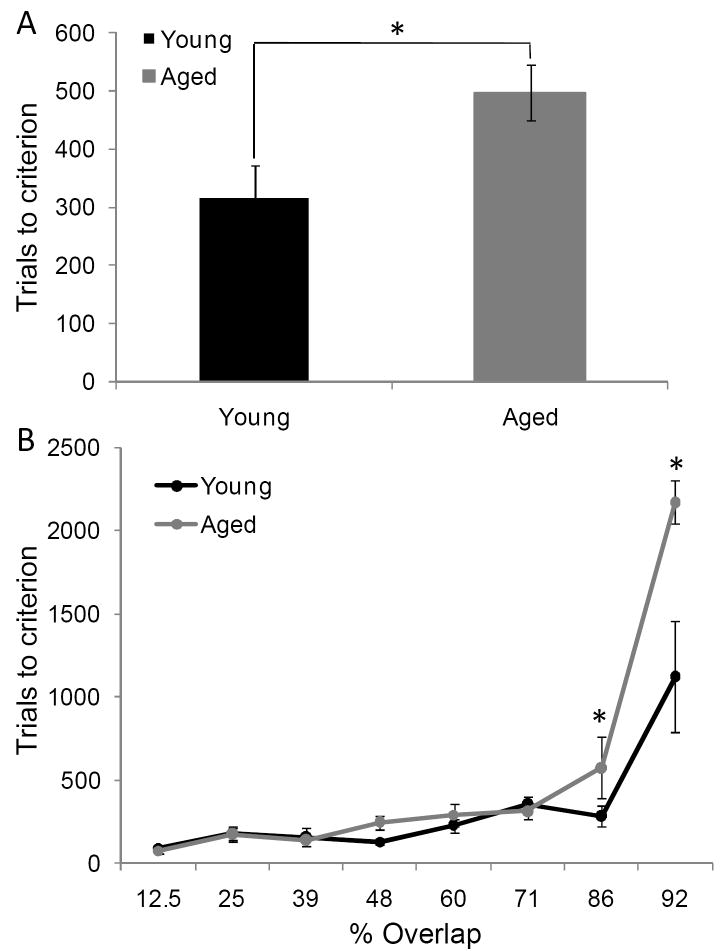
In contrast to the standard OD task, when the object pairs were constructed from LEGOs® so that they shared features, it took the aged monkeys more trials to reach criterion performance compared to the young animals. Figure 7A shows the mean number of trials the young (black) and the aged (grey) monkeys required to reach a performance of 90% correct. Figure 7B shows the mean of the number of trials needed to reach criterion performance as a function of percent overlap for both age groups. It is evident from this plot that as the degree overlap increased, more trials were required for an animal to learn which object of the pair was associated with a reward. In fact, regression analysis with curve estimation revealed there was a significant exponential relationship between percent overlap and trials to criterion in both the young (R2[39] = 0.45, p < 0.001)and the aged monkeys (R2[39] = 0.66, p < 0.001). Additionally, there was a significant effect of age on number of trials to criterion (F[2,64] = 11.13, p < 0.01). Importantly, the interaction effect between age group and percent overlap was also significant (F[7,64] = 5.29, p < 0.001). Planned contrasts revealed that the significant interaction effect was due to the aged monkeys having significantly worse performance, compared to the young animals, on the 86% and 92% overlap conditions (p < 0.05 for both comparisons; repeated contrasts). In contrast, the number of trials to reach criterion was not significantly different between age groups on the conditions with less than 86% overlap (p > 0.05 for all comparisons; repeated contrasts). This indicates that as the feature overlap between object pairs increased, the performance of the aged monkeys was more affected relative to the young monkeys.
Figure 7. The effect of feature overlap on two-choice object discrimination (OD) task performance.
(A) The mean number of trials required for young (black) and aged (grey) monkeys to reach performance criterion of 90% correct on the two-choice OD task when the object pairs shared features. It took the aged monkeys significant more trials to learn which object was rewarded (F[2,64] = 11.13, p < 0.01). (B) The mean number of trials to criterion (Y axis) across the different levels of feature overlap (X axis). There was a significant exponential relationship between percent overlap and trials to criterion in both the young (R2[39] = 0.45, p < 0.001; regression curve estimation for exponential model)and the aged monkeys (R2[39] = 0.66, p < 0.001; regression curve estimation for exponential model). Additionally, there was a significant effect of age on number of trials to criterion (F[2,64] = 11.13, p < 0.01). Specifically, relative to the young animals, the aged monkeys required significantly more trials to reach criterion on the 86% and 92% overlap conditions (p < 0.05 for both comparisons; repeated contrasts). Asterisks indicate p < 0.05 and error bars represent +/-1 standard error of the mean.
Although increasing the percent of feature overlap lead to an age effect in the ability of monkeys to acquire an association between an object and reward, it did not produce an age difference in the ability of monkeys to remember that association following a 48 hour delay. Figure 8 shows the percent correct for the 30 trials that followed the 48 hour delay after the performance criterion was achieved for the young (black) and aged (grey) monkeys. After the two-choice OD was acquired, the young and aged monkeys did not perform significantly different following the delay (F[1,64] = 0.02, p = 0.89). There was a significant main effect of percent overlap on performance, however (F[7,64] = 4.89, p < 0.001). Specifically, monkeys made significantly more errors on the 92% overlap condition compared to the conditions with less overlap (p < 0.02 for all comparisons; Tukey HSD). These data indicate that there was a dissociation between the effects of feature overlap on the ability to discriminate between two stimuli and the ability to remember that discrimination.
Figure 8. Object discrimination (OD) performance following a 48 hour delay.
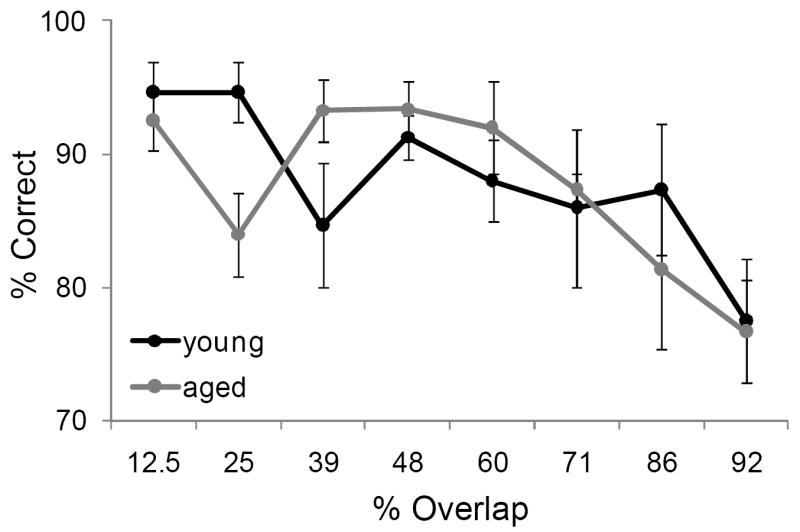
The percent correct (Y axis) over 30 trials for the different levels of feature overlap (X axis). After the two-choice OD was acquired, the young (black) and aged (grey) monkeys did not perform significantly differently from one another following the delay (F[1,64] = 0.02, p = 0.89; ANOVA). Error bars represent +/-1 standard error of the mean.
Discussion
Several novel observations arose from the present experiments. First, the current data indicate that, similar to lesions of the perirhinal cortex (Bartko et al., 2007a, 2007b; Bussey et al., 2002, 2003), advanced age results in difficulty discriminating between stimuli that share common features. In other words, aging produces deficits in perirhinal cortex-dependent pattern separation. Second, there was a cross-species consensus between rats and nonhuman primates that older animals have more difficulty, relative to young, performing pattern separation operations. Finally, the performance of monkeys on the object discrimination (OD) task demonstrated a dissociation of the effects of feature overlap between discrimination performance versus remembering the stimuli. This suggests that age-related memory deficits cannot fully account for the observed performance differences between young and old animals. Specifically, the aged monkeys were impaired when required to learn a discrimination between objects with more than 71% feature overlap, but 48 hours after acquiring the discrimination, their retention was equivalent to that of the young monkeys. These data are consistent with ideas suggesting that the perirhinal cortex supports high-level perception in addition to memory (Baxter, 2009; Buckley & Gaffan, 1997, 1998; Bussey et al., 2005; Cowell, Bussey, & Saksida, 2006; Murray & Bussey, 1999; Murray, Bussey, & Saksida, 2007).
Whether the perirhinal cortex is part of a medial temporal lobe memory system exclusively, or whether it also contributes to perception, continues to be a matter of debate (Baxter, 2009; Clark, Reinagel, Broadbent, Flister, & Squire, 2011; Suzuki, 2009; Suzuki & Baxter, 2009). Several observations, however, suggest that the impairment in object discrimination in old animals cannot solely be accounted for by age-related memory deficits. First, the memory load in Experiment 1 was minimal (only a 30 sec delay). At delays of 5 min or less aged rats do not show object recognition memory impairments (Burke et al., 2010; Cavoy & Delacour, 1993). In line with these data, when the test objects did not share features (the ‘Easy’ condition), recognition performance was similar between the two age groups. Moreover, when the raw exploration times during the test phase were examined, it was evident that the reduced discrimination ratio in the old animals during the perceptually ‘Difficult’ condition was due to the aged rats behaving as if the novel object was familiar (Figure 4B). If the behavior of old animals had been indicative of an impairment in memory trace maintenance alone (that is, they did not recall experiencing the stimulus previously), increased exploration of the familiar object should have been observed with the animal behaving as if it was novel during the test phase of the Difficult condition. This did not occur in the current experiment. Interestingly, recognition performance scores on the Easy and Difficult SOR task conditions were not correlated. This may indicate that in the Easy condition successful performance can be achieved with lower-level sensory areas while the latter condition requires the perirhinal cortex.
Object discrimination (OD) performance in the monkeys tested here (Experiment 2) provides additional evidence that the age-associated discrimination deficits cannot entirely be accounted for by memory problems. When the object pairs did not share common features, both the young and aged monkeys were quickly able to learn which object was associated with reward (Figure 6). Moreover, after a 48 hour delay young and aged monkeys showed similarly high recall of the rewarded object. Importantly, the aged monkeys were impaired at learning which object was rewarded only when the pair of stimuli shared a significant number of features (>71% feature overlap). Once the object-reward association was learned, however, the young and old monkeys were equally able to recall which object was rewarded 48 hours later, regardless of the level of feature overlap (Figure 8). Therefore, the current data indicate a possible dissociation of the effects of advanced age on perceptual discrimination ability and memory.
For the SOR task there was a brief delay between the familiarization and the test phases. Likewise, the OD task that the monkeys performed required the animals to remember a rule. Therefore, the current data cannot rule out the possibility that age-related impairments in memory contribute to the observed deficits in both rats and monkeys. Importantly, however, over the same delay intervals performance only differed between age groups when the stimuli shared features. This indicates that age-associated deficits in object recognition memory and object discrimination are reminiscent of the impairments observed in animals with lesions of the perirhinal cortex. This finding emphasizes the importance of this structure in forming conjunctive representations of the individual features that comprise an object (Bussey & Saksida, 2002), which is a necessary function for discriminating between stimuli and could be critical for pattern separation computations that support both memory and perception.
Previous studies with human subjects have also reported that older subjects have difficulty with pattern separation (Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa et al., 2010). Although this impairment has been attributed to age-associated changes in the dentate gyrus and CA3 (Yassa et al., 2010; Yassa, Mattfeld, Stark, & Stark, 2011), the tasks that have been used to identify relationships between pattern separation performance and activity in these subregions of the hippocampus are reminiscent of behaviors that have been shown to require the perirhinal cortex for accurate performance in rats (Bartko et al., 2007a) and monkeys (Bussey et al., 2002). In the human experiments, subjects are presented with pictures of common objects (e.g., pumpkin, wagon, piano). The images are either, novel, familiar, or a ‘lure’ that is novel but similar to a familiar image (Yassa et al., 2010; Yassa et al., 2011). Therefore, performance on these types of tasks requires the subjects to disambiguate the lure from the similar familiar image. It is notable that papers reporting a correlation between dentate gyrus/CA3 activity and pattern separation have limited the analysis of BOLD activation to the hippocampus (Lacy, Yassa, Stark, Muftuler, & Stark, 2010; Yassa et al., 2010; Yassa et al., 2011). Thus, it remains unknown whether a relationship also exists between activity in the perirhinal cortex and the ability to pattern separate the ‘lure’ from related familiar stimuli. In fact, data obtained from monkeys with lesions of the hippocampus or perirhinal cortex suggest that both of these structures play a role in an animal’s ability to discriminate similar stimuli from one another (Nemanic, Alvarado, & Bachevalier, 2004; Zeamer, Meunier, & Bachevalier, 2011). Future experiments should thus focus on whether or not connections between the hippocampus and the perirhinal cortex are required to perform pattern separation operations.
The perceptual-mnemonic feature conjunctive model of perirhinal cortical function provides a framework for understanding the role that this structure may play in pattern separation. According to this model, the perirhinal cortex receives afferent input from lower sensory cortices and forms conjunctions of these stimulus features (Bussey & Saksida, 2002). When two objects are dissimilar, it is possible to make an accurate discrimination judgment based on a single feature. For example, consider two objects constructed from different color LEGOs®. A discrimination judgment could easily be made using color alone, an operation that does not require the perirhinal cortex. Conversely, if the two objects were constructed from similar sets LEGOs® such that they shared features then no single element would make these items distinct from one another. The important information for disambiguating the objects would thus be the unique combination of the LEGOs®. According to the perceptual-mnemonic feature conjunctive model, this type of operation cannot be accomplished without an intact perirhinal cortex (Bartko et al., 2007b; Cowell et al., 2006). When this structure is lesioned (Barense et al., 2007; Bartko et al., 2007a, 2007b; Bussey et al., 2003; McTighe et al., 2010), or compromised by aging (Burke et al., 2010), animals have a reduced ability to use information regarding the configuration of the multiple elements of a stimulus. This reduces their ability to perform pattern separation operations, rendering them vulnerable to interference (Burke et al., 2010; McTighe et al., 2010). Taken together, the data from the experiments reported here suggest that age-associated changes in the perirhinal cortex contribute to a decreased ability for older rodents and primates to perform pattern separation operations and that the dentate gyrus is not the only locus in the brain that subserves this neural computation (e.g., Aimone et al., 2011; Marr, 1969).
Supplementary Material
Acknowledgments
This work was supported by the McKnight Brain Research Foundation, and NIH grants AG003376, NS054465, and HHMI5205889. Additionally, we would like to thank Lan Hoang, Michelle Carroll, and Luann Snyder for help with completing this manuscript. We would also like to thank Julie Vogt, Bob Vogt and Prisca Zimmerman for their non-human primate expertise.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Works Cited
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12(2):99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25(44):10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem. 1996;3(2-3):124–137. doi: 10.1101/lm.3.2-3.124. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388(6639):272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007a;27(10):2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 2007b;14(12):821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav. 1996;53(2):277–283. doi: 10.1016/0091-3057(95)02021-7. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17(1):14–24. [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61(5):667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20(11):4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111(3):467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. J Neurosci. 1998;18(6):2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15(2):355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15(2):365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur J Neurosci. 2003;17(3):649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q J Exp Psychol B. 2005;58(3-4):269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Cavoy A, Delacour J. Spatial but not object recognition is impaired by aging in rats. Physiol Behav. 1993;53(3):527–530. doi: 10.1016/0031-9384(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Reinagel P, Broadbent NJ, Flister ED, Squire LR. Intact performance on feature-ambiguous discriminations in rats with lesions of the perirhinal cortex. Neuron. 2011;70(1):132–140. doi: 10.1016/j.neuron.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. J Neurosci. 2006;26(47):12186–12197. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima MN, Laranja DC, Caldana F, Bromberg E, Roesler R, Schroder N. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol. 2005;40(6):506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99(2):191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Bromer JA. A test apparatus for monkeys. Psychological review. 1938;19(2):434–436. [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18(9):955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27(5):1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2010;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16(6):947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17(15):6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Morris RG. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10(10):408–415. [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Paradoxical false memory for objects after brain damage. Science. 2010;330(6009):1408–1410. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9(5-6):495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24(8):2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160(1):300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4(6):661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104(6):876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Barnes CA, Wenk GL, McNaughton BL. Differential effects of selective immunotoxic lesions of medial septal cholinergic cells on spatial working and reference memory. Behav Neurosci. 1996;110(5):1181–1186. doi: 10.1037//0735-7044.110.5.1181. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the medial temporal lobe: evaluating the current evidence. Neuron. 2009;61(5):657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Baxter MG. Memory, perception, and the medial temporal lobe: a synthesis of opinions. Neuron. 2009;61(5):678–679. doi: 10.1016/j.neuron.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. American Journal of Primatology. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16(5):338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2(2):189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Meunier M, Bachevalier J. Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learn Mem. 2011;18(3):170–180. doi: 10.1101/lm.2076811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.