Abstract
Background:
While many studies have demonstrated positive associations between childhood obesity and adult metabolic risk, important questions remain as to the nature of the relationship. In particular, it is unclear whether the associations reflect the tracking of body mass index (BMI) from childhood to adulthood or an independent level of risk. This systematic review aimed to investigate the relationship between childhood obesity and a range of metabolic risk factors during adult life.
Objective:
To perform an unbiased systematic review to investigate the association between childhood BMI and risk of developing components of metabolic disease in adulthood, and whether the associations observed are independent of adult BMI.
Design:
Electronic databases were searched from inception until July 2010 for studies investigating the association between childhood BMI and adult metabolic risk. Two investigators independently reviewed studies for eligibility according to the inclusion/exclusion criteria, extracted the data and assessed study quality using the Newcastle–Ottawa Scale.
Results:
The search process identified 11 articles that fulfilled the inclusion and exclusion criteria. Although several identified weak positive associations between childhood BMI and adult total cholesterol, low-density lipo protein-cholesterol, triglyceride and insulin concentrations, these associations were ameliorated or inversed when adjusted for adult BMI or body fatness. Of the four papers that considered metabolic syndrome as an end point, none showed evidence of an independent association with childhood obesity.
Conclusions:
Little evidence was found to support the view that childhood obesity is an independent risk factor for adult blood lipid status, insulin levels, metabolic syndrome or type 2 diabetes. The majority of studies failed to adjust for adult BMI and therefore the associations observed may reflect the tracking of BMI across the lifespan. Interestingly, where adult BMI was adjusted for, the data showed a weak negative association between childhood BMI and metabolic variables, with those at the lower end of the BMI range in childhood, but obese during adulthood at particular risk.
Keywords: childhood obesity, metabolic syndrome, cardiovascular disease
Introduction
Overweight and obesity are associated with a range of chronic disease states, including cardiovascular disease (CVD), type 2 diabetes and certain cancers. The World Health Organisation has estimated that around a third of coronary heart disease and ischaemic stroke cases are attributable to excess adiposity.1 Current prevalence of overweight and obesity therefore has significant implications for population morbidity and mortality, and in this regard the rising prevalence of childhood obesity is of particular concern. In the United Kingdom, 2004 figures showed the prevalence of obesity in children aged 5–17 years to be 29%,2 and most estimates suggest a doubling in prevalence over the course of the preceding decade.3, 4 Similarly in the United States, 32% of children and adolescents were observed to be at or above the 85th percentile of the 2000 body mass index (BMI)-for-age growth charts.5, 6 Such data are predictive of adult overweight and obesity rates in the future, as adiposity has been shown to track from childhood into adult life.7, 8 It is generally assumed that an earlier onset and longer duration of obesity is associated with a greater cardiovascular risk, increasing concern about childhood obesity trends.
Although many studies have demonstrated positive associations between childhood obesity and adult cardiovascular risk, important questions remain as to the nature of the relationship.9 For example, it is not clear whether weight loss interventions in adult life can fully ameliorate the risks associated with childhood obesity, or whether an independent effect of childhood obesity remains, irrespective of the degree of adult adiposity. This has major implications for the design and timing of appropriate public health interventions. Our recent systematic review suggested that the observed associations between childhood obesity and adult blood pressure, carotid intima–media thickness or cardiovascular events largely reflected the tracking of BMI from childhood into adult life, and concluded that there was little evidence of an association that was independent of adult BMI.10 The data suggested that avoiding overweight during childhood failed to provide any protection against the effects of obesity in adulthood, and that those who were obese children but went on to be normal weight adults were not at any greater risk of CVD. Interestingly, those who were lean as children seemed to be most susceptible to the risks associated with adult obesity, particularly with respect to high blood pressure. Lauer et al.11 also observed that the strongest predictor of blood pressure was a change from being at the lower end of the BMI scale in children to the higher end in adulthood. Li et al.12 reported that the effect of adult BMI on blood pressure was highest in those who had been in the lowest BMI decile as children. These associations remain unexplained, but may reflect the differing contributions of lean body mass to BMI across the lifespan and the cumulative effect of changes in body composition over time. The nature of these observations indicates a complexity in the relationship between obesity and metabolic risk across the lifespan, which is worthy of further study.
This second systematic review builds upon that published previously, with the aim of assessing the relationship between childhood obesity and a range of metabolic risk factors related to cardiovascular health during adult life. The inclusion criteria for this review focused on the selection of studies focusing on the wider components of the metabolic syndrome, including dyslipidaemia, insulin resistance and metabolic disease outcomes. This has enabled more detailed investigation of the important research questions regarding the long-term impact of childhood obesity, which arose from the previous review.
Materials and methods
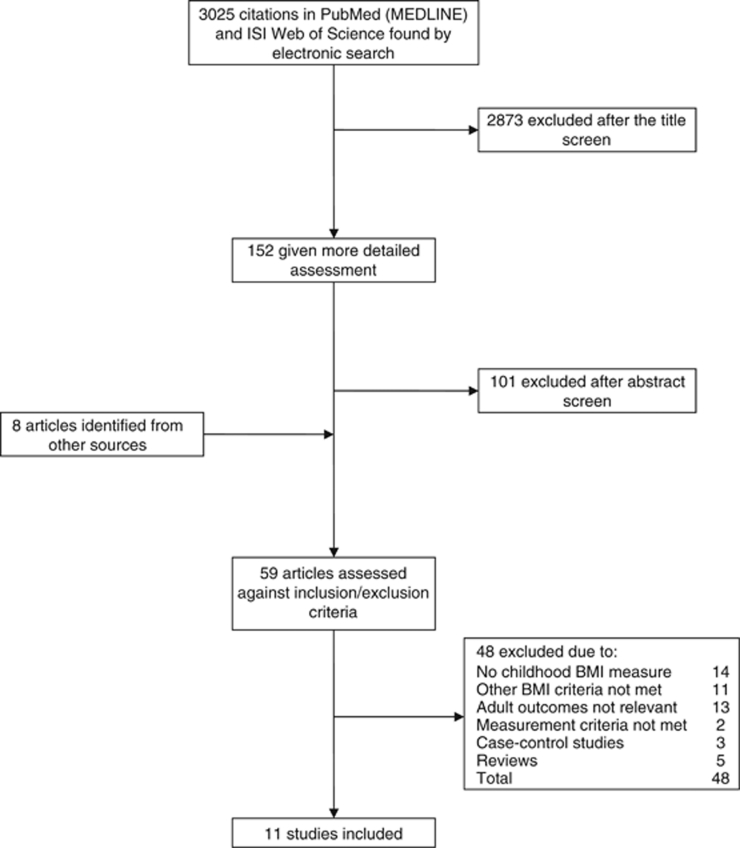
The general methods of systematic review used in this study were as previously described in Dunford et al.10 Briefly, we reviewed the literature reporting results from longitudinal studies investigating the association between childhood obesity and adult metabolic disease risk. A computerised search of the online electronic databases PubMed (MEDLINE) and ISI Web of Science from inception up to 30 June 2010 was carried out, using the terms ‘childhood’, ‘adult’ and ‘obesity’. The selection process and number of articles excluded at each stage are shown in Figure 1.
Figure 1.
Flowchart of the search and selection process.
Two investigators (LJD and SM) independently reviewed studies for eligibility according to the criteria below. Agreement was good (κ=99%) and any differences were agreed by consensus.
The inclusion criteria were as follows:
Childhood measures of BMI at one or more ages in childhood (0–12) or adolescence.13, 14, 15, 16, 17, 18 All measurements taken by health professionals or trained investigators.
Childhood BMI status calculated using US Centre for Disease Control percentile charts5 or International Obesity Task Force charts,13 and childhood overweight and obese defined as within those criteria (Centre for Disease Control: >85th centile=at risk of overweight, >95th centile=overweight; International Obesity Task Force percentiles track back from World Health Organisation adult guidelines14 of ⩾25 kg m−2 overweight and ⩾30 kg m−2 obese). Or childhood BMI treated as a continuous variable and association with adult outcome assessed by regression or correlation.
Adult overweight and obese defined according to World Health Organisation guidelines14 as overweight when BMI ⩾25 kg m−2 and obese when BMI ⩾30 kg m−2.
One or more biomarkers of metabolic disease risk measured at one or more ages in adulthood (defined as >18 years of age), for example, cholesterol, triglycerides and plasma insulin. All measurements were taken by health professionals or trained investigators. Or metabolic disease outcomes/cause of death extracted from secure registers.
Studies carried out in westernised, developed countries.
Articles in English language.
The exclusion criteria were as follows:
Studies where participants were part of an obesity intervention/health promotion programme.
Studies where the individuals involved were all part of a selected group, for example, asthmatics, childhood cancer survivors, preterm babies, and so on.
Studies in which exposure or outcome had been self-reported.
Childhood or adult overweight/obese defined using arbitrary cutoff points.
Reviews, rather than original data.
Data extraction and quality assessment
Data were extracted as described previously.10 In short, regression or correlation coefficients, or hazard ratios or relative risks and confidence intervals were extracted, together with the statistical significance of the associations (where provided). These data are summarised in Table 1.
Table 1. Characteristics of the selected studies grouped by outcome measurement.
| References | Childhood age (years) | Adult age (years) | Population | n | Tracking of BMI | Effect size and significance | Effect of adjusting for adult BMI/% body fat |
|---|---|---|---|---|---|---|---|
| Blood lipids | |||||||
| Freedman et al.19 | 5–17 | 18–37 | Males and females in Louisiana, USA, born 1959–1968; BHS | 2617 | r=0.58 | Cholesterol r=+0.10* Triglycerides r =+0.16* LDL r=+0.11* HDL r=−0.14* | Relationships inversed Cholesterol r=−0.08 Triglycerides r=−0.09 LDL r=−0.09 HDL r=+0.07 |
| Salonen et al.17 | 0–11 | Mean 61.5 | Males and females in Helsinki, Finland, born 1934–1944 | 499 | Not reported | HDL—NS Triglycerides—NS | Not reported |
| Sinaiko et al.24 | 7–18 | 23 | Males and females in Minneapolis, USA, born 1970–71 MCBPS | 679 | r=0.612** | Cholesterol—NS Triglycerides r=+0.19** LDL NS HDL r=−0.18** | Not adjusted |
| Wright et al.20 | 9, 13 | 50 | Males and females in Newcastle, UK, born 1947 NTFS | 412 | r=0.24– 0.39 | All blood lipids NS for men Women—age 13 all blood lipids NS age 9, Cholesterol r=−0.15* age 9, triglycerides r=−0.18* age 9, LDL and HDL NS | Men: age 9, triglycerides r=−0.18* Women: age 9, cholesterol r=−0.17* triglycerides r=−0.27* age 13, triglycerides r=−0.19 All other ages/variables NS |
| Lauer et al.23 | 8–18 | 20–30 | Males and females in Iowa, USA, born 1960–70s | 2446 | r=0.53– 0.84* | Change in cholesterol with change in BMI: Men r=0.20–0.45* Women r=0.10–0.26* from ages 11+, but NS at ages 7–10 | Not adjusted |
| Insulin | |||||||
| Freedman et al.19 | 5–17 | 18–37 | Males and females in Louisiana, USA, born 1959–1968; BHS | 2617 | r=0.58 | r=0.26* | r=−0.15* |
| Freedman et al.18 | 3–17 | Mean 27 | Males and females in Louisiana, USA, born 1959–1968; BHS | 2911 | r=0.58b | Age 3–7, r=+0.27 Age 8–13, r=+0.31 Age 14–17, r=+0.36 | Not adjusted |
| Martin et al.21 | Mean 6 | Mean 71 | Males and females, England and Scotland, born 1920s and 1930s | 456 | r=0.12 | HOMA −8.0% (CI −0.2 to −15.1) per s.d. change in BMI in childhood | Not adjusted |
| Sinaiko et al.24 | 7–18 | 23 | Males and females in Minneapolis, USA, born 1970–1971 MCBPS | 679 | r=0.612** | r=+0.28** | Not adjusted |
| Thearle et al.16 | 5–16 | Mean 25 | Males and females in Gila River Indian Community, USA, born 1960 onwards | 76 | Not reported | Only adjusted figures reported | Insulin action r=−0.43** Acute insulin response r=0.32*** A 5 kg m−2 increase in childhood BMI =−7.4% (CI −12.7 to −1.8%) insulin action* |
| Wright et al.20 | 9, 13 | 50 | Males and females in Newcastle, UK, born 1947 NTFS | 412 | r=0.24– 0.39 | NS | Men: age 9, r=−0.17***; Age 13, r=−0.23*** Women: age 9, r=0.20***; age 13 r=−0.21*** |
| Disease outcomes | |||||||
| Bjorge et al.22 | 14–19a | Up to 62 | Males and females in Norway, born 1944–1961 | 226 678 | Not measured | Risk of death from diabetes mellitus Men : NS Women: results not reported | Not adjusted |
| Morrison et al.28 | Mean 12.8 | Mean 38.4 | Males and females, Princeton, USA, born 1960s | 814 | 0.59 | For every 1 point change in childhood BMI percentile, the OR for having metabolic syndrome was 1.025 (CI 1.018–1.033) | Not adjusted |
| Salonen et al.17 | 0–11 | Mean 61.5 | Males and females in Helsinki, Finland, born 1934–1944 | 499 | Not reported | Higher BMI associated with decreased risk of metabolic syndrome for ages 2–11 OR for having metabolic syndrome for every 1 s.d. change in BMI between ages: 0–2: 0.72 (CI 0.57–0.92) 2–7: 0.63 (0.49 0.81) 7–11: NS | OR for having metabolic syndrome for every 1 s.d. change in BMI between ages: 0–2: 0.75 (CI 0.59–0.95) 2–7: 0.63 (0.49 0.81) 7–11: NS |
| Srinivasan et al.15 | 8–17 | 19–38 | Males and females in Louisiana, USA, born 1959–1968; BHS | 745 | r=0.58b | For every 1 s.d. change in childhood BMI, the OR for having clustering of risk variables for metabolic syndrome was 2.03 | Not adjusted |
Abbreviations: BHS, Bogalusa Heart Study; BO, Boyd Orr Cohort; CI, confidence intervals; HR, hazard ratios; MCBPS, Minneapolis Children's Blood Pressure Study; MS, Muscatine Study; NTFS, Newcastle Thousand Families Cohort; NA, not applicable; NS, not significant; OR, odds ratios; s.d., standard deviation.
Although age 19 is outside the childhood age criteria, the authors report that when the data were broken down into 14–16 and 17–19, there were no differences between them and the overall findings.
Tracking not reported in this study. However, this figure is from the same Bogalusa cohort as reported by Freeman et al.19
*P<0.05.
**P<0.001.
***P<0.01.
Quality of the papers that passed the defined criteria for inclusion in the review was assessed using the Newcastle–Ottawa Scale, based on recommendations by the Cochrane Non-Randomised Studies Methods Working group, as described previously.10 Two investigators (LJD and SM) independently assessed study quality according to the criteria in Table 2. Agreement was good (κ=91.4%) and differences were agreed by consensus. Results from the quality assessment are summarised in Table 3.
Table 2. Assessment of quality for a cohort study (adapted from Newcastle–Ottawa Scale).
| Selection | |
| Representativeness of the intervention cohort: | |
| • truly representative of children in the contemporary western world | |
| somewhat representative of children in the contemporary western world | |
| • selected group of patients, for example, only certain socioeconomic groups/areas | |
| • no description of the derivation of the cohort | |
| Selection of the non-intervention cohort (in this case ‘normal weight’ during childhood): | |
| • drawn from the same community as the intervention cohort (in this case ‘at risk of overweight’ or ‘overweight/obese’ during childhood) | |
| • drawn from a different source | |
| • no description of the derivation of the non-intervention cohort | |
| Ascertainment of exposure: | |
| • measurement by trained health professional | |
| secure record | |
| • written self-report | |
| • other/no description | |
| Demonstration that outcome of interest was not present at the start of study: | |
| • yes | |
| • no | |
| Comparability | |
| Comparability of cohorts on the basis of the design or analysis: | |
| • study controls for age, sex and adult BM/percentage body fat | |
| • study controls for socioeconomic status | |
| Outcome | |
| Assessment of outcome: | |
| • independent assessment by trained health-care professional | |
| record linkage | |
| • self-report | |
| • other/no description | |
| Was follow-up long enough for outcomes to occur: | |
| • yes, if mean adult age >35 | |
| • no, if mean adult age ⩽35 | |
| Adequacy of follow-up of cohorts: | |
| • complete follow-up: all subjects accounted for | |
| subjects lost to follow-up unlikely to introduce bias: number lost ≤20%, or description of those lost suggesting no different from those followed | |
| • follow-up rate <80% and no description of those lost | |
| • no statement | |
Stars were awarded if the criteria shown in italics were met.
Table 3. Summary of results and quality scores.
| References | Effect | Adjusted effect |
Newcastle–Ottawa
Scale |
||
|---|---|---|---|---|---|
| Selection (max. 4*) | Comparability (max. 2*) | Assessment (max. 3*) | |||
| Total cholesterol | |||||
| Freedman et al.19 | + | − | *** | * | ** |
| Lauer et al.23 | + | Not adjusted | *** | * | ** |
| Sinaiko et al.24 | ↔ | Not adjusted | *** | ** | |
| Wright et al.20 | −, ↔ | −, ↔ | *** | * | *** |
| LDL cholesterol | |||||
| Freedman et al.19 | + | − | *** | * | ** |
| Sinaiko et al.24 | ↔ | Not adjusted | *** | ** | |
| Wright et al.20 | ↔ | ↔ | *** | * | *** |
| HDL cholesterol | |||||
| Freedman et al.19 | − | + | *** | * | ** |
| Salonen et al.17 | ↔ | Not reported | *** | ** | ** |
| Sinaiko et al.24 | − | Not adjusted | *** | ** | |
| Wright et al.20 | ↔ | ↔ | *** | * | *** |
| Triglycerides | |||||
| Freedman et al.19 | + | − | *** | * | ** |
| Salonen et al.17 | ↔ | Not reported | *** | ** | ** |
| Sinaiko et al.24 | + | Not adjusted | *** | ** | |
| Wright et al.20 | −, ↔ | −, ↔ | *** | * | *** |
| Insulin | |||||
| Freedman et al.19 | + | − | *** | * | ** |
| Freedman et al.18 | + | Not adjusteda | *** | a | ** |
| Martin et al.21 | −b | Not adjusted | *** | * | *** |
| Thearle et al.16 | Not reported | −c, +d | ** | * | ** |
| Sinaiko et al.24 | + | Not adjusted | *** | ** | |
| Wright et al.20 | ↔ | − | *** | * | *** |
| Disease outcomes | |||||
| Bjørge et al.22 | ↔ | Not adjusted | *** | *** | |
| Morrison et al.28 | + | Not adjusted | *** | *** | |
| Salonen et al.17 | − | − | *** | ** | ** |
| Srinivasan et al.15 | + | Not adjusted | **** | * | ** |
Abbreviations: HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein.
+, positive correlation; ↔, no significant correlation; −, negative correlation; multiple annotations reflect differing associations found within paper, for example, at different age groups; anot reported; bHOMA insulin resistance; cinsulin action; dacute insulin response. Stars (*) were awarded for selection, comparability and assessment according to the criteria in Table 2.
Results
In all, 11 articles fulfilled the selection criteria and a summary of the main characteristics and results is shown in Table 1. All studies, apart from one, were published in the last 10 years.
Quality assessment
Although a satisfactory degree of quality had been assured through the inclusion/exclusion criteria, there was still some variation between the quality assessments made using the Newcastle–Ottawa Scale (Table 3). Based on sample selection, all studies were rated highly with one study attaining four out of four possible stars15 and all others attaining three, apart from Thearle et al.,16 which fulfilled only two of the quality criteria. The studies with three stars all failed to show the absence of the outcome of interest in childhood. Thearle et al.16 studied Pima Indians, therefore failing to attain a star for being truly representative of a westernised cohort. For comparability there was more variation, with only one study17 attaining both of the available stars, six rated one star, three no stars and one study18 providing insufficient information to make an assessment. This variation was due to differences in accounting for confounding factors (Table 2). Age and gender were generally accounted for, but other confounding factors such as smoking or socioeconomic status were unaccounted for in most of the studies. Crucially, only four studies adjusted for current BMI or adult percentage body fat, and as a result the majority of studies was unable to draw valid conclusions regarding the independent impact of childhood obesity upon metabolic parameters.16, 17, 19, 20 For assessment, seven studies attained two stars and four studies rated three. Of those that attained two, all were not awarded the third star because the adult cohort was considered too young (under 35) for the outcome to be demonstrated adequately.
It was difficult to directly compare results across the studies owing to the variety of statistical methods and approaches used and this was the primary reason for not performing a meta-analysis. Where relevant, regression coefficients, odds ratios or estimates of relative risk were extracted from the studies. Most studies treated childhood BMI as a continuous variable for the purpose of statistical analysis, but some used cutoff points (those recommended by US Centre for Disease Control5 or International Obesity Task Force13) to define childhood overweight or obese. In addition, childhood BMI scores were sometimes presented as z-scores, or as the change in BMI or BMI z-scores between age categories.
The exposure variable (childhood BMI) was also measured at substantially different ages, ranging from 2 to 18 years of age between studies. The age at which adult outcomes were measured also varied widely from 18 to 71 years, with historical cohort studies21 tending to include adult outcomes at older ages than prospective studies. The year of birth in the studies ranged from the 1930s21 up to the 1970s.22, 23, 24 Some studies used one measure of childhood BMI, whereas others used longitudinal data from the same study participants, and thus had more than one BMI measure in childhood for each individual.
Generally the adult outcomes were more homogenous, with total cholesterol, triglycerides and high-density lipoprotein (HDL)-cholesterol being measured by standard chemical and/or enzymatic procedures as described in the Lipid Research Clinics Program Manual of Laboratory Operations25 or by Lamont et al.26 low-density protein (LDL)-cholesterol was calculated using the Friedewald equation.27 Fasting insulin was measured using standard radioimmunoassay kits. To determine insulin resistance, Martin et al.21 calculated homeostasis model assessment (HOMA), and Thearle et al.16 calculated acute insulin response and insulin action. Definitions of metabolic syndrome varied between studies. Srinivasan and co-workers15 defined those at risk of syndrome X as being those individuals with the highest quartiles of BMI, blood pressure, fasting insulin and total cholesterol:HDL-cholesterol or triglycerides:HDL-cholesterol ratios. Morrison et al.28 used the definition of metabolic syndrome as described by the National Cholesterol Education Program expert panel on detection, evaluation and treatment of high blood cholesterol in adults.29 Salonen et al.17 used the criteria defined by the International Diabetes Federation 2005. Mortality and morbidity outcomes were identified from the Cause of Death Registry at Statistics Norway.22
Main results
Total cholesterol
Four studies considered the impact of childhood BMI on adult total cholesterol concentrations (Table 1), with two studies19, 23 showing weak to moderate positive correlations. One study20 reported a negative correlation between BMI at age 9 and cholesterol in women in adulthood, but no significant correlation with BMI at age 13 (no correlation observed in men at either age). Sinaiko et al.24 showed no statistically significant association.
All four studies observed significant tracking of childhood BMI into adulthood, but two studies23, 24 did not adjust for adult BMI, thus the independent effects of childhood BMI were not investigated. Of the two studies that did adjust for adult BMI, Freedman et al.19 reported a weak, negative correlation between the two variables after adjustment and Wright et al.20 observed that the weak negative correlation between total cholesterol and BMI at age 9 in women persisted.
LDL- and HDL-cholesterol
Three of the four studies mentioned above also looked at LDL- and HDL-cholesterol concentrations.19, 20, 24 Freedman et al.19 showed a weak positive correlation between childhood BMI and adult LDL-cholesterol, but this became negative once adjusted for adult BMI. They also reported the opposite for HDL-cholesterol, that is, a weak negative correlation, which became positive once adjusted for adult BMI. The two other studies20, 24 did not show any significant correlations between childhood BMI and LDL-cholesterol, and this was still the case after adjustment for adult BMI in the Wright et al. study.20 Wright et al. reported the same findings regarding HDL-cholesterol, that is, no significant correlation between childhood BMI and HDL-cholesterol before or after adjusting for adult BMI. Sinaiko et al.24 did show a significant but weakly negative correlation between childhood BMI and HDL-cholesterol, but did not adjust for adult BMI. This study was rated poorly in the quality assessment (Table 3). Salonen et al.17 included data for HDL-cholesterol as part of defining the metabolic syndrome, but reported no significant correlations between childhood BMI at any age and adult HDL-cholesterol. However, it was not clear if this was adjusted for adult percentage body fat or not.
Triglycerides
Two studies19, 24 reported positive correlations between childhood BMI and adult triglyceride concentrations. One of these24 did not adjust for adult BMI despite observing significant tracking of BMI into adulthood, hence the independent effects of childhood BMI were not investigated. Freedman et al.19 did adjust for adult BMI and found the relationship inversed, that is, that a greater childhood BMI was associated with lower circulating triglyceride concentrations in adulthood. Another study17 reported no significant correlation between the two variables, although it was not clear if this was adjusted for adult body fat. Conversely, Wright et al.20 reported a weak negative correlation between childhood BMI at age 9 and adult triglyceride concentrations in women, which became stronger once adjusted for current BMI. However, no such associations were seen at age 13, or at either time point for males.
Insulin
Six studies considered the impact of childhood BMI on adult insulin concentrations or insulin resistance. Three studies18, 19, 24 reported moderate positive correlations between childhood BMI and insulin concentrations. However, the adjusted data set for Freedman et al.19 showed that this association became negative once adjusted for current BMI. The second paper by Freedman et al.18 did not report whether or not the data were adjusted for adult BMI. Sinaiko et al.24 did not make the adjustment, thus the independent effects of childhood BMI were not assessed. Thearle et al.16 reported moderate positive correlations between childhood BMI and adult acute insulin response and adult insulin action, which were adjusted for adult percentage body fat, therefore supporting an independent effect of childhood BMI on these variables.
Interestingly, the two studies with the oldest adult cohorts had contrasting findings to the literature relating to younger populations. Wright et al.20 observed no significant correlations between childhood BMI and adult insulin concentrations, but a significant negative correlation became apparent once adjusted for adult BMI. Martin et al.21 reported a significantly lower homeostasis model assessment-insulin resistance in adults who were of greater BMI during childhood. Homeostasis model assessment-insulin resistance fell by 8% per standard deviation of increasing childhood BMI. This study reported that the heaviest children were least likely to manifest as insulin resistant in adulthood. This tendency was particularly marked among children who went on to become lean adults.
Disease outcomes
Four studies considered the impact of childhood BMI on risk of type 2 diabetes and metabolic syndrome in adulthood. Two studies showed positive correlations between disease outcomes and childhood BMI. Srinivasan et al.15 observed a positive relationship between childhood BMI and the number of metabolic syndrome criteria risk variables in adulthood. Morrison et al.28 reported that for every one unit increase in childhood BMI percentile, the odds ratio for developing the metabolic syndrome in adulthood was 1.025. The other two studies had the oldest adult cohorts. Bjorge et al.22 reported no significant increase in risk of death from type 2 diabetes in men associated with childhood obesity. It did not report results for women. The final study by Salonen et al.17 reported that higher childhood BMI was associated with lower risk of metabolic syndrome in adult life. This was the only one of the four diabetes studies that adjusted for adult adiposity, thus independent effects of childhood BMI were not considered by most of the studies in this area.
Discussion
In developed countries, circulatory disease is the most common cause of death, accounting for 33% of UK deaths in 2008,30 and in Europe 48% of deaths are due to CVD.2 The burden of disease is increasing owing to ageing populations and the rising prevalence of obesity and its associated co-morbidities. It is imperative to understand how events at important stages across the lifespan influence long-term risk of disease, so that interventions can be targeted to the most appropriate time point. There is a widely held assumption that childhood obesity is an independent risk factor for adult metabolic and CVD. However, our previous review indicated that the relationships are largely dependent on the tracking of BMI from childhood to adulthood. Importantly, the evidence suggested that the risk of raised blood pressure was highest in those who were at the lower end of the BMI scale in childhood and overweight in adulthood. The current review has extended these findings to investigate the relationship between childhood obesity and a range of metabolic risk factors. Using an unbiased review methodology, we found insufficient evidence to support the idea that childhood obesity independently increases risk of dyslipidaemia and insulin resistance in adulthood. Conversely, and in support of our previous analysis of blood pressure data, the review demonstrates that those who were lean as children seemed to be most susceptible to the metabolic risk associated with adult obesity.
Our previous review10 focused on studies assessing CVD end points and two major clinical risk factors for CVD, blood pressure and carotid intima–media thickness. The quality assessment of the studies included raised significant issues regarding the quality of the data presented. In particular, the studies assessing CVD end points were all retrospective in nature, focused on non-contemporary populations and failed to adjust for adult BMI. In contrast, the studies assessing blood pressure and carotid intima–media thickness were more likely to have adjusted for adult BMI, but often involved adult cohorts with an average age considered too young for the outcome to be assessed adequately. The current review aimed to overcome these limitations to some extent by assessing a range of intermediary risk factors.
Blood lipids
Dyslipidaemia is a widely recognised risk factor for progression of atherosclerosis,31 and is a component of the metabolic syndrome and CVD. Adult obesity is generally associated with dyslipidaemia and recent literature suggests that the origins of dyslipidaemia may be determined in childhood, in conjunction with obesity. Several studies have demonstrated links between childhood obesity and raised total cholesterol, LDL-cholesterol, triglycerides and low HDL-cholesterol concentrations32, 33 in adulthood. Importantly, blood lipid concentrations have been shown to track from childhood to adulthood.34
This review demonstrates an overwhelming lack of evidence of an independent positive relationship between childhood BMI and dyslipidemia. Of the two studies that reported positive associations between childhood BMI and adult total cholesterol,19, 23 only one adjusted for adult BMI, and doing so reversed the direction of the relationship,19 suggesting that those with higher BMI in childhood had lower total cholesterol in adulthood if overweight was not maintained. An inverse association after adjustment for adult BMI was also observed in women in the study by Wright et al.20 Lauer et al.23 showed the strongest positive association between childhood BMI and adult cholesterol, but the data were not adjusted for adult BMI, despite a strong degree of tracking of BMI from childhood into adulthood (r=0.53–0.84). Similarly for LDL-cholesterol, the positive association with childhood BMI reported by Freedman et al.19 became negative after adjustment for adult BMI, suggesting that those with higher BMI in childhood had lower LDL-cholesterol as adults if overweight was not maintained. Negative correlations between childhood BMI and HDL-cholesterol were reported by two studies. Once again, adjustment for adult BMI in the Bogalusa Heart Study rendered this relationship positive,19 while the other study demonstrated a high degree of BMI tracking into adulthood (r=0.612), but did not make the adjustment.24 Similar themes emerged in consideration of associations between adult triglyceride concentrations and childhood BMI, with negative association observed in the two studies that made the adjustment for adult BMI.
Our review therefore found very little evidence to suggest that greater BMI in childhood was an independent risk factor for dyslipidaemia in adulthood. This is in agreement with the findings of Porkka et al.,35 who reported that childhood obesity variables (BMI and subscapular skinfold) were poor predictors of adult serum levels and only accounted for between 0 and 6% of adult lipid variability. In contrast to commonly held assumptions and in agreement with our previous review of blood pressure data, several studies observed that those who had a higher BMI during childhood exhibited lower total cholesterol, LDL-cholesterol and triglycerides and higher HDL-cholesterol.
Insulin
Increased adiposity generally leads to systemic hyperinsulinaemia and decreased skeletal muscle sensitivity.36 Insulin resistance is linked to a range of comorbidities, including obesity, dyslipidaemia, type 2 diabetes and atherosclerotic disease.37, 38 Obese children have higher fasting insulin and homeostasis model assessment-insulin resistance compared with age-matched lean children.39, 40 Clinically relevant morbidities, however, remain unusual in children and adolescents.41 Of the six studies that met the selection criteria for this review, three18, 19, 24 reported moderate positive correlations between childhood BMI and adult insulin concentrations. However, only one of these adjusted for adult BMI and this reversed the direction of the association observed, similar to the patterns observed in the blood lipid data. The only study in support of an association that is independent of adult BMI was that by Thearle et al.,16 who reported a positive correlation between childhood BMI and acute insulin response (and a negative correlation with insulin action) when adjusted for adult percentage body fat. Generally, the positive associations between childhood BMI and insulin were slightly stronger than those for blood lipids (range r=+0.26 to +0.36). Although the clinical significance of these trends may not be great, this may indicate a slightly stronger correlation between adult BMI and insulin than that between adult BMI and blood lipids. However, it must be noted that such an assertion is dependent on the studies noted to be of lower quality in this review. The Thearle study16 had some important limitations when considering its relevance to a wider population, as the cohort was very small (n=76) and limited to Pima Indians, a group known to be particularly susceptible to type 2 diabetes.
Interesting negative associations were observed in two other studies. Martin et al.21 reported that the heaviest children were least likely to manifest as insulin resistant in adulthood, particularly if they went on to be lean. Wright et al.20 reported a negative association between childhood BMI and insulin after adjustment for adult BMI. Although study design and paucity of data made it difficult to draw firm conclusions, the balance of available material does not provide sufficient evidence to support an independent detrimental effect of childhood obesity on adult insulin status. Similarly to blood lipids, the data may instead indicate that those who are heavier as children may be less likely to exhibit insulin resistance as adults. We suggest that this may relate to the greater contribution of lean mass to weight and BMI during childhood, and its subsequent involvement in regulating insulin sensitivity.
Disease outcomes
To extend the findings of our previous review of CVD outcomes, the current review assessed the relationship between childhood obesity and risk of metabolic syndrome and type 2 diabetes. The International Diabetes Federation estimates that 285 million people worldwide have diabetes in 2010 and this will rise to 438 million by 2030.42 A similar pattern is envisaged in the United Kingdom, with 2.6 million people diagnosed in 2009, rising to over 4 million by 2025.43 Around 80% of those adults with type 2 diabetes also fulfil the criteria for metabolic syndrome.44
Within the studies that met the inclusion criteria for this review, evidence was presented of a positive correlation between childhood BMI and the number of metabolic syndrome criteria risk variables15 and risk of developing the metabolic syndrome.28 In contrast, Salonen et al.17 reported a decreased risk of metabolic syndrome for those with the highest childhood BMI and the largest study,22 which included over 225 000 subjects, found childhood BMI was not linked to increased risk of type 2 diabetes mortality in men. Taken together, these data fail to provide clear evidence that high childhood BMI leads to increased risk of diabetes and metabolic system morbidity or mortality. Few studies have tested the hypothesis directly and weaknesses were identified in those that did. For example, Morrison et al.28 relied on self-reports of the presence of type 2 diabetes, which may have been associated with under-reporting.
Final conclusions
To conclude, this systematic review has found little evidence to support the view that childhood obesity is an independent risk factor for adult blood lipid status, insulin levels, metabolic syndrome or type 2 diabetes. The research for each is generally insufficient, with the majority of studies failing to adjust for adult BMI and therefore not able to assess the relationship independently of the tracking of BMI and associated biomarkers of metabolic disease across the lifespan. The study by Must et al.45 is often cited as demonstrating a link between adolescent obesity and long-term mortality and morbidity that is independent of adult BMI. However, this study did not meet the criteria for inclusion within this review owing to the use of arbitrary cutoffs for categorising overweight. In addition, the lean cohort only covered a small range at the lower end of what is currently considered to be normal weight and cannot be considered representative of contemporary populations. This systematic review provides a balanced summary of the available data and concludes that there is insufficient evidence to demonstrate links with long-term risk, which are independent of adult BMI.
Interestingly, for those studies that did adjust for adult BMI, the balance of data suggest that there is a weak negative association between childhood obesity and metabolic variables, which could be interpreted as a slight protective effect of obesity in childhood. Similar observations were observed with regard to adult blood pressure in our previous review. However, BMI is a measure of weight relative to height rather than actual adiposity, and fat-free mass makes up a higher proportion of BMI than fat mass in childhood compared with adulthood.46 Relatively weak correlations between BMI and percentage body fat have been observed in children and adolescents.47, 48, 49, 50 Differences in BMI may therefore reflect differences in fat-free mass, particularly among those at the lower end of the BMI range.48 These observations make the negative associations between childhood BMI and adult metabolic risk (independent of adult BMI) particularly interesting, as they may reflect the long-term consequences of differences in the trajectory of lean mass deposition from as early as childhood. More detailed study of the impact of the trajectory of both fat and fat-free mass on future metabolic and cardiovascular risk is required to address this issue. The impact of diet and body composition during the developmental period on long-term metabolic function and disease risk has been demonstrated in a range of epidemiological studies, with biological plausibility and causality demonstrated in experimental studies in animal models.51 We suggest that the susceptibility of long-term metabolic function to diet and body composition extends throughout the growth phase, and that the pattern of inverse associations, which has emerged from this systematic review, may reflect the impact of ‘mismatch’ between the growth phase and adult periods of the life course. Further work is required to establish which physiological systems remain sensitive to permanent change and to identify a threshold age below which the inverse associations arise.
There is substantial evidence that childhood obesity tracks into adulthood and it is clear that adult obesity conveys higher risk of metabolic disease. We are not, therefore, suggesting that childhood obesity is without consequences. Targeting childhood and adolescence for prevention and treatment of obesity is wholly appropriate to establish a healthy weight moving forward into the adult years. However, it is evident from this systematic review that the nature of the relationship between early BMI and adult disease risk is very complex. Those who are at the lower end of the BMI range in childhood, but go on to be obese during adulthood seem to be at particular risk. Focusing on children who are overweight or obese for the promotion of healthy weight management may therefore miss an important at-risk group.
Acknowledgments
This work was supported by Organix Foundation.
Author contributions
SLE and SM originally conceived and supervised the project. LJD performed the literature search and made the selection of papers against the inclusion and criteria paper with SM. All authors took responsibility for quality assessment of the included studies. All authors were involved in the writing of the manuscript and were responsible for the final content of the manuscript.
Footnotes
The authors declare no conflict of interest.
References
- World Health Report. Reducing Risks, Promoting Healthy Life. World Health Organisation: Geneva, 2002. [Google Scholar]
- British Heart Foundation. European Cardiovascular Disease Statistics. British Heart Foundation: UK, 2008. [Google Scholar]
- Chinn S, Rona RJ. Prevalence and trends in overweight and obesity in three cross sectional studies of British children, 1974–94. BMJ 2001; 322: 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundred P, Kitchiner D, Buchan I. Prevalence of overweight and obese children between 1989 and 1998: population based series of cross sectional studies. BMJ 2001; 322: 326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R et al. CDC growth charts: United States. Adv Data 2000; 314: 1–27. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008; 299: 2401–2405. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults—a review of the literature. Prev Med 1993; 22: 167–177. [DOI] [PubMed] [Google Scholar]
- Singh AS, Mulder C, Twisk JWR, van Mechelen W, Chinapaw MJM. Tracking of childhood overweight into adulthood: a systematic review of the literature, 2008; 9: 474–488. [DOI] [PubMed] [Google Scholar]
- Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes 2009; 33: 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford LJ, Langley-Evans SC, McMullen S. Childhood obesity and adult cardiovascular disease risk: a systematic review. Int J Obes 2010; 34: 18–28. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Clarke WR. Childhood risk-factors for high adult blood pressure—the Muscatine Study. Pediatrics 1989; 84: 633–641. [PubMed] [Google Scholar]
- Li L, Law C, Power C. Body mass index throughout the life-course and blood pressure in mid-adult life: a birth cohort study. J Hypertens 2007; 25: 1215–1223. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L et al. 1999 World Health Organization International Society of Hypertension guidelines for the management of hypertension. JHypertens 1999; 17: 151–183. [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood—the Bogalusa Heart Study. Diabetes 2002; 51: 204–209. [DOI] [PubMed] [Google Scholar]
- Thearle MS, Bunt JC, Knowler WC, Krakoff J. Childhood predictors of adult acute insulin response and insulin action. Diabet Care 2009; 32: 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen MK, Kajantie E, Osmond C, Forsen T, Yliharsila H, Paile-Hyvarinen M et al. Role of childhood growth on the risk of metabolic syndrome in obese men and women. Diabet Metab 2009; 35: 94–100. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Inter-relationships among childhood BMI, childhood height, and adult obesity: the Bogalusa Heart Study. Int J Obes 2004; 28: 10–16. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 2001; 108: 712–718. [DOI] [PubMed] [Google Scholar]
- Wright CM, Parker L, Lamont D, Craft AW. Implications of childhood obesity for adult health: findings from thousand families cohort study. BMJ 2001; 323: 1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RM, Holly JMP, Smith GD, Gunnell D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr cohort. J Clin Endocrinol Metab 2006; 91: 3287–3295. [DOI] [PubMed] [Google Scholar]
- Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230 000 Norwegian adolescents. Am J Epidemiol 2008; 168: 30–37. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels—the Muscatine study. Pediatrics 1988; 82: 309–318. [PubMed] [Google Scholar]
- Sinaiko AR, Donahue RP, Jacobs DR, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults—the Minneapolis Children's Blood Pressure Study. Circulation 1999; 99: 1471–1476. [DOI] [PubMed] [Google Scholar]
- Lipid Research Clinics Program. In: Services UDoHaH (ed). Lipid and Lipoprotein Analyses. Lipid Research Clinics Program Manual of Laboratory Operations. US Department of Health and Human Resources: Bethesda, MD, 1982. [Google Scholar]
- Lamont D, Parker L, White M, Unwin N, Bennett SMA, Cohen M et al. Risk of cardiovascular disease measured by carotid intima–media thickness at age 49–51: lifecourse study. BMJ 2000; 320: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W, Fredrick D, Levy RI. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr 2008; 152: 201–206. [DOI] [PubMed] [Google Scholar]
- Cleeman JI, Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA et al. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. Mortality Statistics: Deaths registered in 2008. Office for National Statistics: London, 2008. [Google Scholar]
- McGill HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP et al. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000; 72: 1307S–1315S. [DOI] [PubMed] [Google Scholar]
- Reinehr T, Andler W, Denzer C, Siegried W, Mayer H, Wabitsch M. Cardiovascular risk factors in overweight German children and adolescents: relation to gender, age and degree of overweight. Nutr Metab Cardiovasc Dis 2005; 15: 181–187. [DOI] [PubMed] [Google Scholar]
- Burke V, Beilin LJ, Simmer K, Oddy WH, Blake KV, Doherty D et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes 2005; 29: 15–23. [DOI] [PubMed] [Google Scholar]
- Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum-lipids and lipoproteins from childhood to adulthood—the Bogalusa Heart Study. Am J Epidemiol 1991; 133: 884–899. [DOI] [PubMed] [Google Scholar]
- Porkka KVK, Viikari JSA, Taimela S, Dahl M, Akerblom HK. Tracking and predictiveness of serum-lipid and lipoprotein measurements in childhood—a 12-year follow-up—the cardiovascular risk in young Finns study. Am J Epidemiol 1994; 140: 1096–1110. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635–643. [DOI] [PubMed] [Google Scholar]
- Defronzo RA, Ferrannini E. Insulin resistance—a multifaceted syndrome responsible for niddm, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular-disease. Diabetes Care 1991; 14: 173–194. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365: 1415–1428. [DOI] [PubMed] [Google Scholar]
- Beauloye V, Zech F, Mong HTT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab 2007; 92: 3025–3032. [DOI] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr 2006; 84: 490–496. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr 1998; 132: 191–193. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. Diabetes Atlas 4th edn. International Diabetes Federation: Brussels, 2009. [Google Scholar]
- Diabetes-UK. Diabetes in the UK. Diabetes-UK: London, 2009. [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24: 683–689. [DOI] [PubMed] [Google Scholar]
- Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents—a follow-up of the Harvard growth study of 1922 to 1935. New Engl J Med 1992; 327: 1350–1355. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr 1982; 35: 1169–1175. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997; 99: 804–807. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes 2005; 29: 1–8. [DOI] [PubMed] [Google Scholar]
- Sarria A, Garcia-Llop LA, Moreno LA, Fleta J, Morellon MP, Bueno M. Skinfold thickness measurements are better predictors of body fat percentage than body mass index in male Spanish children and adolescents. Eur J Clin Nutr 1998; 52: 573–576. [DOI] [PubMed] [Google Scholar]
- Schaefer F, Georgi M, Wuhl E, Scharer K. Body mass index and percentage fat mass in healthy German schoolchildren and adolescents. Int J Obes 1998; 22: 461–469. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princip Pract 2010; 19: 87–98. [DOI] [PubMed] [Google Scholar]