Abstract
Background:
Few large population-based studies have compared the incidence of bleeding of gastroduodenal ulcers between patients with and without end-stage renal disease. We investigated the association between ulcer bleeding and end-stage renal disease in patients receiving hemodialysis, and we sought to identify risk factors for ulcer bleeding.
Methods:
We performed a nationwide seven-year population study using data from the National Health Insurance Research Database in Taiwan. We identified 36 474 patients with end-stage renal disease who were receiving hemodialysis, 6320 patients with chronic kidney disease and 36 034 controls matched for age, sex and medication use. We performed log-rank testing to analyze differences in survival time without ulcer bleeding among the three groups. We performed Cox proportional hazard regressions to evaluate the risk factors for ulcer bleeding among the three groups and to identify risk factors in patients receiving hemodialysis.
Results:
Patients receiving hemodialysis and those with chronic kidney disease had a significantly higher incidence of ulcer bleeding than controls had (p < 0.001). Hemodialysis (hazard ratio [HR] 5.24, 95% confidence interval [CI] 4.67–5.86) and chronic kidney disease (HR 1.95, 95% CI 1.62–2.35) were independently associated with an increased risk of ulcer bleeding. Diabetes mellitus, coronary artery disease, cirrhosis and use of nonsteroidal anti-inflammatory drugs were risk factors for ulcer bleeding in patients with end-stage renal disease who were receiving hemodialysis
Interpretation:
Patients with end-stage renal disease who are receiving hemodialysis had a high risk of ulcer bleeding. Diabetes mellitus, coronary artery disease, cirrhosis and the use of nonsteroidal anti-inflammatory drugs were important risk factors for ulcer bleeding in these patients.
Taiwan has the highest incidence and prevalence of end-stage renal disease in the world.1 The approximately 40 000 patients with end-stage renal disease consume 7% (about NT$26 billion) of Taiwan’s health insurance budget for dialysis treatment, especially because 90% of these patients receive hemodialysis rather than peritoneal dialysis.2 In Western and Asian countries, previous studies have suggested that the prevalence of peptic ulcer disease among patients with end-stage renal disease is not higher than in the general population;3–5 however, recent reports show a higher prevalence among patients receiving long-term hemodialysis6,7 and a higher rate of bleeding after the development of ulcers in these patients.8
The pathogenesis and risk factors for ulcers or ulcer bleeding in patients with end-stage renal disease are unclear.9–11 We performed a nationwide population-based cohort study to investigate the association between hemodialysis and bleeding of gastroduodeanl ulcers and to identify the risk factors for ulcer bleeding in patients with end-stage renal disease.
Methods
Data sources
National Health Insurance is a mandatory health insurance program in Taiwan that provides comprehensive coverage for medical care. Up to 96% of the population was enrolled by 2002.12 Claims data are collected in the National Health Insurance Research Database at the National Health Research Institutes. For research purposes, the institute released to the public a cohort dataset for one million randomly selected individuals and a dataset for 46 576 patients with some catastrophic illnesses (e.g., end-stage renal disease, cancer) who were alive in 2000. The institute collected all records for these individuals from 1995 to 2006. There were no statistically significant differences in age, sex and health care costs between the one million randomly sampled individuals and those enrolled in our study. Comprehensive health care data include the enrolment files, claims data, catastrophic illness files and a registry for drug prescriptions.
Each patient’s original identification number was encrypted for privacy in the cohort dataset. The encryption procedure was consistent between datasets so that all claims belonging to each patient could be linked. Because the dataset consisted of de-identified secondary data released to the public for research purposes, this study was exempt from full review by the institutional review board.
Study groups
Our study included almost all patients in Taiwan receiving long-term hemodialysis, as well as age- and sex-matched controls. We did not include patients who had started hemodialysis in the three months before enrolment and those with acute renal failure because of a high rate of ulcer bleeding in patients with critical medical conditions and acute renal failure.15,16
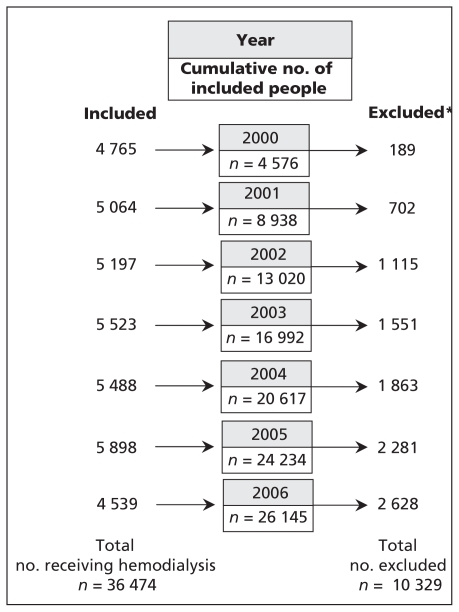
In Taiwan, patients with end-stage renal disease who are in need of long-term (more than three months) renal replacement therapy can apply for a catastrophic illness registration card from the National Health Research Institutes. We identified 36 474 patients with end-stage renal disease who had received a diagnosis of chronic renal failure (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 585) and who had received hemodialysis for more than three months from Jan. 1, 2000 through 2006. We excluded patients receiving peritoneal dialysis and those who had received a kidney transplant before or after enrolment. Figure 1 shows the flow of patients during the course of this study.
Figure 1:
Flow of patients with end-stage renal disease undergoing hemodialysis during the seven-year study period. In total, 26 145 patients were alive at the end of follow-up in 2007. *All patients who were excluded from follow-up had died during the study period (28.3% of patients undergoing hemodialysis).
We included controls without kidney disease (absence of ICD-9-CM codes: 580.x–588.x, 250.4x, 274.1x, 283.11, 403.x1, 404.x2, 404.x3, 440.1, 442.1, 447.3, 572.4, 642.1x and 646.2x) from the database in a 1:1 ratio. We matched the controls with patients receiving hemodialysis in terms of age, sex and use of some ulcerogenic medications (nonsteroidal anti-inflammatory drugs [NSAIDs], acetylsalicylic acid [ASA], steroids, ticlopidine and warfarin) and year of enrolment. Medications were identified and classified by use of the National Drug Classification System and the Anatomic Therapeutic Chemical Code, which is an internationally accepted classification system of drugs coordinated by the World Health Organization Collaborating Centre for Drug Statistics Methodology.13
We included a comparison group of 6320 patients with chronic kidney disease (ICD-9-CM codes 582.0, 582.4, 582.8x, 586, 250.4x, 274.1, 403.x1, 404.x2, 404.x3, corresponding to stage 4 and 5 chronic kidney disease) who were identified from the same cohort from the National Health Research Institutes.
We also investigated the covariables age, sex, pre-existing hypertension (ICD-9-CM 401.0–405.99), diabetes mellitus (ICD-9-CM 250.0–250.93), congestive heart failure (ICD-9-CM 428–428.9), coronary artery disease (ICD-9-CM 410–414.9) and cirrhosis of the liver (ICD-9-CM 571.2, 571.5 and 571.6).
Outcomes
The main outcome was the occurrence of ulcer bleeding as the main diagnosis during hospital stay. Bleeding was confirmed by endoscopic examination. The data were obtained from administrative claims (ICD-9-CM 531.0, 531.00, 531.01, 531.2x, 531.4x, 531.6x, 532.0, 532.00, 532.01, 532.2x, 532.4x, 532.6x, 533.0, 533.00, 533.01, 533.2x, 533.4x, 533.6x, 534.0, 534.00, 534.01, 534.2x, 534.4x and 534.6x). We also investigated bleeding-free survival time.
Statistical analysis
All data were expressed as frequency (percentage) or mean and standard deviation. We compared parametric continuous data between groups using the Student t test, and we compared categorical data using the χ2 test and Yates correction or the Fisher exact test. We performed survival analysis using the Kaplan–Meier method, with significance based on the log-rank test. We calculated survival time as being the time from the date of enrolment in the cohort to the date of admission to hospital because of ulcer bleeding. We performed multiple regression analysis using Cox proportional hazard regression analysis. We defined statistical significance as a two-sided p value of less than 0.05.
Results
The demographic data are shown in Table 1. Except for clopidogrel, concurrent use of ulcerogenic medications such as NSAIDs, ASA, steroids, ticlopidine and warfarin was comparable between the hemodialysis group and the control group (p > 0.05). However, comorbidities, including coronary artery disease, hypertension, diabetes, heart failure and cirrhosis were not equivalent between the groups (Table 1).
Table 1:
Demographic data for patients undergoing hemodialysis, patients with chronic kidney disease and controls from 2001 to 2006
| Characteristic | No. (%) of individuals* | ||
|---|---|---|---|
| Matched controls n = 36 034 |
Patients with chronic kidney disease n = 6 320 |
Patients receiving hemodialysis n = 36 474 |
|
| Age, yr, mean (SD) | 63.1 (13.9) | 61.6 (14.2)† | 63.2 (13.8) |
| Male sex | 17 525 (48.6) | 3 501 (55.4)† | 17 675 (48.5) |
| Hypertension | 11 601 (32.2) | 3 843 (60.8)† | 31 216 (85.6)‡ |
| Diabetes | 3 915 (10.9) | 4 255 (67.3)† | 18 796 (51.5)‡ |
| Coronary artery disease | 5 151 (14.3) | 1 618 (25.6)† | 12 527 (34.4)‡ |
| Heart failure | 1 046 (2.9) | 432 (6.8)† | 9 111 (25.0)‡ |
| Cirrhosis | 277 (0.8) | 96 (1.5)† | 1 472 (4.0)‡ |
| Medication use | |||
| ASA | 5 892 (16.4) | 1 567 (24.8)† | 6 089 (16.7) |
| NSAID | 3 064 (8.5) | 980 (15.5)† | 3 144 (8.6) |
| Steroid | 1 676 (4.6) | 195 (3.1)† | 1 750 (4.8) |
| Clopidogrel | 1 134 (3.2) | 101 (1.6)† | 1 493 (4.1)‡ |
| Ticlopidine | 361 (1.0) | 67 (1.1) | 411 (1.1) |
| Warfarin | 198 (0.6) | 50 (0.8) | 238 (0.6) |
| Ulcer bleeding | 480 (1.3) | 163 (2.6)† | 2 361 (6.5)‡ |
| All-cause mortality | 2 831 (7.9) | 915 (14.5)† | 10 329 (28.3)‡ |
Note: ASA = acetylsalicylic acid, NSAID = nonsteroidal anti-inflammatory drug, SD = standard deviation.
Unless stated otherwise.
p < 0.05 for comparison between patients with chronic kidney disease and controls.
p < 0.05 for comparison between patients receiving hemodialysis and controls.
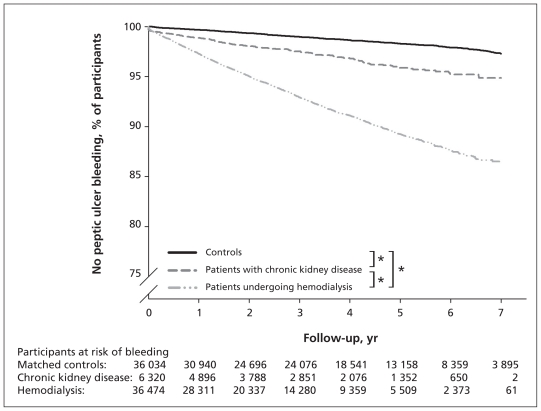
During the seven-year study period, 3004 (3.8%) of the 78 828 people included in this study experienced bleeding of gastroduodenal ulcers. Of these, 2361 were receiving hemodialysis (6.5% of patients with end-stage renal disease), 163 had chronic kidney disease (2.6% of patients with chronic kidney disease) and 480 were controls (1.3% of the controls) (Table 1). The log-rank test and Kaplan–Meier survival analysis showed that there was a significantly higher incidence of ulcer bleeding among patients receiving hemodialysis than among those with chronic kidney disease or among the controls (p < 0.001). The rate of bleeding was significantly higher in the group with chronic kidney disease than in the control group (p < 0.001) (Figure 2).
Figure 2:
Kaplan–Meier estimates of survival time free of peptic ulcer bleeding in patients undergoing hemodialysis, those with chronic kidney disease and controls. *Log rank p < 0.001.
After adjustment for age, sex, hypertension, diabetes, coronary artery disease, heart failure, cirrhosis and the use of NSAIDs, ASA, steroids, clopidogrel, ticlopidine and warfarin, the hazard ratio (HR) for ulcer bleeding was 5.24 times higher (95% confidence interval [CI] 4.67–5.86) in the hemodialysis group and 1.95 times higher (95% CI 1.62–2.35) in the chronic kidney disease group than in the control group (Table 2).
Table 2:
Independent predictors of ulcer bleeding identified by Cox regression analysis
| Variable | Adjusted HR* (95% CI) |
|---|---|
| Age (per year increase) | 1.03 (1.02–1.03) |
| Male sex | 1.23 (1.15–1.32) |
| Hypertension | 1.19 (1.07–1.32) |
| Diabetes | 1.31 (1.21–1.42) |
| Coronary artery disease | 1.14 (1.05–1.24) |
| Heart failure | 1.13 (1.03–1.24) |
| Cirrhosis | 1.86 (1.59–2.18) |
| Medication use | |
| ASA | 1.06 (0.97-–1.16) |
| NSAID | 1.94 (1.76–2.13) |
| Steroid | 1.07 (0.89–1.27) |
| Clopidogrel | 0.99 (0.83–1.17) |
| Ticlopidine | 1.12 (0.84–1.49) |
| Warfarin | 1.11 (0.75–1.67) |
| Renal disease (v. controls) | |
| Patients with chronic kidney disease | 1.95 (1.62–2.35) |
| Patients receiving hemodialysis | 5.24 (4.67–5.86) |
Note: ASA = acetylsalicylic acid, CI = confidence interval, HR = hazard ratio, NSAID = nonsteroidal anti-inflammatory drug.
Each variable was adjusted for every other variable listed.
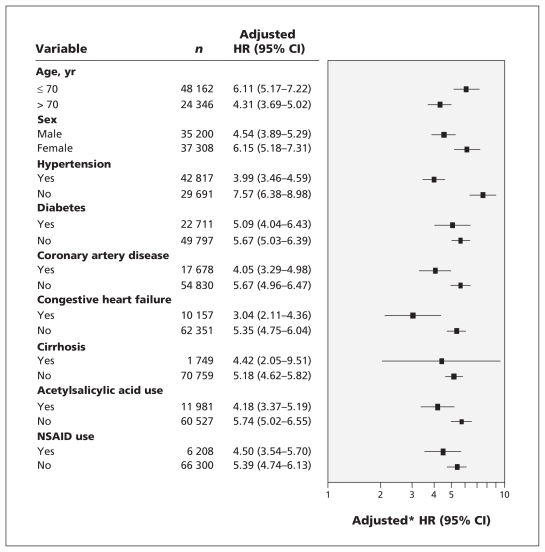
The results of the multivariable analysis are shown in Figure 3. In each stratum, we compared the HRs between patients receiving hemodialysis and controls. Those receiving hemodialysis had a significantly higher risk of ulcer bleeding regardless of age, sex, hypertension, diabetes, coronary artery disease, congestive heart failure, cirrhosis and use of ASA or NSAIDs.
Figure 3:
Adjusted hazard ratios (HRs) for ulcer bleeding in patients receiving hemodialysis. In each stratum, the HRs were compared between patients undergoing hemodialysis and controls. *Each factor was adjusted for all other factors. CI = confidence interval, NSAID = nonsteroidal anti-inflammatory drug.
Cox multivariable regression analysis showed that diabetes, coronary artery disease, cirrhosis and NSAIDs use, but not age, hypertension, heart failure and use of ASA, were risk factors for ulcer bleeding in patients with end-stage renal disease receiving hemodialysis (Table 3).
Table 3:
Risk factors for ulcer bleeding in patients with end-stage renal disease undergoing hemodialysis
| Risk factor | Adjusted HR* (95% CI) |
|---|---|
| Age (per year increase) | 1.01 (0.98–1.04) |
| Male sex | 1.15 (0.99–1.29) |
| Hypertension | 0.99 (0.88–1.12) |
| Diabetes | 1.44 (1.32–1.57) |
| Coronary artery disease | 1.29 (1.18–1.41) |
| Heart failure | 1.09 (0.97–1.21) |
| Cirrhosis | 1.85 (1.57–2.18) |
| ASA use | 0.98 (0.89–1.10) |
| NSAID use | 1.95 (1.74–2.18) |
Note: ASA = acetylsalicylic acid, CI = confidence interval, HR = hazard ratio, NSAID = nonsteroidal anti-inflammatory drug.
Each variable was adjusted for every other variable listed.
Interpretation
We performed a nationwide population-based longitudinal seven-year cohort study. We found that patients undergoing hemodialysis and those with chronic kidney disease had a high risk of ulcer bleeding. Diabetes, coronary artery disease, cirrhosis and use of NSAIDs are important risk factors for ulcer bleeding in patients with end-stage renal disease undergoing hemodialysis.
In a cross-sectional study at a single medical center, Sugimoto and colleagues found that patients undergoing dialysis had higher rates of gastroduodenal ulcers than healthy people with normal renal function (17.8% v. 7.4%).6 In a longitudinal retrospective study, Chen and colleagues found that 18.5% of patients undergoing dialysis (hemodialysis and peritoneal) experienced a symptomatic peptic ulcer during a three-year follow-up period.14 However, they did not include a control group for comparison. We focused on patients undergoing hemodialysis rather than peritoneal dialysis, and we found that patients undergoing hemodialysis had a fivefold higher risk of ulcer bleeding after adjustment for possible confounding variables.
The risk factors and pathogenesis of ulcers and ulcer bleeding in patients with chronic kidney disease and end-stage renal disease include platelet dysfunction, platelet–vessel wall interaction and abnormalities in blood coagulation.11,17 Our results suggest that chronic kidney disease is also an independent risk factor for ulcer bleeding. However, patients undergoing hemodialysis had a higher risk of ulcer bleeding than patients with chronic kidney disease. A possible explanation for this finding is the increased risk of bleeding after the use of anti-coagulants (e.g., heparin) during hemodialysis in patients with existing ulcers or erosions. It is also possible that these patients had impaired healing of ulcers because of intermittent hemodynamic instability in the gastrointestinal tract during hemodialysis.
We found that diabetes, coronary artery disease, cirrhosis and use of NSAIDs were risk factors for ulcer bleeding in patients undergoing hemodialysis. In the general population, a history of ulcer or ulcer bleeding, use of NSAIDs and ASA, and Helicobacter pylori infection are important risk factors for ulcer bleeding.18,19 In a study involving 30 648 patients with end-stage renal disease undergoing hemodialysis, Wasse and colleagues reported that cardiovascular disease, smoking and an inability to walk independently were risk factors for bleeding in the upper gastrointestinal tract.11 Chen and colleagues found that diabetes, congestive heart failure, low albumin levels and receiving peritoneal dialysis were risk factors for peptic ulcers in patients receiving dialysis.14 The dataset used in our study did not include data for smoking and ambulatory status. It also did not include patients undergoing peritoneal dialysis.
The finding that the use of NSAIDs but not ASA is a risk factor is consistent with the findings of Ethier and colleagues, who reported that ASA was not associated with gastrointestinal bleeding in hemodialysis patients,20 and of Jankovic and colleagues, who found that NSAIDs increased the risk of gastrointestinal bleeding in this group.21 Taken together, these results suggest that hemodialysis patients who use NSAIDs and have diabetes, cardiovascular disease or cirrhosis have a higher risk of ulcer bleeding than patients without these factors. In such patients, the use of gastroprotective agents, such as proton-pump inhibitors, should be considered to protect the gastroduodenal mucosa.
Limitations
The findings of this study need to be interpreted in the context of certain limitations. First, the observations were retrospective and based on patients admitted to hospital because of peptic ulcer bleeding. Certain selection biases may exist, and caution must be used when extrapolating the results.
Second, we were unable to assess H. pylori infection, an important risk factor for ulcers and ulcer bleeding,18,22 because the dataset used did not include this information. Nonetheless, previous studies have shown that the prevalence of H. pylori infection in patients with uremia is not higher than in the general population6,23 and that H. pylori infection is not a risk factor for ulcer or recurrent ulcer in uremic patients.14,24
Third, although smoking and alcohol consumption are risk factors for peptic ulcers, information about these factors was not available in the National Health Insurance dataset.
Lastly, we did not evaluate protective factors for peptic ulcer disease, such as the use of gastroprotective agents (proton-pump inhibitors, misoprostol and histamine-2 receptor antagonists). In Taiwan’s National Health Insurance program, the use of proton-pump inhibitors or histamine-2 receptor antagonists is limited to patients with endoscopic reflux esophagitis or peptic ulcer disease.17 Gastroprotective agents are not covered by National Health Insurance for prophylaxis against ulcers or ulcer bleeding, and prescriptions for such prophylactics are not found in the National Health Insurance Research Database.
Conclusion
We found that patients with end-stage renal disease undergoing hemodialysis had a high risk of ulcer bleeding. Diabetes, coronary artery disease, cirrhosis and the use of NSAIDs were important risk factors for ulcer bleeding in this population.
Acknowledgements
The authors thank Miss Pui-Ching Lee (Department of Medicine, Taipei Veterans General Hospital) for statistical consultation.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jiing-Chyuan Luo conceived the study. He, along with Hsin-Bang Leu and Kuang-Wei Huang, designed the study, performed the analysis and interpretation of data, and drafted and revised the manuscript. Chin-Chou Huang, Ming-Chih Hou, Han-Chieh Lin, Fa-Yauh Lee and Shou-Dong Lee contributed to the analysis and interpretation of the data and revising the manuscript. All authors approved the final version of the manuscript submitted for publication.
Funding: This work was supported by grants from the Taipei Veteran General Hospital (grant nos. V98C1-051, V99C1-15 and V100C-026).
References
- 1.US Renal Data System USRDS 2007 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda (MD): National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases; 2007 [Google Scholar]
- 2.Department of Health National health insurance annual statistical report. Taiwan: Department of Health; 2004 [Google Scholar]
- 3.Margolis DM, Saylor JL, Geisse G, et al. Upper gastro-intestinal disease in chronic renal failure. Arch Intern Med 1978;138:1214–7 [PubMed] [Google Scholar]
- 4.Musola R, Franzin G, Mora R, et al. Prevalence of gastro-duodenal lesions in uremic patients undergoing dialysis and after renal transplantation. Gastrointest Endosc 1984;30:343–6 [DOI] [PubMed] [Google Scholar]
- 5.Kang JY, Wu AY, Sutherland IH, et al. Prevalence of peptic ulcer in patients undergoing maintenance hemodialysis. Dig Dis Sci 1988;33:774–8 [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto M, Sakai K, Kita M, et al. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int 2009;75:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khedmat H, Ahmadzad-Asl M, Amini M, et al. Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc 2007;39:1003–7 [DOI] [PubMed] [Google Scholar]
- 8.Cheung J, Yu A, LaBossiere J, et al. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc 2010;71:44–9 [DOI] [PubMed] [Google Scholar]
- 9.Holden RM, Harman GJ, Wang M, et al. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol 2008;3:105–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankovic SM, Aleksic J, Rakovic S, et al. Non-steroidal anti-inflammatory drugs and risk of gastro-intestinal bleeding among patients on hemodialysis. J Nephrol 2009;22:502–7 [PubMed] [Google Scholar]
- 11.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 2003;64:1455–61 [DOI] [PubMed] [Google Scholar]
- 12.Bureau of National Health Insurance 2001 National Health Insurance annual statistical report. Taipei (Taiwan): The Bureau; 2002 [Google Scholar]
- 13.World Health Organizing Collaborating Center for Drug Statistics Methodology ATC index with DDDs 2003. Oslo (Norway): The Center; 2003 [Google Scholar]
- 14.Chen YT, Yang WC, Lin CC, et al. Comparison of peptic ulcer disease risk between peritoneal and hemodialysis patients. Am J Nephrol 2010;32:212–8 [DOI] [PubMed] [Google Scholar]
- 15.Fiaccadori E, Maggiore U, Clima B, et al. Incidence, risk factors, and prognosis of gastro-intestinal hemorrhage complicating acute renal failure. Kidney Int 2001;59:1510–9 [DOI] [PubMed] [Google Scholar]
- 16.Klebl FH, Scholmerich J. Therapy insight: prophylaxis of stress-induced gastro-intestinal bleeding in critically ill patients. Nat Clin Pract Gastroenterol Hepatol 2007;4:562–70 [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Wu CH, Wu MS, et al. A nationwide population-based cohort study shows reduced hospitalization for peptic ulcer disease associated with H. pylori eradication and proton pump inhibitor use. Clin Gastroenterol Hepatol 2009;7:427–31 [DOI] [PubMed] [Google Scholar]
- 18.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in pepticulcer disease: a meta-analysis. Lancet 2002;359:14–22 [DOI] [PubMed] [Google Scholar]
- 19.Hsiang KW, Chen TS, Lin HY, et al. Incidence and possible risk factors for clinical upper GI events in patients administrating selective cyclooxygenase-2 inhibitors: a prospective, observational, cohort study in Taiwan. Clin Ther 2010;32:1294–303 [DOI] [PubMed] [Google Scholar]
- 20.Ethier J, Bragg-Gresham JL, Piera L, et al. Aspirin prescription and outcomes in hemodialysis patients: the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2007;50: 602–11 [DOI] [PubMed] [Google Scholar]
- 21.Jankovic SM, Aleksic J, Rakovic S, et al. Non-steroidal anti-inflammatory drugs and risk of gastro-intestinal bleeding among patients on hemodialysis. J Nephrol 2009;22:502–7 [PubMed] [Google Scholar]
- 22.Luo JC. Gastroprotective strategy in aspirin users. J Chin Med Assoc 2009;72:343–5 [DOI] [PubMed] [Google Scholar]
- 23.Shousha S, Arnaout AH, Abbas SH, et al. Antral Helicobacter pylori in patients with chronic renal failure. J Clin Pathol 1990; 43:397–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JY, Ho KY, Yeoh KG, et al. Peptic ulcer and gastritis in uremia, with particular reference to the effect of Helicobacter pylori infection. J Gastroenterol Hepatol 1999;14:771–8 [DOI] [PubMed] [Google Scholar]