Abstract
The use of selective inhibitors targeting Bcr-Abl kinase is now established as a standard protocol in the treatment of chronic myelogenous leukemia; however, the acquisition of drug resistance is a major obstacle limiting the treatment efficacy. To elucidate the molecular mechanism of drug resistance, we established K562 cell line models resistant to nilotinib and imatinib. Microarray-based transcriptome profiling of resistant cells revealed that nilotinib- and imatinib-resistant cells showed the upregulation of kinase-encoding genes (AURKC, FYN, SYK, BTK and YES1). Among them, the upregulation of AURKC and FYN was observed both in nilotinib- and imatinib-resistant cells irrespective of exposure doses, while SYK, BTK and YES1 showed dose-dependent upregulation of expression. Upregulation of EGF and JAG1 oncogenes as well as genes encoding ATP-dependent drug efflux pump proteins such as ABCB1 was also observed in the resistant cells, which may confer alternative survival benefits. Functional gene set analysis revealed that molecular categories of ‘ATPase activity', ‘cell adhesion' or ‘tyrosine kinase activity' were commonly activated in the resistant clones. Taken together, the transcriptome analysis of tyrosine kinase inhibitors (TKI)-resistant clones provides the insights into the mechanism of drug resistance, which can facilitate the development of an effective screening method as well as therapeutic intervention to deal with TKI resistance.
Keywords: tyrosine kinase inhibitor, chronic myelogenous leukemia, drug resistance, microarray, pathway analysis
Introduction
Chronic myelogenous leukemia (CML) comprises ∼15% of adult-onset leukemia cases.1 The most obvious karyotypic change of the disease is a reciprocal translocation t(9;22)(q34;q11) that gives rise to the Philadelphia chromosome (Ph) and the chimeric BCR-ABL gene. This fusion gene encodes Bcr-Abl oncoprotein with constitutively activated tyrosine kinase activity, which is responsible for uncontrolled cellular proliferation and development of CML and Ph+ ALL.2 As the first commercially available inhibitor of Bcr-Abl tyrosine kinase, imatinib mesylate (Gleevec, STI571) has been used as a frontline therapeutic choice for newly diagnosed CML cases.3 The remarkable rate of cytological remission has been shown in initial clinical surveys and recent follow-up studies.4, 5
One major concern in the first-line imatinib treatment is the drug resistance, the patients often fail to acquire complete cytogenetic response at initial treatment (intrinsic resistance) or fail to maintain the responses during treatment (acquired resistance). Previous studies showed that somatic point mutations involving the kinase domain of Bcr-Abl protein seem to be the primary cause of resistance in clinical cases.6 Genomic amplification and transcriptional activation of the BCR-ABL loci have been also suspected as possible cause of the resistance.7 Other putative mechanisms independent of Bcr-Abl kinase pathway have been also reported, for example, the activation of Src family kinases such as Lyn or Hck,8 transporters involved in drug efflux9 and the antiapoptotic roles conferred by extracellular matrix.10
Increasing the dose of imatinib is one alternative to deal with resistant patients, but it is still controversial whether the resistance can be overcome with the dose escalation.11, 12 More potent second-line tyrosine kinase inhibitors (TKI) such as nilotinib (Tasigna, AMN107) and dasatinib (Sprycel) offer a treatment option for CML patients showing failure or suboptimal response to first-line imatinib treatment.13, 14, 15 However, the patients treated with the second-line TKI also often experience intolerance16 or resistance, which may require the modulation of drug regiments.17, 18
The elucidation of the molecular mechanism of TKI resistance has broad clinical implications such as the early identification of resistant cases, personalized modulation of drug regimens and facilitating the screening of new targets for therapeutic intervention. In this study, we established TKI-resistant in vitro cell line models by exposing K562 cell lines to nilotinib (doses of 50 and 250 n) and imatinib (a dose of 800 n). The expression profiles of TKI-resistant sublines and susceptible K562 parental cell lines were obtained using high-throughput oligonucleotide microarray. We identified gene candidates whose activation may provide survival benefits when endogenous Bcr-Abl oncoprotein becomes inactivated by TKI, and thereby lead to the acquisition of resistance phenotype. Pathway analysis also identified a number of molecular functions activated in the resistant clones, which may provide additional clues about the molecular changes in resistant clones. The transcriptome analysis of TKI-resistant cell lines and their functional analysis in this study can advance the understanding of the mechanisms behind TKI-resistance and facilitate the development of effective diagnostic and therapeutic strategies.
Materials and methods
Cell lines resistant to TKI
Among the Bcr-Abl-positive cell lines, we selected erythroid leukemic K562 cell lines that do not show Bcr-Abl overexpression accompanying the acquisition of imatinib resistance.19 To construct TKI-resistant K562 sublines, the K562 cell lines were exposed to three conditions, 50 and 250 n of nilotinib and 800 n of imatinib. The culture conditions and related experimental protocols are described elsewhere.20 To rule out the mutation-based resistance acquisition, the BCR-ABL loci of three resistant K562 sublines were screened by nucleotide sequencing, and the absence of major clinically relevant point mutations including T315I was confirmed for all three sublines.6 The expression level of BCR-ABL kinase was also checked using real-time reverse transcriptase PCRs to rule out the resistance by transcriptional upregulation as described previously.20
Microarray analysis
RNA was extracted from parental and TKI-resistant K562 cells using Trizol (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. We used Bioanalyzer 2100 (Agilent Technologies, Waldbronn, Germany) to assess the quantity and quality of extracted RNA. For high-throughput expression profiling, we used Applied Biosystems Human Genome Survey Microarray Version 2.0 (Applied Biosystems, Foster, CA, USA) representing 28 000 human genes. For hybridization, digoxigenin-UTP-labeled cRNA was generated and linearly amplified from 5 μg of total RNA using Applied Biosystems Chemiluminescent RT-IVT Labeling Kit V2.0. Array hybridization, chemiluminescence detection and image acquisition were performed using Applied Biosystems Chemiluminescence Detection Kit and Applied Biosystems 1700 Chemiluminescent Microarray Analyzer according to manufacturer's protocol. The hybridization was performed three times per sample. Images of each array hybridization were collected using the AB 1700 Analyzer equipped with high resolution, large-format CCD camera, including two short chemiluminescence images with 5 s exposure length for gene expression analysis. Two fluorescent images were obtained for feature finding and processed for spot normalization. Images were auto-gridded and the chemiluminescent signals were quantified. After the background subtraction, spot intensity data were quantile normalized.
Analysis of expression profiles
Using the signal values corrected for background intensities, the probes were filtered out and 15 000 genes were used for the subsequent analysis. For clustering, we performed one-way analysis of variance (ANOVA) and selected the genes under the P-value <0.10. According to the expression similarities, 12 K-means gene clusters were grouped using the software of Cluster and Treeview.21 For pathway analysis, we collected functional gene sets from three public databases, GO, KEGG and GenMAPP.22, 23, 24 Too large (>200 genes) or small gene sets (<10 genes) were excluded, and a total of 642 functional gene sets were used for subsequent enrichment analysis. The significance of enrichment was calculated using parametric gene set enrichment analysis (PAGE) based on z-statistics.25 To apply PAGE, the signal-to-noise ratio was calculated for all 15 000 genes in the comparison of TKI-resistant versus TKI-susceptible K562 cell lines. For regulatory motif gene sets representing the potential gene targets of known transcription factors, we downloaded 615 gene sets from MSigDB Ver2.5 (c3 symbol set).26 The drug perturbation-related gene sets were obtained from Connectivity Map as described previously.27 In brief, expression log2 ratio was calculated for individual genes, and ordered gene rank list was constructed for each of 281 batches (perturbagen versus vehicle pairs). In the rank list, top-ranking 100 genes (upregulated) and 100 genes at the bottom (downregulated) were selected comprising 562 drug perturbation-related gene sets.
Quantitative RT-PCR
Total RNA extracted from the three TKI-resistant K562 sublines and parental cell lines were used for RT-quantitative PCR (qPCR) analysis for five kinase genes (AURKC, FYN, SYK, BTK and YES1). GAPDH was used as internal control. The first-strand cDNA was synthesized using oligo-dT primer and superscript II reverse transcriptase (Invitrogen), and used for subsequent amplification reaction. RT-qPCR analysis was performed using M × 3000P system, and analysis was done using the software of M × Pro 3.00 (Stratagene, La Jolla, CA, USA). The reaction mixture of 20 μl contains 10 ng of cDNA, 1 × SYBR Green Tbr polymerase mixture (Finnzymes, Vantaa, Finland), 0.5 × ROX and 20 pmol primer pairs. The thermal cycling was as follows: 10 min at 95 °C, followed by 40 cycles of 10 s at 94 °C, 30 s at 55–60 °C and 30 s at 72 °C. To verify the specific amplification, melting curve analysis was performed (55–95 °C, 0.5 °C/s). Relative quantification was performed by the ΔΔCT method. The sequence information of primers used for RT-qPCR is available in Table 1.
Table 1. Primers for RT-qPCR.
| Gene | Amplicon size (bp) | Sequence | |
|---|---|---|---|
| AURKC | 180 | Sense | 5′-AATGTGTACCTGGCTCGGCTCAAG-3′ |
| Anti-sense | 5′-CCGGCGTGCATCATGGAAATAGTT-3′ | ||
| BTK | 146 | Sense | 5′-AAAGCAGTTCCTTCACCGAGACCT-3′ |
| Anti-sense | 5′-ACCGGACTGGAAATTTGGAGCCTA-3′ | ||
| FYN | 128 | Sense | 5′-AGCAAGACAAGGTGCAAAGTTCCC-3′ |
| Anti-sense | 5′-TTCCTTTGGTGACCAGCTCTGTGA-3′ | ||
| SYK | 147 | Sense | 5′-ATGGAAAGTTCCTGATCCGAGCCA-3′ |
| Anti-sense | 5′-AGAGCGTGTCGAACTTCTTTCCCT-3′ | ||
| YES1 | 157 | Sense | 5′-AAGCTGCACTGTATGGTCGGTTTA-3′ |
| Anti-sense | 5′-GGGCACGGCATCCTGTATCCTC-3′ | ||
| GAPDH | 301 | Sense | 5′-GCGGGGCTCTCCAGAACATCA-3′ |
| Anti-sense | 5′-CCAGCCCCAGCGTCAAAGGTG-3′ | ||
Abbreviation: RT-qPCR, real-time quantitative PCR.
Results
Identification of genes associated with TKI resistance
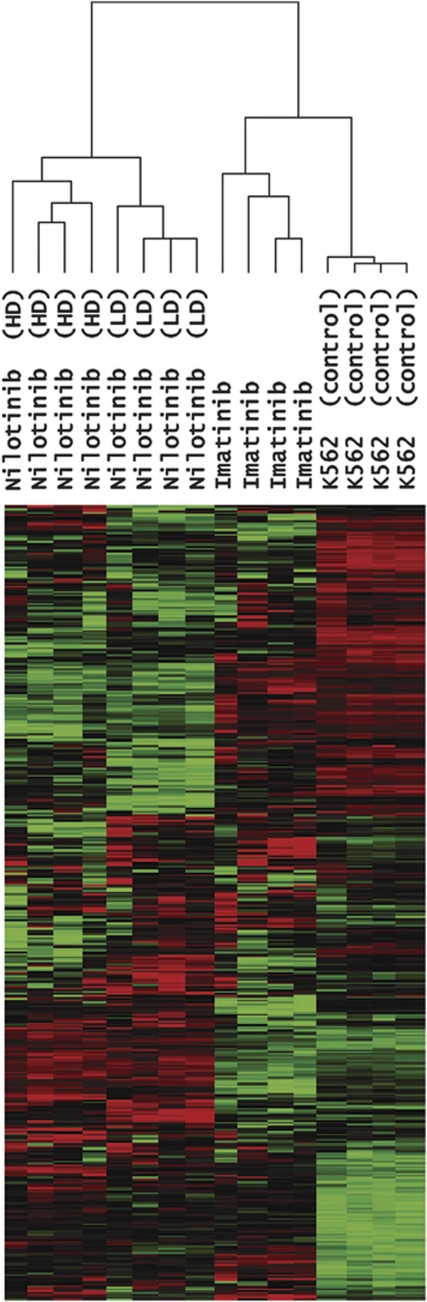
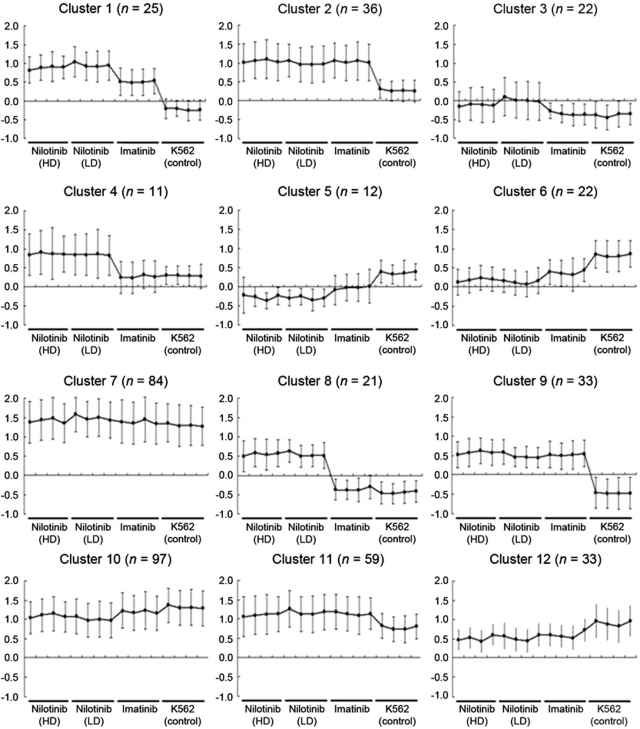
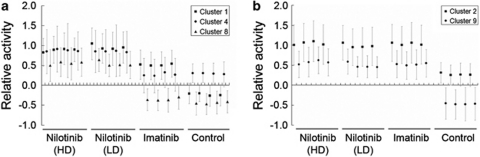
To identify the genes associated with TKI resistance, we compared the gene expression profiles of TKI-resistant K562 sublines versus TKI-susceptible parental K562 control and selected 455 differently expressed genes showing P<0.10 (one-way ANOVA). Unsupervised hierarchical clustering of the 455 genes is illustrated in Figure 1. To categorize the 455 genes according to the expression changes, we performed K-means clustering (n=12). The expression patterns and detailed information of 12 gene clusters are available in Figure 2 and Supplementary Table 1, respectively. Among the clusters, we selected five gene clusters showing relative upregulation in TKI-resistant sublines; three clusters with nilotinib-specific upregulation (Cluster 1, 4 and 8; Figure 3a) and the other two clusters showing the upregulation both in nilotinib- and imatinib-resistant sublines (Cluster 2 and 9; Figure 3b). The genes belonging to these five clusters are listed in Table 2.
Figure 1.
Unsupervised hierarchical clustering of the 455 genes, which showed differential expression between TKI-resistant K562 sublines and TKI-susceptible parental K562.
Figure 2.
Gene expression patterns of 12 gene clusters categorized from the 455 differentially expressed genes.
Figure 3.
Gene clusters with transcriptional upregulation in TKI-resistant K562 sublines. (a) Three gene clusters show the upregulation in two nilotinib-resistant sublines as compared with imatinb-resistant or parental K562 sublines. (b) The transcriptional upregulation in both nilotinib- and Imatinib-treated sublines is observed in two gene clusters (Cluster 2 and 9).
Table 2. Five gene clusters showing transcriptional upregulation in TKI-resistant K562 sublines.
| Upregulation | Cluster | Gene symbols |
|---|---|---|
| Nilotinib | 1 | TMEM51, PLA2G4A, KIF1A, C2orf14, OSBPL6, LOC152078, ENTPD3, ENC1, MAP7, LAMB1, CD36, ABCB1, C9orf125, CASP4, HMGA2, NTS, TRHDE, LOC144766, TEX9, ZNF447, AURKC, FAM9B, NXF5, NXF2, SSX3 |
| 4 | IL1R1, COLEC11, ZNF354A, C6orf85, SPTAN1, KCNMB4, ARHGDIB, MGC16044, FAM9A, VCY, VCY1B | |
| 8 | ARHGAP15, COBLL1, ANTXR1, ITGA4, FMNL2, TNFSF10, FLJ31033, APIN, ZDHHC11, SLCO4C1, CD109, ZNF462, CHST3, MRPS16, MS4A4A, PAK1, DACH1, DCT, STRA6, BC036928, ASB9 | |
| Nilotinib and Imatinib | 2 | SERINC2, KYNU, GAD1, ANKRD20B, CTDSPL, FLJ20647, SNCA, PPP3CA, ELOVL7, PANK3, SLC12A7, TAP1, HEY1, RGS20, ZFHX4, LOC441395, C10orf38, AKR1CL2, C10orf71, CD44, IFITM3, PTPRR, SPRY2, MGAT2, PRKCH, C14orf39, COTL1, PMP22, ZNF420, ZNF85, ZNF567, LILRB1, ZNF626, SYTL4, SSX6, TCEAL4 |
| 9 | AIM2, PTPRC, FAM5C, TNFRSF9, NR5A2, CD1D, CDC42EP3, MEIS1, IFIH1, GCA, PTX3, BOMB, EGF, DAB2, LOC493869, LAMA4, TPBG, SYK, TLE4, SFXN3, MCAM, ADM, MLSTD1, LRMP, NIN, CHES1, ABCA8, ZNF527, HKR1, JAG1, CPXM, SLC35E4, COVA1 |
Abbreviation: TKI, tyrosine kinase inhibitors.
The five gene clusters are selected among 12 K-means clusters and illustrated with genes. Three clusters (Cluster 1, 4 and 8; upper) contain genes upregulated in nilotinib-resistant cell lines and two other clusters (Cluster 2 and 9; below) contain genes showing upregulation in both nilotinib- and imatinib-resistant cell lines.
We observed a number of kinase-encoding genes that are upregulated in TKI-resistant cell lines, which include AURKC (Cluster 1), PRKCH (Cluster 2), PAK1 (Cluster 8) and SYK (Cluster 9). It is notable that the upregulation of different types of kinases may confer alternative survival benefits in place of the Bcr-Abl oncoprotein, which is suppressed by TKI. The molecules whose altered cellular activities may lead to increased cell survival are also observed. For example, we observed that a number of cancer-related genes such as EGF, JAG1 and CDC32EP3 are upregulated in the resistant clones, which can provide additional survival advantages. The upregulation of apoptosis-related genes such as CASP4 (in Cluster 1), TNFSF10 (in Cluster 8) and TNFRSF9 (in Cluster 9) suggests that apoptosis is one of prevailing cellular events in TKI-treated cell lines, which may increase higher cellular turnover rates and also facilitate the selection of clones with survival benefits. Some of the upregulated transcripts in TKI-resistant cell lines are known to involve in cell adhesion (ITGA4 and MCAM) and cytoskeleton (LAMB1, KIF1A, MAP7 and LAMA4). Of interests, ABCB1 gene (in Cluster 1) encodes ATP-dependent drug efflux pump. The overexpression of this transporter molecule may inhibit the intracellular accumulation of drug, which is one of the potential mechanism to obtain drug resistance.9, 28
Pathway analysis of expression profiles associated with TKI resistance
For pathway analysis, we used PAGE method that measures the enrichment of differentially expressed genes to predetermined functional gene sets.25 Table 3 lists the annotated molecular functions of significantly enriched (P<0.001) functional gene sets and their leading edge gene subsets. The top upregulated molecular function is ‘ATPase activity' that includes a number of ATP-binding cassette transporters such as ABCB1 and TAP1. This indicates that TKI-resistant sublines have globally activated transporter activities; their coordinated upregulation suggests that ‘ATPase activity' might have a key role in the acquisition of the resistance to TKI. The upregulation of genes belonging to solute carrier (SLC) gene family is responsible for the enrichment of ‘amino acid transport' category. The roles of the ‘amino acid transport' genes in TKI resistance have not been appreciated except for some examples of organic cation transporter.29 However, these findings suggest that the acquisition of TKI resistance accompanies the transcriptional activation of diverse transmembrane transporter molecules, altering the drug efflux/influx and cellular susceptibility in a given dose of drugs.
Table 3. Molecular functionalities associated with TKI-resistant expression profiles.
| Typea | Functional annotationb | Gene size | P-valuec | Genesd |
|---|---|---|---|---|
| Upregulated | GO/ATPase activity, coupled to transmembrane movement of substances | 22 | 1.7E-06 | ABCA8, ABCB1, TAP1, ABCC1, ABCB9, ABCD4 |
| GO/amino-acid transport | 28 | 1.2E-05 | SLC12A7, SLC38A6, XK, SLC38A1, SLC43A1, MGC15523, SLC7A8 | |
| GO/immune response | 163 | 0.0001 | IFITM3, TAP1, IFIH1, TNFRSF9, AIM2, PSMB9, IL18RAP, IFITM2, PSME1, IFI16, IFIT3, CD97, DAF, CNIH, CCL5, ISGF3G, IL18R1, PSME2, MR1, EBI2, TNFSF10, LIF, IKBKE, FCGR2B, ACSL1 | |
| GO/amino-acid-polyamine transporter activity | 23 | 0.0002 | SLC12A7, SLC38A6, SLC38A1, MGC15523, SLC7A8 | |
| GenMAPP/Integrin-mediated cell adhesion | 55 | 0.0005 | FYN, SEPP1, ITGB5, RAP1B, CAPN2, RAC2, CAV1, TLN1, AKT1, ITGA4, CAV2, VCL, PAK1 | |
| GO/cell adhesion | 189 | 0.0006 | CD44, TPBG, MCAM, LAMA4, LAMB1, CD36, ITGB5, CPXM, CD9, TNXB, PTPRF, CD97, SELPLG, CD47, PCDH10, CCL5, IGSF1, PCDH17, FAT, URP2, ITGA4, NELL2, FEZ1, C16orf9, VCL, PKP2, CASK | |
| GenMAPP/Smooth_muscle_contraction | 81 | 0.0007 | RGS20, PRKCH, IGFBP4, TNXB, ADM, ITPR2, PRKCZ, GSTO1, CREB3, ACTA2, GJA1, NOS3, ATF5, ATP2A3, NFKB1 | |
| GO/non-membrane spanning protein tyrosine kinase activity | 11 | 0.0009 | SYK, FYN | |
| Downregulated | GO/sterol biosynthesis | 19 | 0.0002 | FDFT1, SC4MOL, NSDHL, MVK, HMGCR, IDI1, CYP51A1, DHCR24, SC5DL |
| GenMAPP/Cholesterol_Biosynthesis | 14 | 0.0003 | FDFT1, SC4MOL, NSDHL, MVK, HMGCR, IDI1, LSS, CYP51A1, SC5DL | |
| GO/steroid biosynthesis | 30 | 0.0003 | HSD17B7, HSD17B8, FDFT1, SC4MOL, HSD17B1, NSDHL, MVK, HMGCR, IDI1, LSS, CYP51A1, DHCR24, SC5DL | |
| GO/cholesterol biosynthesis | 16 | 0.0006 | FDFT1, NSDHL, MVK, HMGCR, IDI1, CYP51A1, DHCR24 |
Abbreviation: TKI, tyrosine kinase inhibitors.
The signatures were distinguished for upregulated and downregulated gene sets in TKI-resistant sublines.
Three databases (GO, KEGG and GenMAPP) used to collect the gene sets are denoted in the respective gene sets.
The significance for enrichment is calculated using parametric gene set enrichment analysis algorithm based on z-statistics, and unadjusted P<0.10 was considered significance.
Among the genes belonging to the gene set, the ‘leading edge subset' are listed for genes whose corresponding signal-to-noise ratio is above mean+s.d. (upregulated) or below mean−s.d. (downregulated).
We also observed that ‘cell adhesion' and ‘integrin-mediated cell adhesion' categories are upregulated in TKI-resistant sublines. It has been previously shown that the extracellular signals such as fibronectin-induced integrin signaling can convey antiapoptotic signals to BCR-ABL-positive cells in in vitro settings30 as potential resistance-acquiring mechanism. The upregulated integrin molecules in our study (ITGB5 and ITGA4) may have similar functional implication. In addition, we observed the relative upregulation of various immune-related genes, as well as downregulation of genes belonging to ‘chrolesterol biosynthesis' functional categories, which constitute unique functional categories associated with TKI resistance.
Resistance-associated transcriptional upregulation of kinase molecules
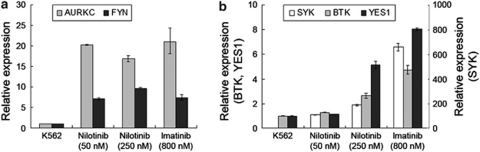
We have already observed the upregulation of various kinase-encoding genes in cluster analysis (Table 2). Pathway analysis also identified the upregulation of two non-receptor tyrosine kinases, SYK and FYN, which is responsible for the enrichment of ‘tyrosine kinase activity' category (Table 3). Thus, we focused on the transcriptional upregulation of kinase molecules as one of the remarkable expression signatures associated with TKI resistance. The activation of tyrosine kinase, especially Src family kinases including FYN, has been implicated in the acquisition of TKI resistance in a number of models8, 31 by mimicking the oncogenic effects of Bcr-Abl kinase.32 The conformational resemblance between Src family kinase and Abl kinase leads to an assumption that dual kinase inhibitors such as dasatinib can be more potential with off-target effects to Src kinase. Similarly, it was proposed that the inhibitor of Aurora kinase can inhibit T315I mutant Bcr-Abl kinase, which is refractory to conventional TKIs.33, 34 Thus, the screening of kinases associated with TKI resistance can advance our understanding to the mechanism of drug resistance and also facilitate to select the appropriate TKI inhibitors in a given clinical context. Our findings suggest that the activation of multiple kinase molecules is a common event in TKI-resistant BCR-ABL(+) cell lines and also highlights the potential utility of using multi-kinase inhibitors to modulate imatinib resistance. We further experimentally verified the expression change of the five kinases (AURKC, FYN, SYK, BTK and YES1) in TKI-resistant and TKI-susceptible K562 cell lines using RT-qPCR. Consistent upregulation of AURKC and FYN was observed in all three TKI-resistant cell lines tested, while the other three kinases, SYK, BTK and YES1, showed dose-dependent upregulation pattern (Figure 4).
Figure 4.
Expression levels of five kinases in TKI-resistant K562 sublines. (a) The expression levels of five kinases in four K562 sublines are measured using real-time quantitative PCR. The relative expression levels of AURKC and FYN for three TKI-resistant sublines are illustrated as compared with those of TKI-susceptible parental cell lines. (b) The expression levels of SYK, BTK and YES1 are demonstrated in the same manner.
The analysis of regulatory motifs and drugs associated with TKI-resistant expression profiles
Regulatory motif gene sets (for example, a set of genes whose cis-regulatory sequences are enriched for a specific regulatory motif; MSigDB C3 category) were also used in pathway analysis to infer the potential regulator involved in TKI resistance. In the comparison of TKI-resistant versus TKI-susceptible K562 sublines, the potential transcription targets of ‘V$ICSBP_Q6' was most significantly upregulated in TKI-resistant sublines (P=5.2 × 10−7) (Table 4). The related regulatory motif gene sets, ‘V$IRF_Q6' (1.8 × 10−5) and ‘V$IRF_Q6' (1.9 × 10−4) also showed substantial enrichment as upregulated genes in TKI-resistant sublines. It is known that Bcr-Abl kinase suppresses the Irf8 transcription factors (interferon consensus sequence-binding protein or ICSBP). This suppression reactivates antiapoptotic genes such as BCL2 or BCLX, whose transcription is normally suppressed by the ISCBP-mediated transcriptional control.35 The upregulation of potential transcription targets of ISCBP or IRF may represent a treatment effect of TKI, but it is expected that TKI-resistant clones will restore some functions mediated by ICSBP, such as apoptosis. Consistent with this expectation, clustering analysis showed that a number of apoptosis-related genes (CASP4, TNFSF10 and TNFRSF9) were upregulated in resistant clones. In addition, the transcription factors previously assumed to have roles in leukemogenesis such as Evi1 were also enriched in TKI-resistance expression profiles,36 which may increase the cellular proliferation after the acquisition of TKI resistance.
Table 4. Putative transcriptional regulators and chemicals associated with expression profiles in TKI-resistant sublines.
| Gene set | Condition | Gene set annotation | Gene size | P-value |
|---|---|---|---|---|
| Regulatory motif gene set | Resistance-up | V$ICSBP_Q6 | 122 | 5.2E−07 |
| STTTCRNTTT_V$IRF_Q6 | 99 | 1.8E−05 | ||
| V$EVI1_02 | 67 | 3.0E−05 | ||
| YAATNRNNNYNATT_UNKNOWN | 38 | 6.6E−05 | ||
| TTANWNANTGGM_UNKNOWN | 24 | 7.7E−05 | ||
| V$OCT1_06 | 125 | 0.0001 | ||
| V$IRF1_01 | 119 | 0.0002 | ||
| V$TCF11_01 | 109 | 0.0003 | ||
| V$WHN_B | 128 | 0.0005 | ||
| V$CDX2_Q5 | 111 | 0.0005 | ||
| V$CART1_01 | 95 | 0.0006 | ||
| Resistance-down | V$ETF_Q6 | 61 | 2.1E−06 | |
| V$E2F_Q2 | 96 | 7.6E−05 | ||
| Connectivity map gene set | Resistance-up | Tamoxifen (1.0E−06M)_Down | 49 | 4.8E−09 |
| Rosiglitazone_Down | 41 | 4.4E−08 | ||
| Sodium phenylbutyrate (1.0E−03M, HL60, medium)_Up | 57 | 2.7E−07 | ||
| Cobalt chloride (1.0E−04M)_Up | 44 | 2.3E−06 | ||
| Rofecoxib (PC3)_Up | 32 | 4.1E−05 | ||
| Butein (PC3)_Down | 64 | 5.5E−05 | ||
| Troglitazone_Down | 41 | 5.8E−05 | ||
| Pyrvinium (1.3E−06M)_Up | 50 | 9.3E−05 | ||
| Gefitinib (HL60)_Up | 42 | 0.0001 | ||
| Blebbistatin (1.7E−05M)_Up | 39 | 0.0002 | ||
| Sodium phenylbutyrate (1.0E−03M, PC3, medium)_Up | 33 | 0.0002 | ||
| SC-58125 (HL60)_Up | 47 | 0.0003 | ||
| Rofecoxib_Up | 38 | 0.0004 | ||
| Monorden (1.0E−07M, PC3)_Up | 63 | 0.0008 | ||
| Resistance-down | Imatinib (PC3)_Up | 48 | 0.0002 | |
| Pirinixic acid (1.0E−04M, SKMEL5)_Up | 58 | 0.0003 |
Abbreviation: TKI, tyrosine kinase inhibitors.
We also used the expression profiles associated with drug perturbation of in vitro cell lines (Connectivity Map) to investigate potential chemical agents that may mimic or modulate the TKI resistance in terms of gene expression (Table 4).37 Among the drug perturbation-related gene sets, ‘imatinib (PC3)_Up' showed downregulation in TKI-resistant sublines (that is, the upregulated genes in the imatinib-treated in vitro PC3 cell line are relatively downregulated in our TKI-resistant K562 sublines). This may also represent the inhibition effects of TKI, indicating that the expression signatures of TKI-resistant clones are largely composed of those from TKI inhibition as shown in the example of upregulation of apoptosis category. The correlation analysis of the expression profiles with in silico drug-screening results should be interpreted with care. However, our preliminary analysis shows that genes that can be suppressed with widely used anticancer or antidiabetic drugs such as tamoxifen and rosiglitazone are upregulated in resistance-related expression profiles, which will require further effort to investigate the potential drug interactions or the possibility that these agents can affect the adverse effects or resistance acquisition of TKI.
We next performed the pathway analysis by comparing two different TKI-resistant cell lines (nilotinib versus imatinib). Table 5 lists the molecular functions enriched to the genes relatively upregulated in nilotinib- or imatinib-resistant cell lines, respectively. We observed that ‘prostaglandin biosynthesis' and ‘glycerolipid metabolism' are relatively upregulated in nilotinib-resistant sublines compared with imatinib-resistant clones. The overexpression of cyclooxygenase-2 and increased prostaglandin E2 production have been observed with imatinib treatment,38 which suggests the inhibitor to cyclooxygenase-2 can modulate the imatinib-resistant cases.39 Our pathway analysis indicates that the differential expression between nilotinib- and imatinib-resistant cases was primarily associated with ‘prostaglandin metabolism' category, which indicates that the disturbance to eicosanoid metabolism is less severe in nilotinib-resistant cases, or such disturbance accompanies different pathway molecules.
Table 5. Comparison of expression profiles between TKIs.
| Condition | Geneset ID | Gene size | P-value |
|---|---|---|---|
| Nilotinib versus imatinib | GenMAPP/Prostaglandin_synthesis_regulation | 13 | 0.0006 |
| KEGG/Glycerolipid metabolism | 23 | 0.0007 | |
| Imatinib versus nilotinib | GO/steroid biosynthesis | 30 | 2.4E−07 |
| GenMAPP/Cholesterol_Biosynthesis | 14 | 5.1E−07 | |
| GO/sterol biosynthesis | 19 | 1.3E−06 | |
| GO/cholesterol biosynthesis | 16 | 6.5E−05 | |
| KEGG/Biosynthesis of steroids | 11 | 0.0003 | |
| GenMAPP/Glycolysis_and_Gluconeogenesis | 26 | 0.0004 | |
| GO/lipid biosynthesis | 47 | 0.0006 | |
| GO/glycolysis | 36 | 0.0007 | |
| High versus low dose of nilotinib | GO/amino acid biosynthesis | 19 | 6.9E−20 |
| KEGG/”Glycine, serine and threonine metabolism | 11 | 9.7E−09 | |
| KEGG/Alanine and aspartate metabolism | 13 | 1.1E−07 | |
| GO/transaminase activity | 12 | 1.2E−06 | |
| GO/growth factor activity | 53 | 2.7E−05 | |
| GO/tRNA binding | 11 | 5.3E−05 | |
| GO/serine-type endopeptidase inhibitor activity | 20 | 0.0001 | |
| GO/endopeptidase inhibitor activity | 27 | 0.0001 | |
| GO/hormone activity | 30 | 0.0003 | |
| GO/cell-cell signaling | 102 | 0.0003 | |
| GO/integrin complex | 15 | 0.0004 | |
| GO/soluble fraction | 107 | 0.0004 | |
| GO/steroid metabolism | 33 | 0.0005 | |
| GO/NADH dehydrogenase (ubiquinone) activity | 38 | 0.0006 | |
| GO/NADH dehydrogenase activity | 37 | 0.0007 | |
| GO/cell motility | 65 | 0.0008 | |
| GenMAPP/Electron_Transport_Chain | 88 | 0.0009 | |
| Low versus high dose of nilotinib | GO/rRNA processing | 42 | 1.7E−10 |
| GO/translation initiation factor activity | 61 | 1.1E−08 | |
| GO/nucleolus | 54 | 1.6E−07 | |
| GenMAPP/Translation_Factors | 44 | 1.4E−06 | |
| GO/helicase activity | 109 | 4.0E−06 | |
| GO/regulation of translational initiation | 26 | 5.0E−06 | |
| GO/ATP-dependent helicase activity | 70 | 5.8E−06 | |
| GO/ribosome biogenesis | 30 | 6.8E−06 | |
| GO/translation factor activity, nucleic acid binding | 22 | 8.8E−06 | |
| GenMAPP/G1_to_S_cell_cycle_Reactome | 57 | 9.2E−06 | |
| GO/mRNA processing | 174 | 2.4E−05 | |
| GO/sterol biosynthesis | 19 | 5.3E−05 | |
| GenMAPP/mRNA_processing_Reactome | 109 | 7.3E−05 | |
| GO/translational initiation | 25 | 0.0002 | |
| GO/cholesterol biosynthesis | 16 | 0.0002 | |
| GO/RNA splicing | 58 | 0.0002 | |
| GenMAPP/Cholesterol_Biosynthesis | 14 | 0.0003 | |
| GO/nuclear pore | 41 | 0.0005 | |
| GO/nucleoside-triphosphatase activity | 93 | 0.0005 | |
| GO/DNA replication | 98 | 0.0006 | |
| GO/mRNA export from nucleus | 31 | 0.0006 | |
| GO/DNA repair | 171 | 0.0006 | |
| GO/tRNA processing | 38 | 0.0009 |
Abbreviation: TKI, tyrosine kinase inhibitors.
Discussion
The use of imatinib as the first-line chemoagent in treating newly developed CML has achieved a high-profile success achieving 80% of response rate in chronic phase CML cases. However, the development of resistance has been a major obstacle that severely limits the clinical utility of this drug.
In this study, the global transcriptome analysis of K562 in vitro cell line model that acquired drug resistance to 1st and 2nd line TKI of imatinib and nilotinib identified a number of candidate genes and potential molecular functions associated with the TKI resistance. The expression profiles of TKI-resistant cell clones are largely composed of two features; one represents the upregulation of genes that may confer survival benefits (for example, kinases and known oncogenes) while the other represents the effects of TKI inhibition (for example, apoptosis). It is notable that we observed the upregulation of various kinds of kinase-encoding genes in TKI-resistant clones (AURKC, FYN, SYK, BTK and YES1). It is expected that the activated kinase molecules can confer the alternative survival signatures when the endogenous Bcr-Abl oncoprotein becomes inactivated by TKI inhibitors. The use of alternative kinase can be an efficient way to obtain drug resistance for the clones that are already addicted to oncogenic Bcr-Abl protein. This feature has also important clinical implications in that the currently available (or those on clinical evaluation) kinase inhibitors have target selectivity and the effort is ongoing to discover synergistic inhibitor combination.40 It is already reported that inhibitors that can target different kinase molecules can have beneficial effects in controlling the imatinib-resistant cases,33, 34 and dual Src/Abl inhibitors such as dasatinib can also help overcome the developed resistance to imatinib.17 We also observed a number of genes whose activation may help the clones to obtain the resistance phenotype such as known cancer-related genes (EGF and JAG1) and transporter-encoding genes (ABCB1 and TAP1). These molecules may represent a set of potential biomarkers, which will facilitate the evaluation and screening of potential resistance cases and targets for therapeutic intervention.
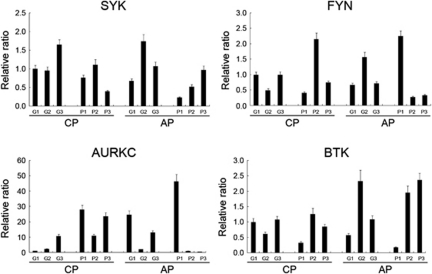
We have observed expression profiles of the four kinase genes (AURKC, FYN, SYK and BTK) in imatinib-resistant patients and good responders (Figure 5). All three chronic phase poor responders showed relative upregulation of AURKC expression compared with good responders. However, the other three kinase genes did not show prominent difference of expression between the poor and good responders. Further study is needed with a larger clinical sample size and we are in the process of conducting it.
Figure 5.
Expression profiles of the four kinase genes in imatinib-resistant patients and good responders. Twelve CML samples were collected, six poor responders (P) containing three chronic phase (CP) and three acute phase (AP); six good responders (G) containing three CPs and three APs. qRT-PCR was performed for the 12 samples as described in Materials and methods section using the same primers listed in Table 1. All three chronic phase poor responders showed relative upregulation of AURKC expression compared with good responders. However, the other three kinase genes did not show prominent difference of expression between the poor and good responders.
Acknowledgments
This study was supported by grants from the Korea Health 21 R&D Project (0405-BC02-0604-0004) and the Korea Healthcare technology R&D Project (A092258), Ministry for Health and Welfare, Republic of Korea.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–350. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- Bartram CR, de Klein A, Hagemeijer A, van Agthoven T, Geurts van Kessel A, Bootsma D, et al. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983;306:277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- Bueno-da-Silva AE, Brumatti G, Russo FO, Green DR, marante-Mendes GP. Bcr-Abl-mediated resistance to apoptosis is independent of constant tyrosine-kinase activity. Cell Death Differ. 2003;10:592–598. doi: 10.1038/sj.cdd.4401210. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Talpaz M, O'Brien S, Garcia-Manero G, Verstovsek S, Giles F, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase following imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour E, Deininger M, Hochhaus A. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2011;25:201–210. doi: 10.1038/leu.2010.215. [DOI] [PubMed] [Google Scholar]
- Cannella L, Breccia M, Stefanizzi C, Napoleone L, Santopietro M, Alimena G. Dasatinib overcomes imatinib and nilotinib failure in Philadelphia chromosome positive chronic myeloid leukemia with different mechanisms of resistance. Leuk Lymphoma. 2009;50:848–850. doi: 10.1080/10428190902829425. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24:1299–1301. doi: 10.1038/leu.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- Chung YJ, Kim TM, Kim DW, Namkoong H, Kim HK, Ha SA, et al. Gene expression signatures associated with the resistance to imatinib. Leukemia. 2006;20:1542–1550. doi: 10.1038/sj.leu.2404310. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32 (Database issue:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Kawashima S, Nakaya A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002;30:42–46. doi: 10.1093/nar/30.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TM, Yim SH, Jeong YB, Jung YC, Chung YJ. PathCluster: a framework for gene set-based hierarchical clustering. Bioinformatics. 2008;24:1957–1958. doi: 10.1093/bioinformatics/btn357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlägel U, von Bonin M, et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18:401–408. doi: 10.1038/sj.leu.2403257. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- van der KH, Goetz AW, Miething C, Duyster J, Aulitzky WE. Adhesion to fibronectin selectively protects Bcr-Abl+ cells from DNA damage-induced apoptosis. Blood. 2001;98:1532–1541. doi: 10.1182/blood.v98.5.1532. [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol. 2003;4:75–85. doi: 10.1016/s1470-2045(03)00979-3. [DOI] [PubMed] [Google Scholar]
- Warmuth M, Bergmann M, Priess A, Häuslmann K, Emmerich B, Hallek M. The Src family kinase Hck interacts with Bcr-Abl by a kinase-independent mechanism and phosphorylates the Grb2-binding site of Bcr. J Biol Chem. 1997;272:33260–33270. doi: 10.1074/jbc.272.52.33260. [DOI] [PubMed] [Google Scholar]
- Cheetham GM, Charlton PA, Golec JM, Pollard JR. Structural basis for potent inhibition of the Aurora kinases and a T315I multi-drug resistant mutant form of Abl kinase by VX-680. Cancer Lett. 2007;251:323–329. doi: 10.1016/j.canlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Ecsedy J, Medina E, Mahalingam D, Padmanabhan S, Nawrocki ST, et al. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukemia and significantly increases the efficacy of nilotinib J Cell Mol Med 2010. e-pub ahead of print 21 November 2010. [DOI] [PMC free article] [PubMed]
- Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kurokawa M, Tanaka T, Tanaka K, Hangaishi A, Mitani K, et al. Increased Evi-1 expression is frequently observed in blastic crisis of chronic myelocytic leukemia. Leukemia. 1996;10:788–794. [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Johnson FM, Yang P, Newman RA, Donato NJ. Cyclooxygenase-2 induction and prostaglandin E2 accumulation in squamous cell carcinoma as a consequence of epidermal growth factor receptor activation by imatinib mesylate. J Exp Ther Oncol. 2004;4:317–325. [PubMed] [Google Scholar]
- Arunasree KM, Roy KR, Anilkumar K, Aparna A, Reddy GV, Reddanna P. Imatinib-resistant K562 cells are more sensitive to celecoxib, a selective COX-2 inhibitor: role of COX-2 and MDR-1. Leuk Res. 2008;32:855–864. doi: 10.1016/j.leukres.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.