Abstract
Comorbidities have been demonstrated to affect progression-free survival (PFS) and overall survival (OS), although their impact in multiple myeloma (MM) patients is as yet unsettled. We (1) assessed various comorbidities, (2) compared established comorbidity indices (CIs; Charlson comorbidity index (CCI), hematopoietic cell transplantation-specific comorbidity index (HCT-CI)), Kaplan Feinstein (KF) and Satariano index (SI) and (3) developed a MM-CI (Freiburger comorbidity index, FCI) in 127 MM patients. Univariate analysis determined moderate or severe pulmonary disease (hazard ratio (HR): 3.5, P<0.0001), renal impairment (via estimated glomerular filtration rate (eGFR); HR: 3.4, P=0.0018), decreased Karnofsky Performance Status (KPS, HR: 2.7, P=0.0004) and age (HR: 2, P=0.0114) as most important variables for diminished OS. Through multivariate analysis, the eGFR ⩽30 ml/min/1.73m2, impaired lung function and KPS ⩽70% were significant for decreased OS, with HRs of 2.9, 2.8 and 2.2, respectively. Combination of these risk factors within the FCI identified significantly different median OS rates of 118, 53 and 25 months with 0, 1 and 2 or 3 risk factors, respectively, (P<0.005). In light of our study, comorbidities are critical prognostic determinants for diminished PFS and OS. Moreover, comorbidity scores are important treatment decision tools and will be valuable to implement into future analyses and clinical trials in MM.
Keywords: multiple myeloma, comorbidities, comorbidity scores, prognosis
Introduction
Despite today's novel therapeutic options,1, 2 multiple myeloma (MM) remains an incurable disease in the majority of patients with highly variable outcome, depending on various risk factors.2, 3 The classification of MM is based on Salmon and Durie (S&D) and International Staging System, including primarily disease-related risk. Nevertheless, patient-related factors, like comorbidities and abnormal organ function, describing additional hazards on outcome, are not as yet integrated in prognostic models. Risk models are of importance, however, as myeloma patients are typically in their sixth to seventh decade of life and often fragile. As numerous treatment options with differing intensity have also become available,4 this adds to the current complexity to choose the best therapeutic option for defined patients. Prior studies have shown that comorbidities have substantial impact on overall survival (OS), such as in patients with myelodysplastic syndromes,5, 6, 7 acute myeloid leukemia8, 9 or for allogeneic stem cell transplantation (SCT).10 As not all comorbidities may affect the outcome, risk factors within these scores are often weighted according to their severity;11 nevertheless, whether these hazards are equally important in different diseases and patient groups are unsettled.
Renal impairment as one essential comorbidity occurs in 20–40% of myeloma patients, depending on the definition of renal function.12, 13 As compared with the estimated glomerular filtration rate (eGFR), serum creatinine is influenced by multiple factors, exposes limits to detect mild and moderate renal impairment14, 15 and differs among individuals. For these reasons, the relationship between creatinine and GFR varies substantially and creatinine values exceed those of the GFR.15 Therefore, the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative and International Myeloma Working Group recommend estimating the GFR via ‘Modification of Diet in Renal Disease' equation.16, 17
We and others have previously confirmed the prognostic importance of renal impairment for diminished progression-free survival (PFS) and OS in cancer patients in general and in MM patients in particular.13, 14, 18, 19 Moreover, we have shown that advanced stage, elevated beta-2 microglobulin, deteriorated Karnofsky Performance Status (KPS), and comorbidities represent univariate risk factors in MM.18, 20, 21 As cancer patients with comorbidities are often excluded from clinical trials, and there is little evidence how to translate results from cooperative studies to older or frail patients, it is increasingly recognized that well-performed cohort analyses are important, reflecting a more typical rather than highly selected patient group. To further understand the role and impact of comorbidities and primary disease on patient outcome,18, 22 we here (1) determined the value of single risk factors, (2) compared established comorbidity indices (CIs) and (3) developed a simply assessable MM–comorbidity score, these assessments recently being attributed as highly important for myeloma patients.23
Materials and methods
Patient description and study design
After approval by the departmental review board, individual consecutive MM patients treated at our institution between January 1997 and July 2003 were retrieved from our electronic database system for tumor documentation.24 Patient data included age, KPS, hypertension, diabetes, secondary malignancies, pain, liver, heart and lung disease, renal impairment, and other relevant concomitant conditions. Definition of various comorbidities was performed as described (Supplementary Methods). The analysis was carried out according to the guidelines of the Declaration of Helsinki Principles and Good Clinical Practice. All patients gave their written informed consent for institutional-initiated research studies and analyses of clinical outcome studies conforming to our institutional review board guidelines.
Treatment schedule
Patients were treated with standard chemotherapy or autologous SCT (ASCT) according to our institutional MM pathway.1 Patients not eligible for autologous SCT received MP-thalidomide (melphalan 0.25 mg/kg, days 1–4, prednisone 2 mg/kg, days 1–4, thalidomide 100 mg/day), MP alone or high-dose dexamethasone.1 Autologous SCT was recommended for medically fit, symptomatic patients up to the age of 70 years. Induction consisted of four ID cycles (idarubicin 8 mg/m2, dexamethasone 20 mg/m2 days 1–4, 9–12, 17–19) within the German Study group (DSMM V) trial. Mobilization (epirubicin 100 mg/m2 day 1, etoposide 150 mg/m2 days 1–3, ifosfamide 2500 mg/m2 days 1–3) and conditioning (melphalan 200 mg/m2 or 140 mg/m2 with creatinine values >2.0 mg/dl) was performed as described.1, 2
Statistical analysis
Data analyses were performed using the SAS statistical software version 9.1. (SAS Institute Inc., Cary, NC, USA). Comparisons of binary variables were conducted by means of continuity adjusted χ2-tests; for continuous variables, Wilcoxon's two-sample tests were used. A P-value of <0.05 was considered as statistically significant. Overall survival was defined as the time from diagnosis to death from any cause, and PFS as the time from diagnosis to death from any cause or cancer recurrence. Data for patients alive (alive without recurrence, respectively) at the time of the analysis were censored at the last follow-up. Probabilities of PFS and OS were calculated using Kaplan–Meier estimator for each variable. Univariate Cox proportional hazards regression models were performed to evaluate the prognostic significance of each comorbidity factor and results are presented as estimated hazard ratios (HRs) with 95% confidence intervals. To include sufficient patients, lung disease, KPS, cardiac disease and eGFR were summarized from initially three or four patients into two groups. Prognostic factors showing a univariate P<0.1 were entered in a multivariate Cox model. Moreover, a non-weighted prognostic model (sum score) was constructed, whereby HR and Kaplan–Meier curves with 0 to 3 risk factors were assessed.
We also compared the Charlson comorbidity index (CCI),25 hematopoietic cell transplantation-specific CI (HCT-CI),10 Kaplan Feinstein (KF)26 and Satariano index (SI).27 Their definition, development, comorbid conditions, weighted vs non-weighted status and rating differences are summarized in Supplementary Table 1. We thereby (1) compared CIs that predict OS in hematological malignancies (CCI), or as assessed for SCT recipients (HCT-CI); (2) included weighted (KF, CCI, HCT-CI) vs non-weighted scores (SI) and (3) evaluated differently scored CIs (KF assigns the highest comorbidity to an end-score, whereas CCI, HCT-CI and SI add their comorbidities to a sum score). We analyzed median comorbidity scores of each CI and determined PFS and OS differences in ‘low-risk' vs ‘high-risk' patients (scoring ⩽ vs > median CI points).
Results
Patient characteristics
In our patients, immunoglobulin G was the most common myeloma type, 17% had light-chain MM and 1% had non-secretory MM. Stages II/III disease by Salmon and Durie or International Staging System was present in 91% and 41%, respectively, and stage B disease was found in 15% of the patients. Although the creatinine levels appeared normal with 0.8 mg/dl, the median eGFR was decreased with 88 ml/min/1.73m2, corresponding to chronic kidney disease (CKD) stage 2. Of note, 51% of patients were in CKD stages 2–5 and 27% in CKD stages 3–5. Our MM patients showed a median age of 60 years (range: 27–83 years; Table 1). Median PFS and OS were 2.9 and 5.8 years, respectively.
Table 1. Patient characteristics (n=127).
| Variables | n (%) | Median (range) |
|---|---|---|
| Age (years) | 60 (27–83) | |
| Sex, M: F | 70 (55): 57 (45) | |
| Type of myeloma | ||
| IgG | 67 (52) | |
| IgA | 31 (24) | |
| IgM | 2 (2) | |
| IgD | 2 (2) | |
| Biclonal (G, A) | 2 (2) | |
| Light-chain MM | 22 (17) | |
| Non-secretory | 1 (1) | |
| Kappa/lambda | 81 (64)/45 (36) | |
| Intramedullary/extramedullary | 118 (93)/9 (7) | |
| AL/AH amyloidosis | 2 (2) | |
| Salmon and Durie stage | ||
| I | 11 (9) | |
| II/III | 116 (91) | |
| A/B | 108 (85)/19 (15) | |
| ISS stage (n=75) | ||
| I | 44 (59) | |
| II | 11 (15) | |
| III | 20 (26) | |
| KPS (%) | 90 (40–100) | |
| BMI (kg/m2) | 24 (15–36) | |
| Beta-2 microglobulin (mg/dl) | 79 (62) | 3 (1.1–23) |
| PC BM infiltration rate (%) | 72 (57) | 31 (0–90) |
| Creatinine (mg/dl) | 0.8 (0.4–7.4) | |
| eGFR (MDRD, ml/min/1.73m2) | 88 (6–182) | |
| CKD stages | ||
| 1: eGFR ⩾90 ml/min/1.73m2 | 62 (49) | |
| 2: eGFR 89–60 ml/min/1.73m2 | 31 (24) | |
| 3: eGFR 59–30 ml/min/1.73m2 | 22 (17) | |
| 4: eGFR 29–15 ml/min/1.73m2 | 6 (5) | |
| 5: eGFR <15 ml/min/1.73m2 | 6 (5) | |
| Cytogenetics (FISH) | 56 (44) | |
| Deletion 13q14 | 16 (29) | |
| Standard therapy : auto PBSCT | 65 (51): 62 (49) | |
Abbreviations: AH, amyloid heavy; AL, amyloid light; auto-PBSCT, autologous peripheral blood stem cell transplantation; BMI, body mass index; CKD, chronic kidney disease stages according to the K/DOQI guidelines defined by MDRD; eGFR, estimated glomerular filtration rate; F, female; FISH, fluorescent in situ hybridization; Ig, immunoglobulin; ISS, International Staging System; KPS, Karnofsky Performance Status; M, male; MDRD, Modification of Diet in Renal Disease; MM, multiple myeloma; PC BM infiltration rate, plasma cell bone marrow infiltration rate; estimated GFR (ml/min/1.73m2)=186 × (serum creatinine level (in milligrams per decilitre))−1.154 × (age (in years))−0.203 × (0.742, if female, 1.21, if black).
Univariate analysis
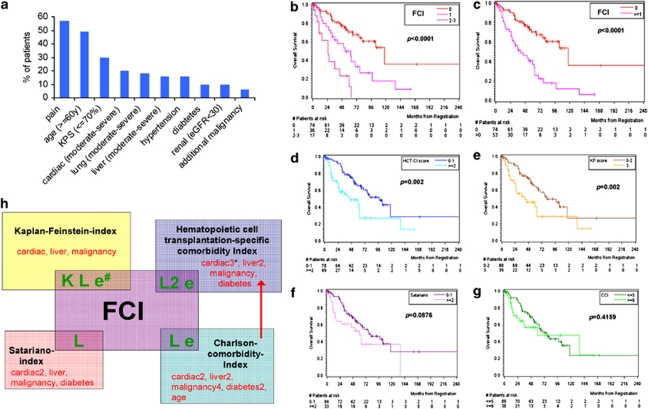
We evaluated comorbid conditions in their specific frequency, similarly as assessed in various CIs (Figure 1a). Pain (57%) and a diminished KPS (30%) showed frequent impairment. Other common comorbidities were cardiac (20%), lung (18%) and liver disease (16%), hypertension (16%), diabetes (10%) and renal impairment (10%). Additional malignancies occurred in 6% of the patients.28 All of our assessed comorbidity conditions are also captured in the KF, HCT-CI, CCI and SI, except for pain (Figure 1a, Supplementary Table 1).
Figure 1.
Analysis of comorbidities, and survival with different comorbidity scores in MM patients. (a) Distribution of specific comorbidities and patient characteristic features. Pain (57%) and a diminished KPS (30%) were most frequently impaired attributes in our MM cohort. Common organ comorbidities were cardiac (20%), lung (18%) and moderate-to-severe liver disease (16%), hypertension (16%), diabetes (10%) and renal impairment (10%). Additional malignancies occurred in 6%. Age ⩾60 years was present in 49% of the patients. All of our assessed comorbidity conditions are also captured in the KF, HCT-CI, CCI and SI, accept for pain (see also Supplementary Table 1). (b) On the basis of our univariate and multivariate results, a prognostic model was constructed, combining the KPS (⩽70%), lung impairment and eGFR (<30 ml/min/1.73 m2) in a comorbidity sum score (FCI). This allowed to define largely different patient groups: OS was significantly different among patients with no (−), 1(−), 2 or 3 (−) risk factors, with median survival times of 118 (n=74), 53 (n=36) and 25 months (n=17), respectively, (P=0.0033 and P<0.0001). (c) FCI stratification into two patient risk groups: OS was again significantly different in patients with no (−) vs 1–3 (−) risk factors, with median OS of 117 (n=74) vs 41 months (n=53, P<0.0001), respectively. (d–g) OS differences of low-risk vs high-risk patients as stratified via HCT-CI (d), KF (e), SI (f) and CCI (g). The differences among risk groups as scored via HCT and KF were significant (P<0.05), whereas via SI and CCI less distinctive. (h) The established four CIs (KF, HCT-CI, SI and CCI) are compared with the FCI. The number of weighted factors is given behind each comorbidity factor. The number of evaluated comorbidities in our univariate and multivariate analyses that led to the FCI covered 8/12, 10/17, 10/20 and 7/7 comorbidities as included in the established KF, HCT-CI, CCI and SI, respectively. The figure depicts why the FCI, KF and HCT-CI were more valuable in MM than in SI: the KF includes the appraisal of a reduced KPS (K), lung disease (L) and renal impairment (e) that were all highly valuable in our MM cohort; both the HCT-CI and CCI also include lung disease and renal impairment in their score, whereas the SI includes only lung impairment in its comorbidity assessment.
Of note, univariate analysis proved that only pulmonary, renal and KPS impairment, and age were significant for both PFS and OS (Table 2). Additional malignancies significantly impaired PFS, but did not substantially decrease OS.28 Hepatic or cardiac disease, hypertension, pain or diabetes did not substantially diminish PFS or OS (Table 2).
Table 2. Univariate analysis of prognostic factors on PFS and OS.
| Comorbidity | Definition | n | Median PFS (months) | P-valuea | Median OS (months) | P-valuea | HR (95% Confidence interval) | P-valuea |
|---|---|---|---|---|---|---|---|---|
| Lung disease | No/mild | 104 | 43 | 0.0014 | 103 | <0.0001 | 3.47 (2–6.14)c | <0.0001c |
| Moderate | 13 | 21 | 37 | |||||
| Severe | 10 | 11 | 25 | |||||
| eGFRMDRD (ml/min/1.73m2) | ⩾90 | 60 | 50 | 0.005 | 98 | 0.0008 | 3.44 (1.58–7.49)c | 0.0018c |
| 60 to < 90 | 38 | 35 | 63 | |||||
| 30 to < 60 | 17 | 22 | 30 | |||||
| < 30 | 12 | 15 | 15 | |||||
| KPS | 100% | 35 | 61 | <0.0001 | — | 0.0003 | 2.69 (1.56–4.63)c | 0.0004c |
| 80–90% | 53 | 33 | 98 | |||||
| ⩽ 70% | 39 | 27 | 41 | |||||
| Age (years) | ⩽59 | 65 | 61 | 0.0003 | 98 | 0.01 | 1.99 (1.17–3.41) | 0.0114 |
| >59 | 62 | 26 | 53 | |||||
| Additional malignancy apart from MMb | No | 119 | 36 | 0.0041 | 69 | 0.9605 | 1.04 (0.25–4.28) | 0.9599 |
| Yes | 8 | 10 | — | |||||
| Hepatic impairment | No/mild | 107 | 36 | 0.5081 | 76 | 0.6328 | 1.18 (0.6–2.35) | 0.6331 |
| Moderate/severe | 20 | 27 | 69 | |||||
| Cardiac impairment | No/mild | 102 | 36 | 0.5299 | 76 | 0.997 | 1.01 (0.52–1.95)c | 0.9831c |
| Moderate | 10 | 27 | 46 | |||||
| Severe | 15 | 35 | 62 | |||||
| Hypertension | No | 106 | 35 | 0.7161 | 76 | 0.5949 | 0.81 (0.36–1.79) | 0.5958 |
| Yes | 21 | 33 | 63 | |||||
| Pain | No | 54 | 50 | 0.5038 | 98 | 0.2105 | 1.41 (0.82–2.41) | 0.2127 |
| Yes | 73 | 32 | 60 | |||||
| Diabetes | No | 114 | 35 | 0.8145 | 69 | 0.9782 | 0.99 (0.36–2.74) | 0.9783 |
| Yes | 13 | 45 | 60 |
Abbreviations: eGFR, estimated glomerular filtration rate; HR, hazard ratio; KPS, Karnofsky Performance Status; MDRD, Modification of Diet in Renal Disease; MM, multiple myeloma; OS, overall survival; PFS, progression-free survival.
Log-rank test.
Any additional malignancy, apart from the MM, occurring prior, synchronous or after the MM diagnosis, which because of any of the 8 out of the 127 MM patients with additional malignancy, was not further subdivided within these groups.
These risk factors were summarized as two groups: lung disease, no/mild vs moderate/severe; eGFR <30 vs ⩾30 ml/min; KPS: >70 vs ⩽70% cardiac impairment, no/mild vs moderate/severe.
Multivariate analysis and risk stratification via Freiburger comorbidity index
After variable selection, the KPS ⩽70%, moderate or severe lung disease and eGFR<30 ml/min/1.73m2 were most relevant multivariate factors for OS (Table 3). On the basis of the univariate and multivariate results, a prognostic model was generated, combining the KPS, lung impairment and eGFR in a sum score (Freiburger comorbidity index; FCI). This allowed to define largely different groups: with 0, 1 and 2 or 3 risk factors, HR substantially increased from 1 to 2.5 and 6.5 and median OS was 118, 53 and 25 months, respectively, (Table 3 and Figures 1b and c).
Table 3. Multivariate analysis of prognostic factors and risk stratification by combination of KPS⩽70%, moderate or severe lung disease and eGFR<30.
| Comorbidity factors | Definition | n | HR (95% Confidence interval) | P-valuea |
|---|---|---|---|---|
| KPS | >70% | 88 | 2.17 (1.23–3.82) | 0.0077 |
| ⩽70% | 39 | |||
| Lung disease | No/mild | 104 | 2.78 (1.53–5.04) | 0.0008 |
| Moderate/severe | 23 | |||
| eGFRMDRD (ml/min/1.73m2) | ⩾30 | 115 | 2.93 (1.33–6.46) | 0.0075 |
| <30 | 12 | |||
|
FCI |
Median OS (months) |
n |
HR (95% Confidence interval) |
P-valuea |
| 0 | 118 | 74 | 1.0 (reference) | — |
| 1 | 53 | 36 | 2.5 (1.4–4.5) | 0.0033 |
| 2 or 3 | 25 | 17 | 6.5 (3.2–13.2) | < 0.0001 |
Abbreviations: eGFRMDRD, estimated glomerular filtration rate by MDRD (Modification of Diet in Renal Disease); FCI, Freiburger comorbidity index; HR, hazard ratio; KPS, Karnofsky Performance Status; OS, overall survival.
χ2-test.
Systematic comparison of various CIs and PFS/OS in ‘low-risk' vs ‘high-risk' groups
Of our 10 risk factors, as assessed via univariate and multivariate analyses, (Table 2), 8 out of 12 comorbidities are also scored within the KF, 10 out of 17 in the HCT-CI, 10 out of 20 in the CCI and all seven in the SI.
Median scores in our cohort were from 0 to 1 for the FCI, HCT-CI and SI, 2 for the KF and 5 for the CCI (Table 4), the latter because of the assignment of two points for the presence of a concomitant hematologic malignancy and inclusion of age.
Table 4. PFS and OS of various analyzed comorbidity indices (HCT-CI, KF, SI, CCI and FCI) in ‘low-risk' vs ‘high-risk' scoring patients.
| Score | Maximum score | Median score (range) | n Low-risk vs n high-riska | Median PFS (months) | P-valueb | Median OS (months) | P-valueb |
|---|---|---|---|---|---|---|---|
| HCT-CI | 26 | 1 (0–10) | Low=78 | 46 | 0.001 | 98 | 0.002 |
| High=49 | 24 | 44 | |||||
| KF | 3 | 2 (0–3) | Low=88 | 45 | 0.0016 | 81 | 0.007 |
| High=39 | 24 | 41 | |||||
| SI | 7 | 1 (0–4) | Low=94 | 39 | 0.0838 | 77 | 0.0876 |
| High=33 | 22 | 60 | |||||
| CCI | 33 + age | 5 (2–12) | Low=89 | 50 | 0.003 | 76 | 0.4159 |
| High=38 | 29 | 60 | |||||
| FCI | 3 | 0 (0–3) | Low=74 | 51 | 0.0003 | 117 | <0.0001 |
| High=53 | 25 | 41 |
Abbreviations: CCI, Charlson comorbidity index; FCI, Freiburger comorbidity index; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; KF, Kaplan Feinstein; OS, overall survival; PFS, progression-free survival; SI, Satariano index.
Low score: ⩽median, high score: >median.
Log-rank test.
A P-value <0.05 was considered as statistically significant.
To facilitate comparisons, all CIs were also collapsed into two groups (‘low-risk' vs ‘high-risk' Table 4 and Figures 1b–g): ‘low-risk' patients revealed substantially longer PFS and higher OS rates than ‘high-risk' patients. Survival differences reached significance via HCT-CI, KF, CCI and FCI for PFS, and via HCT-CI, KF and FCI also for OS. The SI proved least valuable (Figures 1b–g).
Figure 1h depicts the FCI as compared with the other established CIs, illustrating why the FCI, KF, HCT-CI and CCI seemed more valuable in MM: the KF also scores the KPS, lung and renal impairment, risks that were especially valuable in this analysis. Both HCT-CI and CCI include lung and renal impairment, whereas the SI includes only lung impairment in its risk assessment. Thus, the cautious comparison of the FCI with the four well-known CIs suggested that the FCI allows risk prediction for PFS and OS equally well as the HCT-CI and KF, with the advantage of the former to be effortlessly assessable.
Patient characteristics in different age categories
Patients were grouped into three age categories of <60, 60–69 and ⩾70 years (Supplementary Table 2). Patients with higher age showed stage B disease more often (in line with increasing patients with eGFR<30) and rising beta-2 microglobulin levels. Moreover, cardiac impairment (16%, 20%, 29%), hypertension (11%, 18%, 29%), diabetes (7%, 11%, 19%) and pain (41%, 53%, 83%, respectively) increased. Of note, moderate or severe lung disease and hepatic impairment did not substantially enlarge within higher ages.
Of note, ‘high-risk' patients as scored with the FCI, HCT-CI and KF decreased in the age category of ⩾70 years. In contrast, ‘high-risk' patients scored via SI and CCI increased with age, the latter more substantial due to the inclusion of age as an additional weighted condition with extra points for every age decade starting at 50 years. The increase of ‘high-risk' patients with use of the SI could be related to the fact that this CI covers especially age-dependent comorbidities.
Organ function and comorbidity according to treatment
Although peripheral blood SCT and standard therapy were not stratified to be compared in this analysis, both therapeutic options are depicted in Supplementary Table 3. Patients receiving standard therapy were older and showed a decreased KPS. The median eGFR was 76 vs 107 ml/min/1.73m2, respectively. Cardiac impairment, hypertension, diabetes mellitus, hepatic impairment and pain were similar in both groups.
‘High-risk' CI patients were increased in patients receiving standard therapy, although only via FCI, HCT-CI, CCI and KF, these differences were most substantial, but not via SI.
Patient characteristics within CKD stages
Comparison of CKD stages 1–2, 3 and 4–5 revealed that age, impaired KPS, beta-2 microglobulin and some other comorbidities (lung and cardiac impairment, hypertension) increased with renal deterioration, whereas this was less prominent for diabetes or hepatic impairment.
Patients defined as ‘high-risk' because of ⩾ median CI scores assessed via FCI, HCT-CI, CCI, KF and SI increased with higher CKD stages, although the SI showed the less noticeable effect, due to the fact that renal function is not included. These observations highlight that with increasing renal impairment in MM additional underlying comorbidities were also evident (Supplementary Table 4).
Discussion
Numerous risk features have been evaluated in MM to improve its prognostic appraisal, and predictive markers are eagerly tested worldwide.29, 30, 31, 32 Apart from organ function,14, 18 also comorbidity assessment in other diseases,7, 10, 33, 34, 35 but not in MM, has been acknowledged as important. This has recently been stressed,23 as there is a vastly enlarged arsenal of treatment options for MM patients today, so that comorbidity assessments—beside disease-related risk factors—may immensely assist in the allocation of available therapies. Especially in case of stratification between standard, intensive or clinical trial options, clinical judgment by the physician and patient preference require standardized decision tools to balance the treatment profits and risks of toxicity.
Traditionally, risk classifications in MM are based on disease-related factors, although patient-related factors, such as impaired performance status or organ function, may also influence outcome,31, 36 this being highly relevant as MM develops primarily in elderly patients. Our observations demonstrated the high impact of patient-related conditions as additional risks in MM: in line with our and prior data,37, 38 we could identify renal impairment as most influential for survival, followed by lung and KPS impairment. Lung impairment has previously been described to affect survival39 and to be associated with SCT toxicity;40 KPS has been identified as crucial for patient outcome in various diseases,41 underlining its value to be accurately recorded.18, 41
Previous trials have also assessed the impact of advanced age on survival,20, 21 this being linked to higher age-related comorbidities and diminished functional status.21 This is relevant, as the impact of age becomes increasingly important with age escalation.21, 42, 43 Of interest in our multivariate analysis was that age proved less significant as compared with renal, KPS or lung impairment, and that the comparison of different age groups revealed that specific risks can be easily over- or underestimated and that age alone may be an insufficient decision tool for anti-MM treatment. Our data illustrated well that biological age can substantially differ from the chronological patient age and why age was a univariate, but not multivariate risk in our analysis. One may argue that age was found less relevant, because our median patient age was 60 years, which relates typically to large university and referral centers. Although we cannot exclude diminished statistical power to detect a more substantial age impact because of limited patient numbers in much older cohorts, more than half of our patients were older than 60 years and approximately 20% even ⩾70 years.
Besides our assessment of prognostic conditions, different comorbidity scores were also thoroughly evaluated. Among these, the CCI and HCT-CI are widely used to predict outcome in hematological malignancies,7, 41 in line with our results that the CCI proved significant for PFS, and the HCT-CI for both PFS and OS. Farina et al.41 confirmed that the HCT-CI predicts PFS and OS in lymphoma patients after reduced intensity conditioning allogeneic SCT. Another study has demonstrated the utility of the CCI and HCT-CI for predicting transplantation-related toxicity and prolonged hospital stay.40 In addition, renal impairment is assessed in the HCT-CI, which was of importance in this and our previous analyses.14, 18 Interestingly, the CCI revealed a lesser predictive power than the HCT-CI. Explanations for their different OS impact are that the HCT-CI has been developed from the CCI and established in hematologic malignancies, whereas the CCI has been used in various, rather than specific diseases.10 Another reason for the increased sensitivity and specificity of the HCT-CI to predict patient outcome, including in transplant candidates, is the enhancement of comorbidity definition, particular in adding pulmonary and liver function test with higher weights compared with the CCI.10 As pulmonary disorders are profoundly weighted in the HCT-CI, this explains its predictive value in our MM cohort also. Besides the HCT-CI, the KF was valuable for survival in our cohort, this most likely being related to the inclusion of patients' performance status, lung and renal impairment, as well as grading the derangement.44, 45 The use of our FCI allowed to define distinct risk groups: with 0, 1 and 2 or 3 risk factors, OS was substantially different with 118, 53 and 25 months, respectively. In terms of risk allocation in ‘low-risk' vs ‘high-risk' patients, the cautious comparison of the FCI with the four other CIs revealed most striking group differences for the FCI, HCT-CI and KF and least valuable group distinction for the CCI and SI. We could thereby highlight that specific CIs—namely the FCI, HCT-CI and KF—best reflect MM patients' performance status and organ function, and that the chronological age alone may fail to predict the clinical outcome.23
Our analysis represents the first systematically performed organ and functional assessment in myeloma patients, and includes the first comparative evaluation of four previously established CIs in MM. We created a new risk assessment tool in MM, as previously established CIs were developed for entirely different diseases. Translating the organ and functional status into a novel, simply assessable FCI, which we developed independently of the performed myeloma treatment, allowed to define three distinct risk groups with largely different OS. However, the validation of this approach and utility in routine use has to be further investigated in prospective and randomized studies in terms of therapy-related toxicity, lengths of hospital stay and survival. Nevertheless, the primary purpose of this analysis was to introduce a new sum score of risk factors to predict outcome in MM, which was successfully accomplished. The direct comparison of the FCI with the four established CIs may be criticized, as the assessed comorbidities do not cover all derangements as included in the other scores, and the FCI needs to be reassessed in an independent training and test sets, which is underway. Another criticism may cover the use of different therapies that can interact with specific risks, although we intentionally aimed to determine a treatment-independent risk score that can be utilized in various treatment groups. Finally, evaluation of various cytogenetic abnormalities as important molecular risks needs to be included in subsequent analyses.
We conclude that the present study provides an initial important step for the utilization of comorbidity assessment in MM and should facilitate treatment decision-making in the near future. We suggest that assessing the comorbidity status in MM, rather than considering specific age cutoffs alone, may allow to better define patients' status, tolerability of treatment and to learn about best treatment allocations in upcoming patient cohorts.
Acknowledgments
We thank Professor Karl Blume, Stanford, and Dr Josefina Udi and Barbara Metzke, University of Freiburg, for critical reading of the manuscript and valuable suggestions, Hans Schall for diligent data management with use of our eTBD and Volker Schmidt for IT support. We are indebt to the anonymous internal reviewer of our department and external reviewer support for important recommendations. This article is dedicated to Professor Dr Karlheinz Engelhardt with sincere gratitude for his inspiring ideas, for his thriving support and for his stimulating and never-ending interest in medicine, science, ethics, artful reading and writing and music. He is an ever-admired example of a highly dedicated and talented physician. This work is supported by the Deutsche Krebshilfe grant: 1095969 (to ME and MK).
Author contributions
MK and ME designed research, analyzed data and wrote the manuscript; MT analyzed data; GI performed statistical analyses and wrote the manuscript; BK provided laboratory data; BD and RW critically read, discussed, wrote and corrected the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Denz U, Haas PS, Wasch R, Einsele H, Engelhardt M. State of the art therapy in multiple myeloma and future perspectives. Eur J Cancer. 2006;42:1591–1600. doi: 10.1016/j.ejca.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Mertelsmann R. 160 Years of multiple myeloma: progress and challenges. Eur J Cancer. 2006;42:1507–1509. doi: 10.1016/j.ejca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Rajkumar SV, Gertz MA, Fonseca R, Lacy MQ, Bergsagel PL, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- Deschler B, Binek K, Ihorst G, Marks R, Wasch R, Bertz H, et al. Prognostic factor and quality of life analysis in 160 patients aged > or=60 years with hematologic neoplasias treated with allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:967–975. doi: 10.1016/j.bbmt.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- Sperr WR, Wimazal F, Kundi M, Baumgartner C, Nosslinger T, Makrai A, et al. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann Oncol. 2009;21:114–119. doi: 10.1093/annonc/mdp258. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- Etienne A, Esterni B, Charbonnier A, Mozziconacci MJ, Arnoulet C, Coso D, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485–1493. doi: 10.1038/leu.2008.131. [DOI] [PubMed] [Google Scholar]
- Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48:337–341. doi: 10.1080/10428190601126602. [DOI] [PubMed] [Google Scholar]
- Kleber M, Cybulla M, Bauchmuller K, Ihorst G, Koch B, Engelhardt M. Monitoring of renal function in cancer patients: an ongoing challenge for clinical practice. Ann Oncol. 2007;18:950–958. doi: 10.1093/annonc/mdm055. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- Kleber M, Ihorst G, Deschler B, Jakob C, Liebisch P, Koch B, et al. Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. Eur J Haematol. 2009;83:519–527. doi: 10.1111/j.1600-0609.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002-Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Bolejack V, Crowley J, Blade J, Miguel JS, Kyle RA, et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol. 2010;28:1599–1605. doi: 10.1200/JCO.2009.25.2114. [DOI] [PubMed] [Google Scholar]
- Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2010;2010:237–247. doi: 10.1182/asheducation-2010.1.237. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- Markert A, Thierry V, Kleber M, Behrens M, Engelhardt M. Chemotherapy safety and severe adverse events in cancer patients: strategies to efficiently avoid chemotherapy errors in in- and outpatient treatment. Int J Cancer. 2009;124:722–728. doi: 10.1002/ijc.23991. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J Chronic Dis. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- Hasskarl J, Ihorst G, De Pasquale D, Schrottner P, Zerweck A, Wasch R, et al. Association of multiple myeloma with different neoplasms: systematic analysis in consecutive patients with myeloma. Leuk Lymphoma. 2010;52:247–259. doi: 10.3109/10428194.2010.529207. [DOI] [PubMed] [Google Scholar]
- Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C-reactive protein and beta-2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992;80:733–737. [PubMed] [Google Scholar]
- Gassmann W, Pralle H, Haferlach T, Pandurevic S, Graubner M, Schmitz N, et al. Staging systems for multiple myeloma: a comparison. Br J Haematol. 1985;59:703–711. doi: 10.1111/j.1365-2141.1985.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Tricot G, Barlogie B, Jagannath S, Bracy D, Mattox S, Vesole DH, et al. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood. 1995;86:4250–4256. [PubMed] [Google Scholar]
- Moro-Sibilot D, Aubert A, Diab S, Lantuejoul S, Fourneret P, Brambilla E, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005;26:480–486. doi: 10.1183/09031936.05.00146004. [DOI] [PubMed] [Google Scholar]
- Artz AS, Pollyea DA, Kocherginsky M, Stock W, Rich E, Odenike O, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Nosslinger T, Tuchler H, Germing U, Sperr WR, Krieger O, Haase D, et al. Prognostic impact of age and gender in 897 untreated patients with primary myelodysplastic syndromes. Ann Oncol. 2009;21:120–125. doi: 10.1093/annonc/mdp264. [DOI] [PubMed] [Google Scholar]
- Fonseca R. Strategies for risk-adapted therapy in myeloma. Hematology Am Soc Hematol Educ Program. 2007. pp. 304–310. [DOI] [PubMed]
- Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65:175–181. doi: 10.1034/j.1600-0609.2000.90221.x. [DOI] [PubMed] [Google Scholar]
- Knudsen LM, Nielsen B, Gimsing P, Geisler C. Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. Eur J Haematol. 2005;75:27–33. doi: 10.1111/j.1600-0609.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility. Biol Blood Marrow Transplant. 2009;15:447–453. doi: 10.1016/j.bbmt.2008.12.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte L, Iqbal T, Zaidi MA, McDiarmid SA, Huebsch LB, Tay J, et al. Utility of comorbidity assessment in predicting transplantation-related toxicity following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14:1039–1044. doi: 10.1016/j.bbmt.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Farina L, Bruno B, Patriarca F, Spina F, Sorasio R, Morelli M, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23:1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67:124–132. doi: 10.1016/j.critrevonc.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:1047–1057. doi: 10.1016/s0360-3016(01)02741-9. [DOI] [PubMed] [Google Scholar]
- Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.